Isolation and Structural Characterization of a Novel Antioxidant Mannoglucan from a Marine Bubble Snail, Bullacta exarata (Philippi)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Purification of Polysaccharides
| Composition | Samples | |||
|---|---|---|---|---|
| a CBEPS | BEPS-I | BEPS-IA | BEPS-IB | |
| Yield (%) | 7.3 | 3.6 | 1.03 | 2.2 |
| Neutral sugar (%) | 80.12 | 89.31 | 76.29 | 98.76 |
| Protein (%) | 10.7 | 2.4 | 2.7 | 2.1 |
| Sulfate (%) | 1.2 | 1.5 | 0.47 | 1.23 |
| Mw (kDa) | -- b | -- b | 127 | 94 |
| Molar ratio of monosaccharides | ||||
| Mannose | 0.74 | 1.69 | 0.32 | 0.52 |
| Glucose | 0.43 | 1.26 | 0.57 | 1 |
| Galactose | 0.32 | 0.03 | 0.04 | 0.03 |
| Fucose | 0.18 | 0.12 | ND | 0.01 |
| Rhamnose | 0.56 | 0.22 | 0.31 | ND |
| Arabinose | 0.17 | 0.07 | 0.03 | ND |

2.1.1. IR Spectrum and Elucidation of BEPS-IB
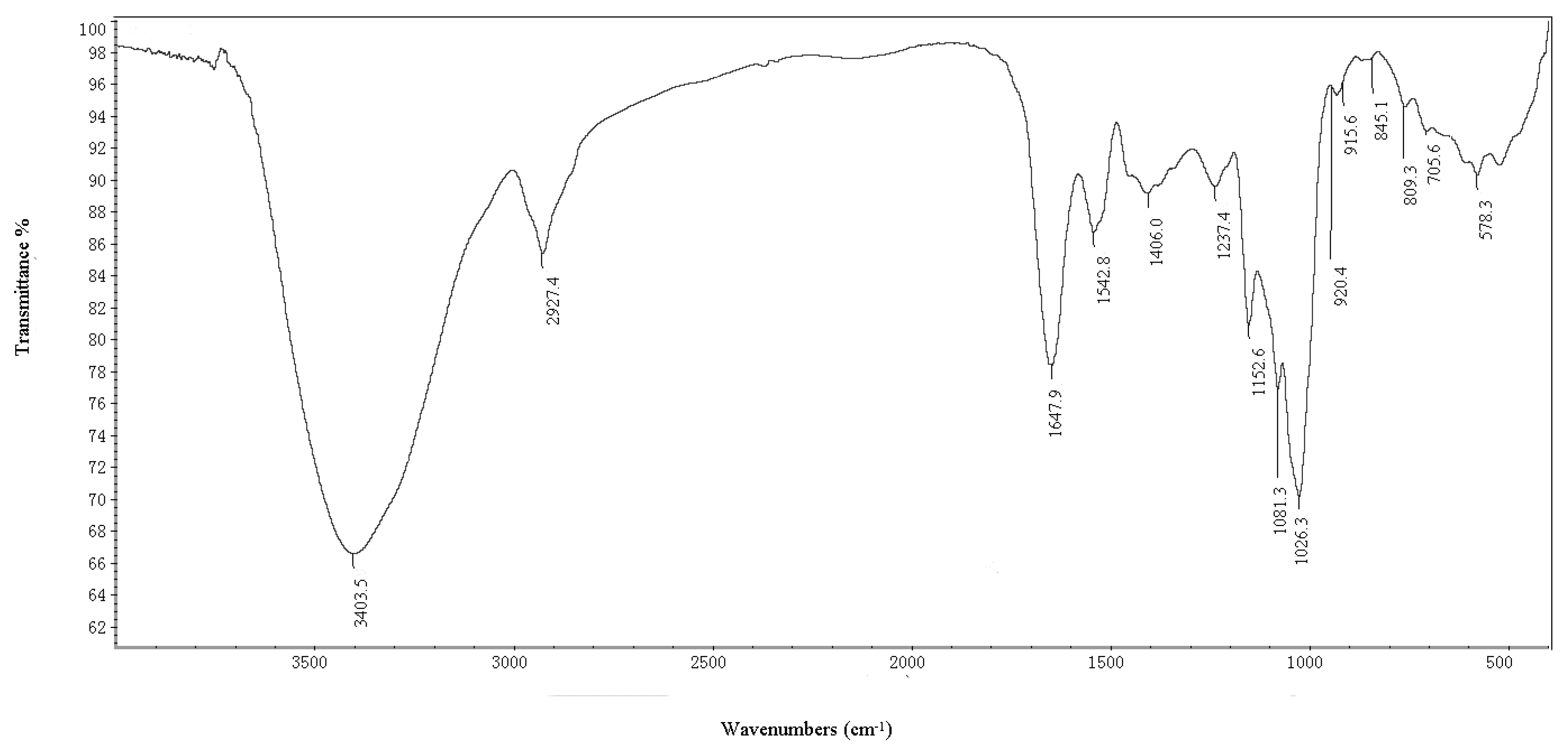
2.1.2. Methylation Analysis
2.1.3. Structure Characteristics of BEPS-IB from NMR
| Methylated sugar | Retention | Molar ratio | Mass fragment (m/z) | Type of linkage |
|---|---|---|---|---|
| time (min) | ||||
| 2,3,4,6-tetra-O-Me-Glc a | 14.80 | 1.32 | 43, 45, 71, 87, 101, 117, 129, 145, 161, 205 | Glc-(1→ |
| 2,4,6-tri-O-Me-Man | 16.95 | 3.16 | 43, 45, 87, 101, 117, 129, 161, 233 | →3)-Man-(1→ |
| 2,3,4-tri-O-Me-Glc | 17.31 | 6.43 | 43, 45, 71, 87, 101, 117, 129, 161, 173, 189, 233 | →6)-Glc-(1→ |
| 2,4-tri-O-Me-Man | 19.16 | 0.97 | 43, 87, 101, 117, 129, 189 | →3,6)-Man-(1→ |
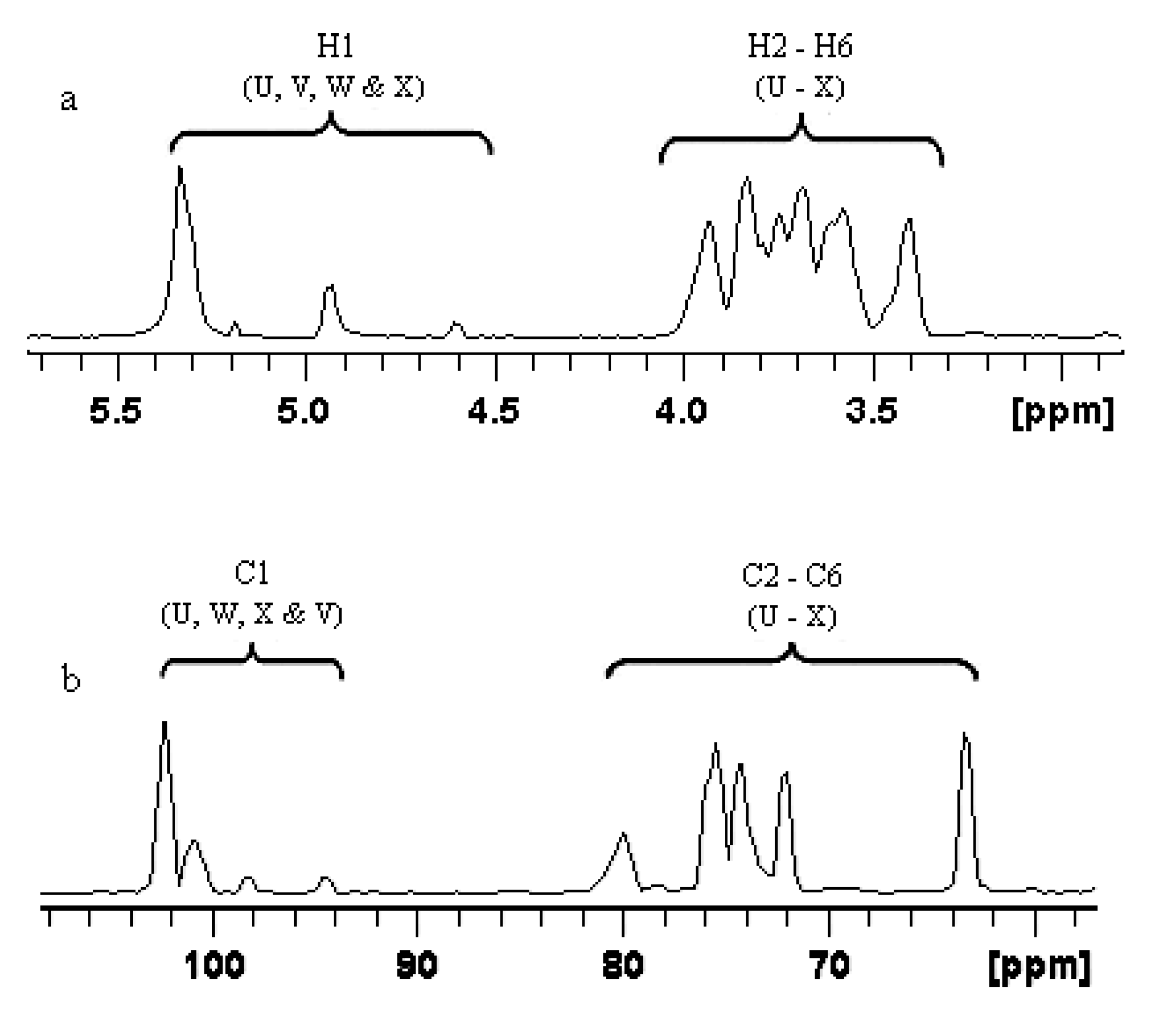
