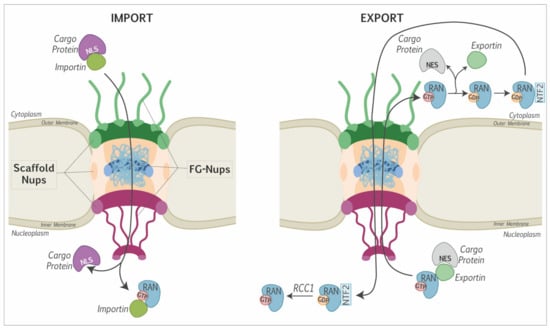
Abstract
Picornaviruses are positive-stranded RNA viruses. Even though replication and translation of their genome take place in the cytoplasm, these viruses evolved different strategies to disturb nucleocytoplasmic trafficking of host proteins and RNA. The major targets of picornavirus are the phenylalanine-glycine (FG)-nucleoporins, which form a mesh in the central channel of the nuclear pore complex through which protein cargos and karyopherins are actively transported in both directions. Interestingly, while enteroviruses use the proteolytic activity of their 2A protein to degrade FG-nucleoporins, cardioviruses act by triggering phosphorylation of these proteins by cellular kinases. By targeting the nuclear pore complex, picornaviruses recruit nuclear proteins to the cytoplasm, where they increase viral genome translation and replication; they affect nuclear translocation of cytoplasmic proteins such as transcription factors that induce innate immune responses and retain host mRNA in the nucleus thereby preventing cell emergency responses and likely making the ribosomal machinery available for translation of viral RNAs.
3. Conclusions and Discussion
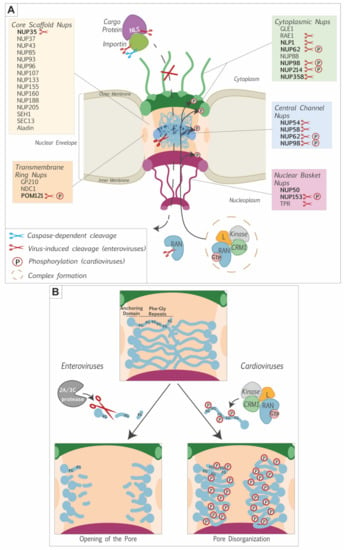
Picornaviruses belonging to different genera evolved different strategies to target the nuclear pore complex and to perturb nucleocytoplasmic trafficking of proteins and RNA. The main targets of these viruses are the phenylalanine-glycine-rich domains of FG-Nups. However, some picornaviruses also act on components of the soluble phase of the NPC by targeting RAN and/or the nuclear transport receptors (Figure 2A).
A likely purpose of nucleocytoplasmic disturbance is the recruitment, to the cytosol, of nuclear proteins that promote viral replication and/or translation (Figure 3). Interestingly, cytoplasmic relocalization of nuclear proteins not always depends on NPC perturbation as illustrated by the case of the La autoantigen. In poliovirus-infected cells, La autoantigen has been shown to migrate into the cytoplasm and to bind the 5′ non-coding region of the poliovirus genome, thereby stimulating IRES-dependent translation [,,]. Shiroki et al. showed that the NLS sequence of the La autoantigen was cleaved out by the viral protease 3Cpro, thereby inducing its cytoplasmic localization (Figure 2A and Figure 3) [].
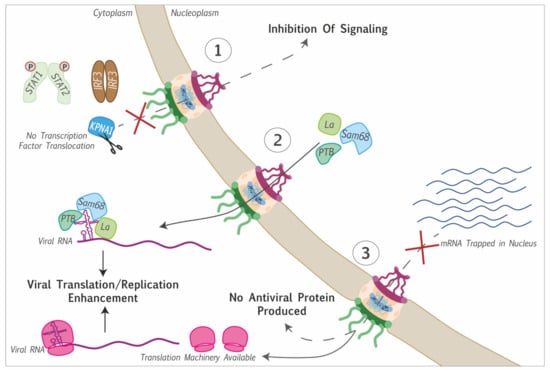
Figure 3.
