Coinfection of HPVs Is Associated with Advanced Stage in Colorectal Cancer Patients from Qatar
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
2.2. HPV Detection by PCR
2.3. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics of the Cohort
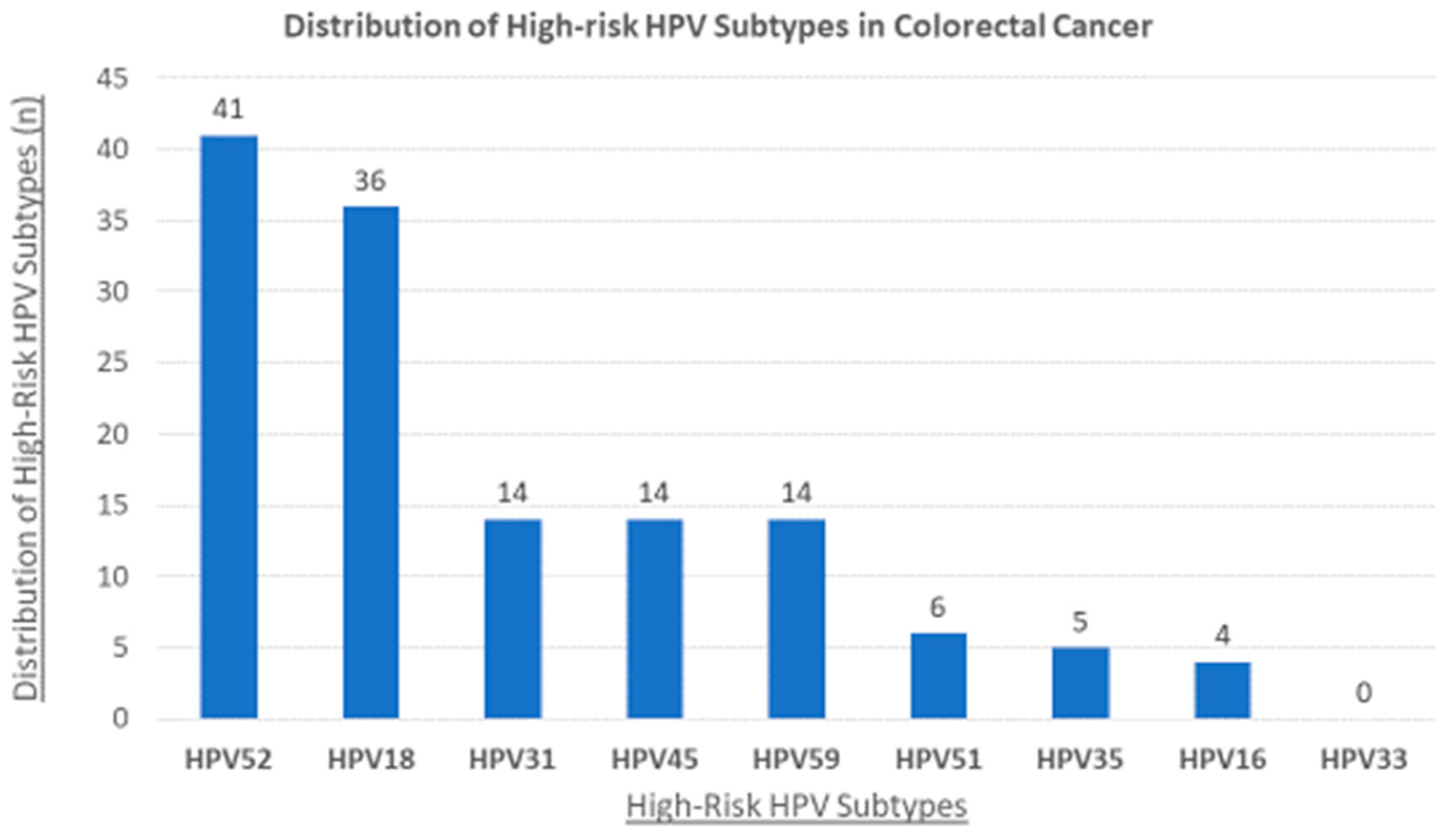
3.2. The Status of High-Risk HPVs and Their Association with Clinicopathological Characteristics
4. Discussion
4.1. Detection of HPV among CRC Samples from a Qatari Population
4.2. Correlation between the Presence of HPV and Clinicopathological Characteristics of the CRC Patients
4.3. Copresence of HR-HPV Subtypes in CRC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Heo, J.S.; Lee, J.; Lee, J.Y.; Lee, M.-Y.; Lim, S.H.; Lee, W.Y.; Kim, S.H.; Park, Y.A.; Cho, Y.B.; et al. The impact of KRAS mutations on prognosis in surgically resected colorectal cancer patients with liver and lung metastases: A retrospective analysis. BMC Cancer 2016, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Marongiu, L.; Allgayer, H. Viruses in colorectal cancer. Mol. Oncol. 2022, 16, 1423–1450. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. The search for infectious causes of human cancers: Where and why. Virology 2009, 392, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- White, M.K.; Pagano, J.S.; Khalili, K. Viruses and human cancers: A long road of discovery of molecular paradigms. Clin. Microbiol. Rev. 2014, 27, 463–481. [Google Scholar] [CrossRef]
- Tommasino, M. The human papillomavirus family and its role in carcinogenesis. Semin. Cancer Biol. 2014, 26, 13–21. [Google Scholar] [CrossRef]
- Stanley, M.A. Genital human papillomavirus infections: Current and prospective therapies. J. Gen. Virol. 2012, 93, 681–691. [Google Scholar] [CrossRef]
- Lacey, C.J.N.; Lowndes, C.M.; Shah, K.V. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006, 24, S35–S41. [Google Scholar] [CrossRef]
- Jamshidi, M.; Shekari, M.; Nejatizadeh, A.A.; Malekzadeh, K.; Baghershiroodi, M.; Davudian, P.; Dehghan, F.; Jamshidi, F. The impact of human papillomavirus (HPV) types 6, 11 in women with genital warts. Arch. Gynecol. Obstet. 2012, 286, 1261–1267. [Google Scholar] [CrossRef]
- Bernard, H.U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; zur Hausen, H.; de Villiers, E.M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Aimagambetova, G.; Ukybassova, T.; Kongrtay, K.; Azizan, A. Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination—Review of Current Perspectives. J. Oncol. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Q.; Gupta, I.; Vranic, S.; Al Moustafa, A.E. Human papillomaviruses and epstein–barr virus interactions in colorectal cancer: A brief review. Pathogens 2020, 9, 300. [Google Scholar] [CrossRef]
- Syrjänen, K.J. HPV infections and oesophageal cancer. J. Clin. Pathol. 2002, 55, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Delgado-García, S.; Martínez-Escoriza, J.-C.; Alba, A.; Martín-Bayón, T.-A.; Ballester-Galiana, H.; Peiró, G.; Caballero, P.; Ponce-Lorenzo, J. Presence of human papillomavirus DNA in breast cancer: A Spanish case-control study. BMC Cancer 2017, 17, 320. [Google Scholar] [CrossRef]