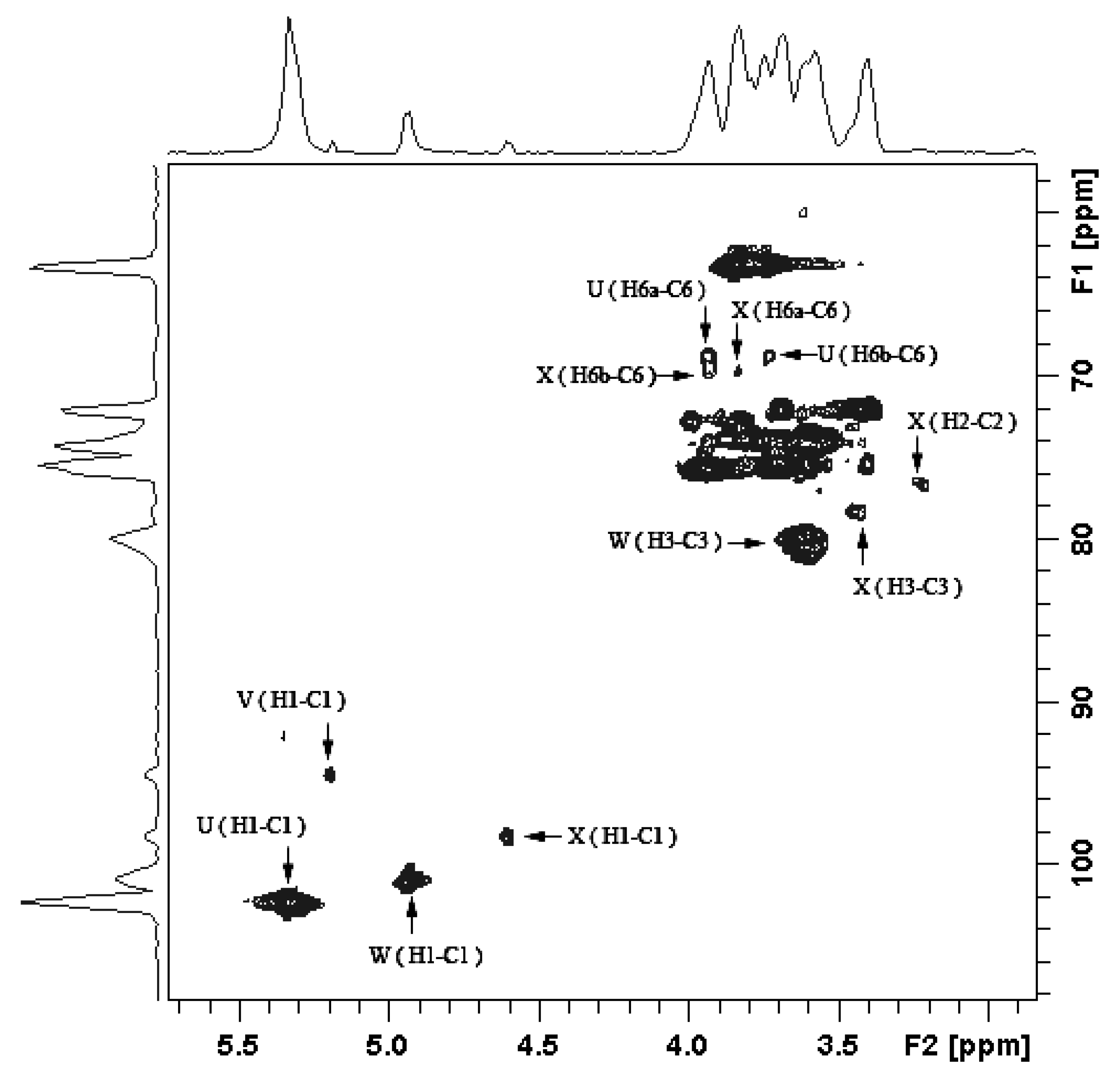
| Glycosidic linkage | Chemical shifts (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| H-1 (C-1) | H-2 (C-2) | H-3 (C-3) | H-4 (C-4) | H-5 (C-5) | H-6 (C-6) | ||
| U | →6)-α-Glcp-(1→ | 5.338 (102.2) | 3.587 (72.1) | 3.742 (76.8) | 3.632 (73.2) | 3.964 (76.8) | 3.937/3.736 (68.9) |
| V | α-Glcp-(1→ | 5.192 (94.6) | 3.517 (73.8) | 3.811 (72.4) | 3.342 (73.2) | 3.623 (75.3) | 3.831/3.792 (63.2) |
| W | →3)-α-Man-(1→ | 4.943 (100.8) | 3.542 (75.4) | 3.605 (80.1) | 3.614 (74.8) | 3.963 (76.3) | 3.972/3.752 (69.2) |
| X | →3,6)-α-Man-(1→ | 4.603 (98.2) | 3.211 (76.5) | 3.729 (78.5) | 3.412 (73.9) | 3.574 (76.7) | 3.833/3.925 (69.6) |
| Residue | Sugar linkage | Anomeric atom (δH/δC) | Observed connectivities | |||
|---|---|---|---|---|---|---|
| δH/δC | Residue | Atom | ||||
| U | →6)-α-Glcp-(1→ | 5.338 | 69.6 | U: H-1 | X: C-6 | |
| 73.2 | U: H-1 | U: C-4 | ||||
| 102.3 | 3.83 | U: C-1 | X: H-6a | |||
| 3.63 | U: C-1 | U: H-4 | ||||
| V | α-Glcp-(1→ | 5.192 | 80.1 | V: H-1 | W: C-3 | |
| 73.8 | V: H-1 | V: C-2 | ||||
| 72.4 | V: H-1 | V: C-3 | ||||
| 94.6 | 3.61 | V: C-1 | W: H-3 | |||
| 3.52 | V: C-1 | V: H-2 | ||||
| 3.81 | V: C-1 | V: H-3 | ||||
| W | →3)-α-Man-(1→ | 4.943 | 78.5 | W: H-1 | X: C-3 | |
| 76.3 | W: H-1 | W: C-5 | ||||
| 100.8 | 3.96 | W: C-1 | W: H-5 | |||
| X | →3,6)-α-Man-(1→ | 4.603 | 68.9 | X: H-1 | U: C-6 | |
| 3.94 | X: C-1 | U: H-6a | ||||
| 98.2 | 3.21 | X: C-2 | X: H-2 | |||
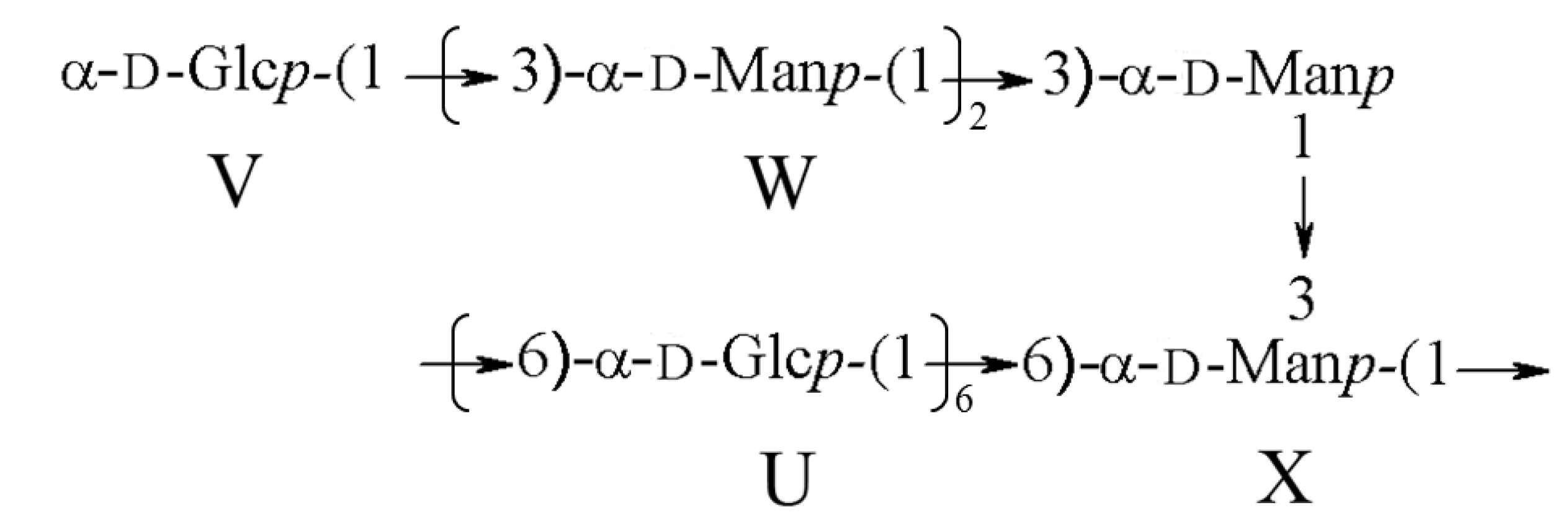
2.2. Antioxidant Activity

