Abstract
Background and aims: The increasing number of diagnosed hepatitis E virus (HEV) infections in Europe has led to the implementation of the testing of blood products in various countries. Many nations have not yet implemented such screening. To assess the need for HEV screening in blood products worldwide, we conducted a systematic review and meta-analysis assessing HEV RNA positivity and anti-HEV seroprevalence in blood donors. Methods: Studies reporting anti-HEV IgG/IgM or HEV RNA positivity rates among blood donors worldwide were identified via predefined search terms in PubMed and Scopus. Estimates were calculated by pooling study data with multivariable linear mixed-effects metaregression analysis. Results: A total of 157 (14%) of 1144 studies were included in the final analysis. The estimated HEV PCR positivity rate ranged from 0.01 to 0.14% worldwide, with strikingly higher rates in Asia (0.14%) and Europe (0.10%) in comparison to North America (0.01%). In line with this, anti-HEV IgG seroprevalence in North America (13%) was lower than that in Europe (19%). Conclusions: Our data demonstrate large regional differences regarding the risk of HEV exposure and blood-borne HEV transmission. Considering the cost–benefit ratio, this supports blood product screening in high endemic areas, such as Europe and Asia, in contrast to low endemic regions, such as the U.S.
1. Introduction
Globally, hepatitis E is the most common cause of enterically transmitted hepatitis []. Anti-HEV IgG seroprevalence rates of 18% up to 30% were reported in the Netherlands and Germany in 2013, and even higher rates of over 50% were reported in some areas of France []. In humans, genotypes 1–4 are of the highest relevance. Genotypes 1 and 2 exclusively infect humans and are endemic in tropical areas, such as Asia, the south of Africa, and parts of Central America. As a source of fecal–oral transmission, contaminated drinking water can lead to local outbreaks with sometimes fulminant courses []. By contrast, HEV genotype 3 is the predominant genotype in Europe and Australia as well as North and South America. Genotype 3 is transmitted zoonotically. Consumption of raw pork seems to be the most relevant risk factor in genotype 3 regions []. Genotype 4 is also transmitted through pork meat but occurs primarily in Asia and plays virtually no role in Europe []. The majority of HEV infections are asymptomatic and self-limiting. Only a small proportion of patients develop elevated transaminases and hepatic dysfunction. In the case of HEV genotype 3 infections, older men and patients with preexisting liver disease are considered to be particularly at risk for a severe course []. Persistent HEV infections (chronic hepatitis E) have been found in various immunocompromised patient populations. These include solid organ transplant patients as well as stem cell transplant patients, HIV-infected patients, chemotherapy-receiving patients, and patients with chronic inflammatory diseases who are permanently receiving immunosuppressive therapy [,]. Patients suffering from chronic hepatitis E are at risk of developing life-threatening cirrhosis over the next five years []. Up to 50% of organ transplant recipients who acquire an HEV infection develop chronic hepatitis E, putting them at high risk for developing cirrhosis []. In addition to foodborne transmission, HEV can be transmitted parenterally, via transfusion of blood products [,,,]. Immunosuppressed patients receiving blood transfusions are particularly at risk. In the UK, the Netherlands, Japan, Austria, Germany, and France, general testing of blood products for HEV has been introduced in recent years. Despite the fact that recorded HEV infections are on the rise in many countries and the risk of chronification of hepatitis E in immunocompromised patients is high, most countries worldwide do not routinely test blood products for HEV. These countries are still evaluating the situation and need valid data depicting the risk of blood-borne HEV infections in their country in comparison to that in other nations.
To evaluate the risk of HEV-positive blood products as well as the risk of HEV exposition in blood donors, a systematic review and meta-analysis was performed comparing the rate of HEV RNA positivity and anti-HEV seroprevalence in blood donors worldwide. As HEV genotype 3 infections are endemic in both North America and Europe, it is adequate to focus on the comparison of PCR and serology positivity rates on these two continents; by contrast, several genotypes are endemic in Asia (genotypes 1, 3, and 4) and Africa (genotypes 1, 2, and 3), and therefore, an inhomogeneous picture exists for these continents. Even if different HEV genotype 3 subtypes are present in North America and Europe, the focus on these two continents allows a relevant comparison.
2. Methods
2.1. Search Strategy and Selection Criteria
The literature search was conducted in two different types of databases: PubMed and Scopus. In both databases, the literature search was performed by using the terms “Hepatitis e” or “HEV” in combination with the terms “blood donors” or “transfusion” or “blood donation” or “blood testing”. A total of 1144 articles were identified and screened for duplicates and reviews, and all duplicates and reviews were removed. Published articles were thoroughly reviewed for possible inclusion. This analysis is reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
2.2. Inclusion and Exclusion Criteria
The inclusion criteria were as follows: identification of the test used (ELISA or PCR) and confirmation of use according to the manufacturer’s instructions; inclusion of only specified blood donors (e.g., studies reporting on healthy individuals or volunteers were excluded). Only articles written in English and study cohorts with more than 50 blood donors were included in the final analysis. Studies that did not meet these study quality criteria were excluded from further analysis.
2.3. Data Extraction
Data were stratified by author, journal, year of publication, continent, country, diagnostic assay, number of blood donors, anti-HEV IgG and IgM seroprevalence, and HEV-RNA positivity in different data sets. Additionally, we categorized blood donors as female or male. If different diagnostic assays were used in one study or if different study cohorts were divided according to gender, a study could contain several data sets.
2.4. Study Quality
The identified articles were assessed for study quality according to a set scheme. Data were assessed on the basis of their methodological quality according to the Joanna Briggs Institute’s well-established critical appraisal tool for prevalence studies. Studies were assessed by A.W. and discussed with T.H. accordingly. Any disagreements were resolved by discussing with a third investigator (S.P.).
2.5. Statistical Analysis
The HEV RNA positivity rate was estimated by pooling the study data separately for each country and continent with a linear mixed effects regression analysis using restricted maximum likelihood. We included the test, year of publication, and methodological quality score as further independent variables. If possible, interaction terms, e.g., for the publication year and test, were also included. Heterogeneity was checked via the quantity I2, and publication bias was conducted via a funnel plot. Odds ratios along with 95% confidence intervals are given. The analysis was checked using R (version 3.6.1) and the metafor package. For the rainforest plots, the R package metaviz was used.
3. Results
Out of 1144 articles, 157 (14%) fulfilled the quality standards and were used in the final analysis, as shown in the flow chart (Figure 1). Detailed information on the studies included and their characteristics are provided as supplementary tables (Supplementary Tables S1 and S2).
Figure 1.
Study flow chart.
3.1. Overall HEV RNA Positivity Rates in Blood Donors
A total of 55 data sets from 44 studies reported the rate of viremia in 3,375,573 blood donors. The HEV RNA positivity varied between continents, and the rate ranged from 0.01% in Australia and North America to 0.14% in Asia (Figure 2). North America (0.01%) had a lower HEV PCR positivity rate in comparison with Europe (0.10%) (OR = 0.14 (95% CI 0.03–0.58), p-value = 0.007). Furthermore, the HEV RNA positivity rate varied greatly between single nations, ranging from 0.01% in Australia, the USA, Canada, Austria, and Japan to up to 0.5% in Cambodia, 0.31% in Serbia, and 0.28% in Germany (Figure 3).
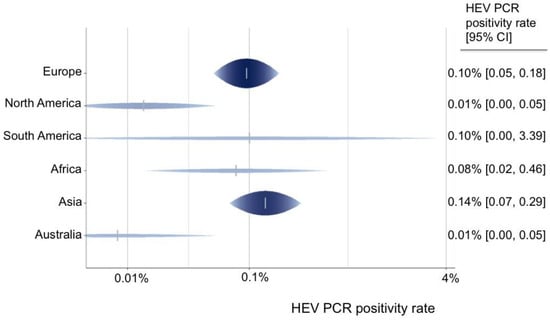
Figure 2.
Predicted HEV PCR positivity rate for all continents.
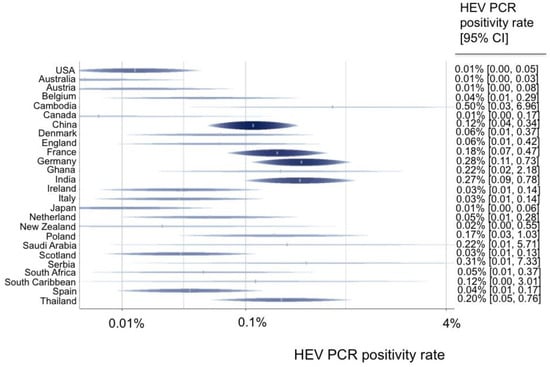
Figure 3.
Predicted HEV PCR positivity rates for all countries.
Regarding the year of the study, the rate of HEV PCR positivity increased over time from the years 1994 to 2020 (OR = 0.87 (95% CI 0.79–0.96, p-value = 0.007)). However, a detailed examination of these results showed that this was only due to data from Europe and did not apply worldwide. The distribution of gender was described in 6 of 44 studies. Adjusted estimates revealed a significantly lower rate of PCR positivity in female versus male blood donors (OR = 0.37 (95% CI 0.20–0.69), p-value = 0.002).
