Chitin-Lignin Material as a Novel Matrix for Enzyme Immobilization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Evaluation
2.1.1. FTIR Spectroscopy
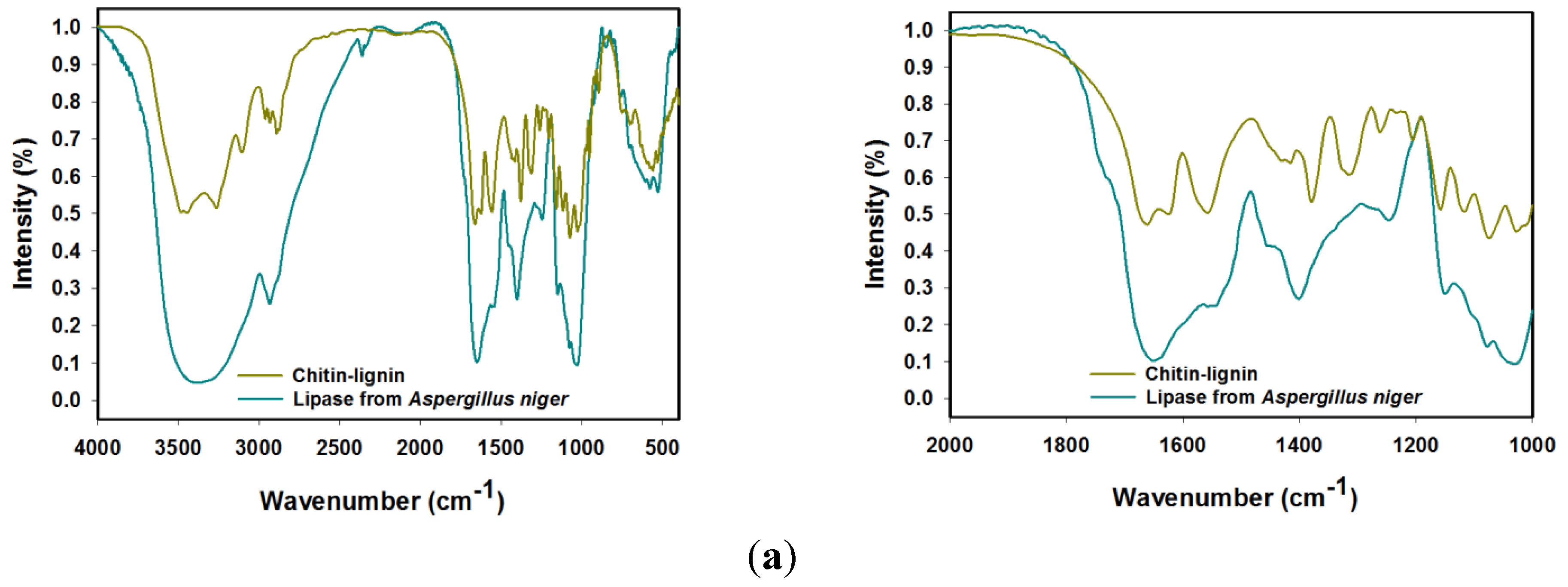
| Lipase from Aspergillus niger | Chitin-Lignin Material | Products after Immobilization | Vibrational Assignment |
|---|---|---|---|
| 3460 | 3444 | 3457 | O-H stretching |
| 3242 | 3257 | 3264 | N-H stretching |
| - | 3111 | 3112 | CAr-H stretching |
| 2931 | 2965, 2930, 2877 | 2966, 2935, 2879 | CHx stretching |
| - | 1674 | 1676 | C=O stretching |
| 1647 | 1625 | 1639 | amide I stretching |
| 1546 | 1556 | 1552 | amide II bending |
| 1448 | 1432 | 1438 | CH2 bending |
| - | 1420 | 1417 | CAr-CAr stretching |
| 1402 | 1388 | 1401 | O–H stretching |
| - | 1323 | 1329 | C-O (syringyl unit) streching |
| 1257 | 1268 | 1261 | amide III bending |
| 1151, 1073, 1037 | 1158, 1116, 1077, 1022 | 1162, 1113, 1081, 1027 | C-O-C (ring), C-O stretching |
| - | 953 | 957 | CH3 bending |
| - | 903 | 905 | β-1,4-glycosidic bonds |
| - | 745 | 745 | aromatic C-H(guaiacyl unit), bending |
| 576 | 558 | 571 | N-H bending |
| 531 | 527 | 530 | C-C scissoring |
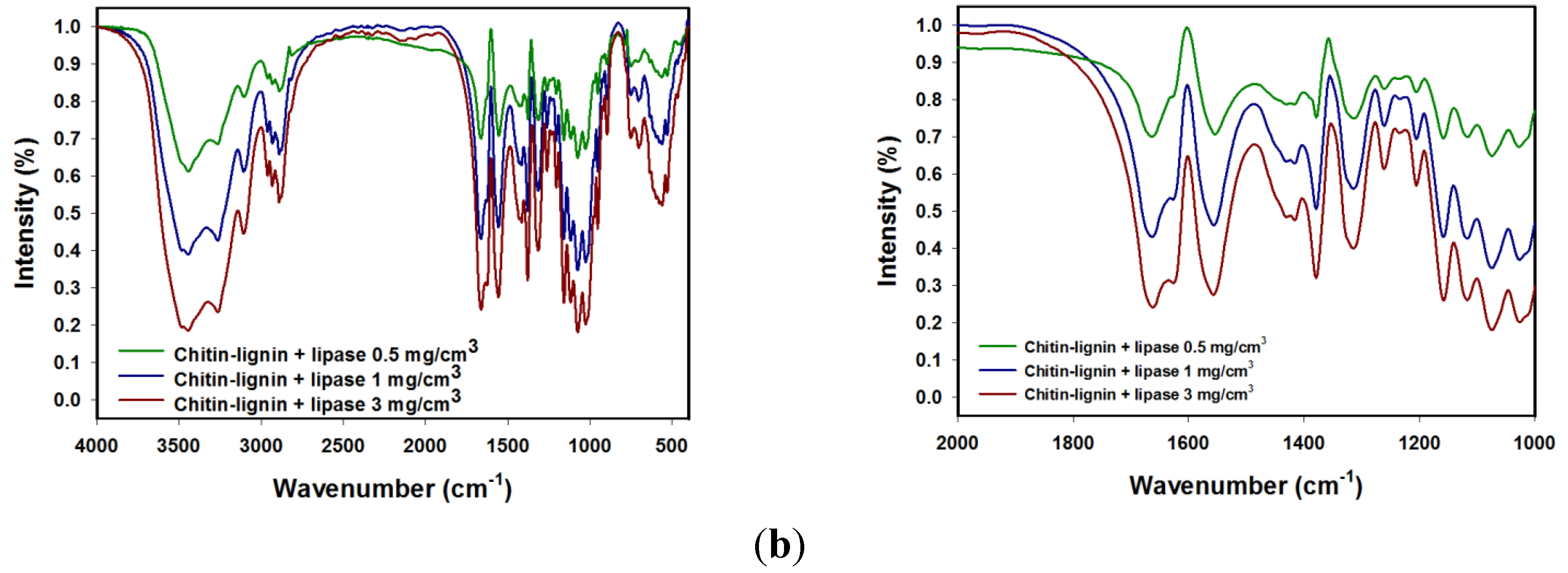
2.1.2. 13C CP MAS NMR Spectroscopy
2.1.3. Elemental Analysis
| Enzyme Solution Concentration (mg/cm3) | Immobilization Time | Elemental Content (%) | |||
|---|---|---|---|---|---|
| N | C | H | S | ||
| Chitin-lignin matrix | 5.07 | 33.86 | 4.93 | 0.03 | |
| 0.5 | 1 min | 5.23 | 35.42 | 5.40 | 0.02 |
| 2 h | 5.58 | 37.17 | 5.67 | 0.01 | |
| 24 h | 6.41 | 37.77 | 5.73 | 0.03 | |
| 1.0 | 1 min | 5.75 | 38.31 | 5.54 | 0.01 |
| 2 h | 5.96 | 38.77 | 5.78 | 0.03 | |
| 24 h | 6.66 | 39.81 | 5.95 | 0.02 | |
| 3.0 | 1 min | 5.96 | 39.01 | 5.91 | 0.03 |
| 2 h | 6.03 | 39.30 | 6.05 | 0.02 | |
| 24 h | 6.77 | 39.92 | 6.07 | 0.02 | |
2.1.4. XPS Analysis
| Sample Name | Atomic % | N/C Ratio | O/C Ratio | ||
|---|---|---|---|---|---|
| C | O | N | H | S | |
| Lipase | 58.2 | 30.7 | 11.1 | 0.19 | 0.53 |
| Chitin-lignin matrix | 61.4 | 32.6 | 6.0 | 0.10 | 0.53 |
| Chitin-lignin + lipase | 62.5 | 30.0 | 7.5 | 0.12 | 0.48 |
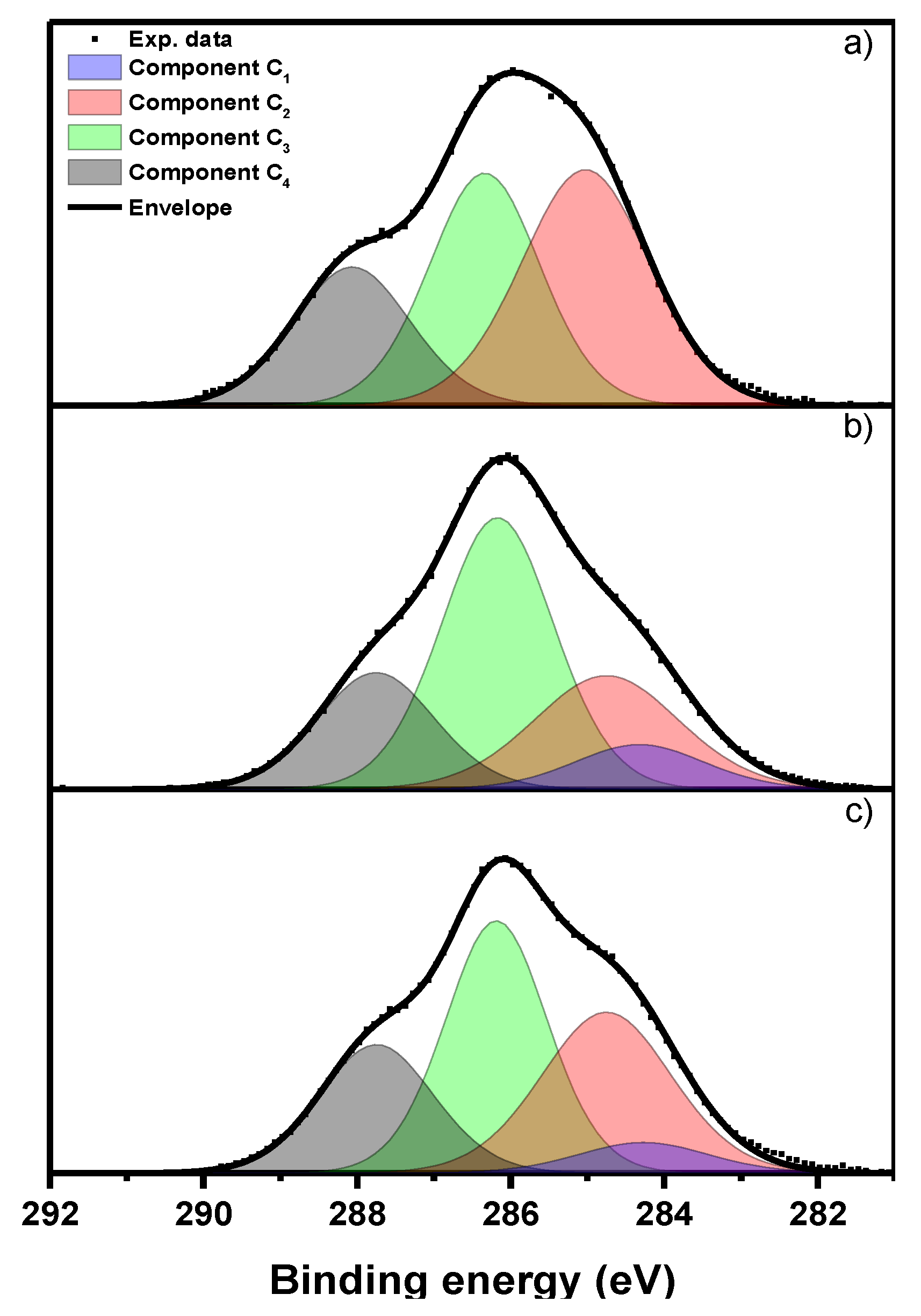
| Sample Name | Total C 1s Peak Intensity (%) | |||
|---|---|---|---|---|
| C1 | C2 | C3 | C4 | |
| Lipase | - | 42 | 36 | 22 |
| Chitin-lignin | 9 | 25 | 46 | 20 |
| Chitin-lignin + lipase | 6 | 32 | 39 | 23 |

2.1.5. Electrokinetic Characteristic

2.1.6. Quantity of Immobilized Enzyme
