Neurodevelopmental Outcome in Very Preterm Infants Randomised to Receive Two Different Standardised, Concentrated Parenteral Nutrition Regimens
Abstract
:1. Introduction
2. Methods
Analytical Methods
3. Results
4. Discussion
- This study design ensured parenteral protein and energy intakes were incrementally introduced from birth so that the intervention did not achieve higher protein and energy intakes until day 4;
- There are potentially greater effects of the intervention in the 24–26-week stratum;
- Hypophosphatemia and hypokalaemia were avoided during hyperalimentation via higher supplementation rates in the SCAMP population;
- The higher glucose intake in the SCAMP infants did not result in significantly higher rates of hyperglycaemia or insulin use when compared to the controls.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Senterre, T.; Rigo, J. Reduction in postnatal cumulative nutritional deficit and improvement of growth in extremely preterm infants. Acta Paediatr. 2012, 101, e64–e70. [Google Scholar] [CrossRef] [PubMed]
- Cormack, B.E.; Bloomfield, F.H. Increased protein intake decreases postnatal growth faltering in ELBW babies. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F399–F404. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A.; Younes, N.; Lemons, J.A.; Fanaroff, A.A.; Donovan, E.F.; Wright, L.L.; Katsikiotis, V.; Tyson, J.E.; Oh, W.; Shankaran, S.; et al. Longitudinal growth of hospitalized very low birthweight infants. Pediatrics 1999, 104, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Pupp, I.; Lofqvist, C.; Polberger, S.; Niklasson, A.; Fellman, V.; Hellström, A.; Ley, D. Influence of insulin-like growth factor 1 and nutrition during phases of postnatal growth in very preterm infants. Pediatr. Res. 2011, 69, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Stoltz Sjostrom, E.; Ohlund, I.; Ahlsson, F.; Engström, E.; Fellman, V.; Hellström, A.; Källén, K.; Norman, M.; Olhager, E.; Serenius, F.; et al. Nutrient intakes independently affect growth in extremely preterm infants: Results from a population-based study. Acta Paediatr. 2013, 102, 1067–1074. [Google Scholar] [CrossRef]
- Clark, R.H.; Thomas, P.; Peabody, J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003, 111, 986–990. [Google Scholar] [CrossRef]
- Wood, N.S.; Costeloe, K.; Gibson, A.T.; Hennessy, E.M.; Marlow, N.; Wilkinson, A.R. The EPICure study: Growth and associated problems in children born at 25 weeks of gestational age or less. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F492–F500. [Google Scholar] [CrossRef]
