Abstract
Background: Mounting evidence suggests that maternal obesity and gestational weight gain (GWG) may increase the risk of cancer in their offspring; however, results are inconsistent. The purpose of this research is to determine the association between maternal body mass index (BMI) and GWG and the risk of cancer in offspring through a systematic and comprehensive meta-analysis. Methods: A systematic literature search of several databases was conducted on 1 October 2022 to identify relevant studies. The quality of the included studies was evaluated using the Newcastle–Ottawa scale. The overall risk estimates were pooled using a random-effects meta-analysis. Results: Twenty-two studies with more than 8 million participants were included. An increased risk of total cancer was found in offspring whose mothers had a high GWG (odds ratio [OR]: 1.10; 95% CI: 1.01–1.19; p: 0.040) but not in offspring whose mothers had a low GWG (OR: 1.06; 95% CI: 0.96–1.17; p: 0.030), when compared with offspring whose mothers had a suitable GWG. In addition, no statistically significant association was found between maternal underweight (OR: 1.05; 95% CI: 0.97–1.13; p: 0.630), overweight/obesity (OR: 1.07; 95% CI: 0.99–1.16; p: 0.020), and risk of total cancer in offspring. Conclusions: Our study proposes evidence that maternal BMI and GWG may be associated with the risk of cancer in offspring, although statistical significance was found only for high GWG. Further well-designed research is required to clarify the potential relevance of maternal BMI and GWG on offspring cancer, especially for specific cancers.
1. Introduction
As a common cause of death worldwide, cancer brings increasing health and economic burden. It has become a persistent public health challenge and an important obstacle to the increase in human life expectancy [1,2]. In recent years, new cancer cases and deaths are increasing every year. The latest data show that the global cancer burden is as high as 19.3 million new cases and 10.0 million deaths in 2020 [3]. Therefore, it is imperative to identify cancer risk factors and target prevention in high-risk populations, which will be a benefit to improving global health.
Studies have shown that factors such as drinking, smoking, and being overweight/obese contribute to a higher risk of cancer [4,5,6]. The association and possible mechanisms of self-overweight and obesity in promoting self-cancer have been extensively studied by many researchers [7,8,9,10,11,12]. Furthermore, the potential relevance of maternal obesity on cancer in offspring has attracted more and more attention over the past few years. Mounting evidence suggests that maternal obesity may increase the risk of cancer in offspring. Meanwhile, a possible link is also observed between maternal gestational weight gain (GWG) and cancer risk in offspring [13,14,15,16]. Considering the relative prevalence of overweight/obesity in pregnant women (the prevalence of overweight/obesity women during pregnancy ranges from 12.3% to 63.5%) and the serious financial and health burden of cancer, even a small risk can lead to a severe disease burden [17,18,19]. However, the available evidence regarding maternal overweight/obesity and GWG and the risk of cancer in offspring is inconsistent [14,16,20,21,22,23]. In this situation, the use of comprehensive methods (for example, meta-analysis) to evaluate the data provided in scientific studies will help to clarify the relationship between maternal body mass index (BMI) and GWG and offspring cancer.
So far, only one relevant meta-analysis published in 2010 focused on the relationship between maternal BMI and risk of testicular cancer in male offspring [24], in which no significant association was found. However, this meta-analysis was not exhaustive because the risks of other major cancers were not reported, such as leukemia, brain cancer, and breast cancer. In addition, several studies with large samples have been published after this meta-analysis. Therefore, it is necessary to resummarize the available evidence to determine the association between maternal BMI and offspring cancer.
To this end, we aim to perform a systematic and comprehensive meta-analysis regarding the relationship between maternal BMI and the risk of offspring cancer. Our study also quantitatively summarized present evidence on the association between maternal GWG and offspring cancer risk since there was still no relevant meta-analysis. Our meta-analysis may help to access the risk of cancer in offspring associated with maternal factors.
2. Methods
2.1. Search Strategy
This meta-analysis was presented following Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement and Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines [25,26]. Web of Science, PubMed, and Embase were systematically searched by two authors on 1 October 2022. The search terms were as follows: (1) BMI, body mass index, obese, obesity, overweight, and weight; (2) weight gain, change, increase, trajector*, and growth; (3) maternal, perinatal, pregnancy*, trimester, gestational, gestation*, pregnant, conception, gravidity, pre-pregnancy, prepregnancy, pre-conception, antenatal, and prenatal; (4) cancer, tumor*, tumor*, melanoma, neoplasm*, phyma*, nub*, retinoblastoma, lymphoma, leukemia, neuroblastoma, extraosseous sarcomas, and hematological malignancy*. Reference lists of all selected literature were searched to identify further relevant literature.