Consequences of protein and RNA trafficking perturbation induced by Picornaviruses. (1) Cytoplasmic retention of transcription factors (STAT1/2, IRF3) thereby inhibiting transcriptional induction of cellular genes: e.g., karyopherin subunit α1 cleavage prevents the translocation of STAT1/2 into the nucleus. (2) Nuclear proteins are delocalized to the cytoplasm, where they interact with the viral genome to promote viral genome translation or replication. (3) Blocking of mRNA export, preventing the translation of antiviral proteins, and making the translation machinery available for viral mRNA translation.
Another likely purpose of NPC targeting by picornaviruses is the inhibition of innate immunity signaling. Most antiviral innate immunity pathways depend on the nuclear translocation of transcription factors that are activated by cytoplasmic kinases in response to viral infection. Preventing the access of such transcription factors to the nucleus thus prevents transcriptional upregulation of genes coding for innate immunity mediators such as interferon. One such example is the interferon regulatory factor 3 (IRF3) that displayed aberrant localization and phosphorylation during cardiovirus infection and failed to induce interferon-α/β gene transcription [,,]. Other transcription factors implicated in the interferon signaling pathway are the STAT proteins. Karyopherin subunit α1 was shown to be degraded in a 3Cpro-dependent manner in FMDV-infected cells and a caspase-dependent manner after enterovirus A71 infection. This karyopherin is responsible for the translocation of phosphorylated STAT1 into the nucleus. So, by triggering KPNA1 degradation, both FMDV and enterovirus A71 prevent the translocation of STAT1/2 into the nucleus, thus antagonizing the transcriptional upregulation of interferon-stimulated genes [,] (Figure 3). In contrast, enterovirus A71-triggered the upregulation of karyopherin 2α gene transcription. This upregulation likely contributes as a proinflammatory signal, as this karyopherin allows the nuclear translocation of proteins such as p65, IRF1, TP53, or ERK1/2 [].
At last, nucleocytoplasmic transport disruption during picornavirus infection was reported to block host polyA+ mRNA export. By doing so, the virus may prevent the translation of cell mRNAs coding for antiviral proteins. Moreover, this would leave ribosomes directly available for viral mRNA translation, as these mRNAs are generated in the cytoplasm and therefore, do not undergo nuclear export (Figure 3).
Picornaviruses are not the only pathogens that interact with NPC components. Viruses that replicate in the nucleus, such as DNA viruses and RNA viruses from the Orthomyxoviridae family (e.g., Influenza virus), need to get their genome in the nucleus. Therefore, these viruses’ proteins and genomes interact with NPC components and use nucleocytoplasmic transport to get their genome into the nucleus []. Nonetheless, other RNA viruses, such as picornaviruses, that replicate in the cytoplasm also target the NPC. Striking examples are Dengue and Zika viruses of the Flaviviridae family since they encode a protease called NS3, which also targets FG-nucleoporins such as NUP153 and NUP98 to trigger mislocalization of cellular components between the nucleus and the cytoplasm []. Another timely example is the coronavirus SARS-CoV-2: ORF6 encoded by this virus interacts and misplaces NUP98 and RAE1 []. This induces a bidirectional perturbation of the nucleocytoplasmic traffic, a retention of mRNA in the nucleus [], and a blockade of STAT1/2 dimer translocation into the nuclei resulting in interferon signaling inhibition []. These effects are very similar to the ones induced by picornaviruses.
Interestingly, non-virus pathogens were also shown to target the nucleocytoplasmic traffic machinery. Bacteria such as Salmonella, Coxiella, and Orientia counteract innate immune defenses and notably NFκB activation by targeting exportins, importins, or RAN [,,]. Thus, pathogens as different as picornaviruses and bacteria evolved diverse manners to target the NPC and to perturb the nucleocytoplasmic traffic, probably in part with the common goal to escape innate immunity.
