Abstract
Arnica L. genus (Asteraceae) comprises perennial herbs native to the temperate and boreal parts of the northern hemisphere. Arnica montana is the main species. It shows different biological activities, such as antioxidant, anti-inflammatory, antibacterial, antifungal, and antitumor effects. The Arnica formulations are mainly used for pain management. This systematic review is aimed at summarizing the studies focusing on the use of Arnica products on pain and inflammatory signs due to traumatic injuries related to sport and surgical interventions as well as to arthritis and other inflammatory conditions. Both phytotherapeutic and homeopathic formulations are taken into account. This paper only includes manuscripts published in mainstream journals. A literature search from Scopus, Web of Science, and PubMed databases has been carried out using a combination of the keywords “Arnica”, “trauma”, “sport”, “injury”, “injuries”, and “pain”. According to the search strategy and inclusion criteria for this study, 42 eligible papers, focusing on both Arnica alone and formulations containing a mixture of plant extracts, have been finally selected. This review critically discusses the in vitro, in vivo, and clinical studies dealing with Arnica products, reporting both positive and negative outcomes, thus providing perspectives for future research on the plant pharmacological potential.
Keywords:
Arnica; bruises; edema; homeopathy; inflammation; pain; phytotherapy; sport; surgery; trauma 1. Introduction
Arnica L. genus belongs to the Asteraceae family and, according to The World Flora Online (WFO) database (https://www.worldfloraonline.org/, accessed on 12 March 2024), it currently comprises 33 known species: A. acaulis Britton, Sterns & Poggenb., A. angustifolia Vahl, A. attenuata (Greene) Maguire, A. cernua Howell, A. chamissonis Less., A. cordifolia Hook., A. dealbata (A. Gray) B.G.Baldwin, A. denudata Greene, A. discoidea Benth., A. fulgens Pursh, A. gracilis Rydb., A. griscomii Fernald, A. intermedia Turcz., A. lanceolata Nutt., A. latifolia Bong., A. lessingii Greene, A. lonchophylla Greene, A. longifolia D.C. Eaton, A. louiseana Farr, A. mallotopus Makino, A. mollis Hook., A. montana L., A. nevadensis A. Gray, A. ovata Greene, A. parryi A. Gray, A. porsildiorum B. Boivin, A. rydbergii Greene, A. sachalinensis (Regel) A. Gray, A. sororia Greene, A. spathulata Greene, A. unalaschcensis Less., A. venosa H.M. Hall, and A. viscosa A. Gray [1].
Plants belonging to this genus are perennial herbs native to the temperate and boreal parts of the northern hemisphere [2].
Arnica montana and, to a latter extent, other Arnica species have been largely utilized as a popular remedy to treat pain and bruises [3].
A. montana is usually referred to as simply “arnica”, and it is also known as “mountain tobacco” or “leopard’s bane” [4]. This species has been traditionally used to treat different ailments, and it has been demonstrated to show antioxidant [5,6], anti-inflammatory [7,8,9], antibacterial [10,11], antifungal [12], and antitumor activity [13,14]. This species is found in Europe and has been introduced into the Tropical Asia region [1].
Arnica products are widely used for the management of pain due to a number of conditions, such as musculoskeletal diseases, arthritis, and surgery [15]. Given the very interesting biological activities that characterize A. montana species, previous reviews have summarized various aspects of the phytochemical and pharmacological properties of this species. In 2014, Brito and colleagues reviewed the efficacy of the topical administration of A. montana in the treatment of pain, swelling, and bruises [3]. The authors highlighted that the efficacy at doses below 10% was not supported by any evidence, and underlined the need for further studies to assess the effectiveness and safety of higher doses. One year later, in 2015, the effects of homeopathic dilutions of A. montana on traumatic tissue injury were described by Charlton, who highlighted a number of pieces of conflicting information, including bias/rhetoric for and against the use of this remedy for the treatment of traumatic tissue injuries [16].
Especially in the past years, many papers dealing with the potential use and beneficial properties of Arnica have been published in homeopathic journals, and the methodological quality of the investigations described was often poor [17]. Although homeopathic remedies are frequently used by many health professionals, there is an ongoing debate concerning their effectiveness. Currently, no unifying explanation on the mechanisms that could explain how homeopathy works exists [18]. This review, as underlined in the following methodology section, takes into account the manuscripts published in mainstream journals and indexed in the main databases.
This systematic review aims to offer an overview of the in vitro, in vivo, and clinical studies on the use of both phytotherapeutic and homeopathic Arnica formulations on pain and inflammatory signs due to traumatic injuries related to sport and surgical interventions as well as to inflammatory conditions including arthritis and other diseases. Positive and negative outcomes are presented and discussed.
2. Methodology
This review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19]. Scopus, Web of Science, and PubMed search engines were used. The keyword “Arnica” was combined with the keywords “trauma”, “sport”, “injury”, “injuries”, and “pain” by using Boolean operators “and” and “or”, in order to obtain more targeted results.
Moreover, a machine learning-based approach was adopted using the MySLR digital platform [20], a semi-automated tool supporting scientists in performing systematic literature reviews (SLRs), available upon registration at https://myslr.unical.it (accessed on 2 March 2024). This tool allowed us to use a methodological approach that included different steps, namely paper selection, their analysis, and the synthesis of the results.
The first step consisted of the selection discussed above. Overall, 572 papers were retrieved, of which 312 were found in the Scopus database, 136 on the Web of Science, and 124 on the PubMed search engine. In the second step of the bibliographic search, duplicates (211) were removed and the remaining papers (361) were manually screened on the basis of their title and abstract in order to select those papers that fitted the eligibility criteria. The following inclusion and exclusion criteria were adopted: studies published in English and articles whose title and/or abstract referred to the specific use of Arnica formulations on traumatic injuries or correlated pain and inflammation were included, while book chapters, previous reviews, retracted articles, and letters to editors were excluded, together with papers not in English.
After this primary screening, 298 manuscripts were manually excluded and 9 full-papers were not retrieved. The remaining 54 full-text records were deeply inspected. Finally, 42 references were included in this review.
Figure 1 describes the selection process of the bibliographic sources cited in this review.
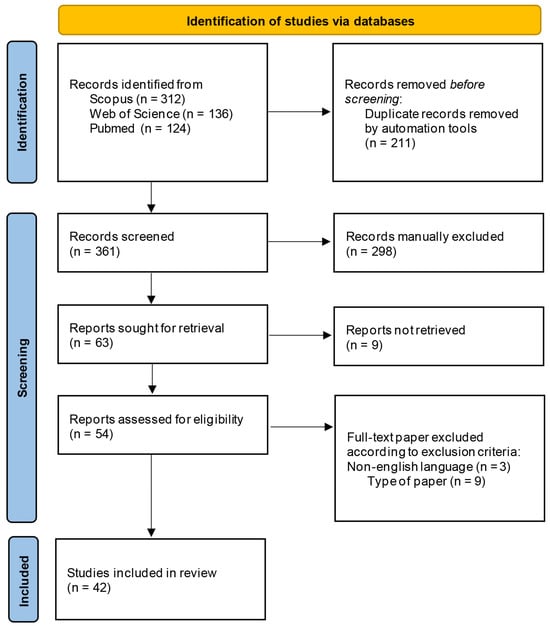
Figure 1.
Selection process of the eligible papers based on the PRISMA 2020 flow diagram.
As already mentioned, a number of papers focusing on Arnica have been published in some homeopathic journals that are not indexed in the main databases [17] and they have not been included in this review. However, starting from 1999 up to the present date, it is possible to observe a constant interest on this topic by mainstream journals, and both in vivo studies and clinical trials have been carried out to investigate the potential health benefits of Arnica on traumatic injuries and inflammatory diseases. Figure 2 reports the distribution of the selected papers by years of publication. Figure 3 illustrates the most significant keywords, visually represented through a “word cloud”.
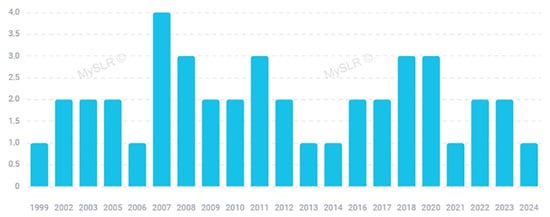
Figure 2.
Papers included in this review distributed by year (created with MySLR).

Figure 3.
Word cloud highlighting the importance of keywords (Produced by MySLR platform).
3. Commonly Used Routes of Administration and Arnica montana Phytochemical Content
A. montana is usually administered topically in pharmacological doses, or orally in very diluted doses that do not present side effects as a homeopathic drug [3]. Indeed, when ingested this herbal remedy can induce vascular dilation, blood stasis, and increased capillary permeability [21]. According to the homeopathy’s main principle “like cures like”, a substance in small amounts is able to treat those symptoms that in the healthy individuals are due to higher doses of the same compound [22]. As a homeopathic remedy, Arnica dilutions are expressed in Roman numeral units. Dilutions 1:10 are labeled with “X” (or “D”, decimal scale); “C” (centesimal scale) corresponds to dilutions 1:100; and dilutions 1:1000 are denoted as “M” (millesimal scale). The unit “LM” (or “Q”, fifty millesimal scale) refers to dilutions 1:50,000. According to these dosages, arnica 10X and arnica 10C have been diluted 10 times 1:10 and 10 times 1:100, respectively [4,23]. The potency of homeopathic arnica, as well as other homeopathic remedies, is considered to be inversely related to the concentration of the remedy. The more times it has been diluted, the more potent the remedy is thought to be [24].