2.2. Exposure and Outcomes
The exposure of interest was maternal BMI and GWG. BMI is classified into the following four groups: underweight (BMI < 18.5 kg/m2), normal weight (BMI: 18.5–25 kg/m2), overweight (BMI: 25–30 kg/m2), and obesity (BMI > 30 kg/m2). GWG categories included low GWG (<10 kg or inadequate GWG according to 2009 the Institute of Medicine [IOM] guidelines), normal GWG (10–15 kg or adequate GWG according to 2009 IOM guidelines), and high GWG (>15 kg or excessive GWG according to 2009 IOM guidelines) [27]. Outcomes of interest were any cancer in offspring, including leukemia, testicular germ-cell cancer, brain cancer, hepatoblastoma, breast cancer, lymphoma, neuroblastoma, retinoblastoma, rhabdomyosarcoma, Wilm’s tumor, etc.
2.3. Eligibility Criteria
Studies were considered eligible if they: (1) were published in English; (2) had a case-control or prospective cohort design; (3) had maternal BMI and/or GWG as the exposure was clearly reported; (4) had use of any cancer in offspring; and (5) reported relative risks (RRs), odds ratios (ORs) and hazard ratios (HRs), with corresponding 95% confidence intervals (CIs) to calculate them. The following studies were excluded: (1) letters, case reports, meeting abstracts, or reviews; (2) redundant publications; or (3) studies with unclear or incomplete data. If two or more studies were from the same population, the most comprehensive or latest one was selected.
2.4. Data Extraction
All studies obtained through the search strategies were evaluated by two reviewers (MJX and CY) independently. Any differences of opinion were settled through discussion, and if necessary, the third reviewer (WTT) was invited to have the final vote. Data collection was performed by using a self-made data extraction table to evaluate and extract the following data for each included piece of literature: the first author and year of publication, geographic region, study design, sample size, study population, age of participants, ascertainment of maternal weight, maternal weight categories, outcomes reported, confounds adjusted, and risk estimates with corresponding 95% CIs.
2.5. Study Quality Assessments
The study quality was assessed independently by two reviewers (MJX and CY), with the Newcastle–Ottawa Scale (NOS) for observational studies [28]. The NOS includes eight items, and the total score is nine.
2.6. Statistical Analyses
Relative risks (RRs) were used to measure the association between maternal BMI/GWG and cancer in offspring. RRs were considered odds ratios (ORs) because of the low incidence of cancer in offspring [29]. According to the previously published study, we used both the hazard ratios and the odds ratios to approximate the relative risk [30,31,32]. For studies that reported two or more kinds of cancer, the pooled OR for the total cancer was calculated using a fixed-effect model within each study. About each specific cancer, the pooled estimate was calculated only when there were two or more relevant studies available.
All analyses were conducted using R version 4.0.3 (The R Foundation for Statistical Computing) and RevMan version 5.3 (The Nordic Cochrane Center, Cochrane Collaboration, Copenhagen, Denmark). The pooled ORs and 95% CIs were computed using a random-effects meta-analysis. The heterogeneity of ORs across studies was assessed using the Cochran Q test and the I2 statistic. The Cochran Q test was used to evaluate whether the variation across studies was compatible with chance, and p < 0.1 was considered to indicate significant heterogeneity. The I2 statistic was a quantitative indicator used to evaluate the percentage of the total variance in prevalence estimates due to statistical heterogeneity rather than chance, or sampling error (I2 > 75% indicated high heterogeneity, 51–75% indicated substantial heterogeneity, 26–50% indicated moderate heterogeneity, and ≤25% indicated low heterogeneity). To explore the sources of heterogeneity, subgroup analyses were conducted based on different categories: maternal BMI (underweight, overweight/obesity), geographic region (e.g., America, Sweden, the United Kingdom, Israel, France, Australia, Canada), study design (case-control study, cohort study), study population (children, adults), ascertainment of maternal weight (self-reported, medical record), and whether confounding factors were adjusted (yes, no). To assess the robustness of the meta-analysis results, sensitivity analysis was conducted by repeating the meta-analysis after excluding each included study. Begg’s test was used to assess the publication bias. Considering the limited number of included studies, subgroup analyses, sensitivity analysis, and Begg’s test were not performed for specific cancers.