Author Contributions
Conceptualization, B.L.-P. and T.M.; writing—original draft preparation, B.L.-P.; writing—review and editing, B.L.-P. and T.M.; supervision, T.M.; funding acquisition, B.L.-P. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
B.L.P was the recipient of a FRIA fellowship of the national fund for scientific research (FNRS). Work in T.M. lab was funded by the EOS joint programme of Fonds de la recherche scientifique—FNRS and Fonds wetenschapelijk onderzoek—Vlaanderen—FWO (EOS ID: 30981113), the National Lottery via the de Duve Institute, and by the Belgian fund for Scientific Research (PDR T.0185.14 and CDR J.0143.18).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Mohsan Saeed (Boston University School of Medicine) for personal communication of unpublished data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Krull, S.; Thyberg, J.; Björkroth, B.; Rackwitz, H.-R.; Cordes, V.C. Nucleoporins as Components of the Nuclear Pore Complex Core Structure and Tpr as the Architectural Element of the Nuclear Basket. Mol. Biol. Cell 2004, 15, 4261–4277. [Google Scholar] [CrossRef] [PubMed]
- Rout, M.P.; Aitchison, J.D.; Suprapto, A.; Hjertaas, K.; Zhao, Y.; Chait, B.T. The yeast nuclear pore complex: Composition, architecture, and transport mechanism. J. Cell Biol. 2000, 148, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.U. The Structure Inventory of the Nuclear Pore Complex. J. Mol. Biol. 2016, 428, 1986–2000. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.Y.H.; Fahrenkrog, B.; Köser, J.; Schwarz-Herion, K.; Deng, J.; Aebi, U. Nanomechanical Basis of Selective Gating by the Nuclear Pore Complex. Science 2007, 318, 640–643. [Google Scholar] [CrossRef]
- Frey, S.; Richter, R.P.; Görlich, D. FG-Rich Repeats of Nuclear Pore Proteins Form a Three-Dimensional Meshwork with Hydrogel-Like Properties. Science 2006, 314, 815–817. [Google Scholar] [CrossRef]
- Rexach, M.; Blobel, G. Protein import into nuclei: Association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell 1995, 83, 683–692. [Google Scholar] [CrossRef]
- Beck, M.; Hurt, E. The nuclear pore complex: Understanding its function through structural insight. Nat. Rev. Mol. Cell Biol. 2017, 18, 73–89. [Google Scholar] [CrossRef]
- Hampoelz, B.; Andres-Pons, A.; Kastritis, P.; Beck, M. Structure and Assembly of the Nuclear Pore Complex. Annu. Rev. Biophys. 2019, 48, 515–536. [Google Scholar] [CrossRef]
- Zell, R.; Delwart, E.; Gorbalenya, A.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef]
- Pelletier, J.; Sonenberg, N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 1988, 334, 320–325. [Google Scholar] [CrossRef]
- Jang, S.K.; Kräusslich, H.G.; Nicklin, M.J.; Duke, G.M.; Palmenberg, A.C.; Wimmer, E. A segment of the 5’ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J. Virol. 1988, 62, 2636–2643. [Google Scholar] [CrossRef]
- Lee, K.-M.; Chen, C.-J.; Shih, S.-R. Regulation Mechanisms of Viral IRES-Driven Translation. Trends Microbiol. 2017, 25, 546–561. [Google Scholar] [CrossRef]
- Martinez-Salas, E.; Francisco-Velilla, R.; Fernandez, J.; Embarek, A.M. Insights into Structural and Mechanistic Features of Viral IRES Elements. Front. Microbiol. 2018, 8, 2629. [Google Scholar] [CrossRef]