According to the European Pharmacopoeia, the A. montana tincture is obtained from the flowers and should contain 0.04% sesquiterpene lactones expressed as dihydrohelenalin tiglate, and it contains one part of the drug in ten parts of 60% (v/v) to 70% (v/v) ethanol. According to European Union, the herbal A. montana preparations include flower tincture 1:10 extracted with ethanol 70% v/v or 1: 5 extracted with ethanol 60% v/v and liquid extract (1:20) obtained with ethanol 50% m/m [14].
Sesquiterpene lactones (STLs) of the helenanolide type, i.e., helenalin, 11a,13-dihydohelenalin, and their short-chain carbonic acid esters, are among the most important phytoconstituents identified in A. montana species, together with flavonoids, fatty acids, monoterpenes, and sesquiterpenes [14]. STLs are the most important phytochemicals of these species, as they are considered the active principles responsible for the pharmacological importance of Arnica. They include different esters of helenalin and 11,13-dihydrohelenalin with low-molecular-weight carboxylic acids, namely acetic, isobutyric, methylbutyric, methacrylic, and tiglic acid. Small amounts of the unesterified parent STLs are also present [25]. Phenolic acids including chlorogenic acid, caffeic acid, and cynarin have been also detected, as well as carotenoids, diterpenes, pyrrolizidine alkaloids, and polyacetylenes. Some coumarins, i.e., umbelliferone and scopoletin, have been identified as well [14,26,27,28,29]. It has been observed that the level of flavonoids and caffeic acid derivatives may also vary depending on the altitude of the growing site [30]. Moreover, the presence of some lignans have been detected by Schmidt and colleagues, who identified these components in A. montana and three other Arnica species, namely A. angustifolia, A. lonchophylla, and A. chamissonis [31]. The essential oils from the roots and rhizomes of A. montana are particularly rich in 2,5-dimethoxy-p-cymene and 2,6-diisopropylanisole [32].
4. In Vitro and In Vivo Studies Concerning Arnica montana L. Formulations
A. montana is the most investigated species belonging to the Arnica genus. The beneficial effects of both phytotherapeutic and homeopathic formulations of A. montana were assessed in different in vitro studies (Table 1).

Table 1.
In vitro studies concerning the effectiveness of Arnica montana L. phytotherapeutic and homeopathic formulations.
One of these interesting studies was carried out by Verre and coworkers, who verified the effects of A. montana tincture and homeopathic dilutions on inflammation markers and oxidative stress in different human and murine cell culture models [33]. The mother tincture was prepared using 45% ethanol (v/v) at a ratio of 1/10, and the homeopathic dilutions 1C, 3C, 5C, and 9C were also tested. The potential anti-inflammatory properties were measured evaluating the levels of the inflammatory markers TNFα, IL-6, COX-2, MCP-1, ICAM-1, as well as oxidative stress compounds (ROS). Both mother tincture and 1C dilution significantly reduced TNFα production in inflamed macrophages and IL-6 and MCP-1 in inflamed human microglial cells. COX-2 expression was also significantly decreased in inflamed murine fibroblasts. Moreover, the expression of ICAM-1 was significantly reduced by all the Arnica formulations tested in inflamed human endothelial cells compared with the vehicle. As regards the effects on the oxidative stress, a significant consistent effect on ROS production in inflamed murine microglial cells was observed, with 1C dilution being the most effective sample [33].
Jäger and colleagues assessed the potential anti-arthritic activity of the flower head tincture, obtained through the extraction of the fresh plant material with ethanol 70% v/v. The extract was tested on human and bovine articular chondrocytes [34]. The Arnica sample was demonstrated to suppress MMP1 and MMP13 mRNA levels in a concentration-dependent manner in both cell lines. These collagenases are enzymes involved in the pathological destruction of cartilage [37,38]. The effects induced by the Arnica sample were supposed to be due to the inhibition of DNA binding of the transcription factors AP-1 and NK-kB [34].
Das and coworkers investigated the ability of A. montana to repair the DNA damage. The experiments were performed on E. coli exposed to ultraviolet irradiation, and the homeopathic dilution 30C was evaluated. Bacteria were treated with the Arnica sample, and exposed to UV light at 25 and 50 J/m2. Compared to the control, treated bacteria showed a lesser DNA damage. Moreover, a decrease in ROS generation and an increase in SOD, CAT, and GSH activities were observed following Arnica treatment, demonstrating a lesser oxidative stress compared to untreated bacteria. The up-regulation of repair genes was also demonstrated using the reverse transcription polymerase chain reaction method [35].
As the oxidative damage is associated with the pathogenesis of various diseases, including inflammation, arthritis, and also trauma [39,40,41], the evaluation of the antioxidant potential of the A. montana sample appears to be useful for the evaluation of its potential health beneficial properties and its anti-inflammatory potential. The antioxidant potential of A. montana was reported by Gawlik-Dziki and colleagues, who assessed the in vitro biological properties of ethanolic extracts from A. montana flower heads and rhizomes. The extracts demonstrated chelating power ability and lipid peroxidation inhibitory potential. Moreover, an in vitro lipoxygenase inhibitory activity was observed [36].
The biological properties of the species were also assessed through in vivo studies, some of which focused on the potential health benefits of Arnica topical formulations (Table 2).

Table 2.
In vivo studies on Arnica montana L. phytotherapeutic and homeopathic formulations effectiveness.
Alfredo and coworkers investigated the effects of a phytotherapeutic formulation of Arnica gel containing 200 mg of tincture per g, both associated with phonophoresis and alone [42]. The study was performed on 40 Wistar male rats. The tibialis anterior muscle was surgically lesioned, and animals were divided into four groups receiving, respectively, no treatment; a session of phonophoresis (3 min) daily for 2 days; phonophoresis plus Arnica gel with the same scheme of application; and a massage with Arnica formulation without phonophoresis. The histological analyses performed on inflamed muscle revealed that the Arnica group presented a lesser density of polymorphonuclear cells when compared to the other ones, while no significant differences were found between phonophoresis and phonophoresis-plus-Arnica groups. The massage with Arnica gel was reported to have a significant anti-inflammatory effect on acute muscle lesion in topic use, while A. montana phonophoresis proved to be ineffective.
Sharma and colleagues investigated the in vivo anti-arthritic potential of A. montana. The flower methanolic extract was tested on collagen-induced arthritis in rats. Animals were treated with a dosage of 75 mg/kg body weight administered orally for 20 days, and the effects of the treatment were compared to those induced by dexamethasone (1 mg/kg) and the vehicle. The Arnica extract was demonstrated to induce a lower expression of tumor necrosis factor-α, interleukins IL-1β, IL-6, and IL-12, nitric oxide, and of the titer of the anti-type II collagen antibody compared with untreated rats, also showing antioxidant potential [43].
Furthermore, A. montana was also explored for its the beneficial properties in the treatment of skin disorders related to inflammatory conditions. Da Silva Prade and colleagues investigated the anti-inflammatory effects on a UVB radiation-induced skin–burn model [8]. Male Swiss mice were first exposed to UVB radiation and then treated with an ointment containing A. montana (250 mg/g) on the ear. The positive control group received dexamethasone. Both edema and the inflammatory response were measured 16 h after the treatment, together with the oxidative stress. The authors reported that A. montana treatment reduced the UVB-induced inflammation, as a reduction in ear edema was observed, together with an inhibition of the myeloperoxidase activation. Moreover, the levels of proinflammatory cytokines, such as IL-1β, IL-6, TNF-α, and IFN-γ, were reduced, and the UVB-induced oxidative damage was ameliorated.
5. Clinical Trials Evaluating the Efficacy of Arnica montana L. Formulations
In addition to the preclinical studies reported in the section above, several clinical trials focusing on the use of phytotherapeutic or homeopathic A. montana formulations have been performed. Table 3 summarizes these studies, reporting the kind of extract/sample, the study design, the treatment, and the obtained results of each study, classifying them according to the type of use and clinical application tested.

Table 3.
Clinical trials concerning the effectiveness of Arnica montana L. phytotherapeutic and homeopathic formulations.
5.1. Muscle Pain and Bruising
Many therapeutic interventions have been tested to alleviate muscle soreness. One of the difficulties for researchers is to find a large pool of participants with similar muscle injuries. The delayed onset muscle soreness (DOMS), a common result of muscular overexertion in unusual exercisers, is useful for researchers to carry out a systematic investigation, as DOMS symptoms are similar to many athletic injuries [24]. A phytotherapeutic formulation of Arnica was tested for its effects on muscle pain and performance. Pumpa and colleagues reported a double-blind, randomized placebo-controlled trial involving 20 well-trained males, and focused on the effectiveness of an A. montana gel containing tincture equiv. to dry flower herb 10 mg per g. The formulation was tested for its ability to reduce pain and improve performance in well-trained males with DOMS. The gel was topically applied to the skin on the quadriceps and gastrocnemius muscles immediately after a downhill running protocol designed to induce DOMS, and applied every 4 waking hours. The formulation was demonstrated to provide pain relief 72 h post-exercise, even if it did not affect any markers of muscle damage or inflammation [44].
Leu and colleagues described a rater-blinded randomized controlled trial on the ability of a topical 20% Arnica formulation to enhance the resolution of laser-induced bruising. In this study, involving healthy volunteers aged between 21 and 65, four bruises of 7 mm diameter each were created on the bilateral upper inner arms, using a 595 nm pulsed-dye laser. The patients were divided into four groups, each randomly receiving 5% vitamin K, 1% vitamin K, 0.3% retinol, 20% Arnica, or white petrolatum. The improvement due to the treatment with 20% Arnica was greater than the placebo and the mixture of 1% vitamin K and 0.3% retinol, even if it was not higher than that induced by 5% vitamin K cream [45].