3. Results
3.1. Identification and Characteristics of Studies
A total of 7578 records were identified after retrieval, of which 7466 were excluded through the screening of titles and abstracts. Based on a review of the full texts of 112 studies, 90 studies were excluded, mainly because they were reviews, nondescendant cancers, lack of information on maternal BMI or GWG, unclear or incomplete data, or duplicated data. Finally, twenty-two studies were identified as eligible and included in the present meta-analysis (Figure 1) [13,14,15,16,20,21,22,23,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
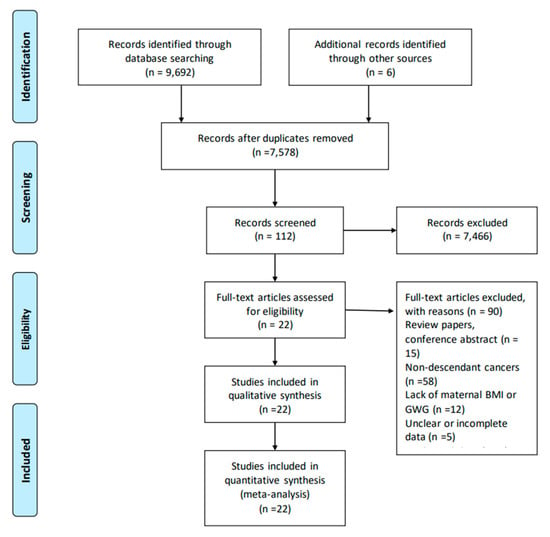
Figure 1.
Flow diagram of search strategy and study exclusion with specific reasons.
The characteristics of twenty-two studies with 8,329,446 participants are summarized in Table 1. The included studies were published between 1998 and 2022, including ten cohort studies and twelve case-control studies. Thirteen studies were conducted in America, three in Sweden, and one each in Israel, the United Kingdom, France, Australia, Canada, and Nordic countries, respectively. Fourteen studies evaluated the outcomes in children, five in adults, and three in young adults or adults. Eleven studies used medical records to collect data on maternal BMI and GWG, while eleven were self-reported. Among the twenty-two studies, nineteen studies provided data on maternal BMI, and thirteen studies provided data on GWG. With regard to specific cancers, six studies reported on leukemia, six on testicular germ-cell cancer, four on brain cancer, three on hepatoblastoma, two on breast cancer, two on lymphoma, two on neuroblastoma, and one each on retinoblastoma, colorectal cancer, rhabdomyosarcoma, and Wilms tumor. Confounding factors such as region, age, education, birth weight, and birth order were controlled in nineteen studies. The quality of all studies included here ranged between six and nine scores.

Table 1.
Selected characteristics of twenty-one studies.
3.2. Meta-Analyses of Maternal BMI and Risk of Cancer in Offspring
Maternal Underweight and Risk of Cancer in Offspring
For maternal underweight, the forest plot of study outcomes is shown in Figure 2. The overall analysis demonstrated that no statistically significant association between maternal underweight and the risk of total cancer in offspring was found (OR: 1.05; 95% CI: 0.97–1.13), with no heterogeneity (I2: 0%, p: 0.630). Begg’s test found no potential publication bias (z: −0.25, p: 0.805). The results of sensitivity analysis suggested that excluding any single research study did not substantially change the overall risk estimates for total cancer, with a range between 1.02 to 1.07 (Supplemental Table S1). Subgroup analyses results of the association between maternal underweight and the risk of total cancer are shown in Table 2. The results of subgroup analyses showed that the variables including geographic region, study design, study population, ascertainment of maternal weight, and whether confounding factors were adjusted were not shown to be associated with the heterogeneity across studies (χ2 range: 0.00–1.67, all p > 0.05).
