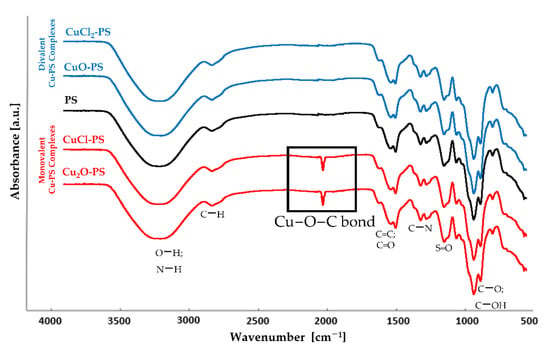
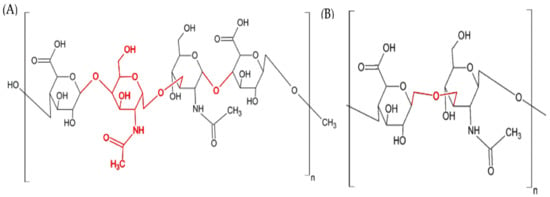
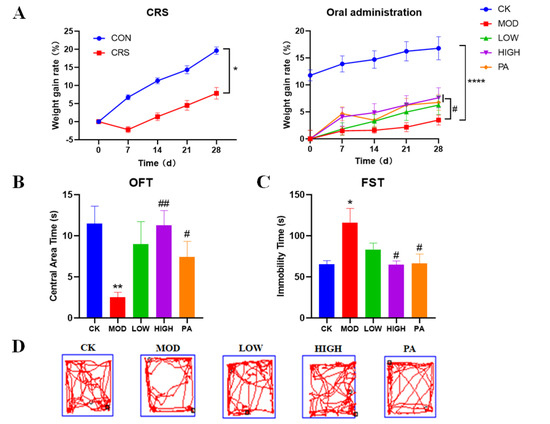
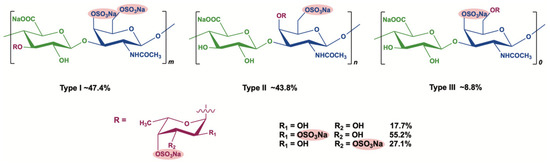
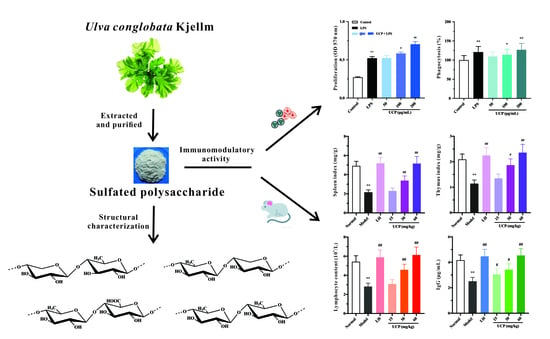
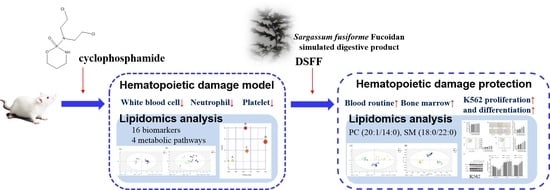
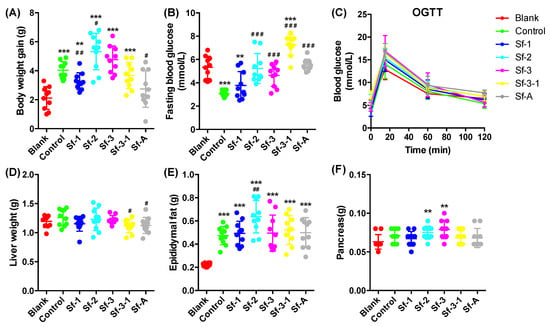
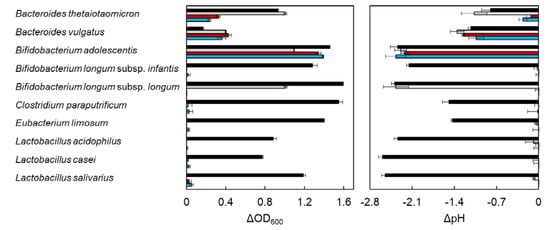
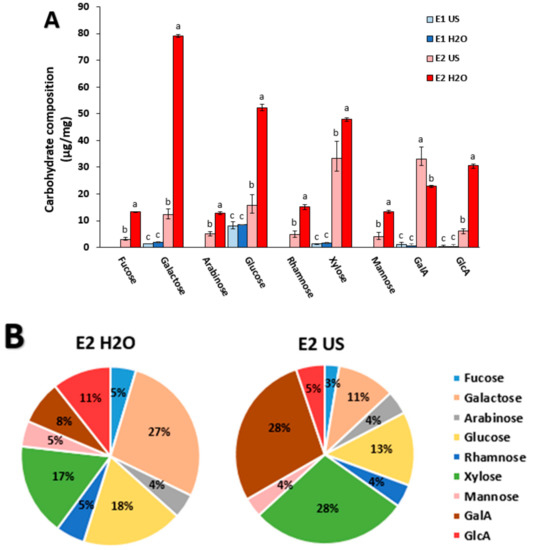
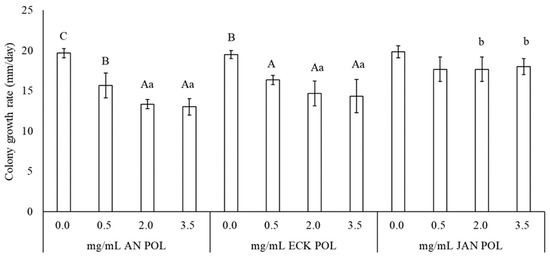
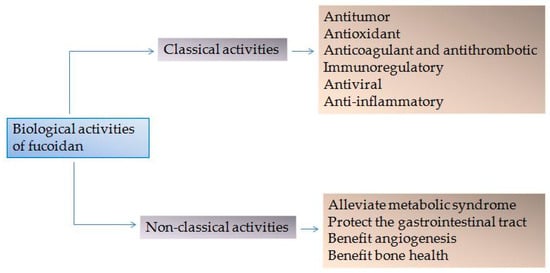
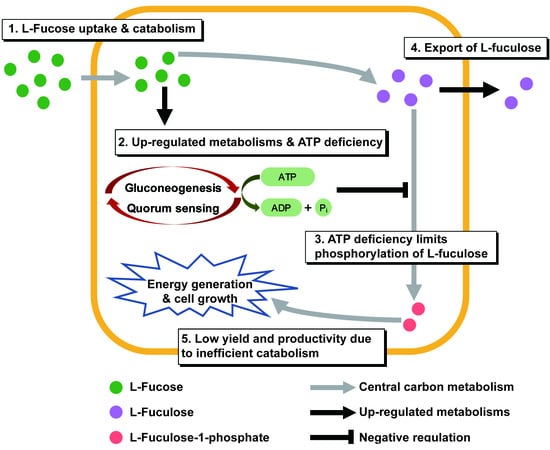
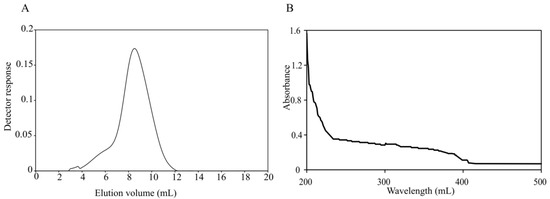
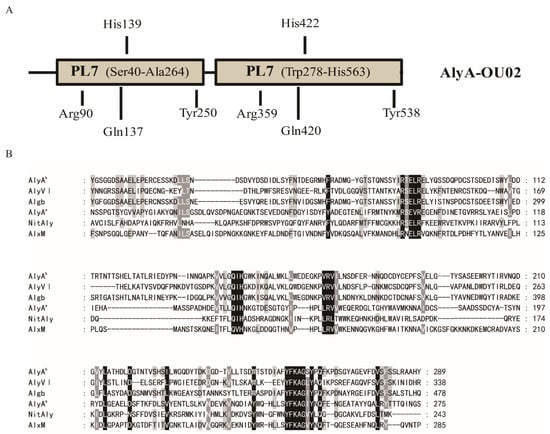
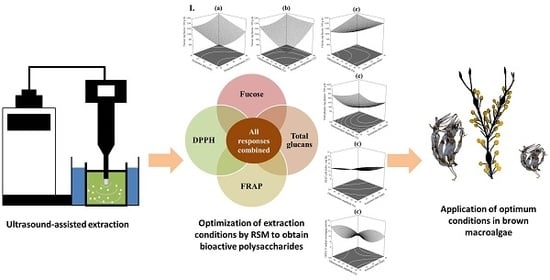
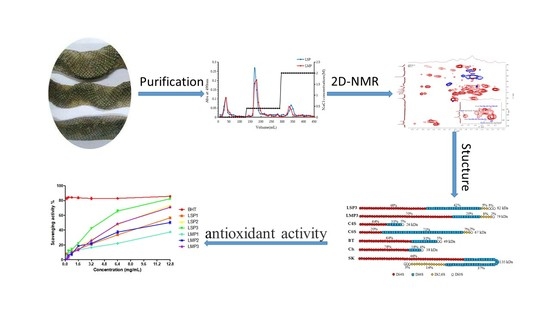
