The Effect of Nattokinase-Monascus Supplements on Dyslipidemia: A Four-Month Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Randomization and Masking
2.3. Procedures
2.4. Outcomes
2.5. Statistical Analysis
3. Results
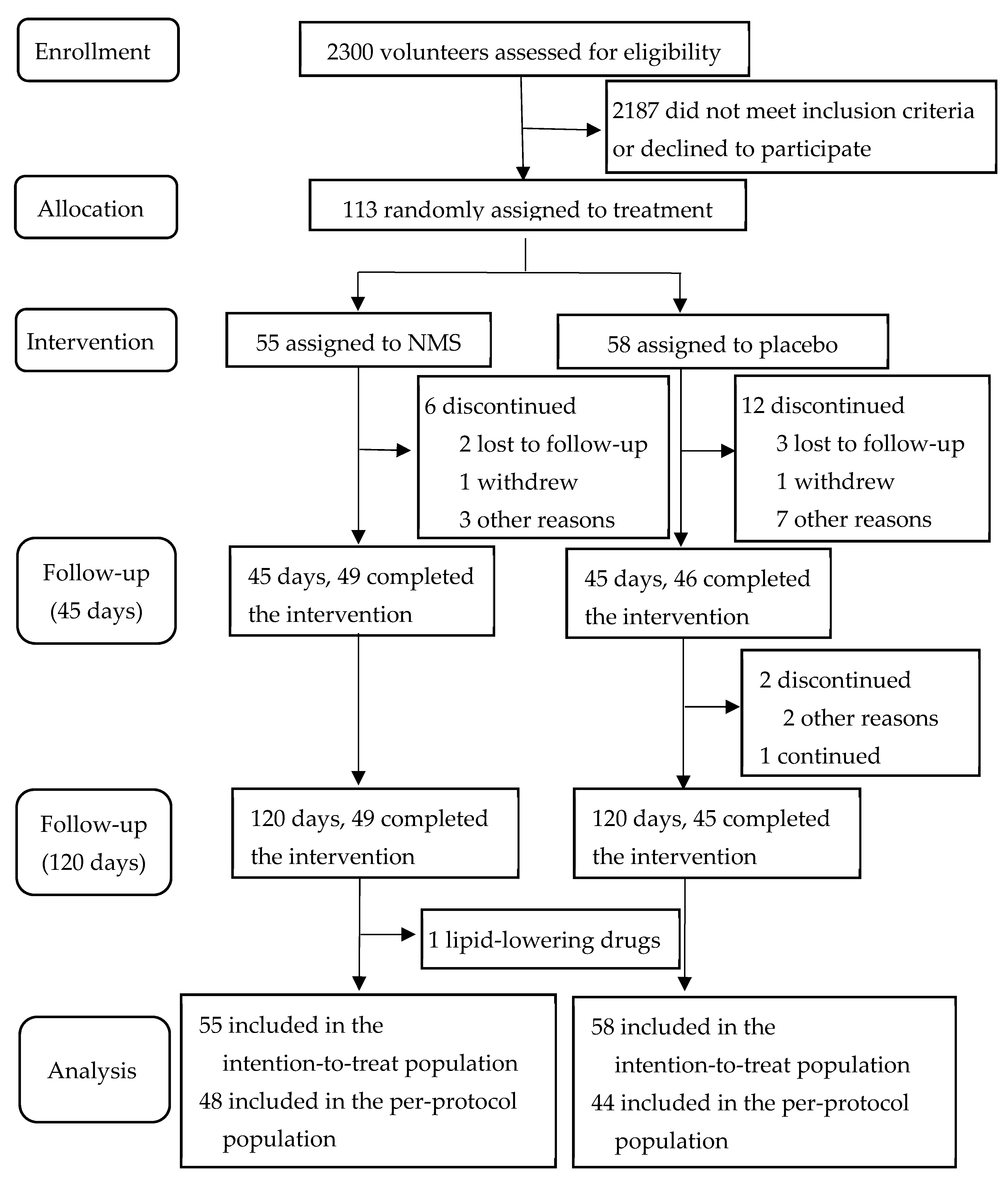
3.1. Subjects and Compliance Rate
3.2. Baseline Characteristics
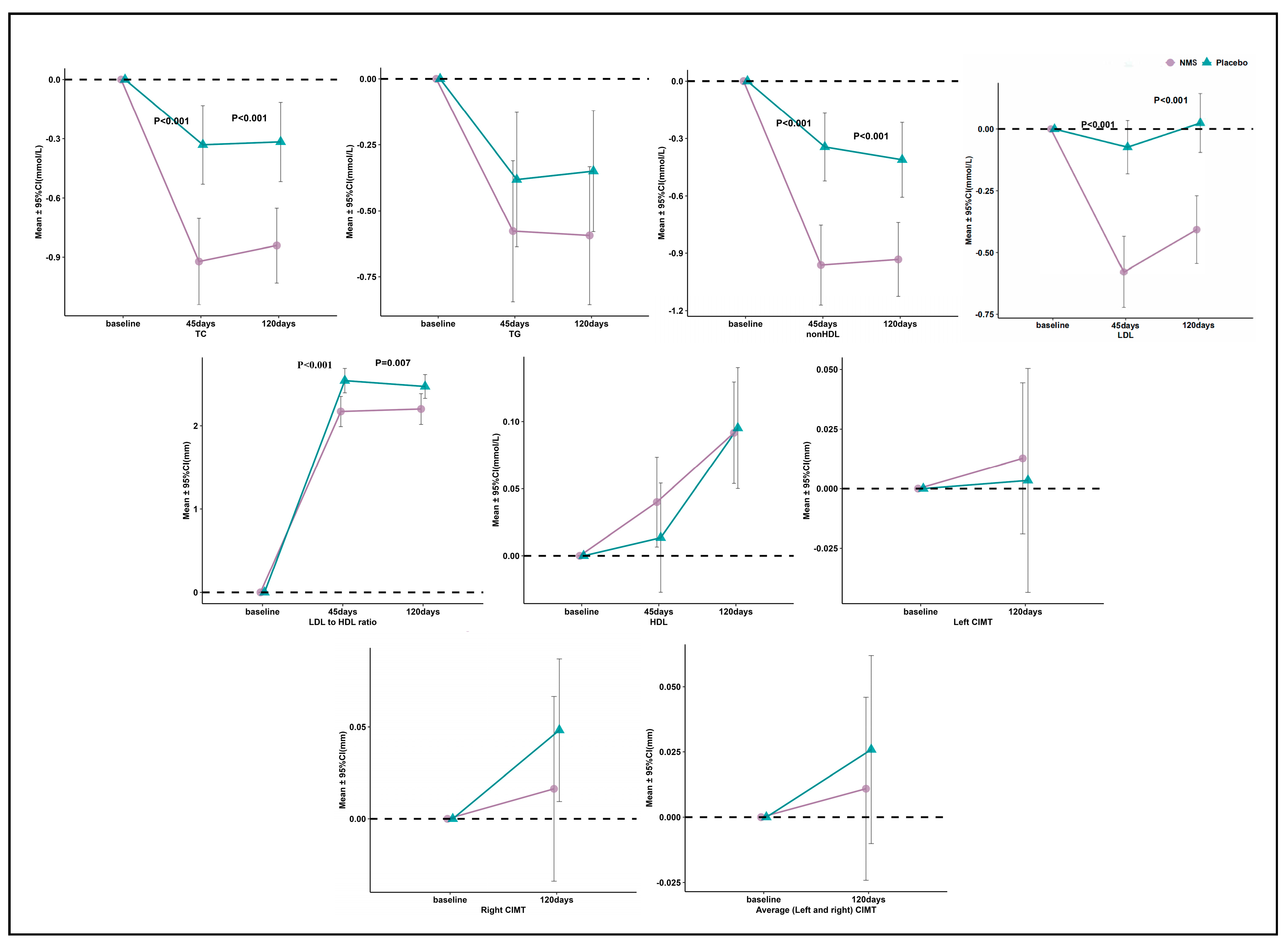
3.3. Primary and Secondary Outcomes
3.4. Laboratory Values and Physical Examination Findings of Interest
3.5. Adverse Events
3.6. Dietary and Physical Activity
3.7. Multi-Factor Analysis
3.8. Subgroup Analysis
4. Discussion
4.1. Significant Effect on Lipids
4.2. Lack of Significant Effect on CIMT
4.3. Safety of NMSs in Humans
4.4. Strengths
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mollace, R.; Macri, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Fini, M.; Volterrani, M.; Mollace, V. Comparative Effect of Bergamot Polyphenolic Fraction and Red Yeast Rice Extract in Rats Fed a Hyperlipidemic Diet: Role of Antioxidant Properties and PCSK9 Expression. Nutrients 2022, 14, 477. [Google Scholar] [CrossRef]
- Michos, E.D.; McEvoy, J.W.; Blumenthal, R.S. Lipid Management for the Prevention of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2019, 381, 1557–1567. [Google Scholar] [CrossRef]
- Farzadfar, F.; Finucane, M.M.; Danaei, G.; Pelizzari, P.M.; Cowan, M.J.; Paciorek, C.J.; Singh, G.M.; Lin, J.K.; Stevens, G.A.; Riley, L.M.; et al. National, regional, and global trends in serum total cholesterol since 1980: Systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3.0 million participants. Lancet 2011, 377, 578–586. [Google Scholar] [CrossRef]
- Luna-Castillo, K.P.; Olivares-Ochoa, X.C.; Hernandez-Ruiz, R.G.; Llamas-Covarrubias, I.M.; Rodriguez-Reyes, S.C.; Betancourt-Nunez, A.; Vizmanos, B.; Martinez-Lopez, E.; Munoz-Valle, J.F.; Marquez-Sandoval, F.; et al. The Effect of Dietary Interventions on Hypertriglyceridemia: From Public Health to Molecular Nutrition Evidence. Nutrients 2022, 14, 1104. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yang, H.; Guo, X.; Zhang, X.; Zheng, L.; Sun, Y. Prevalence of dyslipidemia and associated factors among the hypertensive population from rural Northeast China. BMC Public Health 2015, 15, 1152. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Adhyaru, B.B.; Jacobson, T.A. Safety and efficacy of statin therapy. Nature reviews. Cardiology 2018, 15, 757–769. [Google Scholar] [CrossRef]