- Ohadian Moghadam, S.; Mansori, K.; Nowroozi, M.R.; Afshar, D.; Abbasi, B.; Nowroozi, A. Association of human papilloma virus (HPV) infection with oncological outcomes in urothelial bladder cancer. Infect. Agent. Cancer 2020, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Graflund, M.; Sorbe, B.; Sigurdardóttir, S.; Karlsson, M. HPV-DNA, vascular space invasion, and their impact on the clinical outcome in early-stage cervical carcinomas. Int. J. Gynecol. Cancer 2004, 14, 896–902. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Lukaszuk, K.; Liss, J.; Wozniak, I.; Sliwinski, W.; Emerich, J.; Wojcikowski, C. HPV and histological status of pelvic lymph node metastases in cervical cancer: A prospective study. J. Clin. Pathol. 2004, 57, 472–476. [Google Scholar] [CrossRef]
- Bernard, H.-U.; Calleja-Macias, I.E.; Dunn, S.T. Genome variation of human papillomavirus types: Phylogenetic and medical implications. Int. J. Cancer 2005, 118, 1071–1076. [Google Scholar] [CrossRef]
- Kim, S.-H.; Juhnn, Y.-S.; Kang, S.; Park, S.-W.; Sung, M.-W.; Bang, Y.-J.; Song, Y.-S. Human papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ ERK1,2 and PI3K/Akt. Cell. Mol. Life Sci. 2006, 63, 930–938. [Google Scholar] [CrossRef]
- Suprynowicz, F.A.; Disbrow, G.L.; Krawczyk, E.; Simic, V.; Lantzky, K.; Schlegel, R. HPV-16 E5 oncoprotein upregulates lipid raft components caveolin-1 and ganglioside GM1 at the plasma membrane of cervical cells. Oncogene 2007, 27, 1071–1078. [Google Scholar] [CrossRef]
- Oh, J.-M.; Kim, S.-H.; Cho, E.-A.; Song, Y.-S.; Kim, W.-H.; Juhnn, Y.-S. Human papillomavirus type 16 E5 protein inhibits hydrogen peroxide-induced apoptosis by stimulating ubiquitin-proteasome-mediated degradation of Bax in human cervical cancer cells. Carcinogenesis 2009, 31, 402–410. [Google Scholar] [CrossRef]
- Tomaić, V. Functional roles of E6 and E7 oncoproteins in HPV-induced malignancies at diverse anatomical sites. Cancers 2016, 8, 95. [Google Scholar] [CrossRef]
- Yasmeen, A.; Bismar, T.A.; Kandouz, M.; Foulkes, W.D.; Desprez, P.-Y.; Al Moustafa, A.-E. E6/E7 of HPV Type 16 Promotes Cell Invasion and Metastasis of Human Breast Cancer Cells. Cell Cycle 2007, 6, 2038–2042. [Google Scholar] [CrossRef]
- Yasmeen, A.; Hosein, A.N.; Yu, Q.; Al Moustafa, A.-E. Critical role for D-type cyclins in cellular transformation induced by E6/E7 of human papillomavirus type 16 and E6/E7/ErbB-2 cooperation. Cancer Sci. 2007, 98, 973–977. [Google Scholar] [CrossRef]
- Al Moustafa, A.-E.; Foulkes, W.D.; Wong, A.; Jallal, H.; Batist, G.; Yu, Q.; Herlyn, M.; Sicinski, P.; Alaoui-Jamali, M.A. Cyclin D1 is essential for neoplastic transformation induced by both E6/E7 and E6/E7/ErbB-2 cooperation in normal cells. Oncogene 2004, 23, 5252–5256. [Google Scholar] [CrossRef]