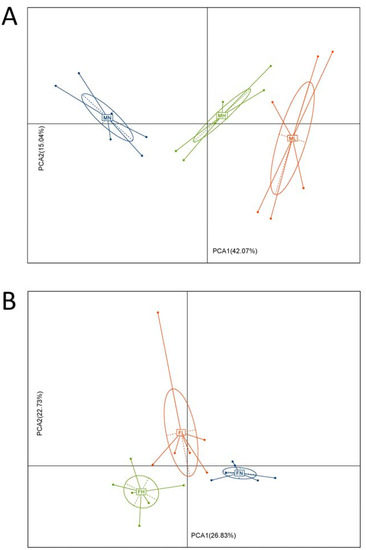
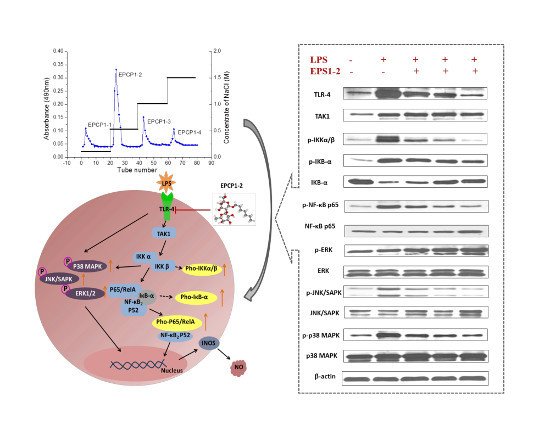
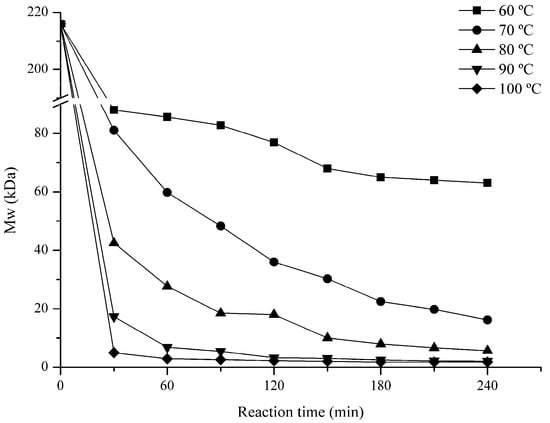
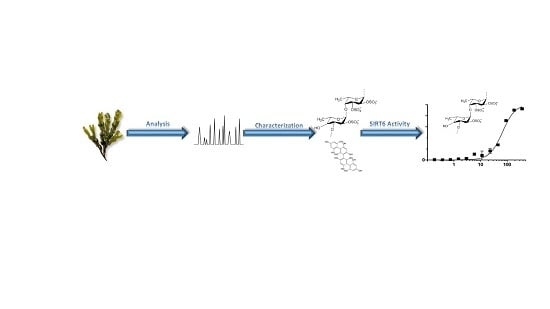
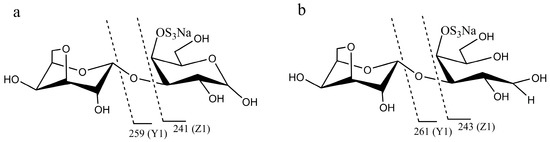
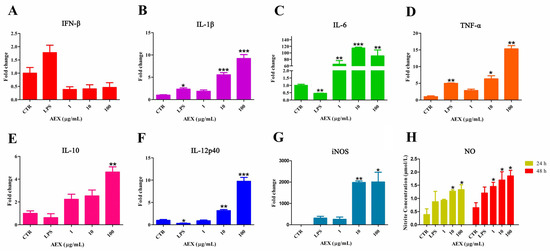
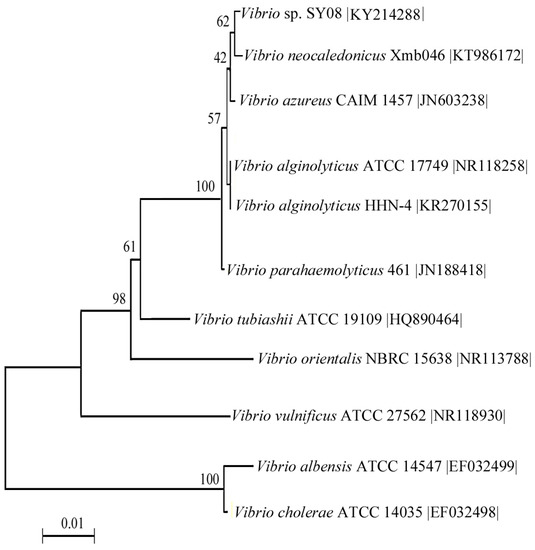
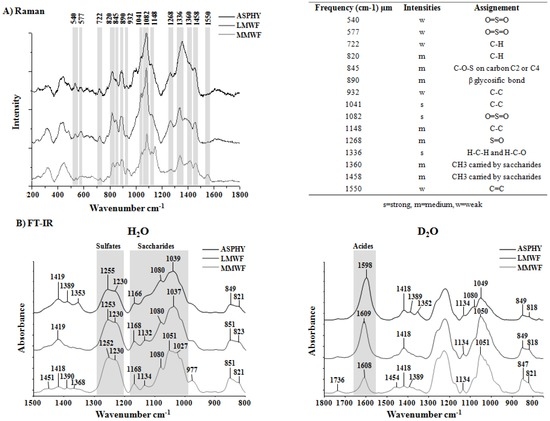
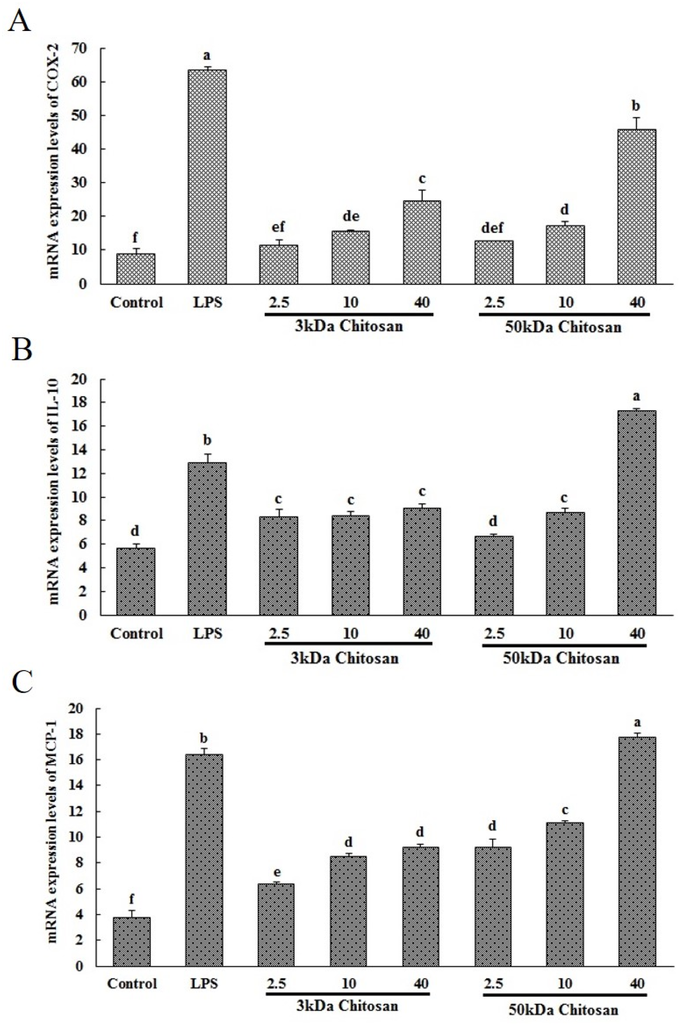
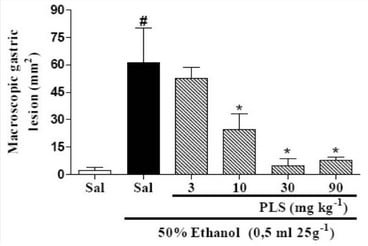
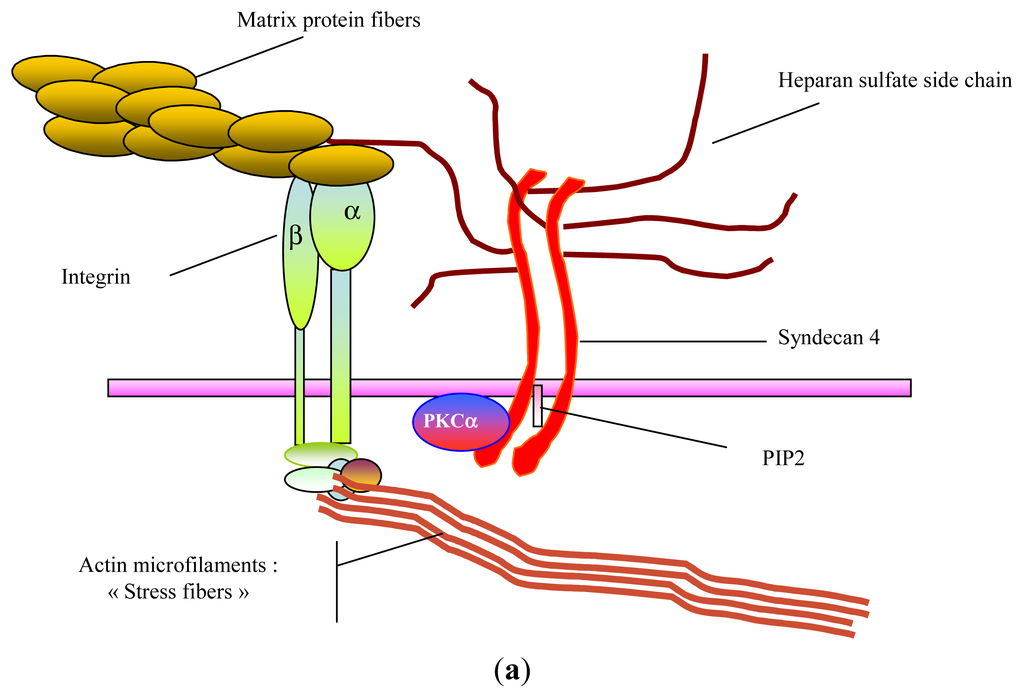
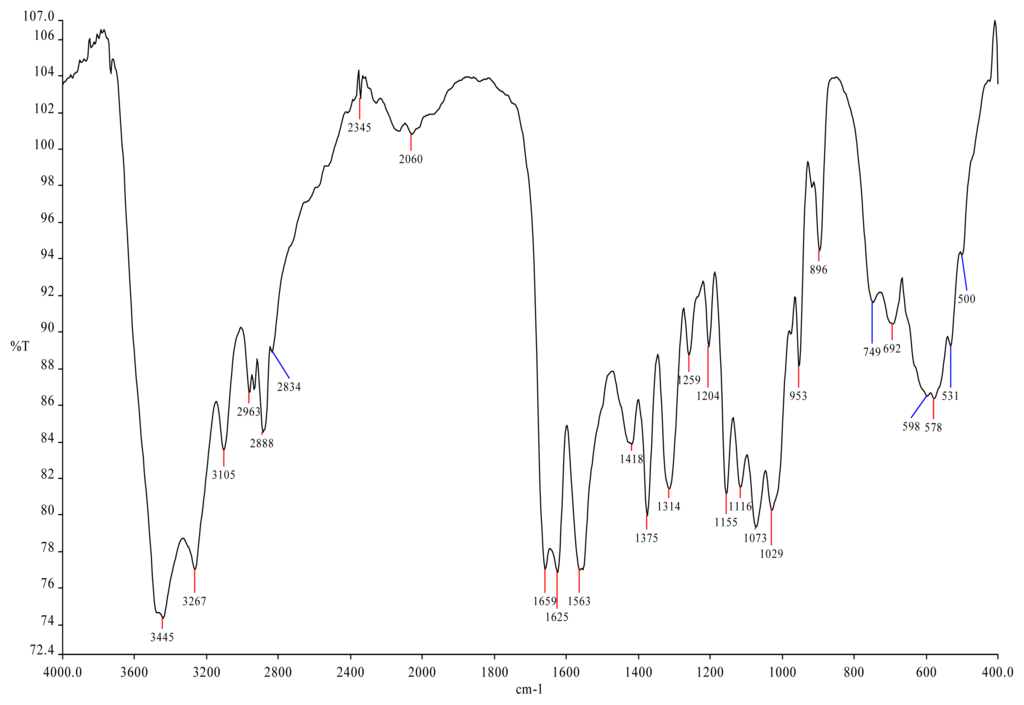
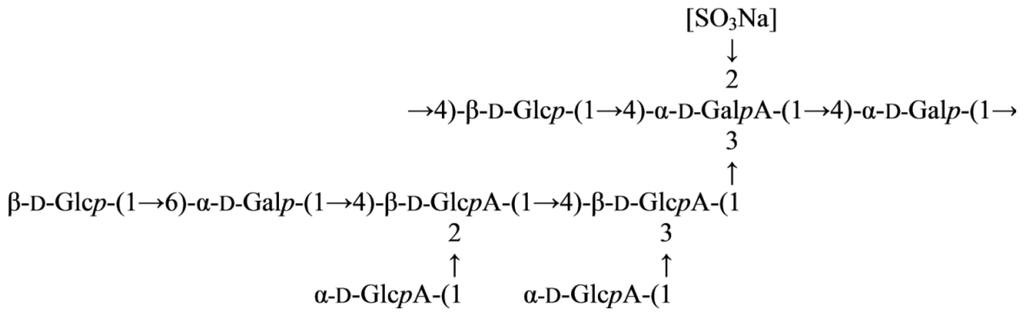Marine Polysaccharides
A topical collection in Marine Drugs (ISSN 1660-3397).
Viewed by 985496Editor