- Pohjola-Sintonen, S.; Julkunen, H. Muscle-related adverse effects of statins. Duodecim 2014, 130, 1622–1627. [Google Scholar] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Zambon, A. Red Yeast Rice for Hypercholesterolemia: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lan, G.; Tian, X.; He, L.; Li, C.; Zeng, X.; Wang, X. Effect of Fermentation Parameters on Natto and Its Thrombolytic Property. Foods 2021, 10, 2547. [Google Scholar] [CrossRef]
- Garrel, D.R.; Verdy, M.; PetitClerc, C.; Martin, C.; Brule, D.; Hamet, P. Milk- and soy-protein ingestion: Acute effect on serum uric acid concentration. Am. J. Clin. Nutr. 1991, 53, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Lien, A.S.; Chen, J.L.; Lin, C.H.; Yang, Y.S.; Yang, S.H. A Randomized Clinical Efficacy Trial of Red Yeast Rice (Monascus pilosus) Against Hyperlipidemia. Am. J. Chin. Med. 2019, 47, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Wang, T.H.; Lee, M.H.; Su, N.W. Biologically active components and nutraceuticals in the Monascus-fermented rice: A review. Appl. Microbiol. Biotechnol. 2008, 77, 965–973. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of monacolins in red yeast rice. EFSA J. Eur. Food Saf. Auth. 2018, 16, e05368. [Google Scholar] [CrossRef]
- Bogsrud, M.P.; Ose, L.; Langslet, G.; Ottestad, I.; Strom, E.C.; Hagve, T.A.; Retterstol, K. HypoCol (red yeast rice) lowers plasma cholesterol—A randomized placebo controlled study. Scand. Cardiovasc. J. SCJ 2010, 44, 197–200. [Google Scholar] [CrossRef]
- Gordon, R.Y.; Becker, D.J. The role of red yeast rice for the physician. Curr. Atheroscler. Rep. 2011, 13, 73–80. [Google Scholar] [CrossRef]
- Benjian, C.; Xiaodan, H.; Huiting, P.; Yishi, L.I.; Yongtao, C.; Huanlin, W.U.; Danping, X.U. Effectiveness and safety of red yeast rice predominated by monacolin K beta-hydroxy acid form for hyperlipidemia treatment and management. J. Tradit. Chin. Med. Chung I Tsa Chih Ying Wen Pan 2022, 42, 264–271. [Google Scholar] [CrossRef]
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.H.; Gerdes, V.E. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef]
- Heber, D.; Yip, I.; Ashley, J.M.; Elashoff, D.A.; Elashoff, R.M.; Go, V.L. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am. J. Clin. Nutr. 1999, 69, 231–236. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Z.; Wang, P.; Zhang, B.; Chen, C.; Zhang, C.; Su, Y. EPA + DHA, but not ALA, Improved Lipids and Inflammation Status in Hypercholesterolemic Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Mol. Nutr. Food Res. 2019, 63, e1801157. [Google Scholar] [CrossRef] [PubMed]