3.2. Overall Anti-HEV IgG and IgM Seroprevalence Rates in Blood Donors
A total of 206 data sets from 125 studies reported the anti-HEV IgG seroprevalence in 225,328 blood donors. Eight anti-HEV IgG assays were used in the various studies. In 56 studies, the Wantai assay was used (44.8%); in 18, DiaPro was used (14.4%); in 11 Abbott, was used; in 11, Mikrogen was used (8.8% each); in 4, MP was used (3.2%); in 1, Adaltis was used; in 1, DSI was used (0.8% each); and in 64 studies, other/inhouse or undefined assays (51.2%) were used.
In 65 data sets, anti-HEV IgM rates were depicted: 25 used Wantai (56.8%), 8 used DiaPro (18.2%), 4 used Mikrogen (9.1%), 2 used MP (4.5%), 1 used DSI (2.3%), and 12 used other/in-house assays/undefined assays (27.3%). Adjusted estimates for group differences regarding the assays demonstrated that the DiaPro (OR = 0.37 (95% CI 0.20–0.69), p-value = 0.002), MP (OR = 0.25 (95% CI 0.11–0.58), p-value = 0.001), Mikrogen (OR = 0.38 (95% CI 0.18–0.79), p-value = 0.01), and various other assays (OR = 0.48 (95% CI 0.31–0.74), p-value = 0.001) showed lower seroprevalence rates in comparison with the Wantai assay.
Depending on the continent, the estimated anti-HEV IgG seroprevalence ranged from 4.79% in Australia to 22.98% in Africa. North America (12.7%, Wantai assay) had a lower IgG seroprevalence in comparison with Europe (19.1%, Wantai assay) (OR = 0.62 (95% CI 0.35–1.09), p-value = 0.094) (Figure 4). We did not observe an association between anti-HEV IgG or IgM seroprevalence and the year of the study. The distribution of gender was reported in 45 of 125 studies. In contrast to HEV PCR positivity, there seemed to be no difference regarding anti-HEV IgG or IgM seroprevalence between genders (OR = 0.74 (95% CI 0.53–1.05), p-value = 0.089; OR = 0.54 (95% CI 0.21–1.35), p-value = 0.186).
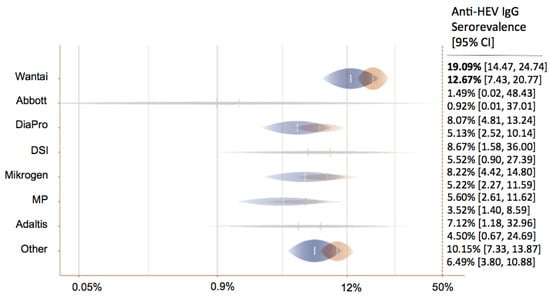
Figure 4.
Predicted anti-HEV IgG seroprevalence in North America (blue) and Europe (orange) for all tests.
4. Discussion
Increasing numbers of reported HEV infections, as well as potentially fatal courses in vulnerable patients, have led to the question of whether all blood products should be tested for HEV to avoid blood-borne HEV transmission. While many European countries have already established general blood donor screening for HEV, this has not yet been decided in the U.S. and many other nations; thus, this meta-analysis could help decision-makers worldwide to choose wisely. This large meta-analysis elucidates the rate of HEV PCR positivity and the anti-HEV seroprevalence in blood donors worldwide for the first time.
The highest HEV PCR positivity rates were found in Asia and Europe, and the lowest rates were found in Australia and North America. Furthermore, the HEV viremia rate in South America (0.1%) was slightly lower than that in Europe. However, since this observation was based on only a few data, it should not be overemphasized. Further studies on South America are necessary to realistically assess the risk there. A recent meta-analysis based on patient anti-HEV seroprevalence data already suggested that the risk of HEV exposure in North America might be lower than that in Europe []. The current study shows, for the first time, that the anti-HEV positivity and viremia rates in blood donors in North America are lower than those in Europe. Possible reasons could be differences in the type of diet, especially with regard to the amount of pork consumption. By focusing on North America vs. Europe, it was clearly shown that in these two HEV genotype 3 regions, there is a completely different endemicity with strongly differing probabilities for HEV exposure (serology results) and blood-borne HEV transmission (PCR results). Because there is a significantly more inhomogeneous distribution of genotypes in Asia, it is more difficult to compare this continent with other continents.
Furthermore, our data regarding single countries reveals a large variability between viremia rates among blood donors in numerous European countries. The highest rates were detected in Serbia, Germany, and France. This observation is in line with that of Hartl et al., who also found the highest anti-HEV seroprevalence in France and Germany in Europe in the general population []. Regarding a possible sex difference, male blood donors showed significantly higher HEV PCR positivity rates than female blood donors. However, this analysis was based only on available data from a few studies (n = 6) from five different countries (Germany, Ireland, Italy, South Africa, and Thailand). Therefore, this observation needs to be confirmed and should not be overemphasized. On the other hand, these studies include 94,386 female (40%) and male (60%) blood donors, which represent a representative cohort. Sex differences could be related to HEV exposure, such as potentially higher rates of consumption of insufficiently heated pork in men [,], hormonal differences affecting the immune system [] or socioeconomic factors, and the distribution of different genders in different occupational groups. The increasing HEV PCR positivity rate we observed was caused by European countries only. Thus, this finding should not be overestimated and cannot be generalized. Perhaps this observation was caused by the increased sensitivity of assays used and does not depict a real epidemiological shift.
This work contributes significantly to the current question of whether blood products worldwide should be tested for HEV. According to the viremia rate in Europe (0.10%) in comparison to North America (0.01%), it is apparent that the risk of HEV transmission by blood products is far lower in North America than in Europe. Therefore, it may be speculated that general blood donor screening for HEV is not indicated in the U.S. in contrast to many European countries (Figure 3). Certainly, the characteristics of the European nations are so inhomogeneous (Figure 3; Supplementary Tables S1 and S2) that they impede a uniform recommendation for general testing throughout Europe. However, according to the data in Supplementary Table S2, nations worldwide can estimate their risk of transfusion-related HEV transmission and then decide, on this basis, whether to introduce general blood donor screening, initiate further studies, or refrain from testing for HEV. Of course, the risk–benefit analysis as well as the costs must also be taken into account. Certainly, the need to have HEV-free blood products is greater in an industrialized nation with numerous immunosuppressed blood product recipients than in a developing country.
A limitation of our study is that the age of the studied individuals was not consistently reported in the studies. Standardized analysis of age-dependent effects and lifetime risk could not be performed adequately due to a lack of data. Furthermore, as ethnicity was available only in a minority of studies, no valid conclusion could be drawn regarding this aspect. However, this is the first examination evaluating the risk of blood-borne HEV transmission (PCR positivity) and the risk of HEV exposure (seropositivity) among blood donors worldwide. Both serology and viremia depicted a lower risk of HEV infections in North America in comparison with Europe. The present study meets the high-quality standards set for meta-analyses. All findings were dependent on the quality of the included studies. To avoid potential bias, all data sets were assessed by experienced scientists according to the Joanna Briggs Institute’s critical appraisal tool, which is well-proven for prevalence studies [].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens12030425/s1, Table S1. Studies of HEV PCR positivity rates of blood donors worldwide [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]; Table S2. Studies of HEV seroprevalence of blood donors worldwide [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
Author Contributions
Conceptualization, S.P. and T.H.; methodology, T.H.; software, A.-K.O.; validation, A.W., T.H. and S.P.; formal analysis, A.W.; investigation, A.W.; resources, S.P.; data curation, A.W.; writing—original draft preparation, A.W.; writing—review and editing, T.H. and S.P.; visualization, A.-K.O.; supervision, S.P. and M.M.A.; project administration, T.H.; funding acquisition, M.M.A. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study has been founded by German Center for Infectious Diseases (DZIF), FKZ 8009701709 (DZIF TI 1.709). We acknowledge financial support from the Open Access Publication Fund of UKE—Universitätsklinikum Hamburg-Eppendorf- and DFG—German Research Foundation.
Informed Consent Statement
Data analyzed in this meta-analysis were a re-analysis of previously existing data published in several studies. For such a meta-analysis, no formal ethical court vote was required according to our ethical court.
Data Availability Statement
Data are available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Hepatitis E virus (HEV), ribonucleic acid (RNA), Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), polymerase chain reaction (PCR), odds ratio (OR), confidence interval (CI).