- Yasmeen, A.; Alachkar, A.; Dekhil, H.; Gambacorti-Passerini, C.; Al Moustafa, A.-E. Locking Src/Abl Tyrosine Kinase Activities Regulate Cell Differentiation and Invasion of Human Cervical Cancer Cells Expressing E6/E7 Oncoproteins of High-Risk HPV. J. Oncol. 2010, 2010, 530130. [Google Scholar] [CrossRef]
- Coluccia, A.M.L.; Benati, D.; Dekhil, H.; De Filippo, A.; Lan, C.; Gambacorti-Passerini, C. SKI-606 decreases growth and motility of colorectal cancer cells by preventing pp60(c-Src)-dependent tyrosine phosphorylation of beta-catenin and its nuclear signaling. Cancer Res. 2006, 66, 2279–2286. [Google Scholar] [CrossRef]
- Al Moustafa, A.-E.; Kassab, A.; Darnel, A.; Yasmeen, A. High-Risk HPV/ErbB-2 Interaction on E-Cadherin/Catenin Regulation in Human Carcinogenesis. Curr. Pharm. Des. 2008, 14, 2159–2172. [Google Scholar] [CrossRef] [PubMed]
- Al Moustafa, A.-E.; Ghabreau, L.; Segal, E.; Yasmeen, A.; Kassab, A.; Akil, N. High-risk human papillomavirus infections in colorectal cancer in the Syrian population and their association with Fascin, Id-1 and P-cadherin expressions: A tissue microarray study. Clin. Cancer Investig. J. 2012, 1, 26. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, Y.B.; Suh, K.W.; Paek, O.J.; Moon, H.Y. Prognostic Impact of Fascin-1 Expression is More Significant in Advanced Colorectal Cancer. J. Surg. Res. 2012, 172, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.-T.; Wang, X.; Zhang, X.; Wong, Y.-C. The multiple roles of Id-1 in cancer progression. Differentiation 2006, 74, 481–487. [Google Scholar] [CrossRef]
- Van Marck, V.; Stove, C.; Jacobs, K.; Van den Eynden, G.; Bracke, M. P-cadherin in adhesion and invasion: Opposite roles in colon and bladder carcinoma. Int. J. Cancer 2010, 128, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.J.; Sparano, J.; Palefsky, J.M. Human Immunodeficiency Virus/AIDS, Human Papillomavirus, and Anal Cancer. Surg. Oncol. Clin. N. Am. 2017, 26, 17–31. [Google Scholar] [CrossRef]
- Assi, R.; Reddy, V.; Einarsdottir, H.; Longo, W.E. Anorectal human papillomavirus: Current concepts. Yale J. Biol. Med. 2014, 87, 537. [Google Scholar]
- Frisch, M.; Glimelius, B.; van den Brule, A.J.C.; Wohlfahrt, J.; Meijer, C.J.L.M.; Walboomers, J.M.M.; Goldman, S.; Svensson, C.; Adami, H.-O.; Melbye, M. Sexually Transmitted Infection as a Cause of Anal Cancer. N. Engl. J. Med. 1997, 337, 1350–1358. [Google Scholar] [CrossRef]
- Beckmann, A.M.; Daling, J.R.; Sherman, K.J.; Maden, C.; Miller, B.A.; Coates, R.J.; Kiviat, N.B.; Myerson, D.; Weiss, N.S.; Hislop, T.G.; et al. Human papillomavirus infection and anal cancer. Int. J. Cancer 1989, 43, 1042–1049. [Google Scholar] [CrossRef]
- Limia, C.M.; Soto, Y.; García, Y.; Blanco, O.; Kourí, V.; López, M.V.; Toledo, M.E.; Pérez, L.; Baños, Y.; Caturla, Y.; et al. Human papillomavirus infection in anal intraepithelial lesions from HIV infected Cuban men. Infect. Agent. Cancer 2017, 12, 5. [Google Scholar] [CrossRef]
- Moscicki, A.B.; Darragh, T.M.; Michael Berry-Lawhorn, J.; Roberts, J.M.; Khan, M.J.; Boardman, L.A.; Chiao, E.; Einstein, M.H.; Goldstone, S.E.; Jay, N.; et al. Screening for anal cancer in women. J. Low. Genit. Tract Dis. 2015, 19, S26. [Google Scholar] [CrossRef] [PubMed]