- Cooke, R.W.; Lucas, A.; Yudkin, P.L.N.; Pryse-Davies, J. Head circumference as an index of brain weight in the fetus and newborn. Early Hum. Dev. 1977, 1, 145–149. [Google Scholar] [CrossRef]
- Cheong, J.L.Y.; Hunt, R.W.; Anderson, P.J.; Howard, K.; Thompson, D.K.; Wang, H.X.; Bear, M.J.; Inder, T.E.; Doyle, L.W. Head growth in preterm infants: Correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics 2008, 121, e1534–e1540. [Google Scholar] [CrossRef]
- Cooke, R.W.; Foulder-Hughes, L. Growth impairment in the very preterm and cognitive and motor performance at 7 years. Arch. Dis. Child. 2003, 88, 482–487. [Google Scholar] [CrossRef]
- Cooke, R.W. Are there critical periods for brain growth in children born preterm? Arch. Dis. Child. Fetal Neonatal Ed. 2006, 91, F17–F20. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K. Growth in the neonatal intensive care unit influences neurodevelopment and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Hack, M.; Breslau, N. Very low birth weight infants: Effects of brain growth during infancy on intelligence quotient at 3 years of age. Pediatrics 1986, 77, 196–202. [Google Scholar] [CrossRef]
- Hack, M.; Breslau, N.; Weissman, B.; Aram, D.; Klein, N.; Borawski, E. Effect of very low birthweight and subnormal head size on cognitive abilities at school age. N. Eng. J. Med. 1991, 325, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Stephens, B.E.; Walden, R.V.; Gargus, R.A.; Tucker, R.; McKinley, L.; Mance, M.; Nye, J.; Vohr, B.R. First week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics 2009, 123, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.; Johnson, M.J.; Leaf, A.A.; Vollmer, B. Nutrition and neurodevelopmental outcomes in preterm infants: A systematic review. Acta Paediatr. 2016, 105, 587–599. [Google Scholar] [CrossRef]
- Bottino, M.; Cowett, R.M.; Sinclair, J.C. Interventions for treatment of hyperglycaemia in very low birthweight infants. Cochrane Database Syst. Rev. 2011, 1, CD007453. [Google Scholar]
- Bonsante, F.; Iacobelli, S.; Latorre, G.; Rigo, J.; Rigo, J.; De Felice, C.; Robillard, P.Y.; Gouyon, J.B. Initial amino acid intake influences phosphorus and calcium homeostasis in preterm infants—It is time to change the composition of the early parenteral nutrition. PLoS ONE 2013, 8, e72880. [Google Scholar] [CrossRef]
- Morgan, C.; McGowan, P.; Herwitker, S.; Hart, A.E.; Turner, M.A. Postnatal head growth in preterm infants: A randomised controlled parenteral nutrition study. Pediatrics 2014, 133, e120–e128. [Google Scholar] [CrossRef]