References
- Debing, Y.; Moradpour, D.; Neyts, J.; Gouttenoire, J. Update on hepatitis E virology: Implications for clinical practice. J. Hepatol. 2016, 65, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Hartl, J.; Otto, B.; Madden, R.G.; Webb, G.; Woolson, K.L.; Kriston, L.; Vettorazzi, E.; Lohse, A.W.; Dalton, H.R.; Pischke, S. Hepatitis E Seroprevalence in Europe: A Meta-Analysis. Viruses 2016, 8, 211. [Google Scholar] [CrossRef]
- Rein, D.B.; Stevens, G.A.; Theaker, J.; Wittenborn, J.S.; Wiersma, S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012, 55, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.; Askar, M.; Stark, K. Case-control study on risk factors for acute hepatitis E in Germany, 2012 to 2014. Euro Surveill. 2018, 23, 17–00469. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, H.; Pischke, S.; Manns, M.P. Pathogenesis and treatment of hepatitis e virus infection. Gastroenterology 2012, 142, 1388–1397.e1381. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Schulze Zur Wiesch, J.; Lütgehetmann, M.; Lohse, A.W.; Pischke, S. The Clinical Perspective on Hepatitis E. Viruses 2019, 11, 617. [Google Scholar] [CrossRef]
- Kamar, N.; Bendall, R.; Legrand-Abravanel, F.; Xia, N.-S.; Ijaz, S.; Izopet, J. Hepatitis E. Lancet 2012, 379, 2477–2488. [Google Scholar] [CrossRef]
- Pischke, S.; Peron, J.M.; von Wulffen, M.; von Felden, J.; Honer Zu Siederdissen, C.; Fournier, S.; Lutgehetmann, M.; Iking-Konert, C.; Bettinger, D.; Par, G.; et al. Chronic Hepatitis E in Rheumatology and Internal Medicine Patients: A Retrospective Multicenter European Cohort Study. Viruses 2019, 11, 186. [Google Scholar] [CrossRef]
- Riveiro-Barciela, M.; Buti, M.; Homs, M.; Campos-Varela, I.; Cantarell, C.; Crespo, M.; Castells, L.; Tabernero, D.; Quer, J.; Esteban, R.; et al. Cirrhosis, liver transplantation and, H.I.V infection are risk factors associated with hepatitis E virus infection. PLoS ONE 2014, 9, e103028. [Google Scholar] [CrossRef]
- Haïm-Boukobza, S.; Ferey, M.P.; Vétillard, A.L.; Jeblaoui, A.; Pélissier, E.; Pelletier, G.; Teillet, L.; Roque-Afonso, A.M. Transfusion-transmitted hepatitis E in a misleading context of autoimmunity and drug-induced toxicity. J. Hepatol. 2012, 57, 1374–1378. [Google Scholar] [CrossRef]
- Hewitt, P.E.; Ijaz, S.; Brailsford, S.R.; Brett, R.; Dicks, S.; Haywood, B.; Kennedy, I.T.R.; Kitchen, A.; Patel, P.; Poh, J.; et al. Hepatitis E virus in blood components: A prevalence and transmission study in southeast England. Lancet 2014, 384, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Huzly, D.; Umhau, M.; Bettinger, D.; Cathomen, T.; Emmerich, F.; Hasselblatt, P.; Hengel, H.; Herzog, R.; Kappert, O.; Maassen, S.; et al. Transfusion-transmitted hepatitis E in Germany, 2013. Euro Surveill. 2014, 19, 20812. [Google Scholar] [CrossRef] [PubMed]
- Ticehurst, J.R.; Pisanic, N.; Forman, M.S.; Ordak, C.; Heaney, C.D.; Ong, E.; Linnen, J.M.; Ness, P.M.; Guo, N.; Shan, H.; et al. Probable transmission of hepatitis E virus (HEV) via transfusion in the United States. Transfusion 2019, 59, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Ozga, A.K.; Westholter, D.; Hartl, J.; Manthey, C.F.; Lutgehetmann, M.; Rauch, G.; Kriston, L.; Lohse, A.W.; Bendall, R.; et al. Hepatitis E seroprevalence in the Americas: A systematic review and meta-analysis. Liver Int. 2018, 38, 1951–1964. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, C.; Mann, S. The Old Man and the Meat: On Gender Differences in Meat Consumption across Stages of Human Life. Foods 2021, 10, 2809. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Pischke, S. HEV in pregnancy: Understanding the crucial role of steroid hormones. Liver Int. 2019, 39, 621–622. [Google Scholar] [CrossRef] [PubMed]
- Joanna-Briggs-Institute. Checklist for Prevalence Studies 2022. cited 2022. Available online: https://jbi.global/critical-appraisal-tools (accessed on 13 September 2022).
- Fu, P.; Lin, B.; Wu, B.; Ke, L.; Yang, T.; Du, Y.; Cheng, L.; Li, Z.; Li, T.; Liu, Y. Hepatitis E virus prevalence among blood donors in Dali, China. Virol. J. 2021, 18, 141. [Google Scholar] [CrossRef]
- Mishra, K.K.; Patel, K.; Trivedi, A.; Patel, P.; Ghosh, K.; Bharadva, S. Risk of hepatitis-E virus infections among blood donors in a regional blood transfusion centre in western India. Transfus. Med. 2021, 31, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Al Dossary, R.A.; Alnafie, A.N.; Aljaroodi, S.A.; Rahman, J.U.; Hunasemarada, B.C.; Alkharsah, K.R. Prevalence of Hepatitis E Virus Infection Among Blood Donors in the Eastern Province of Saudi Arabia. J. Multidiscip. Healthc. 2021, 14, 2381–2390. [Google Scholar] [CrossRef]
- Cordes, A.K.; Goudeva, L.; Lutgehetmann, M.; Wenzel, J.J.; Behrendt, P.; Wedemeyer, H.; Heim, A. Risk of transfusion-transmitted hepatitis E virus infection from pool-tested platelets and plasma. J. Hepatol. 2022, 76, 46–52. [Google Scholar] [CrossRef]
- Spreafico, M.; Raffaele, L.; Guarnori, I.; Foglieni, B.; Berzuini, A.; Valenti, L.; Gerosa, A.; Colli, A.; Prati, D. Prevalence and 9-year incidence of hepatitis E virus infection among North Italian blood donors: Estimated transfusion risk. J. Viral. Hepat. 2020, 27, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Maponga, T.G.; Lopes, T.; Cable, R.; Pistorius, C.; Preiser, W.; Andersson, M.I. Prevalence and risks of hepatitis E virus infection in blood donors from the Western Cape, South Africa. Vox Sang. 2020, 115, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Spada, E.; Costantino, A.; Pezzotti, P.; Bruni, R.; Pisani, G.; Madonna, E.; Chionne, P.; Simeoni, M.; Villano, U.; Marcantonio, C.; et al. Hepatitis E virus infection prevalence among men who have sex with men involved in a hepatitis A virus outbreak in Italy. Blood Transfus. 2019, 17, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, W.C.; Zhu, X.; To, A.P.; Holmberg, J. Hepatitis E virus infection in Hong Kong blood donors. Vox Sang. 2020, 115, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Vercouter, A.S.; Van Houtte, F.; Verhoye, L.; Gonzalez Fraile, I.; Blanco, L.; Compernolle, V.; Meuleman, P. Hepatitis E virus prevalence in Flemish blood donors. J. Viral. Hepat. 2019, 26, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, S.; Gallian, P.; Dimeglio, C.; Assal, A.; Abravanel, F.; Tiberghien, P.; Izopet, J. Viral load and clinical manifestations of hepatitis E virus genotype 3 infections. J. Viral. Hepat. 2019, 26, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Harvala, H.; Hewitt, P.E.; Reynolds, C.; Pearson, C.; Haywood, B.; Tettmar, K.I.; Ushiro-Lumb, I.; Brailsford, S.R.; Tedder, R.; Ijaz, S. Hepatitis E virus in blood donors in England, 2016 to 2017: From selective to universal screening. Euro Surveill. 2019, 24, 1800386. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, T.; Diekmann, J.; Knabbe, C.; Dreier, J. Hepatitis E virus blood donor NAT screening: As much as possible or as much as needed? Transfusion 2019, 59, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Intharasongkroh, D.; Thongmee, T.; Sa-Nguanmoo, P.; Klinfueng, S.; Duang-In, A.; Wasitthankasem, R.; Theamboonlers, A.; Charoonruangrit, U.; Oota, S.; Payungporn, S.; et al. Hepatitis E virus infection in Thai blood donors. Transfusion 2019, 59, 1035–1043. [Google Scholar] [CrossRef]
- Katiyar, H.; Goel, A.; Sonker, A.; Yadav, V.; Sapun, S.; Chaudhary, R.; Aggarwal, R. Prevalence of hepatitis E virus viremia and antibodies among healthy blood donors in India. Indian J. Gastroenterol. 2018, 37, 342–346. [Google Scholar] [CrossRef]