- Authors/Task Force Members; Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016, 253, 281–344. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Kastelein, J.J.P.; Catapano, A.L. Lipids and Lipoproteins in 2020. JAMA 2020, 324, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, L.; Jia, Z.; Xin, W.; Yang, S.; Yang, Q.; Wang, L. A meta-analysis of red yeast rice: An effective and relatively safe alternative approach for dyslipidemia. PLoS ONE 2014, 9, e98611. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, V.; Lopez Hartmann, M.; Remmen, R.; Wens, J.; Apers, S.; Van Royen, P. Red yeast rice lowers cholesterol in physicians—A double blind, placebo controlled randomized trial. BMC Complement. Altern. Med. 2013, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Mazza, A.; Schiavon, L.; Rigatelli, G.; Torin, G.; Montanaro, F.; Lenti, S. The short-term supplementation of monacolin K improves the lipid and metabolic patterns of hypertensive and hypercholesterolemic subjects at low cardiovascular risk. Food Funct. 2018, 9, 3845–3852. [Google Scholar] [CrossRef]
- Magno, S.; Ceccarini, G.; Pelosini, C.; Jaccheri, R.; Vitti, J.; Fierabracci, P.; Salvetti, G.; Airoldi, G.; Minale, M.; Saponati, G.; et al. LDL-cholesterol lowering effect of a new dietary supplement: An open label, controlled, randomized, cross-over clinical trial in patients with mild-to-moderate hypercholesterolemia. Lipids Health Dis. 2018, 17, 124. [Google Scholar] [CrossRef]
- D’Addato, S.; Scandiani, L.; Mombelli, G.; Focanti, F.; Pelacchi, F.; Salvatori, E.; Di Loreto, G.; Comandini, A.; Maffioli, P.; Derosa, G. Effect of a food supplement containing berberine, monacolin K, hydroxytyrosol and coenzyme Q(10) on lipid levels: A randomized, double-blind, placebo controlled study. Drug Des. Dev. Ther. 2017, 11, 1585–1592. [Google Scholar] [CrossRef]
- Yang, N.C.; Chou, C.W.; Chen, C.Y.; Hwang, K.L.; Yang, Y.C. Combined nattokinase with red yeast rice but not nattokinase alone has potent effects on blood lipids in human subjects with hyperlipidemia. Asia Pac. J. Clin. Nutr. 2009, 18, 310–317. [Google Scholar]
- Bots, M.L.; Evans, G.W.; Tegeler, C.H.; Meijer, R. Carotid Intima-media Thickness Measurements: Relations with Atherosclerosis, Risk of Cardiovascular Disease and Application in Randomized Controlled Trials. Chin. Med. J. 2016, 129, 215–226. [Google Scholar] [CrossRef]