| Immobilization Time | Concentration of Enzyme Solution (mg/cm3) | ||
|---|---|---|---|
| 0.5 | 1 | 3 | |
| Amount of Immobilized Enzyme (mg/g) | |||
| 1 min | 1.45 | 5.13 | 6.19 |
| 1 h | 6.23 | 9.76 | 14.97 |
| 2 h | 8.17 | 10.84 | 18.46 |
| 4 h | 8.58 | 11.37 | 18.72 |
| 24 h | 9.22 | 11.84 | 19.31 |
| 96 h | 9.94 | 12.57 | 20.28 |
2.2. Hydrolytic Activity
2.2.1. Determination of Hydrolytic Activity

2.2.2. Thermal Stability
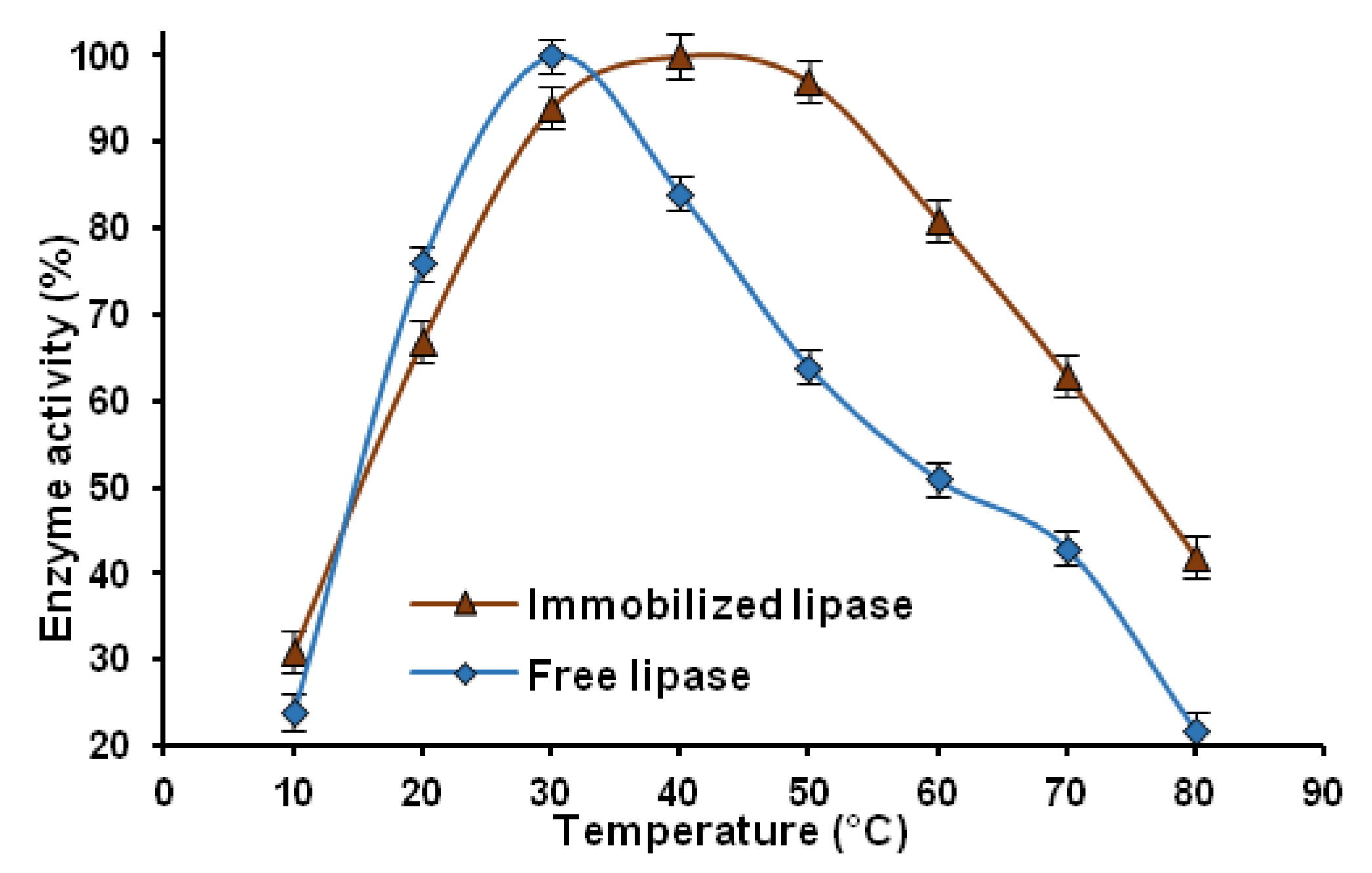
2.2.3. pH Stability
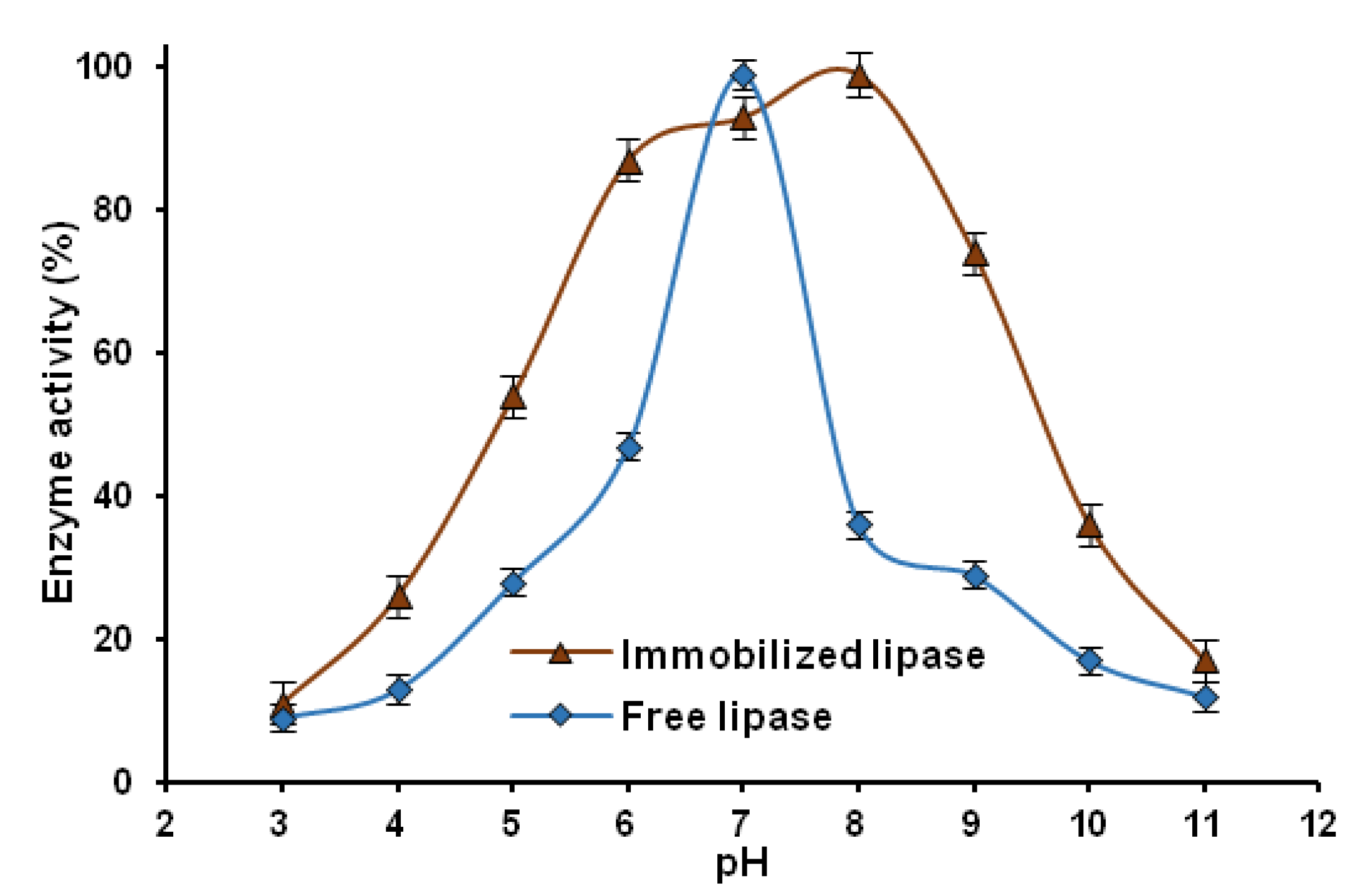
2.2.4. Reusability
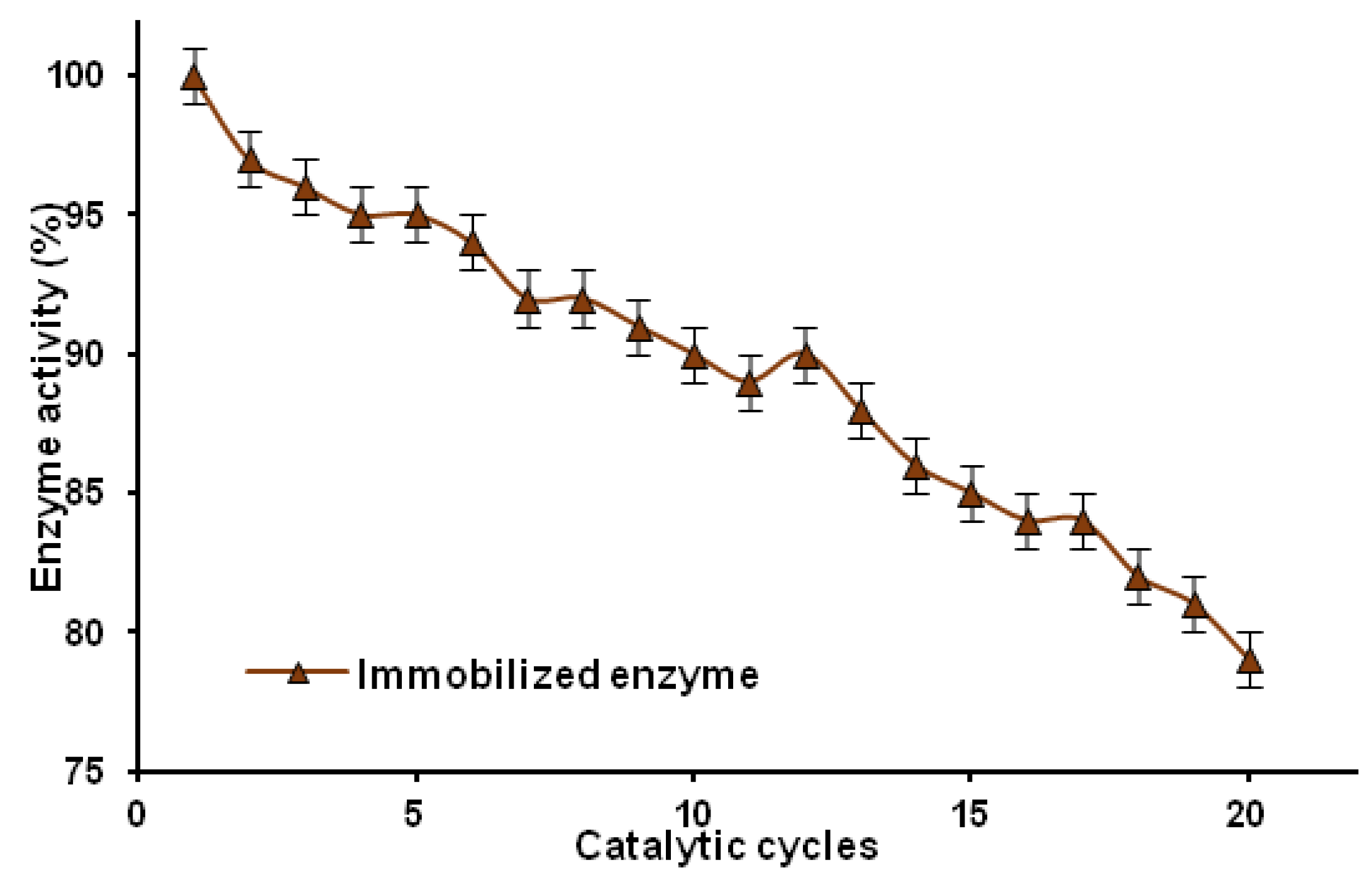
3. Experimental Section
3.1. Materials
3.2. Preparation of Chitin-Lignin Material
3.3. Enzyme Immobilization
3.4. Physicochemical Evaluation
3.5. Evaluation of Hydrolytic Activity
3.5.1. Thermal Stability
3.5.2. pH Stability
3.5.3. Reusability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Evstigneyev, E.; Shevchenko, S.; Mayorova, H.; Platonow, A. Polarographically active structural fragments of lignin. II. Dimeric model compounds and lignins. J. Wood Chem. Technol. 2004, 24, 263–278. [Google Scholar] [CrossRef]
- Lund, H.; Baizer, M.M. Organic Electrochemistry—An Introduction and Guide; Marcel Dekker: New York, NY, USA, 1991. [Google Scholar]
- Milczarek, G.; Inganäs, O. Renewable cathode materials from biopolymer/conjugated polymer interpenetrating networks. Science 2012, 335, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, G. Preparation and characterization of a lignin modified electrode. Electroanalsia 2007, 19, 1411–1414. [Google Scholar] [CrossRef]
- Milczarek, G. Preparation, characterization and electrocatalytic properties of an iodine/lignin modified gold electrode. Electrochim. Acta 2009, 54, 3199–3205. [Google Scholar] [CrossRef]
- Milczarek, G. Lignosulfonate-modified electrodes: Electrochemical properties and electrocatalysis of NADH oxidation. Langmuir 2009, 25, 10345–10353. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, G.; Rębiś, T. Synthesis and electroanalytical performance of a composite material based on poly(3,4-ethylenedioxythiophene) doped with lignosulfonate. Int. J. Electrochem. 2012, 130980, 1–7. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Opra, D.P.; Sinebryukhov, S.L.; Tsvetnikov, A.K.; Ustinov, A.Y.; Sergienko, V.I. Hydrolysis lignin-based organic electrode material for primary lithium batteries. J. Solid State Electrochem. 2014, 17, 2611–2621. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Opra, D.P.; Sinebryukhov, S.L.; Tsvetnikov, A.K.; Ustinov, A.Y.; Sergienko, V.I. Hydrolysis lignin: Electrochemical properties of the organic cathode material for primary lithium battery. J. Ind. Eng. Chem. 2014, 20, 903–910. [Google Scholar] [CrossRef]