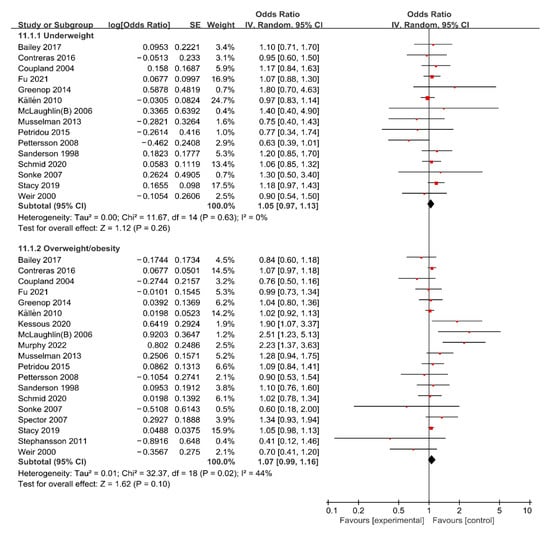
Figure 2.
Forest plot of the association between maternal BMI and the risk of total cancer in offspring [13,14,15,16,20,21,22,23,33,34,35,36,38,39,40,42,43,44,45,46].

Table 2.
Subgroup analyses for the association between maternal BMI and risk of cancer in offspring.
3.3. Maternal Overweight/Obesity and Risk of Cancer in Offspring
For maternal overweight/obesity, the forest plot of study outcomes is shown in Figure 2. The overall analysis showed that no statistically significant association between maternal overweight/obesity and risk of total cancer was found (OR: 1.07; 95% CI: 0.99–1.16), with substantial heterogeneity (I2: 44%, p: 0.020). Begg’s test found no potential publication bias (z: −0.45, p: 0.649). Results of the sensitivity analysis suggested that excluding any single research did not substantially change the overall risk estimates for total APOs, with a range between 1.05 to 1.08 (Supplemental Table S1). Subgroup analyses results of the association between maternal overweight/obesity and risk of total cancer are shown in Table 2. The results of subgroup analyses showed that the variables including maternal BMI, geographic region, study design, study population, ascertainment of maternal weight, and whether confounding factors were adjusted were not shown to be associated with the heterogeneity across studies (χ2 range: 0.02–2.94, all p > 0.05).
Risk estimates between maternal overweight/obesity and the risk of specific cancers are summarized in Supplemental Figure S2. The overall analysis suggested that maternal overweight/obesity was associated with a higher risk of leukemia (OR: 1.18, 95% CI: 1.07–1.30) and a lower risk of testicular germ-cell cancer (OR: 0.78, 95% CI: 0.62–0.99), but not associated with risk of brain cancer (OR: 0.94, 95% CI: 0.69–1.29), hepatoblastoma (OR: 1.31, 95% CI: 0.83–2.08), or breast cancer (OR: 1.05, 95% CI: 0.84–1.31) in offspring.
3.4. Meta-Analysis of Gestational Weight Gain and Risk of Cancer in Offspring
3.4.1. Maternal Low GWG and Risk of Cancer in Offspring
For low GWG, the forest plot of study outcomes is shown in Figure 3. The overall analysis showed that no statistically significant association between low GWG and risk of total cancer was found (OR: 1.06; 95% CI: 0.96–1.17), with substantial heterogeneity (I2: 48%, p: 0.030). Begg’s test found no potential publication bias (z: 1.37, p: 0.170). The results of the sensitivity analysis suggested that excluding any single research study did not substantially change the overall risk estimates for total APOs, with a range between 1.02 to 1.08 (Supplemental Table S1). Subgroup analyses results of the association between low GWG and risk of total cancer are shown in Table 3. The results of subgroup analyses showed that the variables including geographic region, study design, study population, ascertainment of maternal weight, whether confounding factors were adjusted, and whether GWG was classified according to 2009 IOM guidelines were not shown to be associated with the heterogeneity across studies (χ2 range: 0.02–3.20, all p > 0.05).
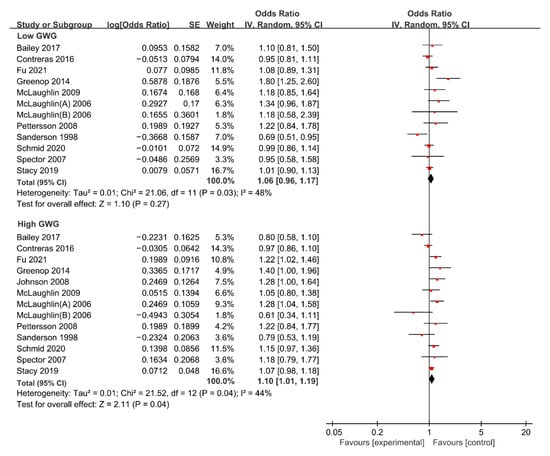
Figure 3.