5.2. Postoperative Pain, Edema, and Wound Healing
Different therapies have been taken into account to improve pain control after surgical procedures, including complementary therapies. Among these, the role of homeopathic Arnica has been investigated, for example, to control pain following ambulatory knee surgery, as after this kind of procedure patients are notoriously encouraged to move the knee [57]. The success of the intervention is closely linked also to the control of postoperative pain, usually based on the use of systemic or intra-articular application of non-steroidal anti-inflammatory drugs (NSAIDs) or opiates [46].
Brinkhaus and colleagues described three randomized, double-blind trials designed to assess the effectiveness of homeopathic Arnica in decreasing swelling and pain following knee surgery (Table 3) [46]. The authors took into account three different conditions: knee arthroscopy, artificial knee joint implantation, and cruciate ligament reconstruction. Participants enrolled in the trials (227, 35, and 57, respectively) were patients of both genders whose age was between 18 and 75 years, and who were suffering from knee diseases needing surgery. These studies aimed to verify the efficacy of arnica 30X administered orally using sucrose globules. Five globules were given to patients three times a day from the day of surgery to the end of the follow-up examination. The authors reported a significantly reduced postoperative swelling in patients receiving homeopathic arnica compared to those receiving the placebo in the trial concerning the cruciate ligament reconstruction, while just a trend towards a lesser swelling was observed in the other two examined conditions.
An interesting study was described by Jeffrey and Belcher [17], who took into account the combined administration of both homeopathic and phytotherapeutic Arnica formulations to relieve pain following carpal tunnel release surgery. The treatment was based on the use of the homeopathic arnica D6 tablet and an ointment containing the 10% w/v of A. montana ethanolic extract. Forty patients were entered into the clinical trial and divided into an Arnica and a placebo group. The administration of Arnica tablets started from the day of surgery for two weeks, and the ointment was administered through a gentle massage three times daily during the same period. A significant reduction in pain was observed after 2 weeks of treatment, while any difference in grip strength and wrist circumference was found between the two groups.
Furthermore, the potential analgesic effects of homeopathic arnica were also verified after tonsillectomy. A randomized, double-blind, placebo-controlled trial, involving 190 patients aged over 18 years and undergoing tonsillectomy was reported by Robertson and colleagues. Patients were divided into two groups: receiving arnica 30 CH or placebo tablets. Two tablets six times on the first postoperative day and then two twice a day for the next seven days were administered. A small but statistically significant decrease in pain scores was observed compared to the placebo [47].
Macedo and colleagues evaluated the efficacy of homeopathic arnica 6 CH in 32 patients who underwent the extraction of impacted third molars. In the described crossover and double-blind study, the authors assessed the impact on edema, pain, and also mouth opening compared to the placebo. The patients received five drops sublingually three times a day before surgery, followed by five drops two times a day at the day of surgery, and three times daily the first day after surgery. Even if any effect was observed on pain and mouth opening, the treatment significantly reduced edema compared to the control group [48].
The homeopathic tablets arnica 30X were tested for their efficacy on the postsurgical sequels following extraction of impacted mandibular third molars as well [49]. Mawardi and coworkers described a case–control pilot study involving 23 patients, which were divided into a treatment and a control group. They received four tablets 1 h before the procedure and four tablets four times per day starting 1 h after the procedure and for the three following days. The treatment induced a significant reduction in pain, bleeding, bruising, and edema, and a decrease in maximum mouth opening compared to the control group.
The homeopathic formulation 200C was also tested for its effects on post-extraction pain management. In a triple-blind randomized controlled trial, 44 children aged between 8 and 12 years requiring two clinical sessions of tooth extraction in two different quadrants of the oral cavity were selected. In this crossover trial, all the children received both arnica and ibuprofen with a washout of 10 days. Authors reported that no differences were observed between arnica and ibuprofen in the post-extraction pain management, suggesting that arnica may be considered as an alternative to ibuprofen in managing post-extraction pain in 8–12-year-old children [21].
The efficacy of homeopathic arnica capsules was tested for its beneficial effects in reducing edema associated with rhinoplasty. Forty-eight patients were randomly treated with arnica or dexamethasone three times a day for 4 days. Even if arnica did not provide any benefit with regard to the extent of ecchymosis, the study suggested that it was effective in reducing edema during the early postoperative period following rhinoplasty [50].
Karow and colleagues focused on the wound healing properties of Arnica after surgery, rather than the decrease in pain. In a randomized double-blinded, parallel-group study involving 88 patients, the authors investigated the efficacy of arnica 4D pills administration in healing the wounds due to hallux valgus surgery [51]. The efficacy of arnica was compared to those of diclofenac sodium (50 mg per os, three times daily). Both this drug and arnica 4D (10 pills three times per day) were administered to patients undergoing surgery for hallux valgus for 4 days following the intervention. Both treatments were equivalent as for wound irritation and patient mobility. On the contrary, arnica showed a lesser analgesic activity but was better tolerated than diclofenac.
Mendes and coworkers evaluated the beneficial effects of Arnica on the cicatrization process of aphtha, the small and painful ulcer on human oral mucosa [52]. Thirty-one patients utilized an ointment containing Arnica tincture. The formulation was topically applied three times daily. The medication demonstrated anti-inflammatory activity and beneficial effects on the healing process of lesions in oral mucosa.
Interestingly, Nejadbagheri and colleagues evaluated the use of A. montana in reducing the pain associated with arteriovenous fistula puncture in patients receiving hemodialysis. In a double-blind single-group randomized clinical trial, patients received an arterial and a venous fistula puncture, randomly allocated to the experiment and the placebo. Before needle insertion, the experiment and the placebo sites were treated for 10 min with a cream containing 5 mg of A. montana extract per 100 mg or vitamin A and D ointment, respectively. The treatment was effective in reducing pain [53].
5.3. Trauma-Induced Pain
Lower back pain is one of the most common health problems worldwide, and it can be due to different factors, such as overuse, muscle strain, trauma, or injuries to the ligaments, muscles, and discs that support the spine [58]. The homoeopathic arnica has been tested in conjunction with physiotherapy to treat traumatic low backache by Alekar and colleagues, who recruited 30 volunteers whose lower back pain was related to a trauma, while all other causes of backache were excluded from the trial. The patients were of both sexes between 20 and 40 years of age (Table 3) [54]. The subjects were divided into three groups: the group A received a dose of arnica 200 twice a day for 15 days, the group B received constitutional medicine (with Bryonia alba Linn., Rhus toxicodendron Linn., and Nux Vomica Linn. being administered on a constitutional basis), and group C received the placebo. Each of the three groups also received a daily session of physiotherapy. Significant improvement in pain scores was observed, with a more pronounced effect in group B. The authors suggested that an individualized approach was more effective in the management of patients. After 15 days of treatment, an assessment was carried out and the repetition of the dose was decided accordingly.
5.4. Osteoarthritis
Arnica has been used for centuries in traditional medicine as a remedy for traumatic injuries and inflammatory conditions of the locomotor system [59].
Knuesel and coworkers reported the results of a 6-week multicenter trial focused on the safety and efficacy of an A. montana gel formulation and involving 79 patients with mild-to-moderate osteoarthritis of the knee (Table 3). Patients were asked to apply a thin layer of A. montana gel to the affected knee two times per day. An amount of 100 g of gel contained 50 g of Arnica fresh plant tincture (drug-to-extract ratio 1:20 in 50% ethanol). The study demonstrated that the topical application of the Arnica gel was effective, being at the same time safe and well tolerated, with the exception of an allergic reaction that occurred in one of the patients [55].
Widrig and colleagues tested the efficacy of a phytotherapeutic Arnica gel formulation (50 g A. montana tincture/100 g, with a drug-to-extract ratio of the tincture 1:20) on osteoarthritis of interphalangeal joints the hand. The performed randomized, double-blind study involved 204 patients, randomly treated with ibuprofen or Arnica gel. Patients were asked to apply the formulation over the affected joints thrice daily for 3 weeks, and were asked not to wash their hands for one hour after application. At the end of the treatments, no differences between the two groups in pain and hand function improvements were observed. Adverse events were reported by six patients using ibuprofen and by five patients treated with Arnica. These results confirmed that arnica gel is not inferior to ibuprofen on the symptoms of osteoarthritis of the hand [56].
6. Case Reports
Some case reports focusing on the pharmacological properties of arnica homeopathic formulations have been also reported (Table 4).

Table 4.
Case reports concerning the effectiveness of Arnica montana L. formulations.
Barkey and colleagues described the use of a homeopathic arnica patch (3X) for its anti-inflammatory effects in a 55-year-old female patient with cellulitis-derived pain and numbness in the hand. Arnica patches were applied on the painful areas of the hand at night and a decrease in pain symptoms was reported after three days [60].
Another case report dealt with a phytotherapeutic formulation of A. montana. Jackson and Tummon Simmons assessed the beneficial outcomes of the topical application of a 200 mg/mL A. montana flowering tops formulation in olive oil (1:5 herb-to-olive oil ratio) in an 82-year-old Caucasian female suffering from severe shoulder deterioration due to osteoarthritis. During the first five weeks, the treatment consisted of the topical application of Arnica oil with a massage for about five minutes followed by acupuncture. On the sixth week, therapeutic ultrasound was also introduced. Some positive outcomes were reported, such as a decreased pain score just after the first week of treatment, which was then further reduced following the introduction of therapeutic ultrasound. Moreover, a decrease in medication usage and an improved functionality were observed [61].