- Morgan, C.; Herwitker, S.; Badhawi, I.; Hart, A.; Tan, M.; Mayes, K.; Newland, P.; A Turner, M. SCAMP: Standardised, Concentrated, Additional Macronutrients, Parenteral nutrition in very preterm infants: A phase IV randomised, controlled exploratory study of macronutrient intake, growth and other aspects of neonatal care. BMC Pediatr. 2011, 11, 53–64. [Google Scholar] [CrossRef]
- Johnson, S.; Moore, T.; Marlow, N. Using the Bayley-III to assess neurodevelopmental delay: Which cut-off should be used? Pediatr. Res. 2014, 75, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Burgess, L.; Morgan, C.; Mayes, K.; Tan, M. Low plasma arginine levels and blood glucose control in very preterm infants receiving two different parenteral nutrition regimens. J. Parenter. Enter. Nutr. 2014, 38, 243–253. [Google Scholar] [CrossRef]
- Ranke, M.B.; Krägeloh-Mann, I.; Vollmer, B. Growth, head growth, and neurocognitive outcome in children born very preterm: Methodological aspects and selected results. Dev. Med. Child. Neurol. 2015, 57, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, M.; Lapinleimu, H.; Lind, A.; Matomäki, J.; Lehtonen, L.; Haataja, L.; Rautava, P.; PIPARI Study Group. Antenatal and postnatal growth and 5-year cognitive outcome in very preterm infants. Pediatrics 2014, 133, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.L.; Gong, A.K.; Schoolfield, J.; Green, B.K.; Daniels, W.; Liechty, E.A.; Ramamurthy, R. Impact of early and high amino acid supplementation on ELBW infants at 2 years. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Burratini, I.; Bellagamba, M.P.; Spagnoli, C.; D’Ascenzo, R.; Mazzoni, N.; Peretti, A.; Cogo, P.E.; Carnielli, V.P. Targeting 2.5 versus 4 g/kg/day of amino acids for extremely low birth weight infants: A randomized clinical trial. J Pediatr. 2013, 163, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Jennings, A.; Przystac, L.; Phornphutkul, C.; Tucker, R.; Vohr, B.; Stephens, B.E.; Bliss, J.M. Growth and Neurodevelopmental Outcomes of Early, High-Dose Parenteral Amino Acid Intake in Very Low Birth Weight Infants: A Randomized Controlled Trial. J. Parenter. Enter. Nutr. 2017, 42, 597–606. [Google Scholar] [CrossRef]
- Uthaya, S.; Liu, X.; Babalis, D.; Doré, C.J.; Warwick, J.; Bell, J.; Thomas, L.; Ashby, D.; Durighel, G.; Ederies, A.; et al. Nutritional Evaluation and Optimisation in Neonates: A randomized, double-blind controlled trial of amino acid regimen and intravenous lipid composition in preterm parenteral nutrition. Am. J. Clin. Nutr. 2016, 103, 1443–1452. [Google Scholar] [CrossRef]
- Poindexter, B.B.; Langer, J.C.; Dusick, A.M.; Ehrenkranz, R.A.; National Institute of Child Health and Human Development Neonatal Research Network. Early provision of parenteral amino acids in extremely low birth weight infants: Relation to growth and neurodevelopmental outcome. J. Pediatr. 2006, 148, 300–305. [Google Scholar] [CrossRef]