3. Materials and Methods
3.1. Materials and Reagents
3.2. Isolation and Purification of Polysaccharides
3.3. Chemical Analysis of Polysaccharide Fractions
3.4. Methylation Analysis
3.5. Measurement of IR and NMR Spectra
3.6. Antioxidant Activity Assays
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Abbreviations
| BEPS | crude polysaccharide extracted from Bullacta exarata |
| BEPS-IB | purified B. exarata polysaccharide of molecular weight 94 kDa |
| Fuc | fucose |
| Gal | galactose |
| Man | mannose |
| Glc | glucose |
| Arb | arabinose |
| Rha | rhamnose |
| Xyl | xylose |
| GalN | galactosamine |
| GlcN | glucosamine |
| GlcA | glucuronic acid |
| GalA | galacturonic acid |
Conflicts of Interest
References
- Babineau, T.J.; Hackford, A.; Kenler, A.; Bistrian, B.; Forse, R.A.; Fairchild, P.G.; Heard, S.; Keroack, M.; Caushaj, P.; Benotti, P. A phase II multicenter, double-blind, randomized, placebo-controlled study of three dosages of an immunomodulator (PGG-glucan) in high-risk surgical patients. Arch. Surg. 1994, 129, 1204–1210. [Google Scholar] [CrossRef]
- Babineau, T.J.; Marcello, P.; Swails, W.; Kenler, A.; Bistrian, B.; Forse, R.A. Randomized phase I/II trial of a macrophage-specific immunomodulator (PGG-glucan) in high-risk surgical patients. Ann. Surg. 1994, 220, 601–609. [Google Scholar] [CrossRef]
- Browder, W.; Williams, D.; Sherwood, E.; McNamee, R.; Jones, E.; DiLuzio, N. Synergistic effect of nonspecific immunostimulation and antibiotics in experimental peritonitis. Surgery 1987, 102, 206–214. [Google Scholar]