Interests: macromolecular chemistry; natural polysaccharides; drug delivery; nanoparticles surface modification; active targeting
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
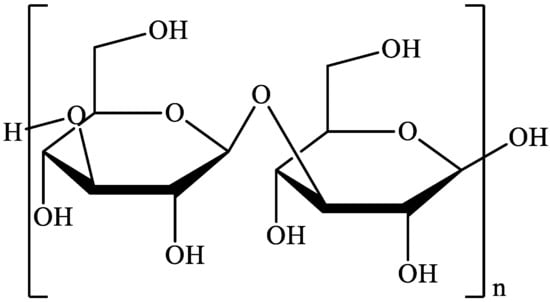
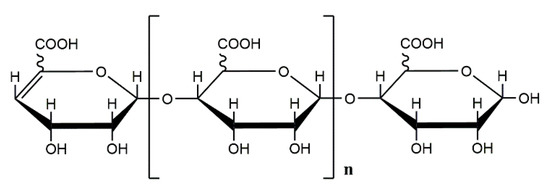
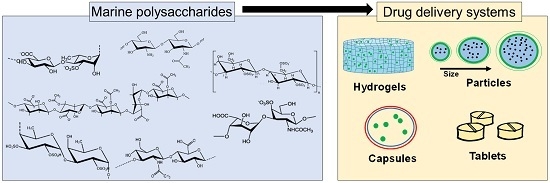
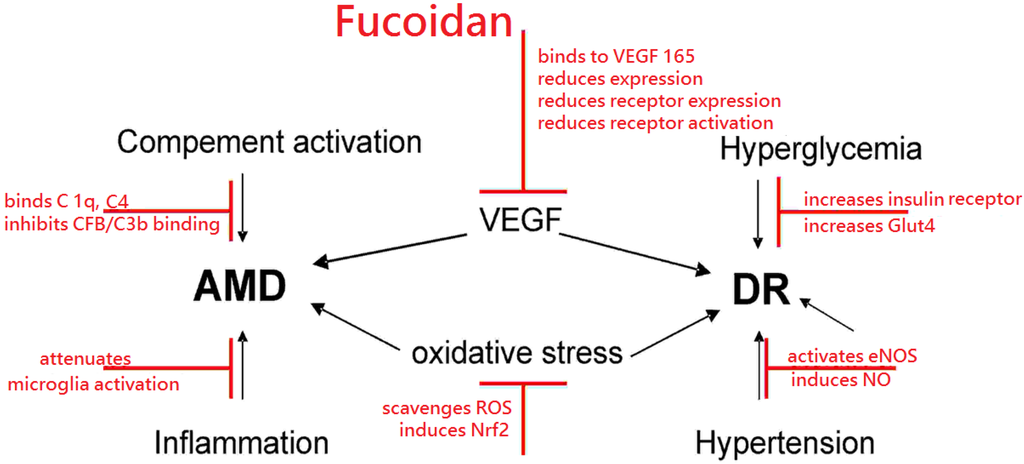
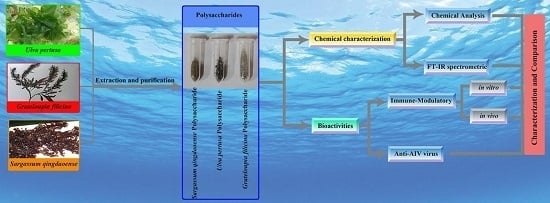
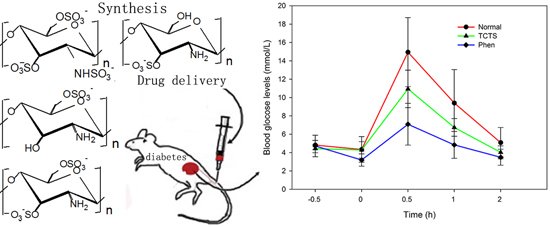
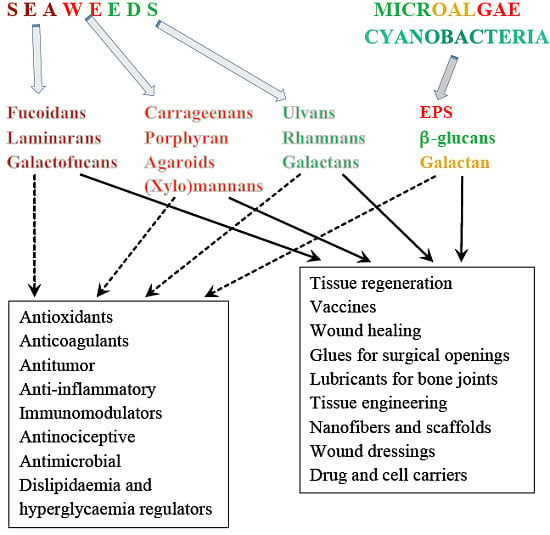
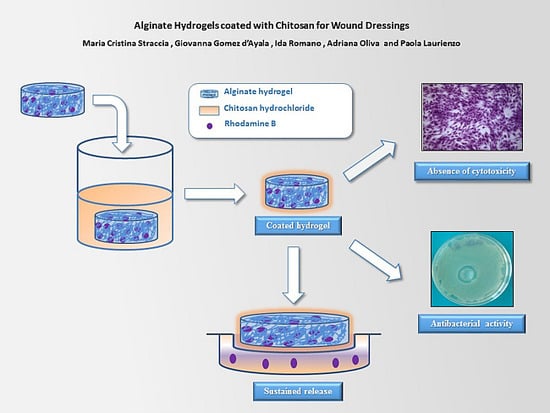
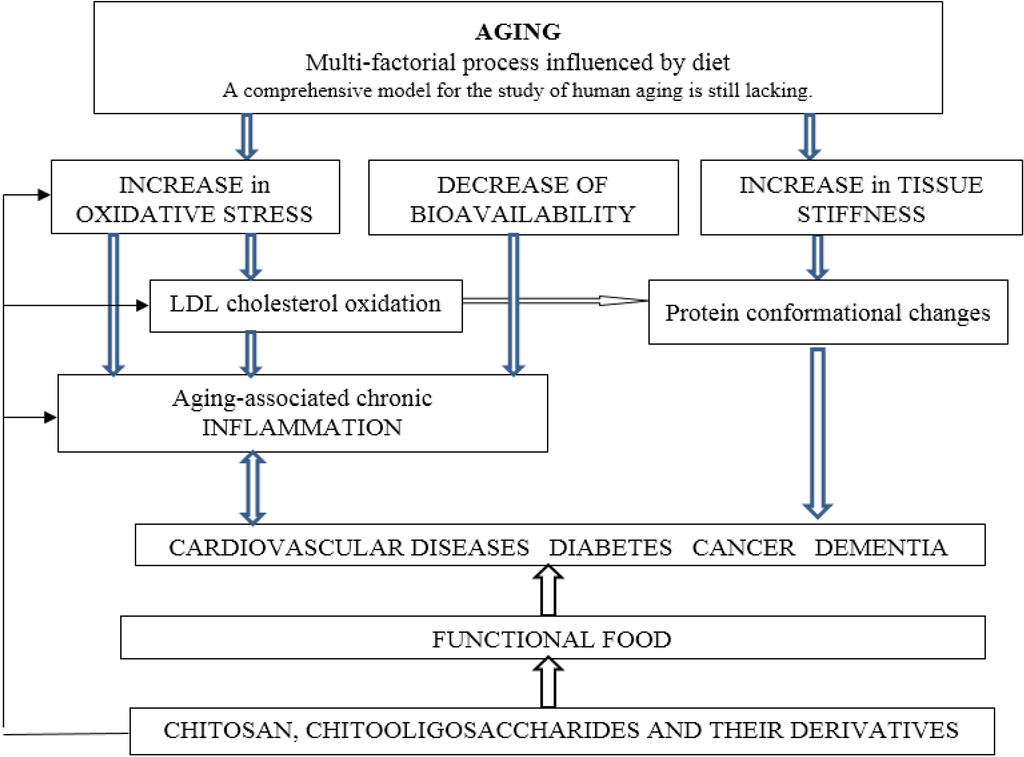
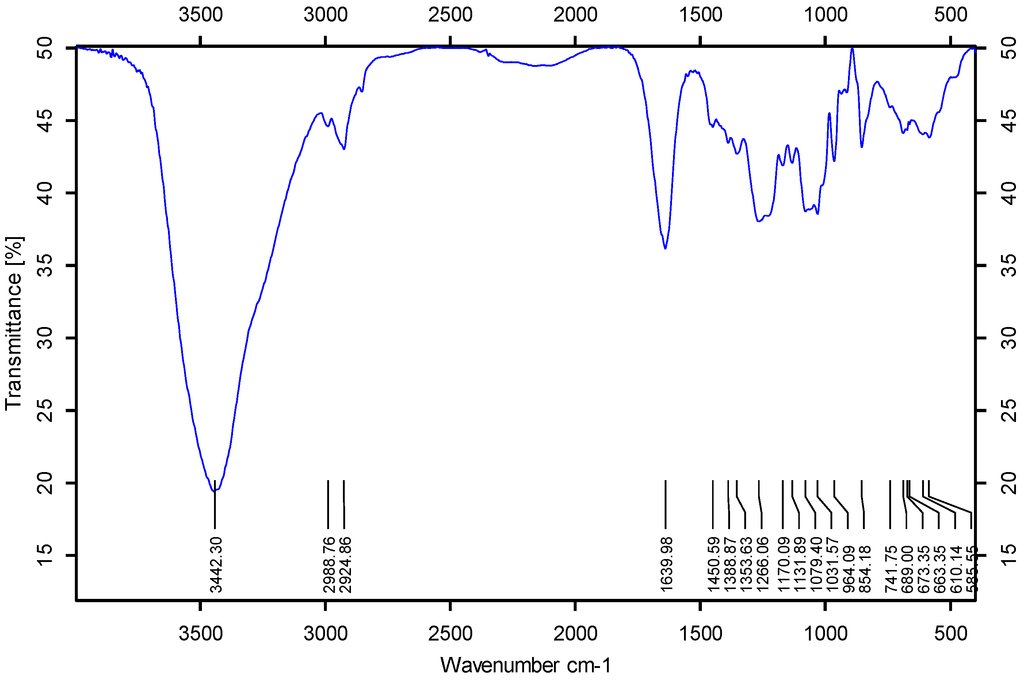
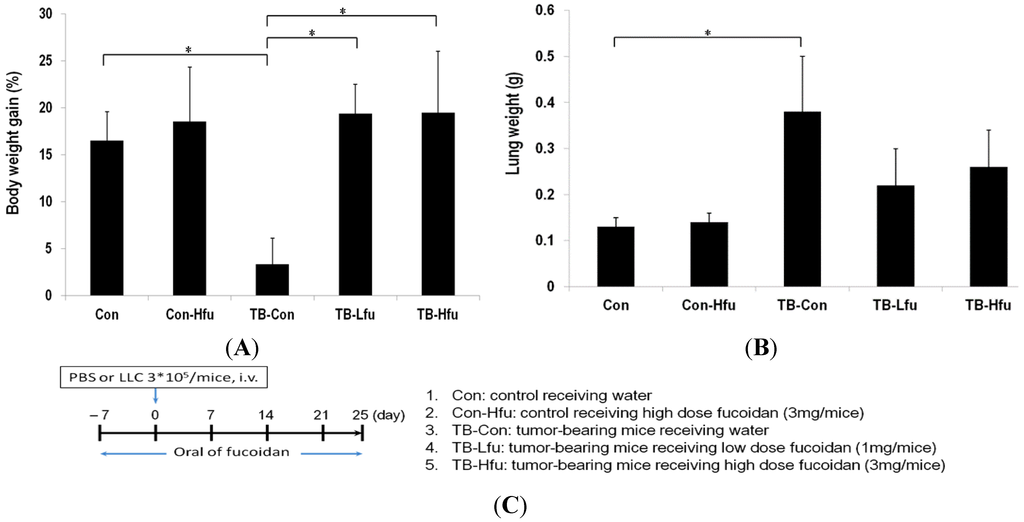
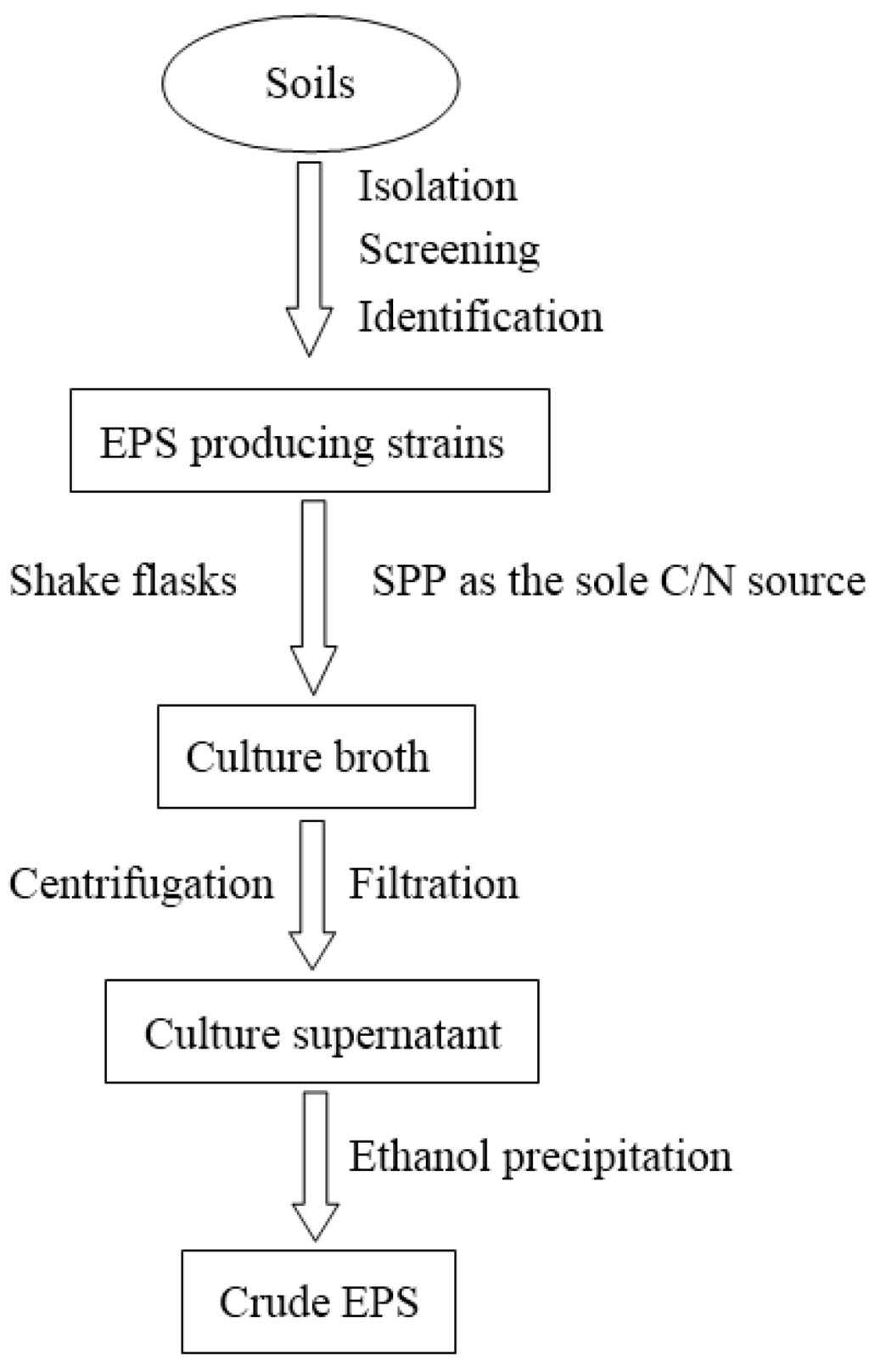