- te Braake, F.W.; van den Akker, C.H.; Riedijk, M.A.; van Goudoever, J.B. Parenteral amino acid and energy administration to premature infants in early life. Semin. Fetal Neonatal Med. 2007, 12, 11–18. [Google Scholar] [CrossRef]
- Morgan, C.; Burgess, L. High protein intake does not prevent low plasma levels of conditionally essential amino acids in very preterm infants receiving parenteral nutrition. J. Parenter. Enter. Nutr. 2017, 41, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.J.; Cooke, R.W.I.; Abernethy, L. Improving head growth in very preterm infants—A randomized controlled trial II: MRI and developmental outcomes in the first year. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F342–F346. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.J.; Cooke, R.W.I. Improving head growth in very preterm infants—A randomized controlled trial I: Neonatal outcomes. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F337–F341. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.A.; Schindler, T.; Jones, L.J.; Sinn, J.K.H.; Bolisetty, S. Higher versus lower amino acid intakes in parenteral nutrition for newborn infants. Cochrane Database Syst. Rev. 2018, 3, CD005949. [Google Scholar] [CrossRef]
- Cormack BE Jiang, Y.; Harding, J.E.; Crowther, C.A.; Bloomfield, F.H. Relationships between Neonatal Nutrition and Growth to 36 Weeks’ Corrected Age in ELBW Babies–Secondary Cohort Analysis from the Provide Trial. Nutrients 2020, 12, 760. [Google Scholar] [CrossRef]
- Bloomfield, F.H.; Jiang, Y.; Harding, J.E.; Crowther, C.A.; Cormack, B.E.; ProVIDe Trial Group. Early Amino Acids in Extremely Preterm Infants and Neurodisability at 2 Years. N. Engl. J. Med. 2022, 387, 1661–1672. [Google Scholar] [CrossRef]
- Paulsen, M.E.; Brown, S.J.; Satrom, K.M.; Scheurer, J.M.; Ramel, S.E.; Rao, R.B. Long-Term Outcomes after Early Neonatal Hyperglycemia in VLBW Infants: A Systematic Review. Neonatology 2021, 118, 509–521. [Google Scholar] [CrossRef]
- Rath, C.P.; Shivamallappa, M.; Muthusamy, S.; Rao, S.C.; Patole, S. Outcomes of very preterm infants with neonatal hyperglycaemia: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 269–280. [Google Scholar] [CrossRef]
- Beardsall, K.; van Haesebrouck, S.; Ogilvy-Stuart, A.; Vanhole, C.; Palmer, C.R.; van Weissenbruch, M.; Midgley, P.; Thompson, M.; Thio, M.; Cornette, L.; et al. Early insulin therapy in very-low-birth-weight infants. N. Eng. J. Med. 2008, 359, 1873–1884. [Google Scholar] [CrossRef]