- Hodis, H.N.; Mack, W.J.; Meiselman, H.J.; Kalra, V.; Liebman, H.; Hwang-Levine, J.; Dustin, L.; Kono, N.; Mert, M.; Wenby, R.B.; et al. Nattokinase atherothrombotic prevention study: A randomized controlled trial. Clin. Hemorheol. Microcirc. 2021, 78, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ao, L.; Hu, X.; Ma, J.; Bao, K.; Gu, Y.; Zhao, J.; Huang, W. Influence of blood glucose fluctuation, C-peptide level and conventional risk factors on carotid artery intima-media thickness in Chinese Han patients with type 2 diabetes mellitus. Eur. J. Med. Res. 2019, 24, 13. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | NMS (n = 55) | Placebo (n = 58) | p |
|---|---|---|---|
| Age (year) | 58.47 ± 8.81 | 59.79 ± 9.10 | 0.228 |
| Male/Female (n) | 14 (25.5)/41 (74.5) | 13 (22.4)/45 (77.6) | 0.705 |
| Smoking (n) | 7 (12.7) | 10 (17.2) | 0.502 |
| Alcohol (n) | 5 (9.1) | 5 (8.6) | 1.000 |
| Lipid-lowering drugs (n) | 0 | 0 | |
| Fish oil (n) | 3 (5.5) | 3 (5.2) | 1.000 |
| Other supplements (n) | 27 (49.1) | 23 (39.7) | 0.313 |
| Traditional Chinese medicine (n) | 7 (12.7) | 6 (10.3) | 0.692 |
| Tea (n) | 18 (32.7) | 31 (53.4) | 0.026 |
| Medical history (n) | 44 (80.0) | 44 (75.9) | 0.596 |
| Hypertension (n) | 23 (41.8) | 28 (48.3) | 0.491 |
| Diabetes (n) | 8 (14.5) | 8 (13.8) | 0.909 |
| Heart disease (n) | 7(12.7) | 4.0 (6.9) | 0.296 |
| Chronic hepatitis (n) | 0 | 0 | |
| Chronic renal insufficiency (n) | 0 (0.0) | 1 (1.7) | 1.000 |
| Kidney ureter or bladder stones (n) | 3 (5.5) | 1 (1.7) | 0.355 |
| BMI (kg/m2) | 26.17 (25.43 to 26.91) | 26.61 (25.84 to 27.38) | 0.412 |
| SBP (mmHg) | 132.09 (127.47 to 136.71) | 132.71 (127.98 to 137.43) | 0.852 |
| DBP (mmHg) | 85.07 (82.15 to 87.99) | 83.47 (80.71 to 86.22) | 0.424 |
| Waist-to-hip ratio (%) | 93.54 (92.09 to 94.99) | 94.18 (92.53 to 95.83) | 0.561 |
| Milk, dairy, and soy nut products (times/day) | 0.80 (0.58 to 1.03) | 0.76 (0.58 to 0.94) | 0.724 |
| Animal food (times/day) | 1.22 (0.95 to 1.48) | 1.34 (1.06 to 1.63) | 0.609 |
| Plant foods (times/day) | 7.37 (6.58 to 8.16) | 7.38 (6.73 to 8.03) | 0.886 |
| Cereals and potatoes (times/day) | 3.70 (3.35 to 4.05) | 3.49 (3.22 to 3.76) | 0.499 |
| Vegetables and fruits (times/day) | 3.67 (3.07 to 4.26) | 3.89 (3.38 to 4.40) | 0.629 |
| Metabolic levels (kcal/day) | 2132.78 (1880.76 to 2384.79) | 1954.52 (1785.49 to 2123.56) | 0.526 |