- Palmenberg, A.C. Proteolytic processing of picornaviral polyprotein. Annu. Rev. Microbiol. 1990, 44, 603–623. [Google Scholar] [CrossRef]
- Donnelly, M.L.; Hughes, L.E.; Luke, G.; Mendoza, H.; Ten Dam, E.; Gani, D.; Ryan, M.D. The ‘cleavage’ activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring ‘2A-like’ sequences. J. Gen. Virol. 2001, 82, 1027–1041. [Google Scholar] [CrossRef]
- Donnelly, M.L.; Luke, G.; Mehrotra, A.; Li, X.; Hughes, L.E.; Gani, D.; Ryan, M.D. Analysis of the aphthovirus 2A/2B polyprotein ‘cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: A putative ribosomal ‘skip’. J. Gen. Virol. 2001, 82, 1013–1025. [Google Scholar] [CrossRef]
- Jesús-González, D.; Adrián, L.; Palacios-Rápalo, S.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Gutiérrez-Escolano, A.L.; del Ángel, R.M. The Nuclear Pore Complex Is a Key Target of Viral Proteases to Promote Viral Replication. Viruses 2021, 13, 706. [Google Scholar] [CrossRef]
- Gustin, K.E. Inhibition of nucleo-cytoplasmic trafficking by RNA viruses: Targeting the nuclear pore complex. Virus Res. 2003, 95, 35–44. [Google Scholar] [CrossRef]
- Lloyd, R.E. Nuclear proteins hijacked by mammalian cytoplasmic plus strand RNA viruses. Virology 2015, 479-480, 457–474. [Google Scholar] [CrossRef]
- Pollack, R.; Goldman, R. Synthesis of Infective Poliovirus in BSC-1 Monkey Cells Enucleated with Cytochalasin B. Science 1973, 179, 915–916. [Google Scholar] [CrossRef]
- Follett, E.A.C.; Pringle, C.R.; Pennington, T.H. Virus Development in Enucleate Cells: Echovirus, Poliovirus, Pseudorabies Virus, Reovirus, Respiratory Syncytial Virus and Semliki Forest Virus. J. Gen. Virol. 1975, 26, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Molla, A.; Paul, A.V.; Wimmer, E. Cell-free, de novo synthesis of poliovirus. Science 1991, 254, 1647–1651. [Google Scholar] [CrossRef]
- Svitkin, Y.V.; Sonenberg, N. Cell-Free Synthesis of Encephalomyocarditis Virus. J. Virol. 2003, 77, 6551–6555. [Google Scholar] [CrossRef][Green Version]
- Barton, D.J.; Flanegan, J.B. Coupled translation and replication of poliovirus RNA in vitro: Synthesis of functional 3D polymerase and infectious virus. J. Virol. 1993, 67, 822–831. [Google Scholar] [CrossRef]
- McBride, A.E.; Schlegel, A.; Kirkegaard, K. Human protein Sam68 relocalization and interaction with poliovirus RNA polymerase in infected cells. Proc. Natl. Acad. Sci. USA 1996, 93, 2296–2301. [Google Scholar] [CrossRef]
- Gustin, K.E.; Sarnow, P. Inhibition of Nuclear Import and Alteration of Nuclear Pore Complex Composition by Rhinovirus. J. Virol. 2002, 76, 8787–8796. [Google Scholar] [CrossRef]
- Waggoner, S.; Sarnow, P. Viral ribonucleoprotein complex formation and nucleolar-cytoplasmic relocalization of nucleolin in poliovirus-infected cells. J. Virol. 1998, 72, 6699–6709. [Google Scholar] [CrossRef]
- Meerovitch, K.; Svitkin, Y.V.; Lee, H.S.; Lejbkowicz, F.; Kenan, D.J.; Chan, E.; Agol, V.I.; Keene, J.D.; Sonenberg, N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J. Virol. 1993, 67, 3798–3807. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.; Howell, M.T.; Patton, J.G.; Jackson, R.J. The involvement of a spliceosome component in internal initiation of human rhinovirus RNA translation. J. Gen. Virol. 1993, 74, 1775–1788. [Google Scholar] [CrossRef] [PubMed]