- Williams, D.L.; Mueller, A.; Browder, W. Glucan-based macrophage stimulators. Clin. Immunother. 1996, 5, 392–399. [Google Scholar] [CrossRef]
- Misaki, A.; Kakuta, M.; Sasaki, T.; Tanaka, M.; Miyaji, H. Studies on interrelation of structure and antitumor effects of polysaccharides: Antitumor action of periodate-modified, branched (1→3)-β-d-glucan of Auricularia auricula-judae, and other polysaccharides containing (1→3)-glycosidic linkages. Carbohydr. Res. 1981, 92, 115–129. [Google Scholar] [CrossRef]
- Kogan, G.; Staško, A.; Bauerová, K.; Polovka, M.; Šoltés, L.; Brezová, V.; Navarová, J.; Mihalová, D. Antioxidant properties of yeast (1→3)-β-d-glucan studied by electron paramagnetic resonance spectroscopy and its activity in the adjuvant arthritis. Carbohydr. Polym. 2005, 61, 18–28. [Google Scholar] [CrossRef]
- Tsiapali, E.; Whaley, S.; Kalbfleisch, J.; Ensley, H.E.; Browder, I.W.; Williams, D.L. Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radic. Biol. Med. 2001, 30, 393–402. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Han, B.; Sun, J.; Wang, D. Isolation and characterization of antitumor polysaccharides from the marine mollusk Ruditapes philippinarum. Eur. Food Res. Technol. 2008, 227, 103–110. [Google Scholar] [CrossRef]
- Vetvicka, V.; Yvin, J.-C. Effects of marine β-1,3-glucan on immune reactions. Int. Immunopharmacol. 2004, 4, 721–730. [Google Scholar] [CrossRef]