- Sinclair, J.C.; Bottino, M.; Cowett, R.M. Interventions for prevention of neonatal hyperglycemia in very low birth weight infants. Cochrane Database Syst. Rev. 2011, CD007615. [Google Scholar] [CrossRef]
- Patidar, N.; Rath, C.P.; Rao, S.; Patole, S. Outcomes of very preterm infants with hyperglycaemia treated with insulin: A systematic review and meta-analysis. Acta Paediatr. 2023, 112, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Mavaheer, A.; Grime, C.; Morgan, C. Increased early protein intake is associated with a reduction in insulin-treated hyperglycaemia in very preterm infants. Nutr. Clin. Prac. 2012, 27, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C. What is the evidence base for using insulin treatment to prevent or treat neonatal hyperglycaemia? Early Hum. Dev. 2015, 91, 655–659. [Google Scholar] [CrossRef]
- Blanco, C.L.; Falck, A.; Green, B.K.; Cornell, J.E.; Gong, A.K. Metabolic responses to early and high protein supplementation in a randomised trial evaluating the prevention of hyperkalaemia in extremely low birth weight infants. J. Pediatr. 2008, 153, 535–540. [Google Scholar] [CrossRef]
- Cormack, B.E.; Jiang, Y.; Harding, J.E.; Crowther, C.A.; Bloomfield, F.H.; ProVIDe Trial Group. Neonatal Refeeding Syndrome and Clinical Outcome in Extremely Low-Birth-Weight Babies: Secondary Cohort Analysis From the ProVIDe Trial. J. Parenter. Enter. Nutr. 2021, 45, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Moltu, S.J.; Strømmen, K.; Blakstad, E.W.; Almaas, A.N.; Westerberg, A.C.; Brække, K.; Rønnestad, A.; Nakstad, B.; Berg, J.P.; Veierød, M.B.; et al. Enhanced feeding in very-low-birth-weight infants may cause electrolyte disturbances and septicemia—A randomized, controlled trial. Clin. Nutr. 2012, 32, 207–212. [Google Scholar] [CrossRef]
- Cormack, B.E.; Embleton, N.D.; van Goudoever, J.B.; Hay, W.W., Jr.; Bloomfield, F.H. Comparing apples with apples: It is time for standardized reporting of neonatal nutrition and growth studies. Pediatr. Res. 2016, 79, 810–820. [Google Scholar] [CrossRef]
- Frondas-Chauty, A.; Simon, L.; Branger, B.; Gascoin, G.; Flamant, C.; Ancel, P.Y.; Darmaun, D.; Rozé, J.C. Early growth and neurodevelopmental outcome in very preterm infants: Impact of gender. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F366–F372. [Google Scholar] [CrossRef]
- Christmann, V.; Roeleveld, N.; Visser, R.; Janssen, A.J.; Reuser, J.J.; van Goudoever, J.B.; van Heijst, A.F. The early postnatal nutritional intake of preterm infants affected neurodevelopmental outcomes differently in boys and girls at 24 months. Acta Paediatr. 2017, 106, 242–249. [Google Scholar] [CrossRef]