7. Clinical Studies on Formulations Containing Arnica and Other Plant Species
Arnica is often used as one of the ingredients of formulations also containing other plant extracts aimed at treating trauma and inflammatory conditions.
Bartolomei and colleagues verified the effectiveness of a mud containing both 3% A. montana essential oil (EO) and 5% menthol to provide pain relief after a high-volume resistance training session [62]. A randomized counterbalanced crossover study was conducted on 10 resistance-trained men that were asked to perform a high-volume resistance workout for lower body squats and leg extensions. The volunteers were aged between 18 and 35, with a minimum of 2 years of resistance training experience. Additional mud or placebos were topically applied above the quadriceps muscle of both legs 3, 19, 27, and 45 h after the workout, and muscle performance, morphology, and soreness were measured before and after the exercise. The observed results allowed us to conclude that muscle morphology was not influenced by mud packs, and that mud with A. montana could be useful to enhance the recovery rate of strength and to reduce muscle soreness after high-volume exercise (Table 5).

Table 5.
Clinical trials, observational studies, and other studies concerning the effectiveness of formulations containing A. montana L. and other plant extracts.
Zanella and colleagues evaluated the effectiveness of Arnica Comp. -Heel® (“Arnica compositum”) ointment in the treatment of symptomatic calcific periarthritis of the shoulder [63]. This homeopathic combined ointment contains a number of plants, namely arnica (A. montana L.), calendula (Calendula officinalis L.), chamomile (Matricaria recutita L.), comfrey (Symphytum officinale L.), milfoil (Achillea millefolium L.), deadly nightshade (Atropa belladonna L.), monkshood (Aconitum napellus L.), daisy (Bellis perennis L.), St John’s wort (Hypericum perforatum L.), narrow-leaved cone flower (Echinacea angustifolia DC.), purple cone flower (Echinacea purpurea L.), witch hazel (Hamamelis virginiana L.), together with mercurico-amidonitrate and calcium sulphide. A pilot study was described, in which 41 patients with calcific periarthritis of the shoulder were treated with a combination of “Arnica compositum” ointment (AC) + Acidum nitricum (AN) + Hekla lava (HL) or with Arnica compositum ointment alone. Patients were asked to apply the formulations on the shoulder and then to cover the shoulder with a bandage, in order to prevent the immediate removal of the material by rubbing on clothing. After a 3-day therapy, the reduction in pain as well as the improvement of shoulder motion were superior in the group treated with AC + AN + HL ointment mixture compared to the group receiving AC ointment alone.
Differently from the study by Macedo [48], who tested a homeopathic dilution containing just A. montana, De Souza and colleagues evaluated the homeopathic preparation Traumeel® S in the control of postoperative outcomes (pain, edema, and trismus) associated with the surgical removal of mandibular third molar teeth [64]. The remedy Traumeel® (Biologische Heilmittel Heel GmbH, Baden-Baden, Germany), has also been marketed in Italy under the name Arnica compositum (Arnica Comp. -Heel®) [74], so it is the same formulation of 12 botanical and 2 mineral substances already described before (Achillea millefolium, Aconitum napellus, A. montana, Atropa belladonna, Bellis perennis, Calendula officinalis, Matricaria recutita, Echinacea angustifolia, Echinacea purpurea, Hamamelis virginiana, Hypericum perforatum, Symphytum officinale, mercurico-amidonitrate, and calcium sulphide) [64,75]. In the preliminary randomized triple-blind clinical trial described by de Souza, 17 patients received Traumeel®S or dexamethasone preoperatively by injection into the masseter muscle. Pain, edema, and trismus (a condition of restricted opening of the mouth) levels were observed after 24 h, 72 h, and 7 days. The results obtained for the treatment were not different from those of dexamethasone at all postoperative evaluations, allowing us to conclude that Traumeel® S might be a good alternative approach to dexamethasone for controlling pain, edema, and trismus after third molar removal [64].
In a pilot clinical trial involving 30 patients, Singer and colleagues tested the efficacy of the same formulation on pain following elective Hallux valgus surgery [65]. A first group received an injection of Traumeel® S (2.2 mL) into the operative incision upon conclusion of surgery, while a second one received both injection and then tablets orally three times a day for 13 days or until pain was negligible. Both single-injection and injection-plus-oral-treatment groups were effective in lowering pain as compared to the control group, suggesting that the formulation was effective in minimizing postoperative pain following repair of H. valgus.
In another study, A. montana was combined with Bellis perennis in a homeopathic formulation tested for the reduction in seroma following mastectomy and immediate breast reconstruction. The medication was based on the combination of A. montana C30 and Bellis perennis C30. This randomized, double-blind, placebo-controlled trial involved 55 patients, who were randomly assigned to the treatment with homeopathic medication or the placebo, administered as spherical saccharose globules. Obtained results demonstrated that the formulation was able to reduce seroma formation and also to decrease opioid intake following mastectomy and reconstruction [66].
Traumeel® S was also investigated for its effectiveness in the treatment of tendinopathies of varying etiology, taking into account both pain- and motility-related variables. Schneider and coworkers reported a nonrandomized observational study involving 357 patients with tendinopathies based on an excessive tendon load rather than inflammation. Patients were treated with Traumeel® S ointment or diclofenac 1% gel for a maximum of 28 days. The study demonstrated that arnica homeopathic formulation was not inferior to diclofenac therapy as regards both pain and motility [67].
Janczewska and colleagues tested the analgesic effectiveness of Traumeel® S ointment in patients with gonarthrosis. The topical treatment was used in combination with magnetic field LED therapy [68]. Magnetic field LED therapy is a physical therapy modality consisting in the combined effects of extremely low-frequency electromagnetic fields and high-power light emitted by light-emitting diodes (LEDs) [76]. Ninety patients with knee osteoarthritis were divided into three groups, and treated with magnetic stimulation plus LED therapy, Traumeel® S ointment, and their combination, respectively. The combined therapy gave positive results in terms of pain reduction, as well as the other two groups, even if the strongest analgesic factor seems to be magnetic and LED therapies [68].
Phytoterapeutic Arnica was also investigated for its effects on ankle distortion. A spray formulation combining Arnica tincture and hydroxyethyl salicylate was tested on 570 patients with acute ankle joint distortion in a randomized double-blind study [69]. Patients were divided into three groups receiving the combined formulation, Arnica, and hydroxyethyl salicylate alone, respectively. The treatment was applied four-to-five times daily for 10 days. A significant pain improvement after the combination spray administration was observed compared to Arnica and hydroxyethyl salicylate groups.
The same combined formulation, a combination spray of Arnica tincture and hydroxyethyl salicylate, was also tested for its analgesic properties and the potential use in sports injuries and diseases of the locomotor apparatus [70]. In this randomized, single-blind trial on 40 healthy volunteers, each subject received both the drug and placebo, filled into white 50 mL plastic bottles with a spray top. The spray was used on a defined skin area, the inner side of the forearm. The combination preparation was more effective than both the placebo and the individual active components.
Madisetti and colleagues tested a topical wound powder containing A. montana 0.01% v/w together with other plant species, including Calendula officinalis L., Mentha arvensis, and Santalum album. The pilot study demonstrated the feasibility, acceptability, and tolerability of a wound care powder for hospice patients with chronic wounds at the end of their life [71]. The effects of this formulation on pain and exudate in 50 patients with ulcers and vascular wounds receiving palliative care at the end of their life was also described in another study, reporting a significant reduction in both pain and exudate [72].
The potential beneficial effects of Arnica containing formulations were also taken into account for the treatment of burns. Huber and coworkers reported a case study in which two individuals used the formulation Combudoron ® (Weleda AG Schwäbisch Gmünd, Germany), containing the ethanolic extracts of Urtica urens and A. montana, for the treatment of laser-induced burns. Four experimental-grade burns (1 cm2) were induced with a laser and the lesions were treated for 15 days with Combudoron gel, Combudoron liquid, placebo gel, or placebo liquid in each of the subjects. The treatment seemed to have positive effects on burns [73].
8. Other Arnica Species
Besides A. montana, another Arnica species has been also evaluated for its potential health benefits in inflammatory diseases and trauma. The tinctures from different parts of Arnica chamissonis Less. were demonstrated to exert an in vitro radical scavenging activity, chelating power ability, and lipid peroxidation inhibitory potential. Moreover, this species showed xanthine oxidase inhibitory activity. This study, by Gawlik-Dziki and colleagues, demonstrated that this species could be also useful in the prevention and treatment of free radical-dependent diseases (Table 6) [36].

Table 6.
Arnica chamissonis Less. effectiveness.
9. In Vivo Studies and Clinical Trials with Negative Outcomes
As underlined by Fanelli [77] and by Matosin and coworkers [78], the discussion of negative outcomes is very important in science, as results that do not confirm the expected effect, being both not statistically significant and in contradiction with the hypothesis, are important for science as the positive ones, as they allow a collective self-correcting process. Negative results are valuable data from the scientific literature as they allow a critical evaluation and validation of our current thinking [78].
In addition to all the positive results previously discussed, some of the studies dealing with the beneficial properties of Arnica also reported negative outcomes (Table 7).

Table 7.
Studies with negative outcomes.