- Wen, G.P.; Chen, C.R.; Song, X.Y.; Tang, Z.M.; Ji, W.F.; Wang, S.L.; Zhang, K.; Zhang, J.; Ou, S.H.; Zheng, Z.Z.; et al. Long-term HEV carriers without antibody seroconversion among eligible immunocompetent blood donors. Emerg. Microbes Infect. 2018, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Spada, E.; Pupella, S.; Pisani, G.; Bruni, R.; Chionne, P.; Madonna, E.; Villano, U.; Simeoni, M.; Fabi, S.; Marano, G.; et al. A nationwide retrospective study on prevalence of hepatitis E virus infection in Italian blood donors. Blood Transfus. 2018, 16, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Thom, K.; Gilhooly, P.; McGowan, K.; Malloy, K.; Jarvis, L.M.; Crossan, C.; Scobie, L.; Blatchford, O.; Smith-Palmer, A.; Donnelly, M.C.; et al. Hepatitis E virus (HEV) in Scotland: Evidence of recent increase in viral circulation in humans. Euro Surveill. 2018, 23, 17–00174. [Google Scholar] [CrossRef] [PubMed]
- Westholter, D.; Hiller, J.; Denzer, U.; Polywka, S.; Ayuk, F.; Rybczynski, M.; Horvatits, T.; Gundlach, S.; Blocker, J.; Schulze Zur Wiesch, J.; et al. HEV-positive blood donations represent a relevant infection risk for immunosuppressed recipients. J. Hepatol. 2018, 69, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, P.; Sulkowska, E.; Gdowska, J.; Kopacz, A.; Liszewski, G.; Kubicka-Russel, D.; Baylis, S.A.; Corman, V.M.; Nocen, E.; Piotrowski, D.; et al. Molecular and serological infection marker screening in blood donors indicates high endemicity of hepatitis E virus in Poland. Transfusion 2018, 58, 1245–1253. [Google Scholar] [CrossRef]
- Hewitt, J.; Harte, D.S.M. Prevalence of hepatitis E virus antibodies and infection in New Zealand blood donors. N. Z. Med. J. 2018, 131, 38–43. [Google Scholar] [PubMed]
- Hoad, V.C.; Seed, C.R.; Fryk, J.J.; Harley, R.; Flower, R.L.P.; Hogema, B.M.; Kiely, P.; Faddy, H.M. Hepatitis E virus RNA in Australian blood donors: Prevalence and risk assessment. Vox Sang. 2017, 112, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.J.; Schafer, W.; Alexander, R.; Elliott, K.; Elliott-Browne, W.; Knowles, J.; Wenzel, J.J.; Simon, T.L. Low hepatitis E virus RNA prevalence in a large-scale survey of United States source plasma donors. Transfusion 2017, 57, 2958–2964. [Google Scholar] [CrossRef]
- Fearon, M.A.; O’Brien, S.F.; Delage, G.; Scalia, V.; Bernier, F.; Bigham, M.; Weger, S.; Prabhu, S.; Andonov, A. Hepatitis E in Canadian blood donors. Transfusion 2017, 57, 1420–1425. [Google Scholar] [CrossRef]
- Gallian, P.; Couchouron, A.; Dupont, I.; Fabra, C.; Piquet, Y.; Djoudi, R.; Assal, A.; Tiberghien, P. Comparison of hepatitis E virus nucleic acid test screening platforms and RNA prevalence in French blood donors. Transfusion 2017, 57, 223–224. [Google Scholar] [CrossRef]
- Minagi, T.; Okamoto, H.; Ikegawa, M.; Ideno, S.; Takahashi, K.; Sakai, K.; Hagiwara, K.; Yunoki, M.; Wakisaka, A. Hepatitis E virus in donor plasma collected in Japan. Vox Sang. 2016, 111, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.C.; Flower, R.L.; Seed, C.R.; Keller, A.J.; Harley, R.; Chan, H.T.; Hoad, V.; Warrilow, D.; Northill, J.; Holmberg, J. Aet al. Hepatitis E virus RNA in Australian blood donations. Transfusion 2016, 56, 3086–3093. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, T.; Diekmann, J.; Eberhardt, M.; Knabbe, C.; Dreier, J. Monitoring of Anti-Hepatitis E Virus Antibody Seroconversion in Asymptomatically Infected Blood Donors: Systematic Comparison of Nine Commercial Anti-HEV IgM and IgG Assays. Viruses 2016, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, J.; Boland, F.; Williams, P.; Donnellan, J.; Hogema, B.M.; Ijaz, S.; Murphy, W.G. Hepatitis E virus infection in the Irish blood donor population. Transfusion 2016, 56, 2868–2876. [Google Scholar] [CrossRef]
- Nouhin, J.; Prak, S.; Madec, Y.; Barennes, H.; Weissel, R.; Hok, K.; Pavio, N.; Rouet, F. Hepatitis E virus antibody prevalence, RNA frequency, and genotype among blood donors in Cambodia (Southeast Asia). Transfusion 2016, 56, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
- Harritshoj, L.H.; Holm, D.K.; Saekmose, S.G.; Jensen, B.A.; Hogema, B.M.; Fischer, T.K.; Midgley, S.E.; Krog, J.S.; Erikstrup, C.; Ullum, H. Low transfusion transmission of hepatitis E among 25,637 single-donation, nucleic acid-tested blood donors. Transfusion 2016, 56, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, I.; Limper, M.; Gerstenbluth, I.; Osterhaus, A.D.M.E.; van Veen, M.G.; Scherbeijn, S.M.J.; van Gorp, E.C.M.; Duits, A.J. Hepatitis E virus infection among blood donors in the South Caribbean: Is screening warranted? Neth. J. Med. 2016, 74, 51–53. [Google Scholar] [PubMed]
- Stramer, S.L.; Moritz, E.D.; Foster, G.A.; Ong, E.; Linnen, J.M.; Hogema, B.M.; Mak, M.; Chia, C.P.; Dodd, R.Y. Hepatitis E virus: Seroprevalence and frequency of viral RNA detection among US blood donors. Transfusion 2016, 56, 481–488. [Google Scholar] [CrossRef]
- Fischer, C.; Hofmann, M.; Danzer, M.; Hofer, K.; Kaar, J.; Gabriel, C. Seroprevalence and Incidence of hepatitis E in blood donors in Upper Austria. PLoS ONE 2015, 10, e0119576. [Google Scholar] [CrossRef]
- Sauleda, S.; Ong, E.; Bes, M.; Janssen, A.; Cory, R.; Babizki, M.; Shin, T.; Lindquist, A.; Hoang, A.; Vang, L.; et al. Seroprevalence of hepatitis E virus (HEV) and detection of HEV RNA with a transcription-mediated amplification assay in blood donors from Catalonia (Spain). Transfusion 2015, 55, 972–979. [Google Scholar] [CrossRef]
- Petrovic, T.; Lupulovic, D.; Jimenez de Oya, N.; Vojvodic, S.; Blazquez, A.B.; Escribano-Romero, E.; Martin-Acebes, M.A.; Potkonjak, A.; Milosevic, V.; Lazic, S.; et al. Prevalence of hepatitis E virus (HEV) antibodies in Serbian blood donors. J. Infect. Dev. Ctries 2014, 8, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Slot, E.; Hogema, B.M.; Riezebos-Brilman, A.; Kok, T.M. Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012 separator. Euro Surveill. 2013, 18, 20550. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, R.Y.; Schechterly, C.A.; Ge, S.; Shih, J.W.; Xia, N.S.; Luban, N.L.; Alter, H.J. An assessment of hepatitis E virus (HEV) in US blood donors and recipients: No detectable HEV RNA in 1939 donors tested and no evidence for HEV transmission to 362 prospectively followed recipients. Transfusion 2013, 53 Pt 2, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Cleland, A.; Smith, L.; Crossan, C.; Blatchford, O.; Dalton, H.R.; Scobie, L.; Petrik, J. Hepatitis E virus in Scottish blood donors. Vox Sang. 2013, 105, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Meldal, B.H.; Sarkodie, F.; Owusu-Ofori, S.; Allain, J.P. Hepatitis E virus infection in Ghanaian blood donors—The importance of immunoassay selection and confirmation. Vox Sang. 2013, 104, 30–36. [Google Scholar] [CrossRef]
- Vollmer, T.; Diekmann, J.; Johne, R.; Eberhardt, M.; Knabbe, C.; Dreier, J. Novel approach for detection of hepatitis E virus infection in German blood donors. J. Clin. Microbiol. 2012, 50, 2708–2713. [Google Scholar] [CrossRef]