- Opra, D.P.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Tsvetnikov, A.K.; Sergienko, V.I. Fabrication of battery cathode material based on hydrolytic lignin. Solid State Phenom. 2014, 213, 154–159. [Google Scholar] [CrossRef]
- Kadla, J.F.; Kubo, S. Lignin-based polymer blends: Analysis of intermolecular interactions in lignin-synthetic polymer blends. Compos. A Appl. Sci. manuf. 2004, 35, 395–400. [Google Scholar] [CrossRef]
- Canetti, M.; Bertini, F. Supermolecular structure and thermal properties of poly(ethylene terephthalate)/lignin composites. Compos. Sci. Technol. 2007, 67, 3151–3157. [Google Scholar] [CrossRef]
- Chen, F.; Dai, H.; Dong, X.; Yang, J.; Zhong, M. Physical properties of lignin-based polypropylene blends. Polym. Compos. 2011, 32, 1019–1025. [Google Scholar] [CrossRef]
- Borysiak, S. Fundamental studies on lignocellulose/polypropylene composites: Effects of wood treatment on the transcrystalline morphology and mechanical properties. J. Appl. Polym. Sci. 2013, 127, 1309–1322. [Google Scholar] [CrossRef]
- Gozdecki, C.; Wilczyński, A.; Kociszewski, M.; Zajchowski, S. Mechanical properties of wood-polypropylene composites with industrial wood particles of different sizes. Wood Fiber. Sci. 2012, 44, 14–21. [Google Scholar]
- Guo, X.; Zhang, S.; Shan, X. Adsorption of metal ions on lignin. J. Hazard. Mater. 2008, 151, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Betancur, M.; Bonelli, P.R.; Velásquez, J.A.; Cukierman, A.L. Potentiality of lignin from the Kraft pulping process for removal of trace nickel from wastewater: Effect of demineralization. Bioresour. Technol. 2009, 100, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Bulgariu, L.; Bulgariu, D.; Malutan, T.; Macoveanu, M. Adsorption of lead(II) ions from aqueous solution onto lignin. Adsorp. Sci. Technol. 2009, 27, 435–445. [Google Scholar] [CrossRef]
- Harmita, H.; Karthikeyan, K.G.; Pan, X.J. Copper and cadmium sorption onto kraft and organosolv lignins. Bioresour. Technol. 2009, 100, 6183–6191. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [PubMed]
- Lei, Y.; Huizhen, Y. Modification of reed alkali lignin to adsorption of heavy metals. Adv. Mater. Res. 2013, 622, 1646–1650. [Google Scholar]