| End Outcomes | NMS (n = 55) | Placebo (n = 58) | NMS vs. Placebo | |
|---|---|---|---|---|
| Mean (95% CI) | Mean Difference (95% CI) | p Value | ||
| Primary outcomes | ||||
| TC | ||||
| Baseline, mmol/L | 5.99 (5.77 to 6.20) | 5.86 (5.62 to 6.10) | 0.13 (0.15 to 0.10) | 0.469 |
| 45 days, mmol/L | 5.06 (4.87 to 5.26) *** | 5.53 (5.35 to 5.70) ** | −0.46 (−0.49 to −0.44) | <0.001 |
| Absolutechange from baseline to 45 days, mmol/L | −0.93 (−1.14 to −0.70) | −0.33 (−0.53 to −0.13) | −0.59 (−0.61 to −0.57) | <0.001 |
| Percentage change from baseline to 45 days, % | −14.75 (−18.04 to −11.46) | −4.40 (−7.66 to −1.14) | −10.35 (−10.38 to −10.33) | <0.001 |
| 120 days, mmol/L | 5.14 (4.92 to 5.37) *** | 5.54 (5.37 to 5.71) ** | −0.40 (−0.45 to −0.34) | 0.006 |
| Absolute change from baseline to 120 days, mmol/L | −0.85 (−1.03 to −0.65) | −0.32 (−0.52 to −0.12) | −0.52 (−0.51 to −0.54) | <0.001 |
| Percentage change from baseline to 120 days, % | −13.75 (−16.81 to −10.69) | −4.16 (−7.27 to −1.05) | −9.58 (−9.54 to −9.63) | <0.001 |
| TG | ||||
| Baseline, mmol/L | 2.66 (2.36 to 2.96) | 2.74 (2.47 to 3.01) | −0.08 (−0.12 to −0.04) | 0.430 |
| 45 days, mmol/L | 2.08 (1.78 to 2.39) *** | 2.36 (2.12 to 2.60) ** | −0.28 (−0.34 to −0.21) | 0.020 |
| Absolute change from baseline to 45 days, mmol/L | −0.58 (−0.84 to −0.31) | −0.38 (−0.64 to −0.13) | −0.20 (−0.21 to −0.18) | 0.169 |
| Percentage change from baseline to 45 days, % | −17.56 (−28.64 to −6.48) | −8.88 (−17.59 to −0.18) | −8.67 (−11.05 to −6.30) | 0.045 |
| 120 days, mmol/L | 2.07 (1.83 to 2.30) *** | 2.39 (2.15 to 2.63) *** | −0.32 (−0.32 to −0.33) | 0.033 |
| Absolute change from baseline to 120 days, mmol/L | −0.59 (−0.85 to −0.33) | −0.35 (−0.58 to −0.12) | −0.24 (−0.28 to −0.21) | 0.120 |
| Percentage change from baseline to 120 days, % | −16.10 (−27.19 to −5.01) | −7.48 (−16.38 to 1.42) | −8.61 (−10.80 to −6.43) | 0.058 |
| LDL-C | ||||
| Baseline, mmol/L | 3.66 (3.49 to 3.84) | 3.56 (3.40 to 3.72) | 0.10 (0.09 to 0.11) | 0.568 |
| 45 days, mmol/L | 3.09 (2.92 to 3.26) *** | 3.49 (3.36 to 3.61) | −0.40 (−0.45 to −0.36) | <0.001 |
| Absolute change from baseline to 45 days, mmol/L | −0.57 (−0.72 to −0.43) | −0.07 (−0.18 to 0.03) | −0.50 (−0.54 to −0.47) | <0.001 |
| Percentage change from baseline to 45 days, % | −15.20 (−18.89 to −11.50) | −0.76 (−3.89 to 2.37) | −14.44 (−15.01 to −13.87) | <0.001 |