Forest plot of the association between maternal GWG and the risk of total cancer in offspring [13,15,16,20,21,22,33,37,38,39,41,42,45]. GWG, gestational weight gain.

Table 3.
Subgroup analyses for the association between GWG and risk of cancer in offspring.
Risk estimates between maternal low GWG and risk of specific cancers including leukemia, brain cancer, breast cancer, testicular germ-cell cancer, hepatoblastoma, and neuroblastoma are summarized in Supplemental Figure S3. The overall analysis suggested that maternal underweight was not associated with the risk of leukemia (OR: 1.02, 95% CI: 0.92–1.14), brain cancer (OR: 1.30, 95% CI: 0.91–1.85), breast cancer (OR: 0.85, 95% CI: 0.60–1.20), testicular germ-cell cancer (OR: 1.24, 95% CI: 0.90–1.72), hepatoblastoma (OR: 1.00, 95% CI: 0.61–1.65) or neuroblastoma (OR: 0.99, 95% CI: 0.68–1.45) in offspring.
3.4.2. Maternal High GWG and Risk of Cancer in Offspring
For high GWG, the forest plot of study outcomes is shown in Figure 3. The overall analysis suggested that high GWG significantly increases the risk of total cancer (OR: 1.10; 95% CI: 1.01–1.19), with substantial heterogeneity (I2: 44%, p: 0.040). Begg’s test found no potential publication bias (z: −0.37, p: 0.714). The results of sensitivity analysis suggested that excluding any single research study did not substantially change the overall risk estimates for total APOs, with a range between 1.08 to 1.12 (Supplemental Table S1). Subgroup analyses results of the association between high GWG and the risk of total cancer are shown in Table 3. The results of subgroup analyses showed that the variables including geographic region, study design, study population, ascertainment of maternal weight, whether confounding factors were adjusted, and whether GWG was classified according to 2009 IOM guidelines were not shown to be associated with the heterogeneity across studies (χ2 range: 0.00–1.32, all p > 0.05).
Risk estimates between maternal high GWG and risk of specific cancers including leukemia, brain cancer, breast cancer, testicular germ-cell cancer, hepatoblastoma, and neuroblastoma are summarized in Supplemental Figure S3. The overall analysis suggested that maternal underweight was not associated with the risk of leukemia (OR: 1.11, 95% CI: 0.97–1.27), brain cancer (OR: 0.93, 95% CI: 0.60–1.45), breast cancer (OR: 1.00, 95% CI: 0.70–1.42), testicular germ-cell cancer (OR: 1.18, 95% CI: 0.86–1.61), hepatoblastoma (OR: 0.88, 95% CI: 0.44–1.78), or neuroblastoma (OR: 1.03, 95% CI: 0.84–1.26) in offspring.
4. Discussion
In this meta-analysis, by combining the results of all available cohort and case-control studies with the method of meta-analysis, we provided evidence that maternal high GWG is associated with an increased risk of total cancer with a relative risk estimate of 1.1. Subgroup analysis showed that maternal overweight/obesity was associated with a higher risk of leukemia and a lower risk of testicular germ-cell cancer while no increase in risk was detected for the other subtypes of offspring cancer. As far as we know, this study is the latest comprehensive meta-analysis evaluating the impact of maternal BMI and GWG on the risk of cancer in offspring. Our results may provide valuable and helpful information for women planning pregnancy, pregnant women, and prenatal care providers, and provide another new idea for the primary prevention of cancer.