- Guo, Q.S.; Yan, Q.; Xiong, J.H.; Ge, S.X.; Shih, J.W.; Ng, M.H.; Zhang, J.; Xia, N.S. Prevalence of hepatitis E virus in Chinese blood donors. J. Clin. Microbiol. 2010, 48, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Herremans, M.; Vennema, H.; Bakker, J.; van der Veer, B.; Duizer, E.; Benne, C.A.; Waar, K.; Hendrixks, B.; Schneeberger, P.; Blaauw, G.; et al. Swine-like hepatitis E viruses are a cause of unexplained hepatitis in the Netherlands. J. Viral. Hepat. 2007, 14, 140–146. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Kamili, S.; Yattoo, G.N. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J. Gastroenterol. Hepatol. 2004, 19, 778–784. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Chobe, L.P. Hepatitis E virus can it be transmitted parenterally. J. Viral. Hepat. 1999, 6, 161–164. [Google Scholar] [CrossRef]
- Costa, M.B.; Gouvea, M.S.G.; Chuffi, S.; Dellavia, G.H.; Ornel, F.; Von Diemen, L.; Kessler, F.; Pinho, J.R.R.; Alvares-da-Silva, M.R. Seroprevalence of hepatitis E virus in risk populations and blood donors in a referral hospital in the south of Brazil. Sci. Rep. 2021, 11, 6011. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.P.; Lee, H.Y.; Khor, C.S.; Abdul-Jamil, J.; Alias, H.; Abu-Amin, N.; Mat-Radzi, M.; Rohimi, N.A.; Mokhtardin, H.N.; AbuBakar, S.; et al. The Risk of Transfusion-Transmitted Hepatitis E Virus: Evidence from Seroprevalence Screening of Blood Donations. Indian J. Hematol. Blood Transfus. 2022, 38, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Baymakova, M.; Terzieva, K.; Popov, R.; Grancharova, E.; Kundurzhiev, T.; Pepovich, R.; Tsachev, I. Seroprevalence of Hepatitis E Virus Infection among Blood Donors in Bulgaria. Viruses 2021, 13, 492. [Google Scholar] [CrossRef] [PubMed]
- Di Lello, F.A.; Blejer, J.; Alter, A.; Bartoli, S.; Vargas, F.; Ruiz, R.; Galli, C.; Blanco, S.; Carrizo, L.H.; Gallego, S.; et al. Seroprevalence of hepatitis E virus in Argentinean blood donors. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Bangueses, F.; Abin-Carriquiry, J.A.; Cancela, F.; Curbelo, J.; Mirazo, S. Serological and molecular prevalence of hepatitis E virus among blood donors from Uruguay. J. Med. Virol. 2021, 93, 4010–4014. [Google Scholar] [CrossRef]
- Capai, L.; Hoze, N.; Chiaroni, J.; Gross, S.; Djoudi, R.; Charrel, R.; Izopet, J.; Bosseur, F.; Priet, S.; Cauchemez, S.; et al. Seroprevalence of hepatitis E virus among blood donors on Corsica, France, 2017. Euro Surveill. 2020, 25, 1900336. [Google Scholar] [CrossRef]
- Yrondi, A.; Salles, J.; Peron, J.M.; Sporer, M.; Taib, S.; Gallini, A.; Noilhan, C.; Dimeglio, C.; Entajan, F.; Crequy, M.; et al. The Prevalence of Hepatitis E in a Patient Cohort Presenting With Addictive Injection Behavior. Front. Psychiatry 2019, 10, 832. [Google Scholar] [CrossRef]
- Arce, L.P.; Muller, M.F.; Martinez, A.; Baiker, A.; Marranzino, G.; Agote, F.; Vizoso-Pinto, M.G. A Novel In-House Enzyme-Linked Immunosorbent Assay for Genotype 3 Hepatitis E Virus Reveals High Seroprevalence in Blood Donors in Northern Argentina. Front. Microbiol. 2019, 10, 2481. [Google Scholar] [CrossRef]
- Capai, L.; Masse, S.; Gallian, P.; Souty, C.; Isnard, C.; Blanchon, T.; Peres, B.; de Lamballerie, X.; Charrel, R.; Falchi, A. Seroprevalence Study of Anti-HEV IgG among Different Adult Populations in Corsica, France, 2019. Microorganisms 2019, 7, 460. [Google Scholar] [CrossRef]
- Jupattanasin, S.; Chainuvati, S.; Chotiyaputta, W.; Chanmanee, T.; Supapueng, O.; Charoonruangrit, U.; Oota, S.; Louisirirotchanakul, S. A Nationwide Survey of the Seroprevalence of Hepatitis E Virus Infections Among Blood Donors in Thailand. Viral Immunol. 2019, 32, 302–307. [Google Scholar] [CrossRef]
- Yasar, O.; Karatayli, E.; Cengiz, G.; Kizilpinar, M.; Yurdcu, E.; Albayrak, R.; Guven, A.; Arslan, O.; Karahan, C.; Otlu, B.; et al. HEV seroprevalence in blood donors in Turkey by two commercial total anti-HEV Ab ELISA kits. J. Med. Virol. 2019, 91, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Moss da Silva, C.; Oliveira, J.M.; Mendoza-Sassi, R.A.; Figueiredo, A.S.; Mota, L.D.D.; Nader, M.M.; Gardinali, N.R.; Kevorkian, Y.B.; Salvador, S.B.S.; Pint, M.A.; et al. Detection and characterization of hepatitis E virus genotype 3 in HIV-infected patients and blood donors from southern Brazil. Int. J. Infect. Dis. 2019, 86, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Miletic, M.; Vuk, T.; Hecimovic, A.; Stojic Vidovic, M.; Jemersic, L.; Jukic, I. Estimation of the hepatitis E assay-dependent seroprevalence among Croatian blood donors. Transfus. Clin. Biol. 2019, 26, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Twagirumugabe, T.; Saguti, F.; Habarurema, S.; Gahutu, J.B.; Bergstrom, T.; Norder, H. Hepatitis A and E virus infections have different epidemiological patterns in Rwanda. Int. J. Infect. Dis. 2019, 86, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Slavov, S.N.; Maconetto, J.D.M.; Martinez, E.Z.; Silva-Pinto, A.C.; Covas, D.T.; Eis-Hubinger, A.M.; Kashima, S. Prevalence of hepatitis E virus infection in multiple transfused Brazilian patients with thalassemia and sickle cell disease. J. Med. Virol. 2019, 91, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gong, P.; Wagner, A.L.; Li, Y.; Wang, G.; Lu, Y. Identification of hepatitis E virus subtype 4f in blood donors in Shanghai, China. Virus Res. 2019, 265, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Dimeglio, C.; Beau, F.; Broult, J.; Gouy, P.; Izopet, J.; Lastere, S.; Abravanel, F. Hepatitis E prevalence in French Polynesian blood donors. PLoS ONE 2018, 13, e0208934. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.S.; Puranik, S.; Sharma, M.; Chakraborty, S.; Devakate, U.R. Hepatitis E virus seroprevalence among blood donors in Pune, India. J. Med. Virol. 2019, 91, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Niederhauser, C.; Widmer, N.; Hotz, M.; Tinguely, C.; Fontana, S.; Allemann, G.; Borri, M.; Infanti, L.; Sarraj, A.; Sigle, J.; et al. Current hepatitis E virus seroprevalence in Swiss blood donors and apparent decline from 1997 to 2016. Euro Surveill. 2018, 23, 1700616. [Google Scholar] [CrossRef]
- Hardtke, S.; Rocco, R.; Ogata, J.; Braga, S.; Barbosa, M.; Wranke, A.; Doi, E.; da Cunha, D.; Maluf, E.; Wedemeyer, H.; et al. Risk factors and seroprevalence of hepatitis E evaluated in frozen-serum samples (2002–2003) of pregnant women compared with female blood donors in a Southern region of Brazil. J. Med. Virol. 2018, 90, 1856–1862. [Google Scholar] [CrossRef]
- Al-Absi, E.S.; Al-Sadeq, D.W.; Younis, M.H.; Yassine, H.M.; Abdalla, O.M.; Mesleh, A.G.; Hadwan, T.A.; Amimo, J.O.; Thalib, L.; Nasrallah, G.K.; et al. Performance evaluation of five commercial assays in assessing seroprevalence of HEV antibodies among blood donors. J. Med. Microbiol. 2018, 67, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Bura, M.; Łagiedo-Żelazowska, M.; Michalak, M.; Sikora, J.; Mozer-Lisewsk, I. Comparative Seroprevalence of Hepatitis A And E Viruses in Blood Donors from Wielkopolska Region, West-Central Poland. Pol. J. Microbiol. 2018, 67, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Mooij, S.H.; Hogema, B.M.; Tulen, A.D.; van Pelt, W.; Franz, E.; Zaaijer, H.L.; Molier, M.; Hofhuis, A. Risk factors for hepatitis E virus seropositivity in Dutch blood donors. BMC Infect. Dis. 2018, 18, 173. [Google Scholar] [CrossRef] [PubMed]
- Juhl, D.; Nowak-Gottl, U.; Blumel, J.; Gorg, S.; Hennig, H. Lack of evidence for the transmission of hepatitis E virus by coagulation factor concentrates based on seroprevalence data. Transfus. Med. 2018, 28, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Fomiatti, L.; Tagliacarne, C.; Velati, C.; Zanetti, A.R.; Castaldi, S.; Romano, L. Seroprevalence of hepatitis E virus among blood donors in northern Italy (Sondrio, Lombardy) determined by three different assays. Blood Transfus. 2017, 15, 502–505. [Google Scholar] [CrossRef]