- Hellen, C.U.; Witherell, G.W.; Schmid, M.; Shin, S.H.; Pestova, T.V.; Gil, A.; Wimmer, E. A cytoplasmic 57-kDa protein that is required for translation of picornavirus RNA by internal ribosomal entry is identical to the nuclear pyrimidine tract-binding protein. Proc. Natl. Acad. Sci. USA 1993, 90, 7642–7646. [Google Scholar] [CrossRef]
- Delhaye, S.; Van Pesch, V.; Michiels, T. The Leader Protein of Theiler’s Virus Interferes with Nucleocytoplasmic Trafficking of Cellular Proteins. J. Virol. 2004, 78, 4357–4362. [Google Scholar] [CrossRef]
- Belov, G.A.; Evstafieva, A.G.; Rubtsov, Y.P.; Mikitas, O.V.; Vartapetian, A.B.; Agol, V.I. Early Alteration of Nucleocytoplasmic Traffic Induced by Some RNA Viruses. Virology 2000, 275, 244–248. [Google Scholar] [CrossRef]
- Kalderon, D.; Roberts, B.L.; Richardson, W.D.; Smith, A.E. A short amino acid sequence able to specify nuclear location. Cell 1984, 39, 499–509. [Google Scholar] [CrossRef]
- Pollard, V.W.; Michael, W.; Nakielny, S.; Siomi, M.C.; Wang, F.; Dreyfuss, G. A Novel Receptor-Mediated Nuclear Protein Import Pathway. Cell 1996, 86, 985–994. [Google Scholar] [CrossRef]
- Kataoka, N.; Bachorik, J.L.; Dreyfuss, G. Transportin-SR, a Nuclear Import Receptor for SR Proteins. J. Cell Biol. 1999, 145, 1145–1152. [Google Scholar] [CrossRef]
- Wen, W.; Meinkotht, J.L.; Tsien, R.Y.; Taylor, S.S. Identification of a signal for rapid export of proteins from the nucleus. Cell 1995, 82, 463–473. [Google Scholar] [CrossRef]
- Ciomperlik, J.J.; Basta, H.A.; Palmenberg, A.C. Three cardiovirus Leader proteins equivalently inhibit four different nucleocytoplasmic trafficking pathways. Virology 2015, 484, 194–202. [Google Scholar] [CrossRef]
- Watters, K.; Inankur, B.; Gardiner, J.; Warrick, J.; Sherer, N.M.; Yin, J.; Palmenberg, A.C. Differential Disruption of Nucleocytoplasmic Trafficking Pathways by Rhinovirus 2A Proteases. J. Virol. 2017, 91, e02472-16. [Google Scholar] [CrossRef]
- Gustin, K.E.; Sarnow, P. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. Embo. J. 2001, 20, 240–249. [Google Scholar] [CrossRef]
- Castelló, A.; Izquierdo, J.M.; Welnowska, E.; Carrasco, L. RNA nuclear export is blocked by poliovirus 2A protease and is concomitant with nucleoporin cleavage. J. Cell Sci. 2009, 122, 3799–3809. [Google Scholar] [CrossRef]
- Ricour, C.; Delhaye, S.; Hato, S.V.; Olenyik, T.D.; Michel, B.; Van Kuppeveld, F.J.M.; Gustin, K.E.; Michiels, T. Inhibition of mRNA export and dimerization of interferon regulatory factor 3 by Theiler’s virus leader protein. J. Gen. Virol. 2009, 90, 177–186. [Google Scholar] [CrossRef]
- Porter, F.W.; Bochkov, Y.; Albee, A.J.; Wiese, C.; Palmenberg, A.C. A picornavirus protein interacts with Ran-GTPase and disrupts nucleocytoplasmic transport. Proc. Natl. Acad. Sci. USA 2006, 103, 12417–12422. [Google Scholar] [CrossRef]
- Lidsky, P.V.; Hato, S.; Bardina, M.V.; Aminev, A.G.; Palmenberg, A.C.; Sheval, E.V.; Polyakov, V.Y.; Van Kuppeveld, F.J.M.; Agol, V.I. Nucleocytoplasmic Traffic Disorder Induced by Cardioviruses. J. Virol. 2006, 80, 2705–2717. [Google Scholar] [CrossRef]