- Mikheiskaya, L.; Molchanova, V.; Ovodova, R.; Santana, V.F. Branched α-1,4-glucan from the gastropod mollusk Strombus gigas. Chem. Nat. Compd. 1988, 24, 29–32. [Google Scholar] [CrossRef]
- Vetvicka, V.; Sima, P. β-Glucan in invertebrates. ISJ 2004, 1, 60–65. [Google Scholar]
- Zhang, H.; Ye, L.; Wang, K. Structural characterization and anti-inflammatory activity of two water-soluble polysaccharides from Bellamya purificata. Carbohydr. Polym. 2010, 81, 953–960. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, M.; Liu, J.; Gan, D.; Zeng, X. Extraction, preliminary characterization, antioxidant and anticancer activities in vitro of polysaccharides from Cyclina sinensis. Carbohydr. Polym. 2011, 84, 851–857. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, J.; Jin, X.; Xie, J. Effect of extract from bullacta exarata on murine melanoma cell B16. Biotechnol. Bull. 2009, 2, 124–128. [Google Scholar]
- Zhang, D.; Wu, H.; Xia, Z.; Wang, C.; Cai, J.; Huang, Z.; Du, L.; Sun, P.; Xie, J. Partial characterization, antioxidant and antitumor activities of three sulfated polysaccharides purified from Bullacta exarata. J. Funct. Foods 2012, 4, 784–792. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, C.; Wu, H.; Xie, J.; Du, L.; Xia, Z.; Cai, J.; Huang, Z.; Wei, D. Three sulfated polysaccharides isolated from the mucilage of mud snail, Bullacta exarata Philippi: Characterization and antitumor activity. Food Chem. 2013, 1, 306–314. [Google Scholar]