No statistically significant association between maternal BMI and overall cancer in offspring was found in this study. However, when looking at specific cancers, maternal overweight or obesity was found to be a risk factor for leukemia and, interestingly, a protective factor for testicular cancer in offspring. In 2010, a meta-analysis performed by Alam et al. suggested that higher maternal weight did not increase testicular cancer risk in offspring, which is inconsistent with our findings [24]. Furthermore, a meta-analysis of 34 studies published in 2022 found pregnancy BMI was positively associated with leukemia risk in offspring (odds ratio [OR] per 5-unit BMI increase = 1.07, 95% CI: 1.04–1.1), which is consistent with our study [47]. In comparison, our meta-analysis not only included pediatric cancer but also cancer in adults, which is thought to be more comprehensive. Previous meta-analyses only considered the highest grades of BMI and included studies that reported only maternal weight rather than BMI. Our study excluded studies that reported only maternal weight rather than BMI or that did not meet the BMI classification criteria, added two large sample studies, and focused on the risk of cancer in overweight or obese offspring. Considering that all included studies adjusted for confounders, although the adjusted confounders were slightly different in each study, maternal overweight or obesity may be a true protective factor for testicular cancer in offspring, but the mechanism is unclear at present. Considering the limited number of references included, more caution should be exercised in interpreting these results and drawing conclusions.
With regard to maternal GWG, a statistically significant association between high GWG and the risk of total cancer in offspring was found. However, when stratified by cancer phenotypes, no significant association was found. Considering the relatively small number of studies included for each specific cancer, the possibility cannot be ruled out that the potential significance of the increased cancer risk cannot be identified due to the limited statistical power. Existing research has shown that insufficient or excessive GWG is not only related to adverse pregnancy outcomes but also has a far-reaching impact on the long-term health of offspring. Excessive GWG is positively associated with a higher risk of C-section, macrocephaly, preeclampsia, gestational diabetes, and postpartum weight retention, while inadequate GWG is also related to a higher risk of placental abruption, small gestational age, preterm birth, and low birth weight [48,49,50,51,52,53]. The latest meta-analysis shows that higher GWG significantly increases the risk of insulin resistance, asthma, wheezing, autism spectrum disorders, atopic dermatitis, overweight, and obesity in offspring [48,54,55,56,57,58]. Unfortunately, the current situation of GWG in pregnant women worldwide is still not optimistic. According to the latest data, the prevalence of GWG above and below the 2009 IOM guidelines was 39.4% and 27.8%, respectively, and only 32.8% of women met the IOM recommendations [59]. Pregnant women are advised to control pregnancy weight gain through a healthy diet and physical activity [60]. It is worth noting that our study included only three studies that classified GWG according to 2009 IOM guidelines. The 2009 IOM guidelines are more appropriate to classify GWG since it takes maternal prepregnancy BMI into account. More studies that classified GWG according to 2009 IOM guidelines are needed in the future to further demonstrate the potential risk of excessive GWG on cancer risk in offspring.
The advantage of our study is the comprehensive search and analysis of all available relevant literature. Before this study, only a meta-analysis published in 2010 assessed the association between maternal prepregnancy BMI and testicular cancer in offspring. Our meta-analysis considered not only maternal BMI, but also the GWG on offspring cancer, and it focused on total cancer and specific cancers. Compared with the previous meta-analysis, our research sample size (8,310,695 participants) is larger, and the analysis is more detailed and comprehensive.
One limitation of our meta-analysis is that no source of heterogeneity was found. We performed a subgroup analysis of factors such as geographic region, study design, study population, ascertainment of maternal weight, whether confounding factors were adjusted, and whether GWG was classified according to 2009 IOM guidelines. Unfortunately, no intergroup differences were found. Due to the limited information provided by the original literature, we cannot further discover the sources of heterogeneity. Although most of the included studies adjusted for confounders, these results are not surprising given that cancer is influenced by many factors, and the confounders adjusted for by different studies differ slightly. Another disadvantage is that due to the limited literature available for each cancer, we only analyzed some specific cancers. Future studies need to refine cancer types to clarify the impact of GWG on different cancers. Moreover, although every effort has been made to minimize the possible deviations in the specific retrieval of major databases, there may still be some unidentified literature. Fortunately, as Begg’s test showed, none of our results found publication bias.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15071601/s1, Table S1: Sensitivity analyses results. Figure S1: Forest plot of the association between maternal underweight and the risk of specific cancer in offspring. Figure S2: Forest plot of the association between maternal overweight/obesity and the risk of specific cancer in offspring. Figure S3: Forest plot of the association between maternal low GWG and the risk of specific cancer in offspring. Figure S4: Forest plot of the association between maternal high GWG and the risk of specific cancer in offspring.