| 120 days, mmol/L | 3.26 (3.06 to 3.46) *** | 3.59 (3.44 to 3.73) | −0.33 (−0.38 to −0.28) | 0.009 |
| Absolute change from baseline to 120 days, mmol/L | −0.40 (−0.54 to −0.27) | 0.03 (−0.09 to 0.14) | −0.43 (−0.45 to −0.41) | <0.001 |
| Percentage change from baseline to 120 days, % | −10.99 (−14.72 to −7.25) | 1.83 (−1.57 to 5.23) | −12.82 (−13.15 to −12.48) | <0.001 |
| non-HDL-C | ||||
| Baseline, mmol/L | 4.54 (4.32 to 4.75) | 4.46 (4.24 to 4.68) | 0.08 (0.08 to 0.07) | 0.767 |
| 45 days, mmol/L | 3.58 (3.37 to 3.79) *** | 4.12 (3.96 to 4.27) *** | −0.54 (−0.60 to −0.48) | <0.001 |
| Absolute change from baseline to 45 days, mmol/L | −0.96 (−1.17 to −0.75) | −0.34 (−0.52 to −0.17) | −0.62 (−0.65 to −0.59) | <0.001 |
| Percentage change from baseline to 45 days, % | −20.49 (−24.74 to −16.23) | −6.06 (−9.81 to −2.31) | −14.43 (−14.93 to −13.92) | <0.001 |
| 120 days, mmol/L | 3.61 (3.38 to 3.83) *** | 4.05 (3.90 to 4.20) *** | −0.44 (−0.52 to −0.37) | 0.002 |
| Absolute change from baseline to 120 days, mmol/L | −0.93 (−1.13 to −0.74) | −0.41 (−0.61 to −0.22) | −0.52 (−0.52 to −0.52) | <0.001 |
| Percentage change from baseline to 120 days, % | −20.18 (−24.26 to −16.09) | −7.45 (−11.28 to −3.63) | −12.72 (−12.98 to −12.47) | <0.001 |
| LDL-C to HDL-C ratio | ||||
| Baseline | 2.63 (2.45 to 2.82) | 2.61 (2.46 to 2.77) | 0.02 (−0.01 to 0.05) | 0.888 |
| 45 days | 2.17 (1.99 to 2.35) *** | 2.54 (2.39 to 2.69) * | −0.37 (−0.40 to −0.34) | 0.001 |
| Absolute change from baseline to 45 days | −0.46 (−0.55 to −0.37) | −0.07 (−0.13 to −0.01) | −0.39 (−0.43 to −0.36) | <0.001 |
| Percentage change from baseline to 45 days, % | −17.74 (−21.18 to −14.30) | −2.03 (−4.39 to 0.33) | −15.71 (−16.79 to −14.62) | <0.001 |
| 120 days | 2.20 (2.02 to 2.38) *** | 2.47 (2.33 to 2.61) ** | −0.27 (−0.31 to −0.23) | 0.007 |
| Absolute change from baseline to 120 days | −0.43 (−0.53 to −0.33) | −0.14 (−0.23 to −0.05) | −0.29 (−0.30 to −0.28) | <0.001 |
| Percentage change from baseline to 120 days, % | −16.31 (−20.09 to −12.53) | −4.34 (−7.64 to −1.03) | −11.97 (−12.45 to −11.50) | <0.001 |
| HDL-C | ||||
| Baseline, mmol/L | 1.45 (1.37 to 1.53) | 1.40 (1.33 to 1.46) | 0.05 (0.03 to 0.07) | 0.533 |
| 45 days, mmol/L | 1.49 (1.41 to 1.57) * | 1.41 (1.35 to 1.47) | 0.08 (0.06 to 0.09) | 0.233 |
| Absolute change from baseline to 45 days, mmol/L | 0.04 (0.01 to 0.07) | 0.01 (−0.03 to 0.05) | 0.03 (0.03 to 0.02) | 0.142 |