- Bura, M.; Bukowska, A.; Bura, A.; Michalak, M.; Mozer-Lisewska, I. Hepatitis E virus antibodies in HIV-infected patients and blood donors from western Poland: A preliminary report. Adv. Clin. Exp. Med. 2017, 26, 577–579. [Google Scholar] [CrossRef]
- Passos-Castilho, A.M.; Reinaldo, M.R.; Sena, A.; Granato, C.F.H. High prevalence of hepatitis E virus antibodies in Sao Paulo, Southeastern Brazil: Analysis of a group of blood donors representative of the general population. Braz. J. Infect. Dis. 2017, 21, 535–539. [Google Scholar] [CrossRef]
- Bura, M.; Lagiedo, M.; Michalak, M.; Sikora, J.; Mozer-Lisewska, I. Hepatitis E virus IgG seroprevalence in HIV patients and blood donors, west-central Poland. Int. J. Infect. Dis. 2017, 61, 20–22. [Google Scholar] [CrossRef]
- Pandolfi, R.; Ramos de Almeida, D.; Alves Pinto, M.; Kreutz, L.C.; Frandoloso, R. In house ELISA based on recombinant ORF2 protein underline high prevalence of IgG anti-hepatitis E virus amongst blood donors in south Brazil. PLoS ONE 2017, 12, e0176409. [Google Scholar] [CrossRef]
- Gupta, B.P.; Lama, T.K.; Adhikari, A.; Shrestha, A.; Rauniyar, R.; Sapkota, B.; Thapa, S.; Shrestha, S.; Gupta, P.P.; Das Manandhar, K. First report of hepatitis E virus viremia in healthy blood donors from Nepal. Virusdisease 2016, 27, 324–326. [Google Scholar] [CrossRef]
- Nasrallah, G.K.; Al Absi, E.S.; Ghandour, R.; Ali, N.H.; Taleb, S.; Hedaya, L.; Ali, F.; Huwaidy, M.; Husseini, A. Seroprevalence of hepatitis E virus among blood donors in Qatar (2013–2016). Transfusion 2017, 57, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Slot, E.; Zaaijer, H.L.; Molier, M.; Van den Hurk, K.; Prinsze, F.; Hogema, B.M. Meat consumption is a major risk factor for hepatitis E virus infection. PLoS ONE 2017, 12, e0176414. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.; Cable, R.; Pistorius, C.; Maponga, T.; Ijaz, S.; Preiser, W.; Tedder, R.; Andersson, M.I. Racial differences in seroprevalence of HAV and HEV in blood donors in the Western Cape, South Africa: A clue to the predominant HEV genotype? Epidemiol. Infect. 2017, 145, 1910–1912. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; He, M.; Wu, B.; Ke, L.; Han, T.; Wang, J.; Shan, H.; Ness, P.; Guo, N.; Liu, Y.; et al. The association of elevated alanine aminotransferase levels with hepatitis E virus infections among blood donors in China. Transfusion 2017, 57, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Abravanel, F.; Lhomme, S.; Fougere, M.; Saune, K.; Alvarez, M.; Peron, J.M.; Delobel, P.; Izopet, J. HEV Infection in French HIV-infected patients. J. Infect. 2017, 74, 310–313. [Google Scholar] [CrossRef] [PubMed]
- De Sabato, L.; Di Bartolo, I.; Montomoli, E.; Trombetta, C.; Ruggeri, F.M.; Ostanello, F. Retrospective Study Evaluating Seroprevalence of Hepatitis E Virus in Blood Donors and in Swine Veterinarians in Italy (2004). Zoonoses Public Health 2017, 64, 308–312. [Google Scholar] [CrossRef]
- Shrestha, A.C.; Flower, R.L.; Seed, C.R.; Rajkarnikar, M.; Shrestha, S.K.; Thapa, U.; Hoad, V.C.; Faddy, H.M. Hepatitis E virus seroepidemiology: A post-earthquake study among blood donors in Nepal. BMC Infect. Dis. 2016, 16, 707. [Google Scholar] [CrossRef]
- Lange, H.; Overbo, J.; Borgen, K.; Dudman, S.; Hoddevik, G.; Urdahl, A.M.; Vold, L.; Sjurseth, S.K. Hepatitis E in Norway: Seroprevalence in humans and swine. Epidemiol. Infect. 2017, 145, 181–186. [Google Scholar] [CrossRef]
- Parsa, R.; Adibzadeh, S.; Behzad Behbahani, A.; Farhadi, A.; Yaghobi, R.; Rafiei Dehbidi, G.R.; Hajizamani, S.; Rahbar, S.; Nikouyan, N.; Okhovat, M.A.; et al. Detection of Hepatitis E Virus Genotype 1 among Blood Donors from Southwest of Iran. Hepat. Mon. 2016, 16, e34202. [Google Scholar] [CrossRef]
- Lucarelli, C.; Spada, E.; Taliani, G.; Chionne, P.; Madonna, E.; Marcantonio, C.; Pezzotti, P.; Bruni, R.; La Rosa, G.; Pisani, G.; et al. High prevalence of anti-hepatitis E virus antibodies among blood donors in central Italy, February to March 2014. Euro Surveill. 2016, 21. [Google Scholar] [CrossRef]
- Hesamizadeh, K.; Sharafi, H.; Keyvani, H.; Alavian, S.M.; Najafi-Tireh Shabankareh, A.; Sharifi Olyaie, R.; Keshvari, M. Hepatitis A Virus and Hepatitis E Virus Seroprevalence Among Blood Donors in Tehran, Iran. Hepat. Mon. 2016, 16, e32215. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, J.M.; Gallian, P.; Dimeglio, C.; Saune, K.; Arnaud, C.; Pelletier, B.; Morel, P.; Legrand, D.; Tiberghien, P.; Izopet, J. A nationwide survey of hepatitis E viral infection in French blood donors. Hepatology 2016, 63, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Traore, K.A.; Ouoba, J.B.; Rouamba, H.; Nebie, Y.K.; Dahourou, H.; Rossetto, F.; Traore, A.S.; Barro, N.; Roques, P. Hepatitis E Virus Prevalence among Blood Donors, Ouagadougou, Burkina Faso. Emerg. Infect. Dis. 2016, 22, 755–757. [Google Scholar] [CrossRef]
- Ricco, G.; Bonino, F.; Lanza, M.; Scatena, F.; Alfieri, C.M.; Messa, P.; Marchisio, E.; Mascolo, G.; Romano, L.; Galli, C.; et al. New immunoassays for total, IgA and IgM antibodies against hepatitis E virus: Prevalence in Italian blood donors and patients with chronic liver or kidney diseases. Dig. Liver Dis. 2016, 48, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, B.; Mazloom Kalimani, F.; Pourfatolah, A.A.; Azimzadeh, M.; Mankhian, A.; Akbarzadeh, S.; Hajiani, G.; Kooshesh, F.; Khamisipour, G. Hepatitis E Virus Seroprevalence Among Blood Donors in Bushehr, South of Iran. Hepat. Mon. 2015, 15, e29219. [Google Scholar] [CrossRef]
- Norder, H.; Karlsson, M.; Mellgren, A.; Konar, J.; Sandberg, E.; Lasson, A.; Castedal, M.; Magnius, L.; Lagging, M. Diagnostic Performance of Five Assays for Anti-Hepatitis E Virus IgG and IgM in a Large Cohort Study. J. Clin. Microbiol. 2016, 54, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Puttini, C.; Riccio, M.; Redi, D.; Tordini, G.; Cenerini, M.; Romanello, F.; De Luca, A.; Carmellini, M.; Fossombroni, V.; Grazia Cusi, M.; et al. Seroprevalence of hepatitis E virus (HEV) infection in blood donors and renal transplant recipients: A retrospective study from central Italy. Infez. Med. 2015, 3, 253–256. [Google Scholar]
- Sarkar, S.; Rivera, E.M.; Engle, R.E.; Nguyen, H.T.; Schechterly, C.A.; Alter, H.J.; Liang, T.J.; Purcell, R.H.; Hoofnagle, J.H.; Ghany, M.G. An Epidemiologic Investigation of a Case of Acute Hepatitis E. J. Clin. Microbiol. 2015, 53, 3547–3552. [Google Scholar] [CrossRef]
- Passos-Castilho, A.M.; de Sena, A.; Geraldo, A.; Spada, C.; Granato, C.F. High prevalence of hepatitis E virus antibodies among blood donors in Southern Brazil. J. Med. Virol. 2016, 88, 361–364. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Saune, K.; Rech, H.; Abravanel, F.; Mengelle, C.; L‘Homme, S.L.; Destruel, F.; Kamar, N.; Izopet, J. Seroprevalence in blood donors reveals widespread, multi-source exposure to hepatitis E virus, southern France, October 2011. Euro Surveill. 2015, 20, 21127. [Google Scholar] [CrossRef]