- Ge, Y.; Li, Z.; Kong, Y.; Song, Q.; Wang, K. Heavy metal ions retention by bi-functionalized lignin: Synthesis, applications, and adsorption mechanisms. J. Ind. Eng. Chem. 2014, 20, 4429–4436. [Google Scholar] [CrossRef]
- Toh, K.; Yokoyama, H.; Takahashi, C.; Watanabe, T.; Noda, H. Effect of herb lignin on the growth of enterobacteria. J. Gen. Appl. Microbiol. 2007, 53, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Ehrlich, H.; Krautter, M.; Hanke, T.; Simon, P.; Knieb, C.; Heinemann, S.; Worch, H. First evidence of the presence of chitin in skeleton of marine sponges. Part II. Glass sponges. (Hexactinellida: Porifera). J. Exp. Zool. B 2007, 308, 473–478. [Google Scholar] [CrossRef]
- Ehrlich, H.; Maldonado, M.; Spindler, K.D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (Demospongia: Porifera). J. Exp. Zool. B 2007, 308, 347–356. [Google Scholar] [CrossRef]
- Brunner, E.; Ehrlich, H.; Schupp, P.; Hedrich, R.; Hunoldt, S.; Kammer, M.; Machill, S.; Paasch, S.; Bazhenov, V.V.; Kurek, D.V.; et al. Chitin-based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J. Struct. Biol. 2009, 168, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Zall, R.R. Absorption of metals by natural polymers generated from seafood processing wastes. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 168–172. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nair, A.; Sanoj Rejinold, N.; Maya, S.; Nair, S.V. Doxorubicin-loaded pH-responsive chitin nanogels for drug delivery to cancer cells. Carbohydr. Polym. 2012, 87, 2352–2356. [Google Scholar] [CrossRef]
- Liu, H.S.; Chen, W.H.; Lai, J.T. Immobilization of isoamylase on carboxymethyl-cellulose and chitin. Appl. Biochem. Biotechnol. 1997, 66, 57–67. [Google Scholar] [CrossRef]
- Chang, R.C.; Shaw, J.F. The immobilization of Candida cylindracea lipase on PVC, chitin and agarose. Bot. Bull. Acad. Sin. 1987, 28, 33–42. [Google Scholar]
- Romo-Sanchez, S.; Arevalo-Villena, M.; Garcia Romero, E.; Ramirez, H.L.; Briones Perez, A. Immobilization of β-glucosidase and its application for enhancement of aroma precursors in muscat wine. Food Bioprocess Technol. 2014, 7, 1381–1392. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzyme Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Vaillant, F.; Millan, A.; Millan, P.; Dormier, M.; Decloux, M.; Reynes, M. Co-immobilized pectinlyase and endocellulase on chitin and nylon supports. Process Biochem. 2000, 35, 989–996. [Google Scholar] [CrossRef]