- Lucas, A.; Morley, R.; Cole, T.J.; Gore, S.M.; Lucas, P.J.; Crowle, P.; Pearse, R.; Boon, A.J.; Powell, R. Early diet in preterm babies and developmental status at 18 months. Lancet 1990, 335, 1477–1481. [Google Scholar] [CrossRef]
- Lucas, A.; Morley, R.; Cole, T.J. Randomised trial of early diet in preterm babies and later intelligence quotient. BMJ 1998, 317, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Sharp, M.; DeMauro, S.B. Counterbalanced Comparison of the BSID-II and Bayley-III at Eighteen to Twenty-two Months Corrected Age. J. Dev. Behav. Pediatr. 2017, 38, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Çelik, P.; Ayranci Sucakli, I.; Yakut, H.I. Turk Which Bayley-III cut-off values should be used in different developmental levels? J. Med. Sci. 2020, 50, 764–770. [Google Scholar] [CrossRef]
- Yi, Y.G.; Sung, I.Y.; Yuk, J.S. Comparison of Second and Third Editions of the Bayley Scales in Children With Suspected Developmental Delay. Ann. Rehabil. Med. 2018, 42, 313–320. [Google Scholar] [CrossRef]
- Anderson, P.J.; Burnett, A. Assessing developmental delay in early childhood—Concerns with the Bayley-III scales. Clin. Neuropsychol. 2017, 31, 371–381. [Google Scholar] [CrossRef]
| Original RCT | Neurodevelopmental FU (Follow up) | |||||
|---|---|---|---|---|---|---|
| Demographics | SCAMP (n = 74) | Control (n = 76) | p | SCAMP (n = 38) | Control (n = 41) | |
| Birthweight (g) | 900 (158) | 884 (183) | 925 (152) | 900 (185) | ||
| Birthweight (SDS) | −0.47 (0.79) | −0.47 (0.73) | −0.41 (0.83) | −0.53 (0.81) | ||
| Gestation (weeks) | 26.8 (1.3) | 26.6 (1.4) | 27.0 (1.2) | 26.8 (1.3) | ||
| Sex (male) | 44 (59.5%) | 39 (51.3%) | 22 (58%) | 24 (59%) | ||
| Nutrient intake | SCAMP (n = 66) | Control (n = 69) | SCAMP (n = 38) | Control (n = 41) | ||
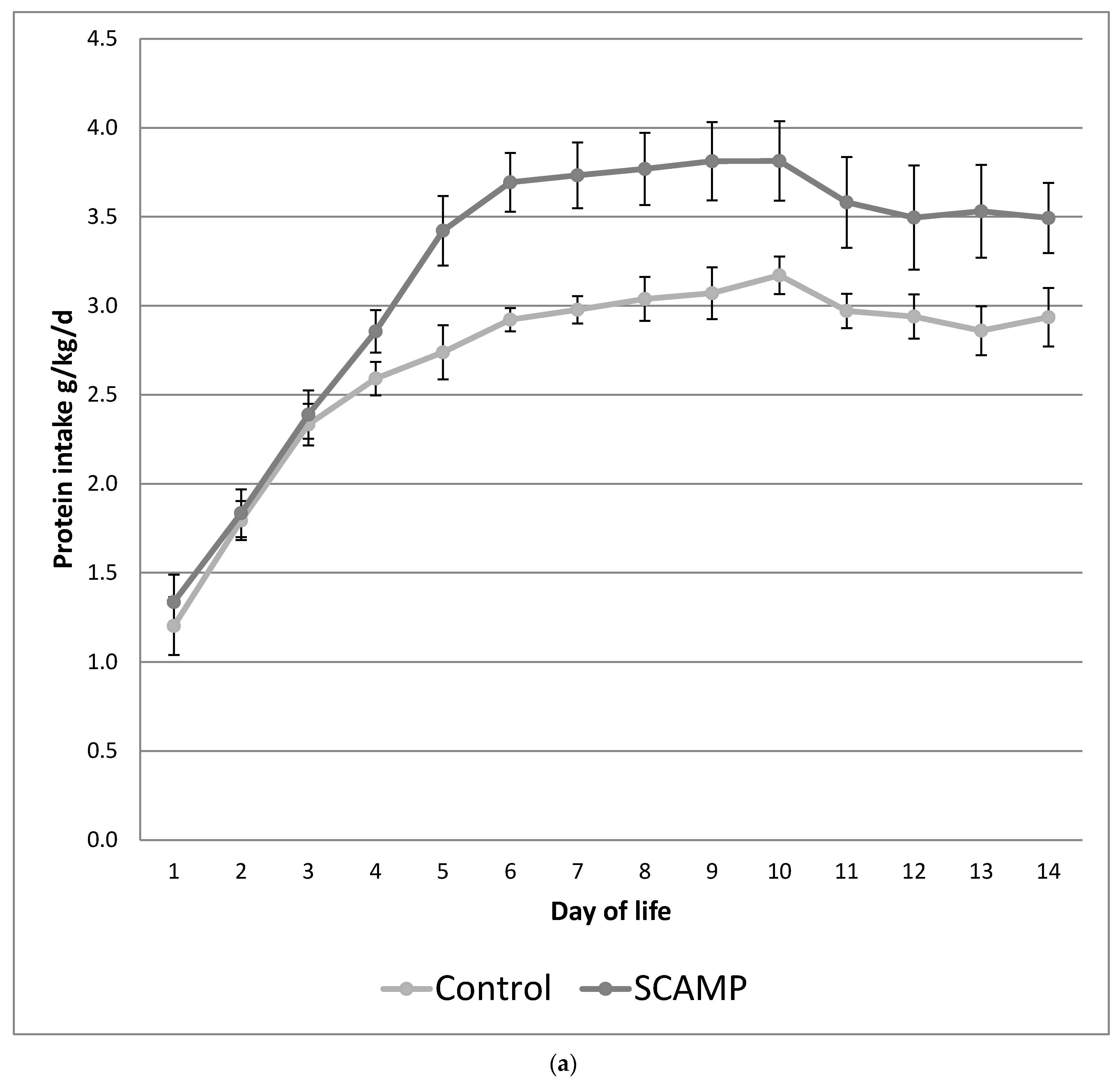
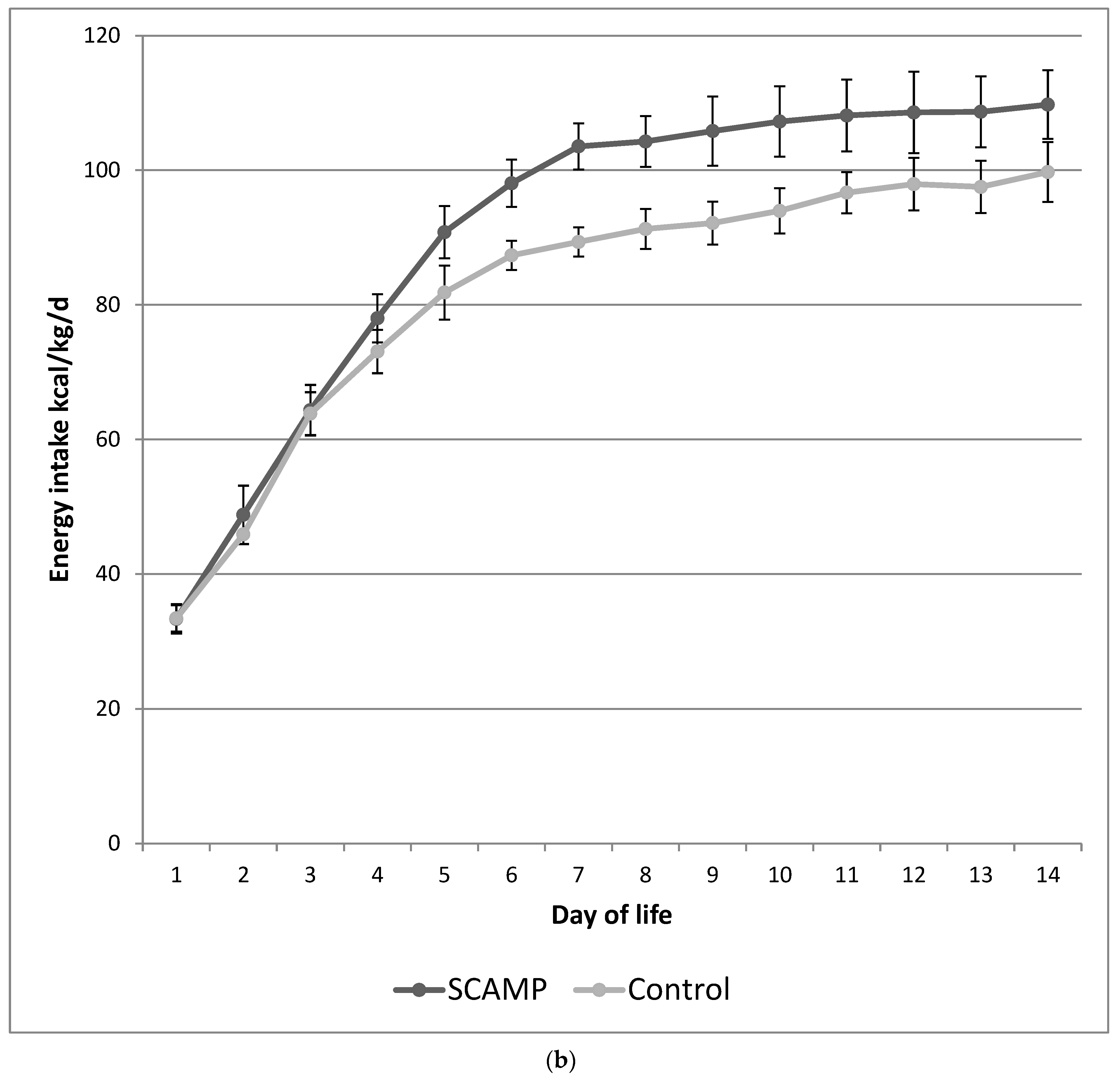
| Total protein d1–14 (g/kg/d) | 3.20 (0.28) | 2.68 (0.24) | <0.001 | 3.21 (0.32) | 2.68 (0.18) | <0.001 |
| PN protein d1–14 (g/kg/d) | 2.82 (0.51) | 2.26 (0.42) | <0.001 | 2.81 (0.55) | 2.24 (0.40) | <0.001 |
| Total energy d1–14 (kcal/kg/d) | 91 (7) | 81 (8) | <0.001 | 91 (7) | 82 (9) | <0.001 |
| PN energy d1–14 (kcal/kg/d) | 76 (14) | 64 (8) | <0.001 | 76 (14) | 64 (12) | <0.001 |
| Total protein d15–28 (g/kg/d) | 3.18 (0.49) | 3.08 (0.56) | 0.24 | 3.19 (0.44) | 3.20 (0.42) | |
| PN protein d15–28 (g/kg/d) | 1.09 (1.20) | 0.85 (1.03) | 0.20 | 1.20 (1.17) | 0.75 (0.85) | |
| Total energy d15–28 (kcal/kg/d) | 112 (15) | 109 (19) | 0.24 | 111 (15) | 113 (14) | |
| PN energy d15–28 (kcal/kg/d) | 31 (33) | 24 (29) | 0.16 | 35 (33) | 21 (24) | |