- Holm, D.K.; Moessner, B.K.; Engle, R.E.; Zaaijer, H.L.; Georgsen, J.; Purcell, R.H.; Christensen, P.B. Declining prevalence of hepatitis E antibodies among Danish blood donors. Transfusion 2015, 55, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Seed, C.R.; Flower, R.L.; Rooks, K.; Keller, A.J. Hepatitis E Virus and Implications for Blood Supply Safety, Australia. Emerg. Infect. Dis. 2014, 20, 1941–1942. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ayed, Y.; Hannachi, H.; Ben-Alaya-Bouafif, N.; Gouider, E.; Triki, H.; Bahri, O. Hepatitis E virus seroprevalence among hemodialysis and hemophiliac patients in Tunisia (North Africa). J. Med. Virol. 2015, 87, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Ding, X.; Lyu, C.; Xiang, L.; Teng, H.; Li, J. Hepatitis E virus seroprevalence among blood donors in Jiangsu Province, East China. Int. J. Infect. Dis. 2014, 26, 9–11. [Google Scholar] [CrossRef]
- Hogema, B.M.; Molier, M.; Slot, E.; Zaaijer, H.L. Past and present of hepatitis E in the Netherlands. Transfusion 2014, 54, 3092–3096. [Google Scholar] [CrossRef] [PubMed]
- Pittaras, T.; Valsami, S.; Mavrouli, M.; Kapsimali, V.; Tsakris, A.; Politou, M. Seroprevalence of hepatitis E virus in blood donors in Greece. Vox Sang. 2014, 106, 387. [Google Scholar] [CrossRef]
- Ren, F.; Zhao, C.; Wang, L.; Wang, Z.; Gong, X.; Song, M.; Zhuang, H.; Huang, Y.; Shan, H.; Wang, J.; et al. Hepatitis E virus seroprevalence and molecular study among blood donors in China. Transfusion 2014, 54 Pt 2, 910–917. [Google Scholar] [CrossRef]
- Jahromi, A.S.; Pourahmad, M. Hepatitis E virus and serum level aminotransferases in blood donors. Rep. Biochem. Mol. Biol. 2013, 2, 48–51. [Google Scholar]
- Ramezani, A.; Velayati, A.A.; Khorami-Sarvestani, S.; Eslamifar, A.; Mohraz, M.; Banifazl, M.; Bidari-Zerehpoosh, F.; Yaghmaei, F.; McFarland, W.; Foroughi, M.; et al. Hepatitis E virus infection in patients infected with human immunodeficiency virus in an endemic area in Iran. Int. J. Std Aids. 2013, 24, 769–774. [Google Scholar] [CrossRef]
- Johargy, A.K.; Mahomed, M.F.; Khan, M.M.; Kabrah, S. Anti Hepatitis E virus seropositivity in a group of male blood donors in Makkah, Saudi Arabia. J. Pak. Med. Assoc. 2013, 63, 185–189. [Google Scholar]
- Ehteram, H.; Ramezani, A.; Eslamifar, A.; Sofian, M.; Banifazi, M.; Ghassemi, S.; Aghakhani, A.; Mashayekhi, P. Seroprevalence of Hepatitis E Virus infection among volunteer blood donors in central province of Iran in 2012. Iran J. Microbiol. 2013, 5, 172–176. [Google Scholar] [PubMed]
- Scotto, G.; Martinelli, D.; Centra, M.; Querques, M.; Vittorio, F.; Delli Carri, P.; Tartaglia, A.; Campanale, F.; Bulla, F.; Prato, R.; et al. Epidemiological and clinical features of HEV infection: A survey in the district of Foggia (Apulia, Southern Italy). Epidemiol. Infect. 2014, 142, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Juhl, D.; Baylis, S.A.; Blumel, J.; Gorg, S.; Hennig, H. Seroprevalence and incidence of hepatitis E virus infection in German blood donors. Transfusion 2014, 54, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Traore, K.A.; Rouamba, H.; Nebie, Y.; Sanou, M.; Traore, A.S.; Barro, N.; Roques, P. Seroprevalence of fecal-oral transmitted hepatitis A and E virus antibodies in Burkina Faso. PLoS ONE 2012, 7, e48125. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.M.T.D.; Oliveira, J.M.D.; Vitral, C.L.; Vieira, K.D.A.; Pinto, M.A.; Souto, F.J.D. Prevalence of hepatitis e virus antibodies in individual exposed to swine in Mato Grosso, Brazil. MemÓRias Do Inst. Oswaldo Cruz 2012, 107, 338–341. [Google Scholar] [CrossRef]
- Cheng, X.F.; Wen, Y.F.; Zhu, M.; Zhan, S.W.; Zheng, J.X.; Dong, C.; Xiang, K.X.; Xia, X.B.; Wang, G.; Han, L.F. Serological and molecular study of hepatitis E virus among illegal blood donors. World J. Gastroenterol. 2012, 18, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Neffati, H.; Ritter, J.; Feki, S.; Dron, A.-G.; Slim, A.; Hassine, M.; Braham, H.; Ramiere, C.; Andre, P.; Aouni, M.; et al. Seroprevalence of hepatitis E virus infection in rural and urban populations, Tunisia. Clin. Microbiol. Infect 2012, 18, E119–E121. [Google Scholar]
- Dremsek, P.; Wenzel, J.J.; Johne, R.; Ziller, M.; Hofmann, J.; Groschup, M.H.; Werdermann, S.; Mohn, U.; Dorn, S.; Motz, M.; et al. Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis E virus-specific immunoglobulin G ELISAs. Med. Microbiol. Immunol. 2012, 201, 189–200. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Bendall, R.; Legrand-Abravanel, F.; Saune, K.; Miedouge, M.; Ellis, V.; Rech, H.; Destruel, F.; Kamar, N.; Dalton, H.R.; et al. Hepatitis E virus antibodies in blood donors, France. Emerg. Infect. Dis. 2011, 17, 2309–2312. [Google Scholar] [CrossRef]
- Dong, C.; Meng, J.; Dai, X.; Liang, J.H.; Feagins, A.R.; Meng, X.J.; Belfiore, N.M.; Bradford, C.; Corn, J.L.; Cray, C.; et al. Restricted enzooticity of hepatitis E virus genotypes 1 to 4 in the United States. J. Clin. Microbiol. 2011, 49, 4164–4172. [Google Scholar] [CrossRef]
- Krumbholz, A.; Mohn, U.; Lange, J.; Motz, M.; Wenzel, J.J.; Jilg, W.; Walther, M.; Straube, E.; Wutzler, P.; Zell, R. Prevalence of hepatitis E virus-specific antibodies in humans with occupational exposure to pigs. Med. Microbiol. Immunol. 2012, 201, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A.; Kenfak-Foguena, A.; Andre, C.; Canellini, G.; Burgisser, P.; Moradpour, D.; Darling, K.E.; Cavassini, M. Hepatitis E virus seroprevalence among blood donors in southwest Switzerland. PLoS ONE 2011, 6, e21150. [Google Scholar] [CrossRef] [PubMed]
- Beale, M.A.; Tettmar, K.; Szypulska, R.; Tedder, R.S.; Ijaz, S. Is there evidence of recent hepatitis E virus infection in English and North Welsh blood donors? Vox Sang. 2011, 100, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Matsubayashi, K.; Sakata, H.; Sato, S.; Kato, T.; Hino, S.; Tadokoro, K.; Ikeda, H. A nationwide survey for prevalence of hepatitis E virus antibody in qualified blood donors in Japan. Vox Sang. 2010, 99, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Masia, G.; Orru, G.; Liciardi, M.; Desogus, G.; Coppola, R.C.; Murru, V.; Argiolas, M.; Orru, G. Evidence of Hepatitis E Virus (HEV) infection in human and pigs in Sardinia, Italy. J. Prev. Med. Hyg. 2009, 50, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.B.; Engle, R.E.; Hjort, C.; Homburg, K.M.; Vach, W.; Georgsen, J.; Purcell, R.H. Time trend of the prevalence of hepatitis E antibodies among farmers and blood donors: A potential zoonosis in Denmark. Clin. Infect. Dis. 2008, 47, 1026–1031. [Google Scholar] [CrossRef]
- Dalton, H.R.; Stableforth, W.; Thurairajah, P.; Hazeldine, S.; Remnarace, R.; Usama, W.; Farrington, L.; Hamad, N.; Sieberhagen, C.; Ellis, V.; et al. Autochthonous hepatitis E in Southwest England: Natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur. J. Gastroenterol. Hepatol. 2008, 20, 784–790. [Google Scholar] [CrossRef]
- Mansuy, J.M.; Legrand-Abravanel, F.; Calot, J.P.; Peron, J.M.; Alric, L.; Agudo, S.; Rech, H.; Destruel, F.; Izopet, J. High prevalence of anti-hepatitis E virus antibodies in blood donors from South West France. J. Med. Virol. 2008, 80, 289–293. [Google Scholar] [CrossRef]
- Assarehzadegan, M.A.; Shakerinejad, G.; Amini, A.; Rezaee, S.A. Seroprevalence of hepatitis E virus in blood donors in Khuzestan Province, southwest Iran. Int. J. Infect. Dis. 2008, 12, 387–390. [Google Scholar] [CrossRef]
- Taremi, M.; Gachkar, L.; MahmoudArabi, S.; Kheradpezhouh, M.; Khoshbaten, M. Prevalence of antibodies to hepatitis E virus among male blood donors in Tabriz, Islamic Republic of Iran. East Mediterr. Health J. 2007, 13, 98–102. [Google Scholar]