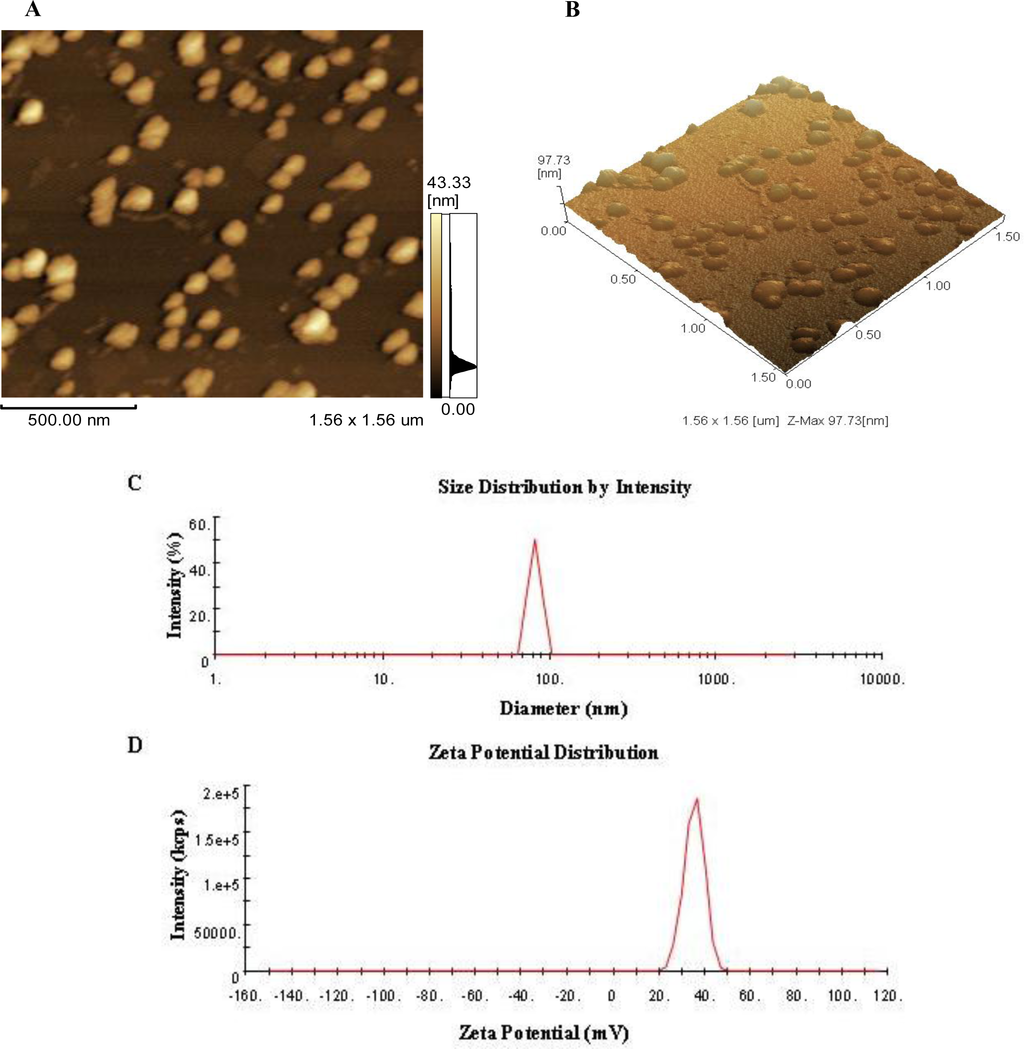
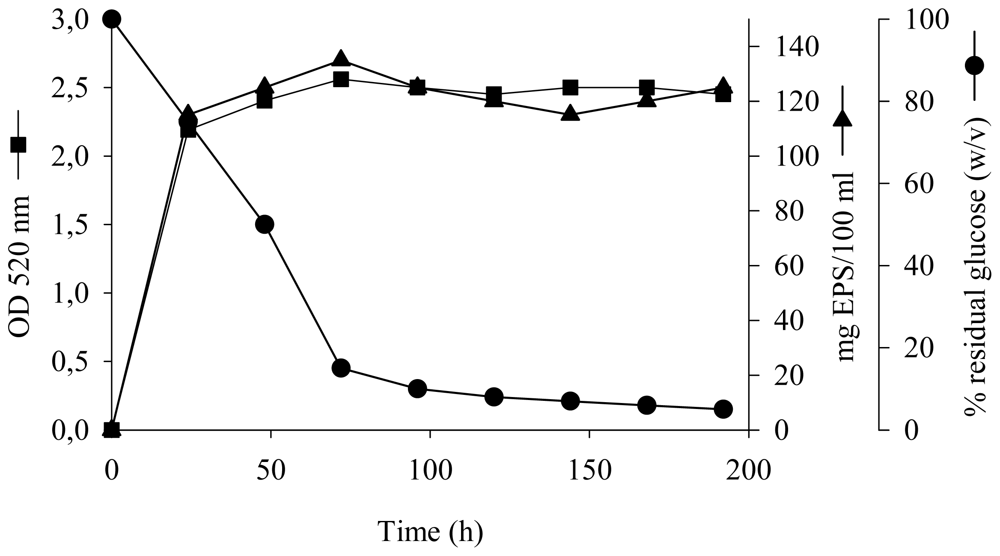
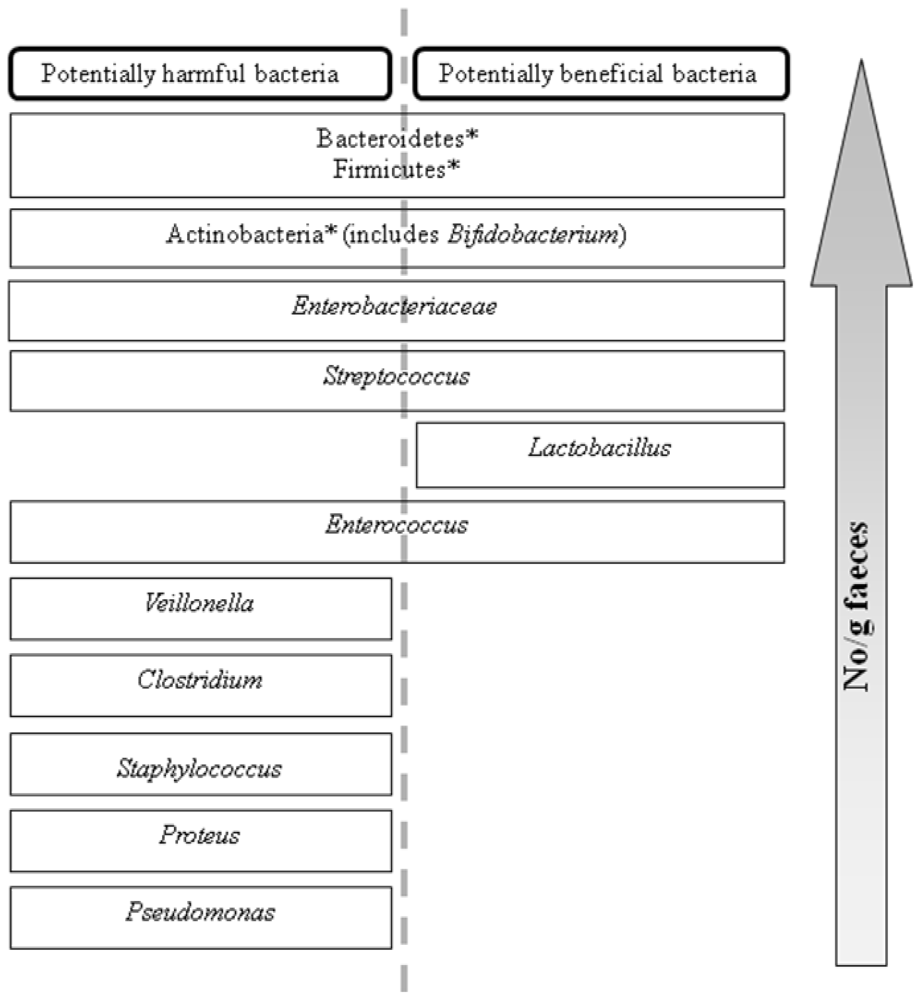
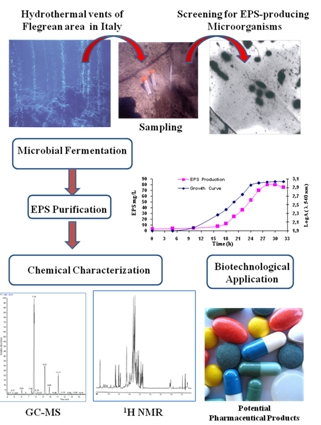
Biopolymers, as natural polysaccharides, are considered benign polymers for what concerns the environment. This is not a new invention, but at best a renaissance: the first type of polymers used by human kind were animal hides, cellulose, silk, wool. Among benefits of natural occurring biopolymers there are potential biocompatibility, renewable resources, low processing costs, tailoring of structure by genetic manipulation, and, as said, environmentally compatibility. Limits are, sometimes, premature