Author Contributions
Conceptualization, L.C., Y.C. and J.M.; methodology, T.W., Y.C. and J.M.; software, Y.C. and J.M.; validation, Y.C. and J.M.; formal analysis, Y.C. and J.M.; investigation, X.Z., Z.Y. and Z.G.; resources, X.L., C.Y. and C.Y.; data curation, X.L. and C.Y.; writing—original draft preparation, J.M. and Y.C.; writing—review and editing, J.M.; visualization, J.M. and Y.C.; supervision, T.W. and L.C.; project administration, L.C.; funding acquisition, L.C. and T.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Hunan Province of China (great number: 2022JJ40207), Changsha Municipal Natural Science Foundation (great number: kq2202470); Hunan Provincial Key Research and Development Program (great number: 2018SK2062). The APC was funded by Hunan Provincial Key Research and Development Program (great number: 2018SK2062).
Institutional Review Board Statement
Ethical approval is not required because it is a secondary study, and all data were from already published studies.
Informed Consent Statement
Not applicable.
Data availability statement
The data behind the article can be found in the text and online supplementary material.
Acknowledgments
Thanks to all the colleagues working in Xiangya School of Public Health, Central South University, and Hunan Provincial Maternal and Child Health Care Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shrestha, G.; Thakur, R.K.; Singh, R.; Mulmi, R.; Shrestha, A.; Pradhan, P.M.S. Cancer burden in Nepal, 1990–2017: An analysis of the Global Burden of Disease study. PLoS ONE 2021, 16, e0255499. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. The global cancer burden: Necessity is the mother of prevention. Nat. Rev. Cancer 2019, 19, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 582–597. [CrossRef] [PubMed]
- Chen, W.; Xia, C.; Zheng, R.; Zhou, M.; Lin, C.; Zeng, H.; Zhang, S.; Wang, L.; Yang, Z.; Sun, K.; et al. Disparities by province, age, and sex in site-specific cancer burden attributable to 23 potentially modifiable risk factors in China: A comparative risk assessment. Lancet Glob. Health 2019, 7, e257–e269. [Google Scholar] [CrossRef]
- Islami, F.; Goding, S.A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Torre, L.A.; Pearson-Stuttard, J.; Islami, F.; Fedewa, S.A.; Sauer, A.G.; Shuval, K.; Gapstur, S.M.; Jacobs, E.J.; et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J. Clin. 2019, 69, 88–112. [Google Scholar] [CrossRef]
- Steele, C.B.; Thomas, C.C.; Henley, S.J.; Massetti, G.M.; Galuska, D.A.; Agurs-Collins, T.; Puckett, M.; Richardson, L.C. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity—United States, 2005–2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1052–1058. [Google Scholar] [CrossRef]
- Ackerman, S.E.; Blackburn, O.A.; Marchildon, F.; Cohen, P. Insights into the Link Between Obesity and Cancer. Curr. Obes. Rep. 2017, 6, 195–203. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Arnold, M.; Leitzmann, M.; Freisling, H.; Bray, F.; Romieu, I.; Renehan, A.; Soerjomataram, I. Obesity and cancer: An update of the global impact. Cancer Epidemiol. 2016, 41, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.C.; Chowdhury-Paulino, I.M.; Giovannucci, E.L.; Mucci, L.A. Prenatal and perinatal factors and risk of cancer in middle and older adulthood among men. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Kessous, R.; Wainstock, T.; Sheiner, E. Pre-pregnancy obesity and childhood malignancies: A population-based cohort study. Pediatr. Blood Cancer 2020, 67, e28269. [Google Scholar] [CrossRef] [PubMed]
- Stacy, S.L.; Buchanich, J.M.; Ma, Z.-Q.; Mair, C.; Robertson, L.; Sharma, R.K.; Talbott, E.O.; Yuan, J.-M. Maternal Obesity, Birth Size, and Risk of Childhood Cancer Development. Am. J. Epidemiol. 2019, 188, 1503–1511. [Google Scholar] [CrossRef]
- Contreras, Z.A.; Ritz, B.; Virk, J.; Cockburn, M.; Heck, J.E. Maternal pre-pregnancy and gestational diabetes, obesity, gestational weight gain, and risk of cancer in young children: A population-based study in California. Cancer Causes Control 2016, 27, 1273–1285. [Google Scholar] [CrossRef]