- Santhiya, D.; Subramanian, S.; Natarajan, K. Surface chemical studies on sphalerite and galena using extracellular polysaccharides isolated from Bacillus polymyxa. J. Colloid Interface Sci. 2002, 256, 237–248. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Mathlouthi, M.; Koenig, J.L. Vibrational spectra of carbohydrates. Adv. Carbohydr. Chem. Biochem. 1987, 44, 7–89. [Google Scholar] [CrossRef]
- Duus, J.Ø.; Gotfredsen, C.H.; Bock, K. Carbohydrate structural determination by NMR spectroscopy: Modern methods and limitations. Chem. Rev. 2000, 100, 4589–4614. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Uchida, K. Role of reactive aldehyde in cardiovascular diseases. Free Radic. Biol. Med. 2000, 28, 1685–1696. [Google Scholar] [CrossRef]
- Stahl, P.D.; Ezekowitz, R.A.B. The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 1998, 10, 50–55. [Google Scholar] [CrossRef]
- Chen, S.; Xue, C.; Yin, L.A.; Tang, Q.; Yu, G.; Chai, W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Staub, A. Removal of protein-Sevag method. Methods Carbohydr. Chem. 1965, 5, 5–6. [Google Scholar]
- Wu, N.; Ye, X.; Guo, X.; Liao, N.; Yin, X.; Hu, Y.; Sun, Y.; Liu, D.; Chen, S. Depolymerization of fucosylated chondroitin sulfate from sea cucumber, Pearsonothuria graeffei, via 60Co irradiation. Carbohydr. Polym. 2013, 2, 604–614. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Needs, P.; Selvendran, R. An improved methylation procedure for the analysis of complex polysaccharides including resistant starch and a critique of the factors which lead to undermethylation. Phytochem. Anal. 1993, 4, 210–216. [Google Scholar] [CrossRef]