| Percentage change from baseline to 45 days, % | 3.28 (0.87 to 5.68) | 1.63 (−1.37 to 4.63) | 1.64 (2.24 to 1.05) | 0.118 |
| 120 days, mmol/L | 1.54 (1.46 to 1.62) *** | 1.49 (1.42 to 1.57) *** | 0.05 (0.04 to 0.05) | 0.428 |
| Absolute change from baseline to 120 days, mmol/L | 0.09 (0.05 to 0.13) | 0.10 (0.05 to 0.14) | 0.00 (0.00 to −0.01) | 0.713 |
| Percentage change from baseline to 120 days, % | 7.03 (4.30 to 9.76) | 7.29 (4.04 to 10.54) | −0.26 (0.26 to −0.79) | 0.758 |
| Second outcomes | ||||
| Right CIMT | ||||
| Baseline, mm | 0.83 (0.78 to 0.89) | 0.81 (0.76 to 0.86) | 0.02 (0.02 to 0.02) | 0.507 |
| 120 days, mm | 0.85 (0.80 to 0.89) | 0.86 (0.81 to 0.91) * | −0.01 (−0.01 to −0.01) | 0.852 |
| Absolute change from baseline to 120 days, mm | 0.02 (−0.03 to 0.07) | 0.05 (0.01 to 0.09) | −0.03 (−0.04 to −0.02) | 0.229 |
| Percentage change from baseline to 120 days, % | 6.48 (−2.17 to 15.13) | 8.44 (2.89 to 14.00) | −1.96 (−5.06 to 1.13) | 0.234 |
| Left CIMT | ||||
| Baseline, mm | 0.88 (0.82 to 0.93) | 0.88 (0.82 to 0.94) | 0.00 (0.00 to −0.01) | 0.951 |
| 120 days, mm | 0.89 (0.83 to 0.94) | 0.88 (0.83 to 0.94) | 0.00 (0.00 to 0.00) | 0.935 |
| Absolute change from baseline to 120 days, mm | 0.01 (−0.02 to 0.04) | 0.00 (−0.04 to 0.05) | 0.00 (0.02 to −0.01) | 0.858 |
| Percentage change from baseline to 120 days, % | 2.77 (−1.60 to 7.15) | 3.06 (−2.54 to 8.66) | −0.28 (0.94 to −1.51) | 0.843 |
| Average (Left and right) CIMT | ||||
| Baseline, mm | 0.86 (0.81 to 0.91) | 0.85 (0.80 to 0.90) | 0.01 (0.01 to 0.01) | 0.769 |
| 120 days, mm | 0.87 (0.82 to 0.91) | 0.87 (0.82 to 0.92) | −0.01 (0.00 to −0.01) | 0.876 |
| Absolute change from baseline to 120 days, mm | 0.01 (−0.02 to 0.05) | 0.02 (−0.01 to 0.06) | −0.02 (−0.01 to −0.02) | 0.463 |
| Percentage change from baseline to 120 days, % | 3.00 (−1.77 to 7.78) | 4.80 (0.18 to 9.41) | −1.79 (−1.95 to −1.64) | 0.464 |
| Variables | Model | NMS (n = 55) | Placebo (n = 58) | NMS vs. Placebo | |
|---|---|---|---|---|---|
| Mean (95% CI) | Mean Diff (95% CI) | p Value | |||
| TC | 1 | 5.40 (5.28 to 5.52) | 5.64 (5.53 to 5.76) | −0.24 (−0.08 to −0.41) | 0.004 |
| 2 | 5.95 (4.96 to 6.94) | 6.15 (5.16 to 7.15) | −0.20 (−0.03 to −0.37) | 0.023 | |
| TG | 1 | 2.27 (2.12 to 2.42) | 2.50 (2.35 to 2.64) | −0.23 (−0.01 to −0.44) | 0.037 |
| 2 | 2.84 (1.56 to 4.12) | 3.03 (1.74 to 4.32) | −0.19 (0.03 to −0.41) | 0.095 | |
| LDL-C | 1 | 3.34 (3.24 to 3.43) | 3.55 (3.45 to 3.64) | −0.21 (−0.08 to −0.34) | 0.002 |