- Dalton, H.R.; Fellows, H.J.; Gane, E.J.; Wong, P.; Gerred, S.; Schroeder, B.; Croxson, M.C.; Garkavenko, O. Hepatitis E in new zealand. J. Gastroenterol. Hepatol. 2007, 22, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Boutrouille, A.; Bakkali-Kassimi, L.; Cruciere, C.; Pavio, N. Prevalence of anti-hepatitis E virus antibodies in French blood donors. J. Clin. Microbiol. 2007, 45, 2009–2010. [Google Scholar] [CrossRef] [PubMed]
- Bortoliero, A.L.; Bonametti, A.M.; Morimoto, H.K.; Matsuo, T.; Reiche, E.M.V. Seroprevalence for hepatitis E virus (HEV) infection among volunteer blood donors of the Regional Blood Bank of Londrina, State of Paraná, Brazil. Rev. Inst. Med. Trop. 2006, 48, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Sunaga, J.; Saito, N.; Fujimura, K.; Itoh, Y.; Sasaki, M.; Tsuda, F.; Takahashi, M.; Nishizawa, T.; Okamoto, H. Prevalence of antibodies to hepatitis E virus among Japanese blood donors: Identification of three blood donors infected with a genotype 3 hepatitis E virus. J. Med. Virol. 2004, 73, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Engle, R.E.; Yu, C.; Emerson, S.U.; Meng, X.J.; Purcell, R.H. Hepatitis E virus (HEV) capsid antigens derived from viruses of human and swine origin are equally efficient for detecting anti-HEV by enzyme immunoassay. J. Clin. Microbiol. 2002, 40, 4576–4580. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J.; Wiseman, B.; Elvinger, F.; Guenette, D.K.; Toth, T.E.; Engle, R.E.; Emerson, S.U.; Purcell, R.H. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 2002, 40, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, D.; Rocha Jr, J.E.; Crispim, M.A. Prevalence of hepatitis E virus antibodies among different groups in the Amazonian basin. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 215. [Google Scholar] [CrossRef]
- Trinta, K.S.; Liberto, M.I.M.; Paula, V.S.D.; Yoshida, C.F.; Gaspar, A.M. Hepatitis E virus infection in selected Brazilian populations. MemÓRias Do Inst. Oswaldo Cruz 2001, 96, 25–29. [Google Scholar] [CrossRef]
- Goncales, N.S.L.; Pinho, J.R.R.; Moreira, R.C.; Saraceni, C.P.; Spina, A.M.M.; Stucchi, R.B.; Ribeiro Filho, A.D.; Magna, L.A.; Goncales Junior, F.L. Hepatitis E virus immunoglobulin G antibodies in different populations in Campinas, Brazil. Clin. Diagn Lab. Immunol. 2000, 7, 813–816. [Google Scholar] [CrossRef]
- Lemos, G.; Jameel, S.; Panda, S.; Rivera, L.; Rodriguez, L.; Gavilondo, J.V. Hepatitis E virus in Cuba. J. Clin. Virol. 2000, 16, 71–75. [Google Scholar] [CrossRef]
- Jutavijittum, P.; Jiviriyawat, Y.; Jiviriyawat, W.; Yousukh, A.; Hayashi, S.; Itakura, H.; Toriyama, K. Seroprevalence of antibody to hepatitis E virus in voluntary blood donors in Northern Thailand. Trop. Med. 2000, 42, 135–139. [Google Scholar]
- Karetnyi, Y.V.; Gilchrist, M.J.R.; Naides, S.J. Hepatitis E virus infection prevalence among selected populations in Iowa. J. Clin. Virol. 1999, 14, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Konomi, N.; Miyoshi, C.; La Fuente Zerain, C.; Li, T.-C.; Arakawa, Y.; Abe, K. Epidemiology of hepatitis, B.; C, E, and G virus infections and molecular analysis of hepatitis G virus isolates in Bolivia. J. Clin. Microbiol. 1999, 37, 3291–3295. [Google Scholar] [CrossRef] [PubMed]
- Seow, H.-F.; Mahomed, N.M.B.; Mak, J.-W.; Riddell, M.A.; Li, F.; Anderson, D.A. Seroprevalence of antibodies to hepatitis E virus in the normal blood donor population and two aboriginal communities in Malaysia. J. Clin. Virol. 1999, 59, 164–168. [Google Scholar] [CrossRef]
- Mateos, M.L.; Camarero, C.; Lasa, E.; Teruel, J.L.; Mir, N.; Baquero, F. Hepatitis E virus: Relevance in blood donors and risk groups. Vox Sang. 1998, 75, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Dalekos, G.N.; Zervou, E.; Elisaf, M.; Germanos, N.; Galanakis, E.; Bourantas, K.; Siamopoulos, K.C.; Tsianos, E.V. Antibodies to hepatitis E virus among several populations in Greece: Increased prevalence in an hemodialysis unit. Transfusion 1997, 38, 589–595. [Google Scholar] [CrossRef]
- Abdelaal, M.; Zawawi, T.H.; Al Sobhi, E.; Jeje, O.; Gilpin, C.; Kinsara, A.; Osoba, A.; Oni, G.A. Epidemiology of hepatitis E virus in male blood donors in Jeddah, Saudi Arabia. J. Multidiscip. Heal. 1998, 167, 94–96. [Google Scholar] [CrossRef]
- Pavia, M.; Iiritano, E.; Veratti, M.A.; Angelillo, I.F. Prevalence of hepatitis E antibodies in healthy persons in southern Italy. Infection 1998, 26, 32–35. [Google Scholar] [CrossRef]
- Araujo, F.; Koch, M.C.; Monteiro, F.; Araujo, A.R.; Cunha-Ribeiro, L.M. Hepatitis E in Portuguese haemophiliacs and blood donors. Haemophilia 1997, 3, 219–221. [Google Scholar] [CrossRef]
- Mast, E.E.; Kuramoto, I.K.; Favorov, M.O.; Schoening, V.R.; Burkholder, B.T.; Shapiro, C.N.; Holland, P.V. Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in Northern California. J. Infect. Dis. 1997, 176, 34–40. [Google Scholar] [CrossRef]
- Thomas, D.L.; Yarbough, P.O.; Vlahov, D.; Tsarev, S.A.; Nelson, K.E.; Saah, A.J.; Purcell, R.H. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J. Clin. Microbiol. 1997, 35, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.A.; Findor, J.A.; Daruich, J.R.; Canero Velazco, C.; Bruch Igartua, E.; Schmee, E.; Kohan, A.I. Prevalence of IgG anti-HEV in Buenos Aires, a nonendemic area for hepatitis E. J. Travel. Med. 1997, 4, 100–101. [Google Scholar] [CrossRef] [PubMed]
- Poovorawan, Y.; Theamboonlers, A.; Chumdermpadetsuk, S.; Komolmit, P. Prevalence of hepatitis E virus infection in Thailand. Ann. Trop. Med. Parasitol. 1996, 90, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.C.; Leyva, A.; Garcia, F.; Galan, I.; Piedrola, G.; Heyermann, H.; Maroto, M.C. Seroepidemiological study of hepatitis E virus in different population groups. Eur. J. Clin. Microbiol. Infect. Dis. 1995, 14, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Zaaijer, H.; Mauser-Bunschoten, E.P.; Ten Veen, J.H.; Kapprell, H.P.; Kok, M.; Van den Berg, H.M.; Lelie, P.N. Hepatitis E virus antibodies among patients with hemophilia, blood donors, and hepatitis patients. J. Med. Virol. 1995, 46, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Moaven, L.; Van Asten, M.; Crofts, N.; Locarnini, S.A. Seroepidemiology of hepatitis E in selected Australian populations. J. Med. Virol. 1995, 45, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-F.; Lin, M.-R.; Chue, P.-Y.; Tsai, J.-F.; Shih, C.-H.; Chen, I.-L.; He, J.; Carl, M. Prevalence of antibody to hepatitis E virus among healthy individuals in southern Taiwan. Microbiol. Immunol. 1995, 39, 733–736. [Google Scholar] [CrossRef]
- Zanetti, A.R.; Dawson, G.J. Hepatitis type E in Italy: A seroepidemiological survey. Study Group of Hepatitis E. J. Med. Virol. 1994, 42, 318–320. [Google Scholar] [CrossRef]
- Lavanchy, D.; Morel, B.; Frei, P. Seroprevalence of hepatitis E virus in Switzerland. Lancet 1994, 344, 747–748. [Google Scholar] [CrossRef]
- Gajjar, M.D.; Bhatnagar, N.M.; Sonani, R.V.; Gupta, S.; Patel, T. Hepatitis E seroprevalence among blood donors: A pilot study from Western India. Asian J. Transfus. Sci. 2014, 8, 29–31. [Google Scholar] [CrossRef]
- Utba, N.M. The prevalence of hepatitis E virus in Al-Sadr City—Baghdad. Clin. Lab. 2013, 59, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ibraham, E.H.; Abdelwahab, S.F.; Nady, S.; Hashem, M.; Galal, G.; Sobhy, M.; Saleh, A.S.; Shata, M.T. Prevalence of anti-HEV IgM among blood donors in Egypt. Egypt J. Immunol. 2011, 18, 47–58. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).