- Batra, R.; Gupta, M.N. Non-covalent immobilization of potato (Solanum tuberosum) polyphenol oxidase on chitin. Biotechnol. Appl. Biochem. 1994, 19, 209–215. [Google Scholar]
- Wang, G.; Xu, J.J.; Ye, L.H.; Zhu, J.J.; Chen, H.Y. Highly sensitive sensors based on the immobilization of tyrosinase in chitosan. Bioelectrochemistry 2002, 57, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.M.; Pereira, E.B.; de Castro, H.F. Immobilization of lipase on chitin and its use in nonconventional biocatalysis. Biomacromolecules 2004, 5, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.B.; He, Y.S.; Li, S.L.; Wang, Y.Z. Chitin whiskers: An overwiev. Biomacromolecules 2012, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Filipkowska, U. Desorption of reactive dyes from modified chitin. Environ. Technol. 2008, 29, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzymes immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Wong, P.T.T.; Nong, R.K.; Caputo, T.A.; Godwin, T.A.; Rigas, B. Infrared spectroscopy of exfoliated human cervical cells: Evidence of extensive structural changes during carcinogenesis. Proc. Natl. Acad. Sci. USA 1991, 88, 10988–10992. [Google Scholar] [CrossRef] [PubMed]
- Dousseau, F.; Pezolet, M. Determination of the secondary structure content of proteins in aqueous solutions from their amide I and amide II infrared bands. Comparison between classical and partial least-squares methods. Biochemistry 1990, 29, 8771–8779. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Ge, C.; Qin, P.; Chen, Y.; Xu, Q. Immobilization and catalytic properties of candida lipolytic lipase on surface of organic intercalated and modified MgAl-LDHs. Sol. Sci. 2014, 31, 8–15. [Google Scholar] [CrossRef]
- Cabrera-Padilla, R.Y.; Lisboa, M.C.; Pereira, M.M.; Figueiredo, R.T.; Franceschi, E.; Fricks, A.T.; Lima, A.S.; Silva, D.P.; Soares, C.M.F. Immobilization of Candida rugosa lipase onto an eco-friendly support in the presence of ionic liquid. Bioprocess Biosyst. Eng. 2014. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Zdarta, J.; Szatkowski, T.; Wysokowski, M.; Nowacka, M.; Szwarc-Rzepka, K.; Bartczak, P.; Siwińska-Stefańska, K.; Ehrlich, H.; Jesionowski, T. Silica/lignosulfonate hybrid materials: Preparation and characterization. Cent. Eur. J. Chem. 2014, 12, 719–735. [Google Scholar] [CrossRef]
- Lavall, R.L.; Assis, O.B.G.; Campana-Filho, S.P. β-Chitin from the pens of Loligo sp.: Extraction and characterization. Bioresource Technol. 2007, 98, 2465–2472. [Google Scholar] [CrossRef]