- Björndal, H.; Lindberg, B.; Svensson, S. Mass spectrometry of partially methylated alditol acetates. Carbohydr. Res. 1967, 5, 433–440. [Google Scholar] [CrossRef]
- Li, X.; Zhou, A.; Li, X. Inhibition of Lycium barbarum polysaccharides and Ganoderma lucidum polysaccharides against oxidative injury induced by γ-irradiation in rat liver mitochondria. Carbohydr. Polym. 2007, 69, 172–178. [Google Scholar] [CrossRef]
- Li, X.; Zhou, A.; Han, Y. Anti-oxidation and anti-microorganism activities of purification polysaccharide from Lygodium japonicum in vitro. Carbohydr. Polym. 2006, 66, 34–42. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, D.; Liao, N.; Ye, X.; Hu, Y.; Wu, D.; Guo, X.; Zhong, J.; Wu, J.; Chen, S. Isolation and Structural Characterization of a Novel Antioxidant Mannoglucan from a Marine Bubble Snail, Bullacta exarata (Philippi). Mar. Drugs 2013, 11, 4464-4477. https://doi.org/10.3390/md11114464
Liu D, Liao N, Ye X, Hu Y, Wu D, Guo X, Zhong J, Wu J, Chen S. Isolation and Structural Characterization of a Novel Antioxidant Mannoglucan from a Marine Bubble Snail, Bullacta exarata (Philippi). Marine Drugs. 2013; 11(11):4464-4477. https://doi.org/10.3390/md11114464
Chicago/Turabian StyleLiu, Donghong, Ningbo Liao, Xingqian Ye, Yaqin Hu, Dan Wu, Xin Guo, Jianjun Zhong, Jianyong Wu, and Shiguo Chen. 2013. "Isolation and Structural Characterization of a Novel Antioxidant Mannoglucan from a Marine Bubble Snail, Bullacta exarata (Philippi)" Marine Drugs 11, no. 11: 4464-4477. https://doi.org/10.3390/md11114464