- Einstein, M.H.; Burk, R.D. Persistent human papillomavirus infection: Definitions and clinical implications. Papillomavirus Rep. 2001, 12, 119–123. [Google Scholar]
- Schiffman, M.; Wentzensen, N. From human papillomavirus to cervical cancer. Obstet. Gynecol. 2010, 116, 177–185. [Google Scholar] [CrossRef]
- Gazzaz, F.; Mosli, M.H.; Jawa, H.; Sibiany, A. Detection of human papillomavirus infection by molecular tests and its relation to colonic polyps and colorectal cancer. Saudi Med. J. 2016, 37, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Khabaz, M.N. HPV and the Development of Colorectal Cancer. Glob. J. Health Sci. 2016, 9, 251. [Google Scholar] [CrossRef]
- Gupta, I.; Al Farsi, H.; Jabeen, A.; Skenderi, F.; Al-Thawadi, H.; AlAhmad, Y.M.; Al Moustafa, A.-E.; Vranic, S. High-Risk Human Papillomaviruses and Epstein-Barr Virus in Colorectal Cancer and Their Association with Clinicopathological Status. Pathogens 2020, 9, 452. [Google Scholar] [CrossRef]
- Gupta, I.; Ulamec, M.; Peric-Balja, M.; Ramic, S.; Al Moustafa, A.-E.; Vranic, S.; Al-Farsi, H.F. Presence of high-risk HPVs, EBV, and MMTV in human triple-negative breast cancer. Hum. Vaccin. Immunother. 2021, 17, 4457–4466. [Google Scholar] [CrossRef]
- Compton, C.C.; Fielding, L.P.; Burgart, L.J.; Conley, B.; Cooper, H.S.; Hamilton, S.R.; Hammond, M.E.H.; Henson, D.E.; Hutter, R.V.P.; Nagle, R.B.; et al. Prognostic factors in colorectal cancer: College of American Pathologists consensus statement 1999. Arch. Pathol. Lab. Med. 2000, 124, 979–994. [Google Scholar] [CrossRef]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef]
- Nagi, K.; Gupta, I.; Jurdi, N.; Yasmeen, A.; Vranic, S.; Batist, G.; Al Moustafa, A.-E. Copresence of High-Risk Human Papillomaviruses and Epstein-Barr Virus in Colorectal Cancer: A Tissue Microarray and Molecular Study from Lebanon. Int. J. Mol. Sci. 2021, 22, 8118. [Google Scholar] [CrossRef]
- Buyru, N.; Tezol, A.; Dalay, N. Coexistence of K-ras mutations and HPV infection in colon cancer. BMC Cancer 2006, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Salepci, T.; Yazici, H.; Dane, F.; Topuz, E.; Dalay, N.; Onat, H.; Aykan, F.; Seker, M.; Aydiner, A. Detection of human papillomavirus DNA by polymerase chain reaction and southern blot hybridization in colorectal cancer patients. J. BUON 2009, 14, 495–499. [Google Scholar] [PubMed]
- Damin, D.C.; Caetano, M.B.; Rosito, M.A.; Schwartsmann, G.; Damin, A.S.; Frazzon, A.P.; Ruppenthal, R.D.; Alexandre, C.O.P. Evidence for an association of human papillomavirus infection and colorectal cancer. Eur. J. Surg. Oncol. 2007, 33, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Karbalaie Niya, M.H.; Keyvani, H.; Safarnezhad Tameshkel, F.; Salehi-Vaziri, M.; Teaghinezhad-S, S.; Bokharaei Salim, F.; Monavari, S.H.R.; Javanmard, D. Human Papillomavirus Type 16 Integration Analysis by Real-time PCR Assay in Associated Cancers. Transl. Oncol. 2018, 11, 593–598. [Google Scholar] [CrossRef]