- Jang, M.K.; Kong, B.G.; Jeong, Y.I.; Lee, C.H.; Nah, J.W. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. A 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Wysokowski, M.; Majchrzak, I.; Szatkowski, T.; Nowacka, M.; Siwińska-Stefańska, K.; Szwarc-Rzepka, K.; Bartczak, P.; Ehrlich, H.; Jesionowski, T. Preparation and characterization of multifunctional chitin/lignin materials. J. Nanomater. 2013, 1–13. [Google Scholar] [CrossRef]
- Naidja, A.; Liu, C.; Huang, P.M. Formation of protein-birnessite complex: XRD, FTIR, and AFM analysis. J. Colloid Interface Sci. 2002, 251, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Portaccio, M.; Della Ventura, B.; Mita, D.G.; Manolova, N.; Stoilova, O.; Rashkov, I.; Lepore, M. FT-IR microscopy characterization of sol–gel layers prior and after glucose oxidase immobilization for biosensing applications. J. Sol-Gel Sci. Technol. 2011, 57, 204–211. [Google Scholar] [CrossRef]
- Wysokowski, M.; Klapiszewski, Ł.; Moszyński, D.; Bartczak, P.; Majchrzak, I.; Siwińska-Stefańska, K.; Bazhenov, V.V.; Jesionowski, T. Modification of chitin with kraft lignin and development of new biosorbents for removal of cadmium(II) and nickel(II) ions. Mar. Drugs 2014, 12, 2245–2268. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Tomizuka, N.; Ota, Y.; Yamada, K. Lipase from Candida cylindracea II. Amino acid composition, carbohydrate component, and some physical properties. Agric. Biol. Chem. 1966, 30, 1090–1096. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Zhang, J.; Li, H.; Chen, P.; Gu, Q.; Wang, Z. Thermo-mechanical properties of the composite made of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and acetylated chitin nanocrystals. Carbohydr. Polym. 2013, 95, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kahveci, D.; Chen, M.; Guo, Z.; Xie, E.; Xu, X.; Besenbacher, F.; Dong, M. Enhanced catalytic activity of lipase encapsulated in PCL nanofibers. Langmuir 2012, 28, 6157–6162. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.; Grant, J.T. Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; IM Publications and SurfaceSpectra Limited: Charlton, UK, 2003. [Google Scholar]
- Wang, J.; Wang, Z.; Li, J.; Wang, B.; Liu, J.; Chen, P.; Miao, M.; Gu, Q. Chitin nanocrystals grafted with poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and their effects on thermal behavior of PHBV. Carbohydr. Polym. 2012, 87, 784–789. [Google Scholar] [CrossRef]