- Park, N.; Katikaneni, P.; Skern, T.; Gustin, K.E. Differential Targeting of Nuclear Pore Complex Proteins in Poliovirus-Infected Cells. J. Virol. 2008, 82, 1647–1655. [Google Scholar] [CrossRef]
- Krull, S.; Dörries, J.; Boysen, B.; Reidenbach, S.; Magnius, L.; Norder, H.; Thyberg, J.; Cordes, V.C. Protein Tpr is required for establishing nuclear pore-associated zones of heterochromatin exclusion. Embo. J. 2010, 29, 1659–1673. [Google Scholar] [CrossRef]
- Park, N.; Skern, T.; Gustin, K.E. Specific Cleavage of the Nuclear Pore Complex Protein Nup62 by a Viral Protease. J. Biol. Chem. 2010, 285, 28796–28805. [Google Scholar] [CrossRef]
- Park, N.; Schweers, N.J.; Gustin, K.E. Selective Removal of FG Repeat Domains from the Nuclear Pore Complex by Enterovirus 2A(pro). J. Virol. 2015, 89, 11069–11079. [Google Scholar] [CrossRef]
- Hülsmann, B.B.; Labokha, A.A.; Görlich, D. The Permeability of Reconstituted Nuclear Pores Provides Direct Evidence for the Selective Phase Model. Cell 2012, 150, 738–751. [Google Scholar] [CrossRef]
- Patel, S.S.; Belmont, B.; Sante, J.M.; Rexach, M.F. Natively Unfolded Nucleoporins Gate Protein Diffusion across the Nuclear Pore Complex. Cell 2007, 129, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Belov, G.A.; Lidsky, P.V.; Mikitas, O.V.; Egger, D.; Lukyanov, K.A.; Bienz, K.; Agol, V.I. Bidirectional increase in permeability of nuclear envelope upon poliovirus infection and accompanying alterations of nuclear pores. J. Virol. 2004, 78, 10166–10177. [Google Scholar] [CrossRef]
- Watters, K.; Palmenberg, A.C. Differential Processing of Nuclear Pore Complex Proteins by Rhinovirus 2A Proteases from Different Species and Serotypes. J. Virol. 2011, 85, 10874–10883. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.J.; Hossain, A.R.; Qiu, Y.; Zhang, H.M.; Zhao, G.; Li, C.; Lin, V.; Sulaimon, S.; Vlok, M.; Fung, G.; et al. Cleavage and Sub-Cellular Redistribution of Nuclear Pore Protein 98 by Coxsackievirus B3 Protease 2A Impairs Cardioprotection. Front. Cell. Infect. Microbiol. 2019, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.J.; Younessi, P.; Fulcher, A.J.; McCuaig, R.; Thomas, B.J.; Bardin, P.G.; Jans, D.A.; Ghildyal, R. Rhinovirus 3C Protease Facilitates Specific Nucleoporin Cleavage and Mislocalisation of Nuclear Proteins in Infected Host Cells. PLoS ONE 2013, 8, e71316. [Google Scholar] [CrossRef] [PubMed]
- Ghildyal, R.; Jordan, B.; Li, D.; Dagher, H.; Bardin, P.G.; Gern, J.E.; Jans, D.A. Rhinovirus 3C protease can localize in the nucleus and alter active and passive nucleocytoplasmic transport. J. Virol. 2009, 83, 7349–7352. [Google Scholar] [CrossRef]
- Saeed, M.; Kapell, S.; Hertz, N.T.; Wu, X.; Bell, K.; Ashbrook, A.W.; Mark, M.T.; Zebroski, H.A.; Neal, M.L.; Flodström-Tullberg, M.; et al. Defining the proteolytic landscape during enterovirus infection. PLoS Pathog. 2020, 16, e1008927. [Google Scholar] [CrossRef]
- Porter, F.W.; Palmenberg, A.C. Leader-induced phosphorylation of nucleoporins correlates with nuclear trafficking inhibition by cardioviruses. J. Virol. 2009, 83, 1941–1951. [Google Scholar] [CrossRef]