- Karbasi, A.; Borhani, N.; Daliri, K.; Kazemi, B.; Manoochehri, M. Downregulation of external death receptor genes FAS and DR5 in colorectal cancer samples positive for human papillomavirus infection. Pathol. Res. Pract. 2015, 211, 444–448. [Google Scholar] [CrossRef]
- Mahmoudvand, S.; Safaei, A.; Erfani, N.; Sarvari, J. Presence of Human Papillomavirus DNA in Colorectal Cancer Tissues in Shiraz, Southwest Iran. Asian Pac. J. Cancer Prev. 2015, 16, 7883–7887. [Google Scholar] [CrossRef]
- Afshar, R.M.; Deldar, Z.; Mollaei, H.R.; Arabzadeh, S.A.; Iranpour, M. Evaluation of HPV DNA positivity in colorectal cancer patients in Kerman, Southeast Iran. Asian Pac. J. Cancer Prev. 2018, 19, 193. [Google Scholar] [CrossRef]
- Meshkat, M.; Tayyebi Meibodi, N.; Sepahi, S.; Fadaee, N.; Salehpour, M.; Meshkat, Z. The frequency of human papillomaviruses in colorectal cancer samples in Mashhad, northeastern Iran. TURKISH J. Med. Sci. 2014, 44, 501–503. [Google Scholar] [CrossRef]
- Ranjbar, R.; Saberfar, E.; Shamsaie, A.; Ghasemian, E. The Aetiological Role of Human Papillomavirus in Colorectal Carcinoma: An Iranian Population- Based Case Control Study. Asian Pac. J. Cancer Prev. 2014, 15, 1521–1525. [Google Scholar] [CrossRef]
- Taherian, H.; Tafvizi, F.; Fard, Z.T.; Abdirad, A. Lack of association between human papillomavirus infection and colorectal cancer. Prz. Gastroenterol. 2014, 9, 280–284. [Google Scholar] [CrossRef]
- Tavakolian, S.; Goudarzi, H.; Eslami, G.; Dayyani, F.; Kazeminezhad, B.; Faghihloo, E. Prevalence of human papilloma virus and Epstein–Barr virus in tumorous and adjacent tissues of colorectal cancer in Iran. Gene Rep. 2020, 20, 100774. [Google Scholar] [CrossRef]
- El-Seidi, E.A.; Sorour, A.E.; Gamil, M. Human Papillomavirus in Patients with Colorectal Cancer. Egypt J. Med. Microbiol. 2014, 23, 59–67. [Google Scholar] [CrossRef]
- Hafez, F.S.; Meckawy, G.R.; Alorabi, M.; Shakweer, M.M. Interpretation of P16 expression as a marker of HPV in colorectal cancer. Histol. Histopathol. 2022, 37, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Kadhem Mallakh, M.; Mohammed Mahmood, M.; Hasan Mohammed Ali, S. Immunomolecular Investigation of Human Papillomavirus Genotypes (16, 18) and P63 Expression in Patients with Malignant and Non-malignant Colorectal Tumors. Arch. Razi Inst. 2022, 77, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Malki, M.I.; Gupta, I.; Fernandes, Q.; Aboulkassim, T.; Yasmeen, A.; Vranic, S.; Al Moustafa, A.E.; Al-Thawadi, H.A. Co-presence of Epstein–Barr virus and high-risk human papillomaviruses in Syrian colorectal cancer samples. Hum. Vaccines Immunother. 2020, 16, 2403–2407. [Google Scholar] [CrossRef]
- Alberts, C.J.; Heard, I.; Canestri, A.; Marchand, L.; Fléjou, J.-F.; Piroth, L.; Ferry, T.; Didelot, J.-M.; Siproudhis, L.; Henno, S.; et al. Incidence and Clearance of Anal Human Papillomavirus (HPV)-16 and HPV-18 Infection, and Their Determinants, Among Human Immunodeficiency Virus-Infected Men Who Have Sex With Men in France. J. Infect. Dis. 2019, 221, 1488–1493. [Google Scholar] [CrossRef]