| 2 | 3.10 (2.31 to 3.89) | 3.28 (2.49 to 4.07) | −0.18 (−0.05 to −0.32) | 0.009 | |
| Non-HDL-C | 1 | 3.88 (3.77 to 4.00) | 4.18 (4.07 to 4.29) | −0.29 (−0.14 to −0.45) | <0.001 |
| 2 | 3.91 (2.99 to 4.83) | 4.15 (3.23 to 5.08) | −0.24 (−0.09 to −0.40) | 0.003 | |
| LDL-C to HDL-C ratio | 1 | 2.33 (2.24 to 2.43) | 2.54 (2.45 to 2.63) | −0.21 (−0.07 to −0.34) | 0.003 |
| 2 | 1.65 (0.93 to 2.37) | 1.83 (1.10 to 2.55) | −0.18 (−0.05 to −0.30) | 0.005 | |
| HDL-C | 1 | 1.49 (1.45 to 1.53) | 1.43 (1.39 to 1.47) | 0.06 (0.12 to 0.00) | 0.056 |
| 2 | 2.01 (1.68 to 2.33) | 1.96 (1.63 to 2.29) | 0.05 (0.11 to −0.01) | 0.076 | |
| left cimt | 1 | 0.88 (0.84 to 0.92) | 0.88 (0.84 to 0.92) | 0.00 (0.06 to −0.06) | 0.999 |
| 2 | 0.78 (0.52 to 1.04) | 0.77 (0.51 to 1.04) | 0.01 (0.06 to −0.05) | 0.838 | |
| right cimt | 1 | 0.84 (0.81 to 0.88) | 0.84 (0.80 to 0.87) | 0.00 (0.05 to −0.04) | 0.850 |
| 2 | 0.74 (0.51 to 0.97) | 0.73 (0.50 to 0.96) | 0.02 (0.06 to −0.03) | 0.520 | |
| average cimt | 1 | 0.86 (0.83 to 0.90) | 0.86 (0.83 to 0.89) | 0.00 (0.05 to −0.05) | 0.953 |
| 2 | 0.75 (0.53 to 0.98) | 0.75 (0.52 to 0.97) | 0.01 (0.05 to −0.04) | 0.688 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zeng, X.; Mahe, J.; Guo, K.; He, P.; Yang, Q.; Zhang, Z.; Li, Z.; Wang, D.; Zhang, Z.; et al. The Effect of Nattokinase-Monascus Supplements on Dyslipidemia: A Four-Month Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 4239. https://doi.org/10.3390/nu15194239
Liu X, Zeng X, Mahe J, Guo K, He P, Yang Q, Zhang Z, Li Z, Wang D, Zhang Z, et al. The Effect of Nattokinase-Monascus Supplements on Dyslipidemia: A Four-Month Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 2023; 15(19):4239. https://doi.org/10.3390/nu15194239
Chicago/Turabian StyleLiu, Xiaoming, Xuejiao Zeng, Jinli Mahe, Kai Guo, Panpan He, Qianwen Yang, Zhiwei Zhang, Zhongxia Li, Di Wang, Zheqing Zhang, and et al. 2023. "The Effect of Nattokinase-Monascus Supplements on Dyslipidemia: A Four-Month Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Nutrients 15, no. 19: 4239. https://doi.org/10.3390/nu15194239
APA StyleLiu, X., Zeng, X., Mahe, J., Guo, K., He, P., Yang, Q., Zhang, Z., Li, Z., Wang, D., Zhang, Z., Wang, L., & Jing, L. (2023). The Effect of Nattokinase-Monascus Supplements on Dyslipidemia: A Four-Month Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients, 15(19), 4239. https://doi.org/10.3390/nu15194239