- De Lange, P.J.; Mahy, J.W.G. ToF-SIMS and XPS investigations of fibers, coatings and biomedical materials. Fresenius’ J. Anal. Chem. 1995, 353, 487–493. [Google Scholar] [CrossRef]
- Rouxhet, P.G.; Genet, M.J. XPS analysis of bio-organic systems. Surf. Interface Anal. 2011, 43, 1453–1470. [Google Scholar] [CrossRef]
- Namboodiri, V.M.H.; Chattaopadhyaya, R. Purification and biochemical characterization of a novel thermostable lipase from Aspergillus niger. Lipids 2000, 35, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, D.; Cimerman, A.; Steiner, W. Aspergillus niger lipases: Induction, isolation and characterization of two lipases from a MZKI Al 16 strain. J. Mol. Cat. B Enzym. 1997, 2, 215–222. [Google Scholar] [CrossRef]
- Xiaoming, L.; Breddam, K. A novel carboxylesterase from Aspergillus niger and its hydrolysis of succinimide esters. Carlsberg Res. Commun. 1989, 54, 241–249. [Google Scholar] [CrossRef]
- Rezwan, K.; Studart, A.R.; Vo1ro1s, J.; Gauckler, L.J. Change of ζ potential of biocompatible colloidal oxide particles upon adsorption of bovine serum albumin and lysozyme. J. Phys. Chem. B 2005, 109, 14469–14474. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Meier, L.P.; Rezwan, M.; Voros, J.; Textor, M.; Gauckler, L.J. Bovine serum albumin adsorption onto colloidal Al2O3 particles: A new model based on zeta potential and UV-Vis measurements. Langmuir 2004, 20, 10055–10061. [Google Scholar] [CrossRef] [PubMed]
- Bernsmann, F.; Frisch, B.; Ringwald, C.; Ball, V. Protein adsorption on dopamine–melanin films: Role of electrostatic interactions inferred from ζ-potential measurements versus chemisorption. J. Colloid Interface Sci. 2010, 344, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, J.; Liu, B. A study on the adsorption behavior of protein onto functional microspheres. Chem. Technol. Biotechnol. 2005, 80, 531–536. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation: Why, what and how? Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naby, M.A. Immobilization of Aspergillus niger NRC 107 xylanase and beta-xylosidase, and properties of the immobilzed enzymes. Appl. Biochem. Biotechnol. 1993, 38, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Hu, Y.; Liu, L.; Jiang, L.; Zou, B.; Huang, H. Enhancing catalytic performance of porcine pancreatic lipase by covalent modification using functional ionic liquids. ACS Catal. 2013, 3, 1976–1983. [Google Scholar] [CrossRef]