- de Jesus, S.P.; da Costa, A.C.M.; Barcellos, R.B.; de Medeiros, R.M.; da Silva, C.M.D.; Rossetti, M.L. A high prevalence of human papillomavirus 16 and 18 co-infections in cervical biopsies from southern Brazil. Braz. J. Microbiol. 2018, 49 (Suppl. 1), 220–223. [Google Scholar] [CrossRef]
- Gupta, I.; Jabeen, A.; Al-Sarraf, R.; Farghaly, H.; Vranic, S.; Sultan, A.A.; Al Moustafa, A.-E.; Al-Thawadi, H. The co-presence of high-risk human papillomaviruses and Epstein-Barr virus is linked with tumor grade and stage in Qatari women with breast cancer. Hum. Vaccin. Immunother. 2021, 17, 982–989. [Google Scholar] [CrossRef]
- Motlagh, A.; Azadeh, P.; Hashemi, M.; Shafaghi, B.; Nouri, N.B.; Moulaei, M.; Sheybani, K.H.M.; Fazl, A.A.; Foudazi, M.; Velaei, N. Human Papillomavirus Infection, P53 over Expression and Histopathologic Characteristics in Colorectal Cancer. Govaresh 2007, 12, 126–133. [Google Scholar]
- Tanzi, E.; Bianchi, S.; Frati, E.R.; Amicizia, D.; Martinelli, M.; Bragazzi, N.L.; Brisigotti, M.P.; Colzani, D.; Fasoli, E.; Zehender, G.; et al. Human papillomavirus detection in paraffin-embedded colorectal cancer tissues. J. Gen. Virol. 2015, 96, 206–209. [Google Scholar] [CrossRef]
- Sayhan, N.; Yazici, H.; Budak, M.; Bitisik, O.; Dalay, N. P53 codon 72 genotypes in colon cancer. Association with human papillomavirus infection. Res. Commun. Mol. Pathol. Pharmacol. 2001, 109, 25–34. [Google Scholar] [PubMed]
- Riley, R.R.; Duensing, S.; Brake, T.; Münger, K.; Lambert, P.F.; Arbeit, J.M. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003, 63, 4862–4871. [Google Scholar] [PubMed]
- Hengstermann, A.; Linares, L.K.; Ciechanover, A.; Whitaker, N.J.; Scheffner, M. Complete switch from Mdm2 to human papillomavirus E6-mediated degradation of p53 in cervical cancer cells. Proc. Natl. Acad. Sci. USA 2001, 98, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Huang, C.-C.; Yeh, K.-T.; Chang, S.-H.; Chang, S.-W.; Sung, W.-W.; Cheng, Y.-W.; Lee, H. Human papilloma virus 16 E6 oncoprotein associated with p53 inactivation in colorectal cancer. World J. Gastroenterol. 2012, 18, 4051–4058. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.M.; Baena, A.; Valls, J.; Colucci, M.C.; Mendoza, L.; Rol, M.; Wiesner, C.; Ferrera, A.; Fellner, M.D.; González, J.V.; et al. Distribution of human papillomavirus genotypes by severity of cervical lesions in HPV screened positive women from the ESTAMPA study in Latin America. PLoS ONE 2022, 17, e0272205. [Google Scholar] [CrossRef]
- Mbulawa, Z.Z.A.; Phohlo, K.; Garcia-Jardon, M.; Williamson, A.L.; Businge, C.B. High human papillomavirus (HPV)-35 prevalence among South African women with cervical intraepithelial neoplasia warrants attention. PLoS ONE 2022, 17, e0264498. [Google Scholar] [CrossRef]
- Mpunga, T.; Baena, A.; Valls, J.; Colucci, M.C.; Mendoza, L.; Rol, M.; Wiesner, C.; Ferrera, A.; Fellner, M.D.; González, J.V.; et al. Human papillomavirus genotypes in cervical and other HPV-related anogenital cancer in Rwanda, according to HIV status. Int. J. Cancer 2020, 146, 1514–1522. [Google Scholar] [CrossRef]