- Porter, F.W.; Brown, B.; Palmenberg, A.C. Nucleoporin Phosphorylation Triggered by the Encephalomyocarditis Virus Leader Protein Is Mediated by Mitogen-Activated Protein Kinases. J. Virol. 2010, 84, 12538–12548. [Google Scholar] [CrossRef]
- Freundt, E.C.; Drappier, M.; Michiels, T. Innate Immune Detection of Cardioviruses and Viral Disruption of Interferon Signaling. Front. Microbiol. 2018, 9, 2448. [Google Scholar] [CrossRef]
- Brahic, M.; Bureau, J.-F.; Michiels, T. The genetics of the persistent infection and demyelinating disease caused by theiler’s virus. Annu. Rev. Microbiol. 2005, 59, 279–298. [Google Scholar] [CrossRef]
- Basta, H.; Bacot-Davis, V.R.; Ciomperlik, J.J.; Palmenberg, A.C. Encephalomyocarditis Virus Leader Is Phosphorylated by CK2 and Syk as a Requirement for Subsequent Phosphorylation of Cellular Nucleoporins. J. Virol. 2014, 88, 2219–2226. [Google Scholar] [CrossRef]
- Basta, H.A.; Palmenberg, A.C. AMP-activated protein kinase phosphorylates EMCV, TMEV and SafV leader proteins at different sites. Virology 2014, 462-463, 236–240. [Google Scholar] [CrossRef]
- Bardina, M.V.; Lidsky, P.V.; Sheval, E.V.; Fominykh, K.V.; van Kuppeveld, F.J.M.; Polyakov, V.Y.; Agol, V.I. Mengovirus-Induced Rearrangement of the Nuclear Pore Complex: Hijacking Cellular Phosphorylation Machinery. J. Virol. 2009, 83, 3150–3161. [Google Scholar] [CrossRef]
- Bacot-Davis, V.R.; Palmenberg, A.C. Encephalomyocarditis virus Leader protein hinge domain is responsible for interactions with Ran GTPase. Virology 2013, 443, 177–185. [Google Scholar] [CrossRef]
- Bacot-Davis, V.R.; Ciomperlik, J.J.; Basta, H.A.; Cornilescu, C.C.; Palmenberg, A.C. Solution structures of Mengovirus Leader protein, its phosphorylated derivatives, and in complex with nuclear transport regulatory protein, RanGTPase. Proc. Natl. Acad. Sci. USA 2014, 111, 15792–15797. [Google Scholar] [CrossRef]
- Petty, R.V.; Palmenberg, A.C. Guanine-Nucleotide Exchange Factor RCC1 Facilitates a Tight Binding between the Encephalomyocarditis Virus Leader and Cellular Ran GTPase. J. Virol. 2013, 87, 6517–6520. [Google Scholar] [CrossRef]
- Ciomperlik, J.J.; Basta, H.A.; Palmenberg, A.C. Cardiovirus Leader proteins bind exportins: Implications for virus replication and nucleocytoplasmic trafficking inhibition. Virology 2016, 487, 19–26. [Google Scholar] [CrossRef]
- Du, Y.; Bi, J.; Liu, J.; Liu, X.; Wu, X.; Jiang, P.; Yoo, D.; Zhang, Y.; Wu, J.; Wan, R.; et al. 3Cpro of Foot-and-Mouth Disease Virus Antagonizes the Interferon Signaling Pathway by Blocking STAT1/STAT2 Nuclear Translocation. J. Virol. 2014, 88, 4908–4920. [Google Scholar] [CrossRef]
- Wang, C.; Sun, M.; Yuan, X.; Ji, L.; Jin, Y.; Cardona, C.J.; Xing, Z. Enterovirus 71 suppresses interferon responses by blocking Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling through inducing karyopherin-α1 degradation. J. Biol. Chem. 2017, 292, 10262–10274. [Google Scholar] [CrossRef]
- Peng, N.; Yang, X.; Zhu, C.; Zhou, L.; Yu, H.; Li, M.; Lin, Y.; Wang, X.; Li, Q.; She, Y.; et al. MicroRNA-302 Cluster Downregulates Enterovirus 71–Induced Innate Immune Response by Targeting KPNA2. J. Immunol. 2018, 201, 145–156. [Google Scholar] [CrossRef]