- Emregul, E.; Sungur, S.; Akbulut, U. Polyacrylamide-gelatine carrier system used for invertase immobilization. Food Chem. 2006, 97, 591–597. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Ren, X.Y.; Liu, Y.M.; Wei, Y.; Qing, L.S.; Liao, X. Covalent immobilization of porcine pancreatic lipase on carboxyl-activated magnetic nanoparticles: Characterization and application for enzymatic inhibition assays. Mater. Sci. Eng. C Mater Boil. Appl. 2014, 38, 278–285. [Google Scholar] [CrossRef]
- Melgosa, R.; Sanz, M.T.; Solaesa, A.G.; Bucio, S.L.; Beltran, S. Enzymatic activity and conformational and morphological studies of four commercial lipases treated with supercritical carbon dioxide. J. Supercrit. Fluids 2015, 97, 51–62. [Google Scholar] [CrossRef]
- Zdarta, J.; Sałek, K.; Kołodziejczak-Radzimska, A.; Siwińska-Stefańska, K.; Szwarc-Rzepka, K.; Norman, M.; Klapiszewski, Ł.; Bartczak, P.; Kaczorek, E.; Jesionowski, T. Immobilization of Amano Lipase A onto Stöber silica surface: Process characterization and kinetic studies. Open Chem. 2015, 13, 138–148. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdarta, J.; Klapiszewski, Ł.; Wysokowski, M.; Norman, M.; Kołodziejczak-Radzimska, A.; Moszyński, D.; Ehrlich, H.; Maciejewski, H.; Stelling, A.L.; Jesionowski, T. Chitin-Lignin Material as a Novel Matrix for Enzyme Immobilization. Mar. Drugs 2015, 13, 2424-2446. https://doi.org/10.3390/md13042424
Zdarta J, Klapiszewski Ł, Wysokowski M, Norman M, Kołodziejczak-Radzimska A, Moszyński D, Ehrlich H, Maciejewski H, Stelling AL, Jesionowski T. Chitin-Lignin Material as a Novel Matrix for Enzyme Immobilization. Marine Drugs. 2015; 13(4):2424-2446. https://doi.org/10.3390/md13042424
Chicago/Turabian StyleZdarta, Jakub, Łukasz Klapiszewski, Marcin Wysokowski, Małgorzata Norman, Agnieszka Kołodziejczak-Radzimska, Dariusz Moszyński, Hermann Ehrlich, Hieronim Maciejewski, Allison L. Stelling, and Teofil Jesionowski. 2015. "Chitin-Lignin Material as a Novel Matrix for Enzyme Immobilization" Marine Drugs 13, no. 4: 2424-2446. https://doi.org/10.3390/md13042424
APA StyleZdarta, J., Klapiszewski, Ł., Wysokowski, M., Norman, M., Kołodziejczak-Radzimska, A., Moszyński, D., Ehrlich, H., Maciejewski, H., Stelling, A. L., & Jesionowski, T. (2015). Chitin-Lignin Material as a Novel Matrix for Enzyme Immobilization. Marine Drugs, 13(4), 2424-2446. https://doi.org/10.3390/md13042424