- Badawi, H.; Ahmed, H.; Ismail, A.; Diab, M.; Moubarak, M.; Badawy, A.; Saber, M. Role of human papillomavirus types 16, 18, and 52 in recurrent cystitis and urinary bladder cancer among Egyptian patients. Medscape J. Med. 2008, 10, 232. [Google Scholar]
- Kaliff, M.; Sorbe, B.; Mordhorst, L.B.; Helenius, G.; Karlsson, M.G.; Lillsunde-Larsson, G. Findings of multiple HPV genotypes in cervical carcinoma are associated with poor cancer-specific survival in a Swedish cohort of cervical cancer primarily treated with radiotherapy. Oncotarget 2018, 9, 18786. [Google Scholar] [CrossRef]
- Munagala, R.; Doná, M.G.; Rai, S.N.; Jenson, A.B.; Bala, N.; Ghim, S.J.; Gupta, R.C. Significance of multiple HPV infection in cervical cancer patients and its impact on treatment response. Int. J. Oncol. 2009, 34, 263–271. [Google Scholar] [CrossRef]
- Fernandes, Q.; Allouch, S.; Gupta, I.; Elmakaty, I.; Elzawawi, K.E.; Amarah, A.; Al-Thawadi, H.; Al-Farsi, H.; Vranic, S.; Al Moustafa, A.-E. Human Papillomaviruses-Related Cancers: An Update on the Presence and Prevention Strategies in the Middle East and North African Regions. Pathogens 2022, 11, 1380. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Du, J. Human papillomavirus vaccines: An updated review. Vaccines 2020, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M.; DeMars, L.R. HPV vaccines—A review of the first decade. Gynecol. Oncol. 2017, 146, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Q.; Mestiri, S.; Bedhiafi, T.; Raza, A.; Merhi, M.; Hydrose, S.; El-Ella, D.M.A.; Inchakalody, V.P.; Uddin, S.; Dermime, S. Chapter 1. Vaccines and Immunotherapies against the Human Papillomavirus and Epstein-Barr Virus. In Advances in Health and Disease, 60th ed.; Duncan, L.T., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2022; pp. 1–44. [Google Scholar]
- Cuzick, J. Gardasil 9 joins the fight against cervix cancer. Expert Rev. Vaccines 2015, 14, 1047–1049. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Category | HPV− (n = 31) | HPV+ (n = 69) | p-Value |
|---|---|---|---|---|
| Age in years (median) | 59.0 (47.0, 65.0) | 60.0 (49.0, 67.0) | 0.61 | |
| Gender | Male | 25 (81%) | 41 (59%) | 0.038 |
| Female | 6 (19%) | 28 (41%) | ||
| Tumor Location | Ascending colon | 5 (16%) | 11 (16%) | 0.83 |
| Descending colon | 4 (13%) | 10 (14%) | ||
| Transverse colon | 2 (6%) | 3 (4%) | ||
| Sigmoid colon | 5 (16%) | 18 (26%) | ||
| Rectosigmoid | 7 (23%) | 10 (14%) | ||
| Cecum | 4 (13%) | 6 (9%) | ||
| Rectum | 1 (3%) | 2 (3%) | ||
| Hepatic flexure | 3 (10%) | 4 (6%) | ||
| Splenic flexure | 0 (0%) | 2 (3%) | ||
| Others (ileocecal valve, appendix) | 0 (0%) | 3 (4%) | ||
| Tumor Grade * | Not Applicable | 0 (0%) | 1 (1%) | 0.41 |
| Grade 1 (Well Differentiated) | 2 (6%) | 3 (4%) | ||
| Grade 2 (Moderately Differentiated) | 28 (90%) | 56 (81%) | ||
| Grade 3 (Poorly Differentiated) | 1 (3%) | 9 (13%) | ||
| Tumor Stage ☥ | pT1 | 0 (0%) | 1 (1%) | 0.45 |