Adkison and coworkers investigated the effectiveness of a commercially available Arnica homeopathic cream formulation 1X HPUS-7% [4]. HPUS is an acronym that stands for Homeopathic Pharmacopeia of the United States, and indicates that the remedy meets defined criteria, as stated in the Homeopathic Pharmacopeia, such as strength, quality, purity, packaging, or label requirements [22]. The study by Adkison et al. involved 55 male and female volunteers between the age of 18 and 65 years, with both legs functioning without acute or chronic pain. In this randomized, placebo-controlled, and double-blind study design, each participant received two containers of cream produced by the same manufacturer, marked as “left” and “right”. One of the tubes held a commercially available arnica cream formulation, and the other one held the vehicle–placebo cream without the active ingredient. The subjects were asked to apply the formulations to the legs immediately after the exercise, and also at 24 and 48 h post-exercise, and to rate the pain in each leg at the baseline, and after 24, 48, and 72 h. The average amount of cream applied by each subject was found to be 5 g. A negative outcome was observed in this study, since arnica cream was found to increase leg pain 24 h after calf exercises rather than decreasing it. Any significant difference was instead observed among Arnica and placebo after 48 and 72 h [4].
The absence of effectiveness was reported also in some studies concerning the use of Arnica formulations on postoperative pain and bruising. An in vivo study on the analgesic properties of homeopathic arnica was performed on the control of postoperative pain in cats subjected to a hysterectomy with bilateral salpingo-oophorectomy. The analgesic effects of different formulations and routes of administrations were tested, namely arnica 30X per subcutaneous route (SC), and per oral transmucosal route (OT), and arnica 6X per OT. Their efficacy was compared to those of morphine and ketoprofen. The obtained results demonstrated that arnica showed less efficacy than ketoprofen and morphine. Furthermore, no significant difference was detected either between the different formulations or between the routes of administration [79].
Differently from the randomized, double-blind study from Jeffrey and Belcher, who observed a significant pain reduction in patients undergoing carpal tunnel release surgery [17], negative outcomes related to the use of Arnica were described by Stevinson and colleagues [80]. These authors reported a double-blind, placebo-controlled, randomized trial involving 64 adults undergoing surgery for carpal tunnel syndrome. Patients were treated with homeopathic arnica 30CH or 6CH or placebo tablets, which were administered three times daily for seven days preoperatively and fourteen days postoperatively. Patients were asked not to swallow the tablets, in order to have an oral transmucosal route of administration. No significant effects were observed in reducing postoperative pain, bruising, and swelling compared to the placebo.
Homeopathic Arnica was also a component of a formulation tested for its ability to reduce analgesic morphine consumption following knee ligament reconstruction. This phase III randomized placebo-controlled study was reported by Paris and coworkers and involved 158 patients undergoing knee ligament reconstruction. The complex tested contained A. montana 5 CH, Bryonia alba 5 CH, Hypericum perforatum 5 CH, and Ruta graveolens 3 DH. The activity of the tested formulation was not higher than the placebo [81].
Lauche and colleagues reported a randomized controlled trial focused on the effectiveness of a cupping massage using A. montana oil compared to progressive muscle relaxation in patients with chronic non-specific neck pain [82]. Sixty-one patients were randomly divided into two groups, treated for 12 weeks with a partner-delivered home-based cupping massage with Arnica oil, or underwent progressive muscle relaxation for the same period. Even if both groups showed significantly less pain compared to the baseline, any significant difference was detected among the two groups, with the cupping massage being no more effective than progressive muscle relaxation in reducing chronic non-specific neck pain.
Differently from Pumpa, who successfully tested the effects of an Arnica phytotherapeutic formulation on DOMS [44], Tuten and colleagues did not obtain positive outcomes when testing the sublingual administration of homeopathic arnica 6X pellets [24]. Twenty-three university students aged between 18 and 25 were instructed to take three pellets sublingually before following a bench-stepping protocol in a double-blind randomized study. The obtained results allowed us to conclude that arnica at 6X potency was not an efficacious agent for moderating DOMS.
10. Arnica Absorption and Side Effects
Arnica preparations are generally considered safe, both if used topically on intact skin and orally in homeopathic doses. However, some side effects, such as a rash, dry skin, and itching, have been reported [4].
Moreover, commercialized Arnica preparations are often not standardized, and for this reason it is not possible to accurately recommend a dose [23].
As regards the use of Arnica in homeopathic preparations, the homeopathic remedies are subject to regulatory control in several countries, such as the European Community, USA, Canada, and Australia. However, despite a long experience and the common perception of safe use, comprehensive toxicological data for the most homeopathic remedies are often scarce or even lacking. Moreover, homeopathic medicines can contain a multitude of different homeopathic ingredients, including raw material of botanical origin, environmental materials such as contaminants, food additives, metals, and even starting materials of biological origin such as micro-organism preparations. The abundance and diversity of homeopathic ingredients make an appropriate safety evaluation difficult [22].
Some sensitizing effects have been related to the use of A. montana and related species, above all induced by self-treatment with Arnica tincture. These skin reactions have been demonstrated to be due to the sesquiterpene lactones helenalin, its acetate, and methacrylate [83,84].
The absence of toxic effects was instead reported in an in vivo study by Jürgens and coworkers, who tested the dermal application of Arnica tincture in rats for the potential treatment of cutaneous leishmaniasis [85].
To assess the dermal absorption of the Arnica tincture and its bioactive constituents, STLs, dermal absorption experiments on porcine and human skin (“ex vivo”) in diffusion cells under near-physiological conditions were also performed. In this research, Jürgens and colleagues estimated the amounts of STLs on the skin surfaces, in skin extracts, and in the receptor fluids by UHPLC-HRMS. Within 48 h, a maximum of about 8% and 36% of STLs permeated through porcine and human skin. A significant portion of sesquiterpene lactones did not permeate and could not be extracted from the skin because of an irreversible bounding to skin proteins [86].
11. Future Perspectives
An important aspect about the pharmacological potential of A. montana has been recently raised by Schmidt, who underlined some important differences among A. montana populations from different geographical areas. It is well known that STLs of the helenanolide type, including a variety of esters of helenalin and 11,13-dihydrohelenalin with low-molecular-weight carboxylic acids, are the main active principles responsible for the Arnica biological potential. However, a great difference has been observed between A. montana flowers from plants growing in Central or Eastern Europe, containing above all helenalin esters, and those originating from the Iberic peninsula, which contain almost exclusively 11,13-dihydrohelenalin derivatives. The presence of two A. montana subspecies have also been proposed: subsp. montana, mainly occurring in Central and Eastern Europe, and subsp. atlantica, present in the Iberian Peninsula [25]. From the point of view of the systematic botany, this difference has not been cited in the recognised database, The World Flora Online (WFO) (http://www.worldfloraonline.org/, accessed on 27 August 2024) [1], in which only Arnica montana subsp. montana is currently mentioned as a subspecies of A. montana, while Arnica montana subsp. atlantica A.Bolòs is mentioned as a subspecies of Arnica chamissonis Less. However, the presence of two chemotypes is reported to be evident, and the need to compare their pharmacological activity through in vitro, in vivo, and even clinical studies has been proposed in order to assess their efficacy and safety [25].
Moreover, another aspect to be taken into account should be the utilized extraction technique. It is well known that the qualitative and quantitative profile of the phytochemical composition of an extract from plant material depends on the selection of the proper extraction method [87]. Nowadays, response surface methodology (RSM) in extraction processes represents a useful tool for the optimization of the procedures in analytical chemistry and in phytochemistry [88,89]. Garcia-Oliveira and colleagues, for example, investigated how to improve the extraction of the phenolic compound from A. montana flowers through multivariate optimization of heat- and ultrasound-assisted methods, suggesting a potential industrial application of A. montana flower extracts with an improved resource utilization compared to conventional extraction methods [90]. The same kind of study should be helpfully applied to the extraction of STLs and other bioactive compounds.
12. Concluding Remarks
In their review published in 2015 and including eight clinical trials, Ernst and Pittler concluded that homeopathic Arnica could be not considered more effective than a placebo [91]. Some years ago (2016), Iannitti and colleagues focused on the use of Arnica on just postsurgical pain and inflammation. These authors evidenced that overall A. montana could be considered an alternative to non-steroidal anti-inflammatory drugs [92].
In the present review, following a selection process of eligible papers performed according to the PRISMA guidelines, 42 studies dealing with the effects on pain and inflammatory signs due to both traumatic injuries and inflammatory conditions of Arnica phytotherapeutic and homeopathic formulations have been discussed. Some of these works have been reported as in vitro and in vivo studies, while most of them were related to clinical trials.
Even if some studies report negative outcomes, a relevant number of evidences support the use of Arnica formulations for the treatment of pain, bruises, and swelling that occur after traumatic injuries related to sport and surgical interventions as well as with arthritis and other inflammatory conditions. These positive results refer to Arnica as both a single remedy and as a combination with other active principles and remedies.
Author Contributions
Conceptualization, C.-C.T., M.M. and G.S.; methodology, M.M. and G.S.; data curation, C.-C.T., M.M., M.P., F.C. and G.S.; writing—original draft preparation, C.-C.T., M.M., M.P., F.C. and G.S.; writing—review and editing, C.-C.T., M.M. and G.S.; supervision, G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Adm. | route of administration |
| AP-1 | activator protein-1 transcription factor |
| CAT | catalase |
| COX-2 | cyclooxygenase-2 |
| DOMS | delayed onset muscle soreness |
| Form. | formulation |
| GSH | glutathione |
| H | homeopathic |
| ICAM-1 | intercellular adhesion molecule |
| IFN-γ | interferon gamma |
| IL-12 | interleukin-12 |
| IL-1β | interleukin -1-beta |
| IL-6 | interleukin-6 |
| MCP-1 | monocyte chemoattractant protein-1 |
| MMP1 | collagenase-1 |
| MMP13 | interstitial collagenase-3 |
| NF-kB | transcription factor nuclear factor kappa B |
| O | oral |
| OT | oral transmucosal |
| P | phytotherapeutic |
| ROS | reactive oxygen species |
| SC | subcutaneous |
| SOD | superoxide dismutase |
| STLs | sesquiterpene lactones |
| T | topical |
| TNFα | tumor necrosis factor alpha |
| UHPLC-HRMS | ultra-high-performance liquid chromatography with high-resolution mass spectrometry |
| UV | ultraviolet |
References
- WFO. Available online: https://www.worldfloraonline.org/ (accessed on 19 August 2024).