| Primary outcome measure | SCAMP (n = 66) | Control (n = 69) | SCAMP (n = 38) | Control (n = 41) | ||
| OFC SDS (randomisation) | See reference [19] | −1.57 (0.82) | −1.50 (0.68) | |||
| ΔOFC 28 days (POM) | See reference [19] | 31 (9) | 26 (8) | |||
| ΔOFC SDS 28 days (POM) | See reference [19] | 0.04 (0.61) | −0.27 (0.63) | |||
| OFC SDS 36 weeks CGA | See reference [19] | −0.91 (1.12) | −1.31 (1.25) | |||
| Neurodevelopmental FU (All Infants) | SCAMP (n = 38) | Control (n = 41) | Mean Difference (95% CI) | p-Value |
|---|---|---|---|---|
| Corrected age (months) | 29.2 (3.7) | 30.0 (3.9) | 0.49 | |
| Combined composite score | 84 (15) | 78 (14) | 6 (−0.8 to 12) | 0.09 |
| Cognitive composite score | 87 (15) | 81 (14) | 6 (−0.5 to 12) | 0.08 |
| Language composite score | 81 (18) | 76 (17) | 6 (−2 to 13) | 0.11 |
| Motor composite score | 79 (13) | 76 (15) | 3 (−4 to 9) | 0.38 |
| Neurodevelopmental FU (Gestation 24–26 weeks) | SCAMP (n = 15) | Control (n = 16) | Mean Difference (95% CI) | p-value |
| Corrected age (months) | 28.9 (3.4) | 30.3 (3.5) | 0.32 | |
| Combined composite score | 84 (15) | 78 (14) | 8 (−3 to 19) | 0.14 |
| Cognitive composite score | 85 (17) | 75 (16) | 10 (−2 to 21) | 0.11 |
| Language composite score | 75 (20) | 67 (13) | 7 (−5 to 19) | 0.25 |
| Motor composite score | 74 (15) | 69 (18) | 5 (−7 to 17) | 0.44 |
| Neurodevelopmental FU | All Infants | Gestation 24–26 Weeks | ||||
|---|---|---|---|---|---|---|
| SCAMP (n = 38) | Control (n = 41) | p | SCAMP (n = 15) | Control (n = 16) | p | |
| Corrected age (months) | 29.2 (3.7) | 30.0 (3.9) | 0.49 | 28.9 (3.4) | 30.3 (3.5) | 0.32 |
| Combined score < 80: n (%) | 11 (29) | 21 (51) | 0.07 | 6 (40) | 11 (69) | 0.15 |
| Cognitive score < 85: n (%) | 11 (29) | 18 (47) | 0.17 | 4 (27) | 8 (50) | 0.27 |
| Language score < 85: n (%) | 16 (42) | 29 (71) | 0.013 | 9 (60) | 16 (100) | <0.01 |
| Motor score < 85: n (%) | 21 (55) | 27 (66) | 0.36 | 11 (73) | 10 (63) | 0.70 |
| Mineral/Electrolyte | Original RCT | Neurodevelopmental FU | ||||
|---|---|---|---|---|---|---|
| SCAMP (n = 74) | Control (n = 76) | p | SCAMP (n = 38) | Control (n = 41) | p | |
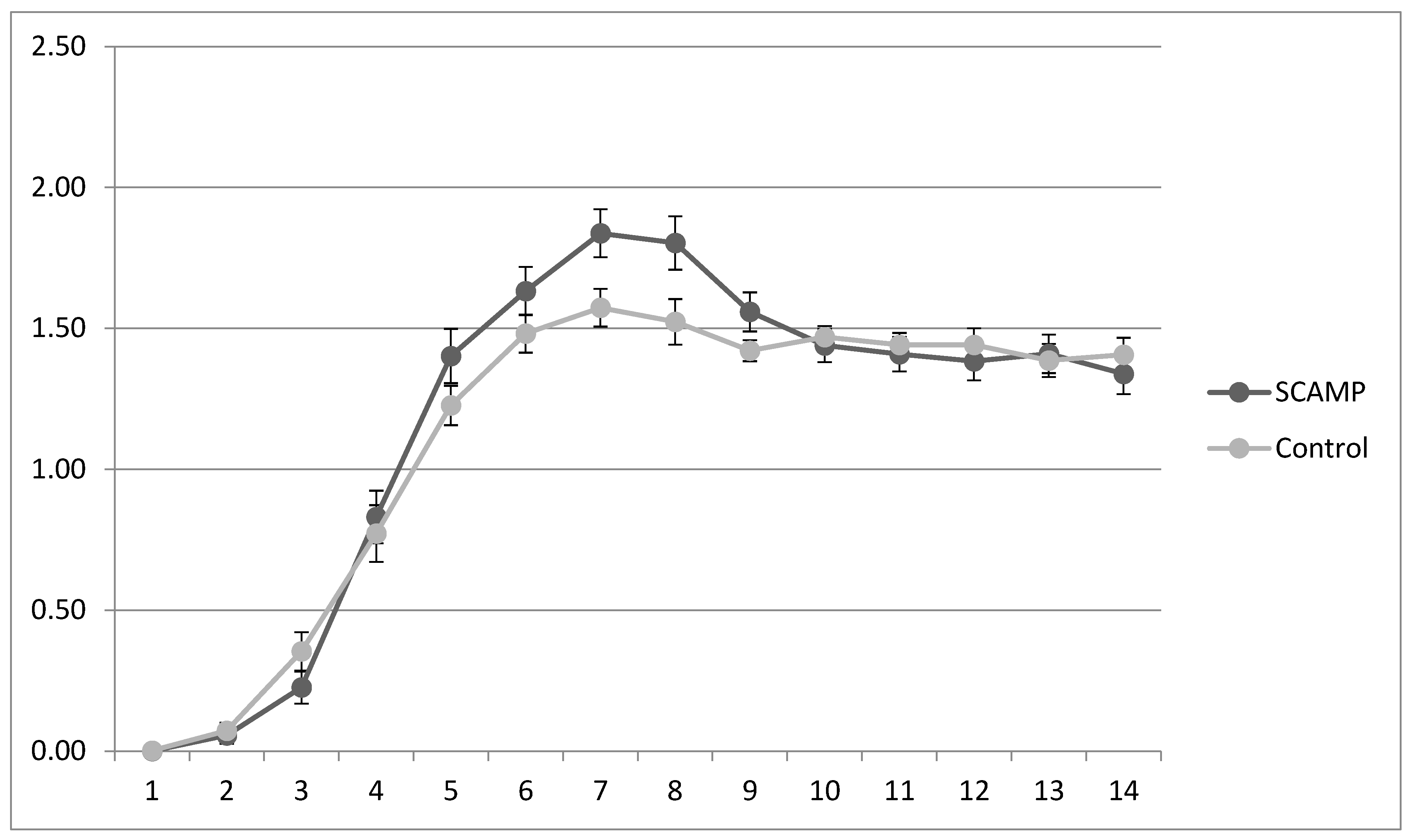
| Mean potassium intake | 1.48 (0.30) | 1.57 (0.29) | 1.49 (0.26) | 1.65 (0.19) | ||
| Mean phosphate intake | 1.21 (0.31) | 1.15 (0.21) | 1.20 (0.27) | 1.15 (0.21) | ||
| Mean calcium intake | 0.89 (0.27) | 0.93 (0.25) | 0.89 (0.21) | 0.98 (0.27) | ||
| Plasma potassium level | 4.79 (0.51) | 4.87 (0.57) | 4.84 (0.44) | 4.99 (0.56) | ||
| Plasma phosphate level | 1.66 (0.19) | 1.71 (0.24) | 1.65 (0.16) | 1.74 (0.26) | ||