degradation and high production costs due to the very high purity required for medical uses. Polysaccharides are not drugs by themselves, but their use in pharmaceutical field, for example as drug carriers or antimicrobial, anti-inflammatory or anticoagulant agents, is increasingly promising. Marine polysaccharides include chitin, chitosan, alginate, agar and carrageenans. Chitosan is a cationic carbohydrate biopolymer derived from chitin, the second most abundant polysaccharides present in nature after cellulose. The main sources of chitin are the shell wastes of shrimps, lobsters and crabs. For its characteristics, chitosan founds particular application as non viral vector in gene delivery. Films from chitosan are very tough and long lasting. Alginates derive from seaweed extraction (pheophyceae), and are mainly used in drug delivery and as hydrogels for immobilizing cells and enzymes, due to the mild conditions of cross-linking through bivalent cations (Ca2+). Agar (or agar-agar) and carrageenans are linear polysaccharides from red seaweeds. They are highly reactive chemically and are peculiar for thermoreversible gel formation. Exopolysaccharides (EPS), substantial components of the extracellular matrix of many cells of marine origin, also have to be mentioned for their potential interest in pharmaceuticals, and new EPS producing bacteria, particularly from extreme marine environments, are being isolated.
The possibility of chemical modification, blending and addition of biodegradable additives allows to tailor the final properties of polysaccharides and opens the doors to wider applications, particularly in pharmaceutical area. This collection is intended to explore any new potentiality of marine polysaccharides, as those above mentioned, deriving from chemical or chemical-physical modifications, and the scaling-up of their pharmaceutical applications.
Dr. Paola Laurienzo
Collection Editor
Manuscript Submission Information
Manuscripts for the topical collection can be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. All papers will be peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on this website. The topical collection considers regular research articles, short communications and review articles. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page.
Please visit the Instructions for Authors page before submitting a manuscript. The article processing charge (APC) for publication in this open access journal is 2900 CHF (Swiss francs).
Keywords
- chitosan
- alginate
- agar
- carrageenans
- exopolysaccharides
- chemical modification
- drug delivery
- gene delivery