- Plants of the World Online. Kew Science. Available online: https://powo.science.kew.org/ (accessed on 19 August 2024).
- Brito, N.; Knipschild, P.; Doreste-Alonso, J. Systematic Review on the Efficacy of Topical Arnica montana for the Treatment of Pain, Swelling and Bruises. J. Musculoskelet. Pain 2014, 22, 216–223. [Google Scholar] [CrossRef]
- Adkison, J.D.; Bauer, D.W.; Chang, T. The Effect of Topical Arnica on Muscle Pain. Ann. Pharmacother. 2010, 44, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, D.; Balabanova, V. Antioxidant and Acetylcholinesterase Inhibitory Potential of Arnica montana Cultivated in Bulgaria. Turk. J. Biol. 2012, 36, 732–737. [Google Scholar] [CrossRef]
- Craciunescu, O.; Constantin, D.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of Antioxidant and Cytoprotective Activities of Arnica montana L. and Artemisia absinthium L. Ethanolic Extracts. Chem. Cent. J. 2012, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Klaas, C.A.; Wagner, G.; Laufer, S.; Sosa, S.; Loggia, R.D.; Bomme, U.; Pahl, H.L.; Merfort, I. Studies on the Anti-Inflammatory Activity of Phytopharmaceuticals Prepared from Arnica Flowers. Planta Med. 2002, 68, 385–391. [Google Scholar] [CrossRef]
- da Silva Prade, J.; Costeira Bálsamo, E.; Romero Machado, F.; Rósula Poetini, M.; Cardoso Bortolotto, V.; Machado Araújo, S.; Londero, L.; Peterini Boeira, S.; Sehn, C.P.; de Gomes, M.G.; et al. Anti-Inflammatory Effect of Arnica montana in a UVB Radiation-Induced Skin-Burn Model in Mice. Cutan. Ocul. Toxicol. 2020, 39, 126–133. [Google Scholar] [CrossRef]
- Yalgi, V.S.; Bhat, K.G. Evaluation of Anti-Inflammatory Properties of Homoeopathic Gel Prearations of Calendula Officinalis, Arnica montana, Echinacea Angustifolia and Hypericum Perforatum by Zymography—An in Vitro Study. Int. J. Homoeopath. Sci. 2020, 4, 38–42. [Google Scholar] [CrossRef]
- Koo, H.; Gomes, B.P.F.A.; Rosalen, P.L.; Ambrosano, G.M.B.; Park, Y.K.; Cury, J.A. In Vitro Antimicrobial Activity of Propolis and Arnica montana against Oral Pathogens. Arch. Oral Biol. 2000, 45, 141–148. [Google Scholar] [CrossRef]
- Nieto-Trujillo, A.; Cruz-Sosa, F.; Luria-Pérez, R.; Gutiérrez-Rebolledo, G.A.; Román-Guerrero, A.; Burrola-Aguilar, C.; Zepeda-Gómez, C.; Estrada-Zúñiga, M.E. Arnica montana Cell Culture Establishment, and Assessment of Its Cytotoxic, Antibacterial, α-Amylase Inhibitor, and Antioxidant In Vitro Bioactivities. Plants 2021, 10, 2300. [Google Scholar] [CrossRef]
- Hiraoka, J.M.; de Oliveira, F.F.; Baldo, E.C.F.; Baldo, M.A.; Fernandes, F.F.; Gullo-Luzente, F.P. Antifungal Effect of Arnica montana and Hamamelis Virginiana against Candida Species. J. Health Sci. Inst. 2023, 41, 153–157. [Google Scholar]
- Žitek, T.; Postružnik, V.; Knez, Ž.; Golle, A.; Dariš, B.; Knez Marevci, M. Arnica montana L. Supercritical Extraction Optimization for Antibiotic and Anticancer Activity. Front. Bioeng. Biotechnol. 2022, 10, 897185. [Google Scholar] [CrossRef] [PubMed]
- Kriplani, P.; Guarve, K.; Baghael, U.S. Arnica montana L.—A Plant of Healing: Review. J. Pharm. Pharmacol. 2017, 69, 925–945. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Miles, V.N.; Holmes, D.T.; Chen, X.; Lei, W. Clinical Trials, Potential Mechanisms, and Adverse Effects of Arnica as an Adjunct Medication for Pain Management. Medicines 2021, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Charlton, F. Let’s Set the Record Straight! Traumatic Tissue Injury Treated with Homeopathic Arnica montana—A Injury Therapists Perspective. Int. J. Complement. Altern. Med. 2015, 1, 00032. [Google Scholar] [CrossRef][Green Version]
- Jeffrey, S.L.A.; Belcher, H.J.C.R. Use of Arnica to Relieve Pain after Carpal-Tunnel Release Surgery. Altern. Ther. Health Med. 2002, 8, 66–68. [Google Scholar]
- Pannek, J.; Pannek-Rademacher, S. Time to Say Good-Bye? Homeopathy, Skeptics and Thoughts on How to Proceed. J. Complement. Integr. Med. 2023, 20, 289–291. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ammirato, S.; Felicetti, A.M.; Rogano, D.; Linzalone, R.; Corvello, V. Digitalising the Systematic Literature Review Process: The MySLR Platform. Knowl. Manag. Res. Pract. 2023, 21, 777–794. [Google Scholar] [CrossRef]
- Thakur, J.H.; Katre, A.N. Comparison of the Efficacy of Homeopathic Drug Arnica and Ibuprofen on Postextraction Pain in Children: A Triple-Blind Randomized Controlled Trial. Int. J. Clin. Pediatr. Dent. 2022, 15, 332–337. [Google Scholar] [CrossRef]
- Buchholzer, M.-L.; Werner, C.; Knoess, W. Current Concepts on Integrative Safety Assessment of Active Substances of Botanical, Mineral or Chemical Origin in Homeopathic Medicinal Products within the European Regulatory Framework. Regul. Toxicol. Pharmacol. RTP 2014, 68, 193–200. [Google Scholar] [CrossRef]
- Kouzi, S.A.; Nuzum, D.S. Arnica for Bruising and Swelling. Am. J. Health. Syst. Pharm. 2007, 64, 2434–2443. [Google Scholar] [CrossRef] [PubMed]
- Tuten, C.; McClung, J. Reducing Muscle Soreness with Arnica montana: Is It Effective? Altern. Complement. Ther. 1999, 5, 369–372. [Google Scholar] [CrossRef]
- Schmidt, T.J. Arnica montana L.: Doesn’t Origin Matter? Plants 2023, 12, 3532. [Google Scholar] [CrossRef]
- Duthen, S.; Gadéa, A.; Trempat, P.; Boujedaini, N.; Fabre, N. Comparison of the Phytochemical Variation of Non-Volatile Metabolites within Mother Tinctures of Arnica montana Prepared from Fresh and Dried Whole Plant Using UHPLC-HRMS Fingerprinting and Chemometric Analysis. Molecules 2022, 27, 2737. [Google Scholar] [CrossRef]
- Ganzera, M.; Egger, C.; Zidorn, C.; Stuppner, H. Quantitative Analysis of Flavonoids and Phenolic Acids in Arnica montana L. by Micellar Electrokinetic Capillary Chromatography. Anal. Chim. Acta 2008, 614, 196–200. [Google Scholar] [CrossRef]
- Pljevljakušić, D.; Rančić, D.; Ristić, M.; Vujisić, L.; Radanović, D.; Dajić-Stevanović, Z. Rhizome and Root Yield of the Cultivated Arnica montana L., Chemical Composition and Histochemical Localization of Essential Oil. Ind. Crops Prod. 2012, 39, 177–189. [Google Scholar] [CrossRef]
- Douglas, J.A.; Smallfield, B.M.; Burgess, E.J.; Perry, N.B.; Anderson, R.E.; Douglas, M.H.; leAnne Glennie, V. Sesquiterpene Lactones in Arnica montana: A Rapid Analytical Method and the Effects of Flower Maturity and Simulated Mechanical Harvesting on Quality and Yield. Planta Med. 2004, 70, 166–170. [Google Scholar] [CrossRef]
- Spitaler, R.; Schlorhaufer, P.D.; Ellmerer, E.P.; Merfort, I.; Bortenschlager, S.; Stuppner, H.; Zidorn, C. Altitudinal Variation of Secondary Metabolite Profiles in Flowering Heads of Arnica montana Cv. ARBO. Phytochemistry 2006, 67, 409–417. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Stausberg, S.; Raison, J.V.; Berner, M.; Willuhn, G. Lignans from Arnica Species. Nat. Prod. Res. 2006, 20, 443–453. [Google Scholar] [CrossRef]
- Sugier, P.; Jakubowicz-Gil, J.; Sugier, D.; Kowalski, R.; Gawlik-Dziki, U.; Kołodziej, B.; Dziki, D. Chemical Characteristics and Anticancer Activity of Essential Oil from Arnica montana L. Rhizomes and Roots. Molecules 2020, 25, 1284. [Google Scholar] [CrossRef]
- Verre, J.; Boisson, M.; Paumier, A.; Tribolo, S.; Boujedaini, N. Anti-Inflammatory Effects of Arnica montana (Mother Tincture and Homeopathic Dilutions) in Various Cell Models. J. Ethnopharmacol. 2024, 318, 117064. [Google Scholar] [CrossRef] [PubMed]