- Meerovitch, K.; Pelletier, J.; Sonenberg, N. A cellular protein that binds to the 5’-noncoding region of poliovirus RNA: Implications for internal translation initiation. Genes Dev. 1989, 3, 1026–1034. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Svitkin, Y.; Sonenberg, N. La Autoantigen Is Necessary for Optimal Function of the Poliovirus and Hepatitis C Virus Internal Ribosome Entry Site In Vivo and In Vitro. Mol. Cell. Biol. 2004, 24, 6861–6870. [Google Scholar] [CrossRef]
- Shiroki, K.; Isoyama, T.; Kuge, S.; Ishii, T.; Ohmi, S.; Hata, S.; Suzuki, K.; Takasaki, Y.; Nomoto, A. Intracellular redistribution of truncated La protein produced by poliovirus 3Cpro-mediated cleavage. J. Virol. 1999, 73, 2193–2200. [Google Scholar] [CrossRef]
- Hato, S.V.; Ricour, C.; Schulte, B.M.; Lanke, K.H.W.; De Bruijni, M.; Zoll, J.; Melchers, W.; Michiels, T.; Van Kuppeveld, F.J.M. The mengovirus leader protein blocks interferon-α/β gene transcription and inhibits activation of interferon regulatory factor 3. Cell. Microbiol. 2007, 9, 2921–2930. [Google Scholar] [CrossRef]
- Mettenleiter, T.C. Breaching the Barrier—The Nuclear Envelope in Virus Infection. J. Mol. Biol. 2016, 428, 1949–1961. [Google Scholar] [CrossRef]
- De Jesús-González, L.A.; Cervantes-Salazar, M.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Farfán-Morales, C.N.; Palacios-Rápalo, S.N.; Pérez-Olais, J.H.; Cordero-Rivera, C.D.; Hurtado-Monzón, A.M.; Ruíz-Jiménez, F.; et al. The Nuclear Pore Complex: A Target for NS3 Protease of Dengue and Zika Viruses. Viruses 2020, 12, 583. [Google Scholar] [CrossRef]
- Kato, K.; Ikliptikawati, D.K.; Kobayashi, A.; Kondo, H.; Lim, K.; Hazawa, M.; Wong, R.W. Overexpression of SARS-CoV-2 protein ORF6 dislocates RAE1 and NUP98 from the nuclear pore complex. Biochem. Biophys. Res. Commun. 2021, 536, 59–66. [Google Scholar] [CrossRef]
- Addetia, A.; Lieberman, N.A.; Phung, Q.; Hsiang, T.Y.; Xie, H.; Roychoudhury, P.; Shrestha, L.; Loprieno, M.A.; Huang, M.L.; Gale, M., Jr.; et al. SARS-CoV-2 ORF6 Disrupts Bidirectional Nucleocytoplasmic Transport through Interactions with Rae1 and Nup98. mBio 2021, 12, e00065-21. [Google Scholar] [CrossRef]
- Miorin, L.; Kehrer, T.; Sanchez-Aparicio, M.T.; Zhang, K.; Cohen, P.; Patel, R.S.; Cupic, A.; Makio, T.; Mei, M.; Moreno, E.; et al. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 28344–28354. [Google Scholar] [CrossRef]
- Rolhion, N.; Furniss, R.C.D.; Grabe, G.; Ryan, A.; Liu, M.; Matthews, S.A.; Holden, D.W. Inhibition of Nuclear Transport of NF-ĸB p65 by the Salmonella Type III Secretion System Effector SpvD. PLoS Pathog. 2016, 12, e1005653. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.M.; Rodino, K.G.; Adcox, H.E.; Carlyon, J.A. Orientia tsutsugamushi uses two Ank effectors to modulate NF-κB p65 nuclear transport and inhibit NF-κB transcriptional activation. PLoS Pathog. 2018, 14, e1007023. [Google Scholar] [CrossRef] [PubMed]
- Burette, M.; Allombert, J.; Lambou, K.; Maarifi, G.; Nisole, S.; Case, E.D.R.; Blanchet, F.P.; Hassen-Khodja, C.; Cabantous, S.; Samuel, J.; et al. Modulation of innate immune signaling by a Coxiella burnetii eukaryotic-like effector protein. Proc. Natl. Acad. Sci. USA 2020, 117, 13708–13718. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).