| pT2 | 6 (19%) | 7 (10%) | ||
| pT3 | 19 (61%) | 51 (74%) | ||
| pT4 | 6 (19%) | 10 (14%) | ||
| Lymph Node Involvement (pN) | pN0 | 15 (48%) | 27 (39%) | 0.69 |
| pN1 | 10 (32%) | 26 (38%) | ||
| pN2 | 6 (19%) | 16 (23%) | ||
| Metastasis | Absent | 28 (90%) | 56 (81%) | 0.25 |
| Present | 3 (10%) | 13 (19%) | ||
| Coinfection of HR-HPV Subtypes (n = 35) | |
|---|---|
| HPV Subtypes | Cases |
| Double-infections (n = 16) 16% | |
| HPV18 and HPV52 | 4 |
| HPV52 and HPV45 | 3 |
| HPV52 and HPV35 | 2 |
| HPV18 and HPV59 | 2 |
| HPV52 and HPV59 | 2 |
| HPV18 and HPV31 | 2 |
| HPV16 and HPV18 | 1 |
| Triple-infections (n = 8) 8% | |
| HPV18, HPV52, and HPV59 | 4 |
| HPV18, HPV35, and 52 | 1 |
| HPV18, HPV45, and HPV52 | 1 |
| HPV18, HPV31, and HPV52 | 1 |
| HPV45, HPV51, and HPV52 | 1 |
| Quadruple-infections (n = 8) 8% | |
| HPV18, HPV31, HPV45, and HPV52 | 3 |
| HPV18, HPV45, HPV52, and HPV59 | 1 |
| HPV18, HPV31, HPV35, and HPV52 | 1 |
| HPV16, HPV18, HPV45, and HPV52 | 1 |
| HPV35, HPV45, HPV52, and HPV59 | 1 |
| HPV18, HPV51, HPV52, and HPV59 | 1 |
| Quintuple-infections (n = 3) 3% | |
| HPV18, HPV31, HPV45, HPV51, and HPV52 | 2 |
| HPV18, HPV31, HPV45, HPV52, and HPV59 | 1 |
| Variables | Odds Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|---|
| Unadjusted | HPV | 1.44 | 0.61–3.42 | 0.399 |
| Adjusted | 1.62 | 0.64–4.11 | 0.305 | |
| Age | 0.95 | 0.92–0.98 | 0.009 | |
| Sex | 0.85 | 0.34–2.12 | 0.731 | |
| Location | 0.99 | 0.83–1.19 | 0.989 | |
| Variables | Odds Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|---|
| Unadjusted | HPV coinfection | 3.87 | 1.48–10.13 | 0.0033 |
| Adjusted | 4.28 | 1.57–11.68 | 0.004 | |
| Age | 0.95 | 0.92–0.98 | 0.009 | |
| Sex | 0.850 | 0.31–2.105 | 0.647 | |
| Location | 0.998 | 0.81–1.19 | 0.856 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, Q.; Gupta, I.; Murshed, K.; Abo Samra, H.; Al-Thawadi, H.; Vranic, S.; Petkar, M.; Babu, G.R.; Al Moustafa, A.-E. Coinfection of HPVs Is Associated with Advanced Stage in Colorectal Cancer Patients from Qatar. Pathogens 2023, 12, 424. https://doi.org/10.3390/pathogens12030424
Fernandes Q, Gupta I, Murshed K, Abo Samra H, Al-Thawadi H, Vranic S, Petkar M, Babu GR, Al Moustafa A-E. Coinfection of HPVs Is Associated with Advanced Stage in Colorectal Cancer Patients from Qatar. Pathogens. 2023; 12(3):424. https://doi.org/10.3390/pathogens12030424
Chicago/Turabian StyleFernandes, Queenie, Ishita Gupta, Khaled Murshed, Hayan Abo Samra, Hamda Al-Thawadi, Semir Vranic, Mahir Petkar, Giridhara Rathnaiah Babu, and Ala-Eddin Al Moustafa. 2023. "Coinfection of HPVs Is Associated with Advanced Stage in Colorectal Cancer Patients from Qatar" Pathogens 12, no. 3: 424. https://doi.org/10.3390/pathogens12030424
APA StyleFernandes, Q., Gupta, I., Murshed, K., Abo Samra, H., Al-Thawadi, H., Vranic, S., Petkar, M., Babu, G. R., & Al Moustafa, A.-E. (2023). Coinfection of HPVs Is Associated with Advanced Stage in Colorectal Cancer Patients from Qatar. Pathogens, 12(3), 424. https://doi.org/10.3390/pathogens12030424