- Jäger, C.; Hrenn, A.; Zwingmann, J.; Suter, A.; Merfort, I. Phytomedicines Prepared from Arnica Flowers Inhibit the Transcription Factors AP-1 and NF-κB and Modulate the Activity of MMP1 and MMP13 in Human and Bovine Chondrocytes. Planta Med. 2009, 75, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Saha, S.K.; De, A.; Das, D.; Khuda-Bukhsh, A.R. Potential of the Homeopathic Remedy, Arnica montana 30C, to Reduce DNA Damage in Escherichia Coli Exposed to Ultraviolet Irradiation through up-Regulation of Nucleotide Excision Repair Genes. Zhong Xi Yi Jie He Xue Bao 2012, 10, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Świeca, M.; Sugier, D.; Cichocka, J. Comparison of in vitro lipoxygenase, xanthine oxidase inhibitory and antioxidant activity of Arnica montana and Arnica chamissonis tinctures. Acta Sci. Pol. Hortorum Cultus 2011, 10, 15–27. [Google Scholar]
- Otterness, I.G.; Bliven, M.L.; Eskra, J.D.; te Koppele, J.M.; Stukenbrok, H.A.; Milici, A.-J. Cartilage Damage after Intraarticular Exposure to Collagenase 3. Osteoarthr. Cartil. 2000, 8, 366–373. [Google Scholar] [CrossRef][Green Version]
- Martel-Pelletier, J. Pathophysiology of Osteoarthritis. Osteoarthritis Cartilage 2004, 12 (Suppl. A), S31–S33. [Google Scholar] [CrossRef]
- Lunec, J.; Blake, D. Oxidative Damage and Its Relevance to Inflammatory Joint Disease. In Cellular Antioxidant Defense Mechanisms, 1st ed.; Chow, C.K., Ed.; CRC Press: Boca Raton, FL, USA, 1988; ISBN 978-0-429-28932-3. [Google Scholar]
- Gelderman, K.A.; Hultqvist, M.; Olsson, L.M.; Bauer, K.; Pizzolla, A.; Olofsson, P.; Holmdahl, R. Rheumatoid Arthritis: The Role of Reactive Oxygen Species in Disease Development and Therapeutic Strategies. Antioxid. Redox Signal. 2007, 9, 1541–1567. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, A.; Egea-Guerrero, J.J.; Murillo-Cabezas, F.; Carrillo-Vico, A. Oxidative Stress in Traumatic Brain Injury. Curr. Med. Chem. 2014, 21, 1201–1211. [Google Scholar] [CrossRef]
- Alfredo, P.P.; Anaruma, C.A.; Pião, A.C.S.; João, S.M.A.; Casarotto, R.A. Effects of Phonophoresis with Arnica montana onto Acute Inflammatory Process in Rat Skeletal Muscles: An Experimental Study. Ultrasonics 2009, 49, 466–471. [Google Scholar] [CrossRef]
- Sharma, S.; Arif, M.; Nirala, R.K.; Gupta, R.; Thakur, S.C. Cumulative Therapeutic Effects of Phytochemicals in Arnica montana Flower Extract Alleviated Collagen-Induced Arthritis: Inhibition of Both pro-Inflammatory Mediators and Oxidative Stress. J. Sci. Food Agric. 2016, 96, 1500–1510. [Google Scholar] [CrossRef]
- Pumpa, K.L.; Fallon, K.E.; Bensoussan, A.; Papalia, S. The Effects of Topical Arnica on Performance, Pain and Muscle Damage after Intense Eccentric Exercise. Eur. J. Sport Sci. 2014, 14, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.; Havey, J.; White, L.E.; Martin, N.; Yoo, S.S.; Rademaker, A.W.; Alam, M. Accelerated Resolution of Laser-Induced Bruising with Topical 20% Arnica: A Rater-Blinded Randomized Controlled Trial. Br. J. Dermatol. 2010, 163, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Brinkhaus, B.; Wilkens, J.M.; Lüdtke, R.; Hunger, J.; Witt, C.M.; Willich, S.N. Homeopathic Arnica Therapy in Patients Receiving Knee Surgery: Results of Three Randomised Double-Blind Trials. Complement. Ther. Med. 2006, 14, 237–246. [Google Scholar] [CrossRef]
- Robertson, A.; Suryanarayanan, R.; Banerjee, A. Homeopathic Arnica montana for Post-Tonsillectomy Analgesia: A Randomised Placebo Control Trial. Homeopathy 2007, 96, 17–21. [Google Scholar] [CrossRef]
- Macedo, S.; Carvalho, J.C.; Luciano, F.; Santos-Pinto, R. Effect of Arnica montana 6 cH on Edema, Mouth Opening and Pain in Patients Submitted to Extraction of Impacted Third Molars. Arztez. Naturheilverfahren Regul. 2005, 46, 381–387. [Google Scholar]
- Mawardi, H.; Ghazalh, S.; Shehatah, A.; Abdelwahid, A.; Aljohani, A.; Felemban, O.; Almazrooa, S.; Elbadawi, L.; Shawky, H. Systemic Use of Arnica montana for the Reduction of Postsurgical Sequels Following Extraction of Impacted Mandibular 3 Molars: A Pilot Study. Evid. Based Complement. Alternat. Med. 2020, 2020, 6725175. [Google Scholar] [CrossRef]
- Totonchi, A.; Guyuron, B. A Randomized, Controlled Comparison between Arnica and Steroids in the Management of Postrhinoplasty Ecchymosis and Edema. Plast. Reconstr. Surg. 2007, 120, 271–274. [Google Scholar] [CrossRef]
- Karow, J.-H.; Abt, H.-P.; Fröhling, M.; Ackermann, H. Efficacy of Arnica montana D4 for Healing of Wounds After Hallux Valgus Surgery Compared to Diclofenac. J. Altern. Complement. Med. N. Y. N 2008, 14, 17–25. [Google Scholar] [CrossRef]
- Mendes, A.M.; Hasse Vilela, D.C.; Jung, M.T.; KawakamOkuyama, S.S.; Naval Machado, M.A.; Soares de Lima, A.A.; de Azevedo, L.R.; Trindade Grégio, A.M. Therapeutic Effect of Arnica Ointment on Cicatrization Process of Aphthas and Lesions in Human Oral Mucosa. Pharmacologyonline 2008, 3, 273–280. [Google Scholar]
- Nejadbagheri, S.; Hosseini, H.S.; Kazemi, M. The Effects of Arnigol Cream on Pain Associated with Arteriovenous Fistula Puncture in Patients Receiving Hemodialysis: A Randomized Double-Blind Clinical Trial Study. Nurs. Midwifery Stud. 2018, 7, 100. [Google Scholar] [CrossRef]
- Alekar, A.; Aphale, P.; Sharma, D.; Palekar, T.; Basu, S. Efficacy of High Dilution Medicines in Combination with Physiotherapy in Trauma Induced Low Back Pain- A Randomized Control Clinical Trial. Int. J. Ayurvedic Med. 2023, 14, 71–75. [Google Scholar] [CrossRef]
- Knuesel, O.; Weber, M.; Suter, A. Arnica montana Gel in Osteoarthritis of the Knee: An Open, Multicenter Clinical Trial. Adv. Ther. 2002, 19, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Widrig, R.; Suter, A.; Saller, R.; Melzer, J. Choosing between NSAID and Arnica for Topical Treatment of Hand Osteoarthritis in a Randomised, Double-Blind Study. Rheumatol. Int. 2007, 27, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Barlow, T.; Downham, C.; Barlow, D. The Effect of Complementary Therapies on Post-Operative Pain Control in Ambulatory Knee Surgery: A Systematic Review. Complement. Ther. Med. 2013, 21, 529–534. [Google Scholar] [CrossRef]
- Urits, I.; Burshtein, A.; Sharma, M.; Testa, L.; Gold, P.A.; Orhurhu, V.; Viswanath, O.; Jones, M.R.; Sidransky, M.A.; Spektor, B.; et al. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr. Pain Headache Rep. 2019, 23, 23. [Google Scholar] [CrossRef]
- Dragos, D.; Gilca, M.; Gaman, L.; Vlad, A.; Iosif, L.; Stoian, I.; Lupescu, O. Phytomedicine in Joint Disorders. Nutrients 2017, 9, 70. [Google Scholar] [CrossRef]
- Barkey, E.; Kaszkin-Bettag, M. A Homeopathic Arnica Patch for the Relief of Cellulitis-Derived Pain and Numbness in the Hand. Glob. Adv. Health Med. 2012, 1, 18. [Google Scholar] [CrossRef]
- Jackson, M.; Tummon Simmons, L. Challenging Case in Clinical Practice: Improvement in Chronic Osteoarthritis Pain with Use of Arnica Oil Massage, Therapeutic Ultrasound, and Acupuncture—A Case Report. Altern. Complement. Ther. 2018, 24, 60–62. [Google Scholar] [CrossRef]
- Bartolomei, S.; Nigro, F.; D’Amico, A.; Cortesi, M.; Di Michele, R. Mud Pack With Menthol and Arnica montana Accelerates Recovery Following a High-Volume Resistance Training Session for Lower Body in Trained Men. J. Strength Cond. Res. 2022, 36, 1909–1915. [Google Scholar] [CrossRef]
- Zanella, S.; Buccelletti, F.; Franceschi, F.; Ramponi, C.; Spagnolli, F.; Sacchetti, G.; Oliva, G.; Lumachi, F. Arnica Compositum, Hekla Lava and Acidum Nitricum Together Are Superior to Arnica Compositum Alone in the Local Treatment of Symptomatic Calcific Periarthritis of the Shoulder: A Pilot Study. Rev. Recent Clin. Trials 2018, 13, 150–155. [Google Scholar] [CrossRef]