| Plasma calcium level | 2.16 (0.15) | 2.20 (0.15) | 2.17 (0.13) | 2.21 (0.13) | ||
| Potassium supplement: n (%) | 38 (51) | 18 (24) | 0.0007 | 15 (39) | 8 (20) | 0.08 |
| Phosphate supplement: n (%) | 54 (73) | 36 (47) | 0.0016 | 27 (71) | 18 (44) | 0.023 |
| Calcium supplement: n (%) | 8 (11) | 6 (8) | 0.59 | 4 (11) | 2 (5) | 0.42 |
| Infants at 24–26 wks gestation | SCAMP (n = 35) | Control (n = 36) | p | SCAMP (n = 15) | Control (n = 16) | p |
| Potassium supplement: n (%) | 21 (60) | 11 (31) | 0.017 | 7 (47) | 2 (13) | 0.053 |
| Phosphate supplement: n (%) | 32 (91) | 18 (50) | 0.0002 | 13 (87) | 8 (50) | 0.053 |
| Calcium supplement: n (%) | 3 (9) | 3 (9) | 1.0 | 4 (27) | 2 (13) | 0.39 |
| Insulin/Glucose | Original RCT | Neurodevelopmental FU | ||||
|---|---|---|---|---|---|---|
| SCAMP (n = 74) | Control (n = 76) | p | SCAMP (n = 38) | Control (n = 41) | p | |
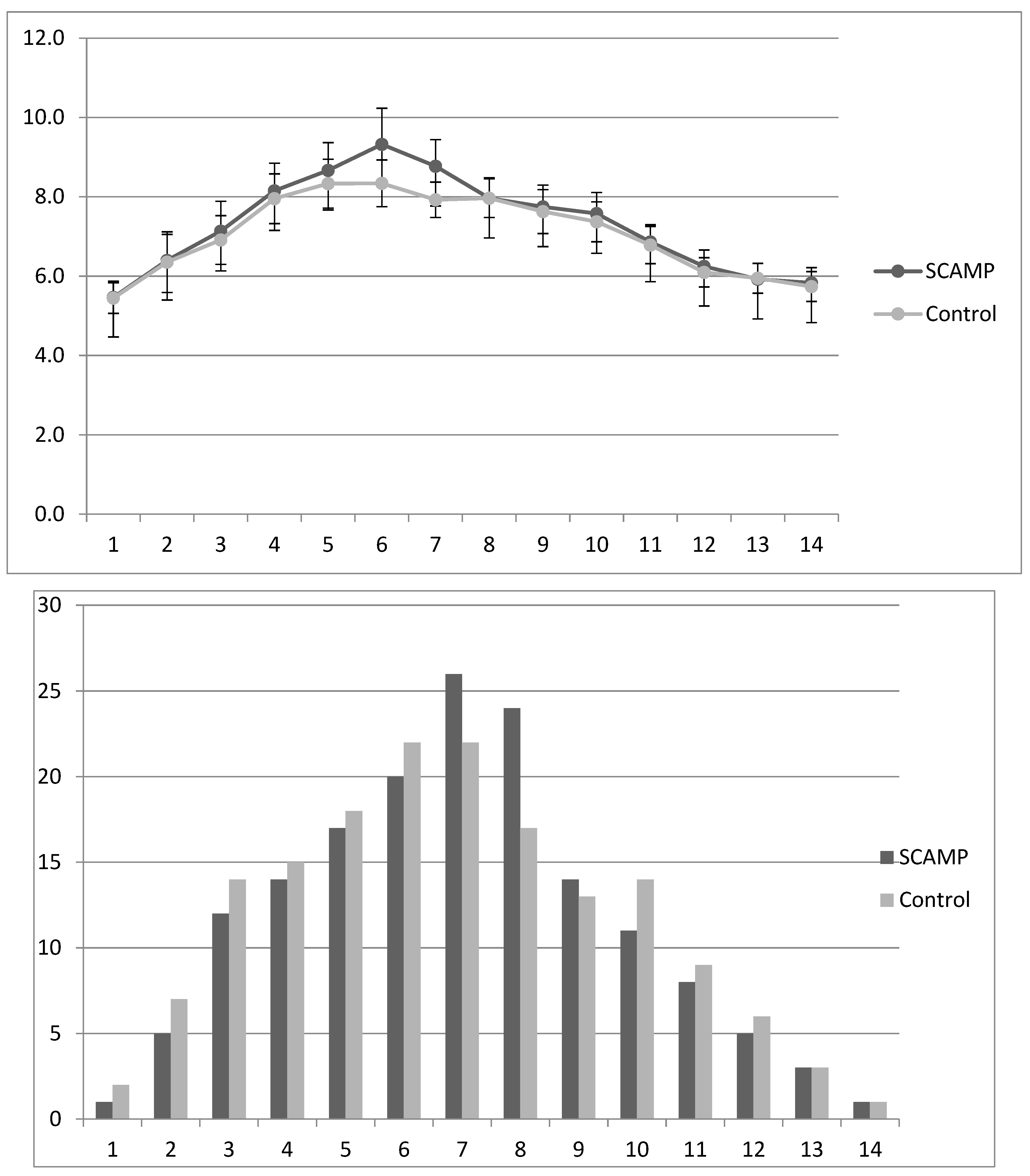
| Mean blood glucose (d1–14) | 7.50 (1.80) | 7.16 (1.71) | 0.24 | 7.32 (1.66) | 7.02 (1.72) | 0.43 |
| Mean blood glucose (d6) | 9.49 (3.76) | 8.37 (2.46) | 0.037 | 9.66 (4.47) | 8.24 (2.84) | 0.093 |
| Insulin use | 39 (53) | 33 (44) | 0.33 | 16 (42) | 18 (44) | 1.0 |
| Infants at 24–26 wks gestation | SCAMP (n = 35) | Control (n = 36) | p | SCAMP (n = 15) | Control (n = 16) | p |
| Mean blood glucose (d1–14) | 8.27 (1.67) | 7.86 (1.67) | 0.30 | 8.30 (1.15) | 7.75 (1.40) | 0.24 |
| Mean blood glucose (d6) | 9.73 (2.54) | 8.84 (2.23) | 0.12 | 10.02 (2.83) | 8.69 (2.59) | 0.18 |
| Insulin use | 27 (77) | 22 (61) | 0.20 | 11 (73) | 11 (69) | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, C.; Parry, S.; Park, J.; Tan, M. Neurodevelopmental Outcome in Very Preterm Infants Randomised to Receive Two Different Standardised, Concentrated Parenteral Nutrition Regimens. Nutrients 2023, 15, 4741. https://doi.org/10.3390/nu15224741
Morgan C, Parry S, Park J, Tan M. Neurodevelopmental Outcome in Very Preterm Infants Randomised to Receive Two Different Standardised, Concentrated Parenteral Nutrition Regimens. Nutrients. 2023; 15(22):4741. https://doi.org/10.3390/nu15224741
Chicago/Turabian StyleMorgan, Colin, Samantha Parry, Julie Park, and Maw Tan. 2023. "Neurodevelopmental Outcome in Very Preterm Infants Randomised to Receive Two Different Standardised, Concentrated Parenteral Nutrition Regimens" Nutrients 15, no. 22: 4741. https://doi.org/10.3390/nu15224741
APA StyleMorgan, C., Parry, S., Park, J., & Tan, M. (2023). Neurodevelopmental Outcome in Very Preterm Infants Randomised to Receive Two Different Standardised, Concentrated Parenteral Nutrition Regimens. Nutrients, 15(22), 4741. https://doi.org/10.3390/nu15224741