- de Souza, G.M.d.; Fernandes, I.A.; Pinheiro, M.L.P.; Falci, S.G.M. Comparative Effectiveness of the Homeopathic Preparation Traumeel S in Third Molar Extraction Surgery: A Preliminary Triple-Blind Clinical Trial. Homeopath. J. Fac. Homeopath. 2021, 110, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.R.; Amit-Kohn, M.; Weiss, S.; Rosenblum, J.; Lukasiewicz, E.; Itzchaki, M.; Oberbaum, M. Efficacy of a Homeopathic Preparation in Control of Post-Operative Pain—A Pilot Clinical Trial. Acute Pain 2007, 9, 7–12. [Google Scholar] [CrossRef]
- Maisel Lotan, A.; Gronovich, Y.; Lysy, I.; Binenboym, R.; Eizenman, N.; Stuchiner, B.; Goldstein, O.; Babai, P.; Oberbaum, M. Arnica montana and Bellis Perennis for Seroma Reduction Following Mastectomy and Immediate Breast Reconstruction: Randomized, Double-Blind, Placebo- Controlled Trial. Eur. J. Plast. Surg. 2020, 43, 285–294. [Google Scholar] [CrossRef]
- Schneider, C.; Klein, P.; Stolt, P.; Oberbaum, M. A Homeopathic Ointment Preparation Compared With 1% Diclofenac Gel for Acute Symptomatic Treatment of Tendinopathy. Explore 2005, 1, 446–452. [Google Scholar] [CrossRef]
- Janczewska, K.; Koszela, K.; Klimkiewicz, R.; Kubsik-Gidlewska, A.; Jankowska, A.; Klimkiewicz, P.; Woldańska-Okońska, M. Analgesic Effectiveness of Physical Therapy Combining the Use of Electromagnetic Fields with Light Radiation Emitted by LEDs along with the Use of Topical Herbal Ointment in Patients with Gonarthrosis. Int. J. Environ. Res. Public Health 2023, 20, 3696. [Google Scholar] [CrossRef]
- Kučera, M.; Kolar, P.; Barna, M.; Kučera, A.; Hladiková, M. Arnica/Hydroxyethyl Salicylate Combination Spray for Ankle Distortion: A Four-Arm Randomised Double-Blind Study. Pain Res. Treat. 2011, 2011, 365625. [Google Scholar] [CrossRef][Green Version]
- Kucera, M.; Horácek, O.; Kálal, J.; Kolár, P.; Korbelar, P.; Polesná, Z. Synergetic Analgesic Effect of the Combination of Arnica and Hydroxyethyl Salicylate in Ethanolic Solution Following Cutaneous Application by Transcutaneous Electrostimulation. Arzneimittelforschung 2003, 53, 850–856. [Google Scholar] [CrossRef]
- Madisetti, M.; Kelechi, T.J.; Mueller, M.; Amella, E.J.; Prentice, M.A. Feasibility, Acceptability, and Tolerability of RGN107 in the Palliative Wound Care Management of Chronic Wound Symptoms. J. Wound Care 2017, 26, S25–S34. [Google Scholar] [CrossRef]
- Kelechi, T.; Prentice, M.; Madisetti, M.; Brunette, G.; Mueller, M. Palliative Care in the Management of Pain, Odor, and Exudate in Chronic Wounds at the End of Life: A Cohort Study. J. Hosp. Palliat. Nurs. 2017, 19, 17–25. [Google Scholar] [CrossRef]
- Huber, R.; Bross, F.; Schempp, C.; Gründemann, C. Arnica and Stinging Nettle for Treating Burns—A Self-Experiment. Complement. Ther. Med. 2011, 19, 276–280. [Google Scholar] [CrossRef]
- Volpi, P.; Bisciotti, G.N. The Conservative Treatment of Muscle Injuries: General Principles. In Muscle Injury in the Athlete: The Italian Consensus Conference Guidelines; Volpi, P., Bisciotti, G.N., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 161–192. ISBN 978-3-030-16158-3. [Google Scholar]
- Vanden Bossche, L.; Vanderstraeten, G. A Multi-Center, Double-Blind, Randomized, Placebo-Controlled Trial Protocol to Assess Traumeel Injection vs Dexamethasone Injection in Rotator Cuff Syndrome: The TRAumeel in ROtator Cuff Syndrome (TRARO) Study Protocol. BMC Musculoskelet. Disord. 2015, 16, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kijak, K.; Cieślar, G.; Kowacka, M.; Skomro, P.; Gronwald, H.; Garstka, A.; Lietz-Kijak, D. Cone Beam Computed Tomography in the Assessment of the Effectiveness of Physical Therapy with the Use of the Electromagnetic Field Combined with Light Radiation Emitted by LEDs in the Treatment of Inflammation of the Paranasal Sinuses—A Case Study. Int. J. Environ. Res. Public Health 2022, 19, 13570. [Google Scholar] [CrossRef]
- Fanelli, D. Negative Results Are Disappearing from Most Disciplines and Countries. Scientometrics 2011, 90, 891–904. [Google Scholar] [CrossRef]
- Matosin, N.; Frank, E.; Engel, M.; Lum, J.S.; Newell, K.A. Negativity towards Negative Results: A Discussion of the Disconnect between Scientific Worth and Scientific Culture. Dis. Model. Mech. 2014, 7, 171–173. [Google Scholar] [CrossRef]
- Rodrigues, D.d.F.; Luna, S.P.L.; Brondani, J.T.; Minto, B.W. Comparison of Morphine, Ketoprofen and Arnica montana 6x and 30x per Oral Transmucosal or Subcutaneous Route for Control of Postoperative Pain in Cats Subjected to Hysterectomy with Bilateral Salpingo-Oophorectomy. Ciênc. Rural 2016, 46, 330–335. [Google Scholar] [CrossRef][Green Version]
- Stevinson, C.; Devaraj, V.S.; Fountain-Barber, A.; Hawkins, S.; Ernst, E. Homeopathic Arnica for Prevention of Pain and Bruising: Randomized Placebo-Controlled Trial in Hand Surgery. J. R. Soc. Med. 2003, 96, 60–65. [Google Scholar] [CrossRef]
- Paris, A.; Gonnet, N.; Chaussard, C.; Belon, P.; Rocourt, F.; Saragaglia, D.; Cracowski, J.L. Effect of Homeopathy on Analgesic Intake Following Knee Ligament Reconstruction: A Phase III Monocentre Randomized Placebo Controlled Study. Br. J. Clin. Pharmacol. 2008, 65, 180–187. [Google Scholar] [CrossRef]
- Lauche, R.; Materdey, S.; Cramer, H.; Haller, H.; Stange, R.; Dobos, G.; Rampp, T. Effectiveness of Home-Based Cupping Massage Compared to Progressive Muscle Relaxation in Patients with Chronic Neck Pain—A Randomized Controlled Trial. PLoS ONE 2013, 8, e65378. [Google Scholar] [CrossRef]
- Hausen, B.M. Arnica allergy. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 1980, 31, 10–17. [Google Scholar]
- Hörmann, H.P.; Korting, H.C. Allergie Acute Contact Dermatitis Due to Arnica Tincture Self-Medication. Phytomedicine 1995, 1, 315–317. [Google Scholar] [CrossRef]
- Jürgens, F.M.; Robledo, S.M.; Schmidt, T.J. Evaluation of Pharmacokinetic and Toxicological Parameters of Arnica Tincture after Dermal Application In Vivo. Pharmaceutics 2022, 14, 2379. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, F.; Herrmann, F.; Robledo, S.; Schmidt, T. Dermal Absorption of Sesquiterpene Lactones from Arnica Tincture. Pharmaceutics 2022, 14, 742. [Google Scholar] [CrossRef] [PubMed]
- Vieira da Silva, B.; Barreira, J.C.M.; Oliveira, M.B.P.P. Natural Phytochemicals and Probiotics as Bioactive Ingredients for Functional Foods: Extraction, Biochemistry and Protected-Delivery Technologies. Trends Food Sci. Technol. 2016, 50, 144–158. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Ilaiyaraja, N.; Likhith, K.R.; Sharath Babu, G.R.; Khanum, F. Optimisation of Extraction of Bioactive Compounds from Feronia Limonia (Wood Apple) Fruit Using Response Surface Methodology (RSM). Food Chem. 2015, 173, 348–354. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Chamorro, F.; Simal-Gandara, J.; Prieto, M.A.; Cassani, L. Improving Phenolic Compound Extraction from Arnica montana Flowers through Multivariate Optimization of Heat and Ultrasound-Assisted Methods. Sustain. Chem. Pharm. 2024, 41, 101722. [Google Scholar] [CrossRef]
- Ernst, E.; Pittler, M.H. Efficacy of Homeopathic Arnica: A Systematic Review of Placebo-Controlled Clinical Trials. Arch. Surg. 1998, 133, 1187–1190. [Google Scholar] [CrossRef]
- Iannitti, T.; Morales-Medina, J.C.; Bellavite, P.; Rottigni, V.; Palmieri, B. Effectiveness and Safety of Arnica montana in Post-Surgical Setting, Pain and Inflammation. Am. J. Ther. 2016, 23, e184–e197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).