- Ratnasiri, A.; Lee, H.C.; Lakshminrusimha, S.; Parry, S.S.; Arief, V.N.; DeLacy, I.H.; Yang, J.-S.; Dilibero, R.J.; Logan, J.; Basford, K.E. Trends in maternal prepregnancy body mass index (BMI) and its association with birth and maternal outcomes in California, 2007–2016: A retrospective cohort study. PLoS ONE 2019, 14, e0222458. [Google Scholar] [CrossRef]
- Chen, C.; Xu, X.; Yan, Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE 2018, 13, e0202183. [Google Scholar] [CrossRef]
- Zhao, R.; Xu, L.; Wu, M.L.; Huang, S.H.; Cao, X.J. Maternal pre-pregnancy body mass index, gestational weight gain influence birth weight. Women Birth. 2018, 31, e20–e25. [Google Scholar] [CrossRef]
- Greenop, K.R.; Blair, E.M.; Bower, C.; Armstrong, B.K.; Milne, E. Factors relating to pregnancy and birth and the risk of childhood brain tumors: Results from an Australian case-control study. Pediatr. Blood Cancer 2014, 61, 493–498. [Google Scholar] [CrossRef]
- Bailey, H.D.; Rios, P.; Lacour, B.; Guerrini-Rousseau, L.; Bertozzi, A.I.; Leblond, P.; Faure-Conter, C.; Pellier, I.; Freycon, C.; Michon, J.; et al. Factors related to pregnancy and birth and the risk of childhood brain tumours: The ESTELLE and ESCALE studies (SFCE, France). Int. J. Cancer 2017, 140, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.G.; Davies, S.M.; Robison, L.L.; Hilden, J.M.; Roesler, M.; Ross, J.A. Birth characteristics, maternal reproductive history, and the risk of infant leukemia: A report from the Children’s Oncology Group. Cancer Epidemiol. Biomark. Prev. 2007, 16, 128–134. [Google Scholar] [CrossRef]
- Musselman, J.R.; Georgieff, M.K.; Ross, J.A.; Tomlinson, G.E.; Feusner, J.; Krailo, M.; Spector, L.G. Maternal pregnancy events and exposures and risk of hepatoblastoma: A Children’s Oncology Group (COG) study. Cancer Epidemiol. 2013, 37, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.S.; Cantwell, M.M.; Cardwell, C.R.; Cook, M.B.; Murray, L.J. Maternal body mass index and risk of testicular cancer in male offspring: A systematic review and meta-analysis. Cancer Epidemiol. 2010, 34, 509–515. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Yaktine, A.L. (Eds.) Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. In Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Q.; Xu, Y.Y. Medical Statistics, 4th ed.; People’s Medical Publishing House: Beijing, China, 2014. [Google Scholar]
- Brouwers, L.; van der Meiden-van, R.A.; Savelkoul, C.; Vogelvang, T.E.; Lely, A.T.; Franx, A.; van Rijn, B.B. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: A systematic review and meta-analysis. BJOG 2018, 125, 1642–1654. [Google Scholar] [CrossRef]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic Syndrome and Risk of Cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef]
- Schmid, D.; Willett, W.C.; Ding, M.; Michels, K.B. Maternal and Infant Anthropometric Characteristics and Breast Cancer Incidence in the Daughter. Sci. Rep. 2020, 10, 2550. [Google Scholar] [CrossRef] [PubMed]
- Petridou, E.T.; Sergentanis, T.N.; Skalkidou, A.; Antonopoulos, C.N.; Dessypris, N.; Svensson, T.; Stephansson, O.; Helle, K.; Karin, E.S. Maternal and birth anthropometric characteristics in relation to the risk of childhood lymphomas: A Swedish nationwide cohort study. Eur. J. Cancer Prev. 2015, 24, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Stephansson, O.; Wahnström, C.; Pettersson, A.; Sørensen, H.T.; Tretli, S.; Gissler, M.; Troisi, R.; Akre, O.; Grotmol, T. Perinatal risk factors for childhood testicular germ-cell cancer: A Nordic population-based study. Cancer Epidemiol. 2011, 35, e100–e104. [Google Scholar] [CrossRef] [PubMed]
- Källén, B.; Finnström, O.; Lindam, A.; Nilsson, E.; Nygren, K.G.; Olausson, P.O. Cancer risk in children and young adults conceived by in vitro fertilization. Pediatrics 2010, 126, 270–276. [Google Scholar] [CrossRef]
- McLaughlin, C.C.; Baptiste, M.S.; Schymura, M.J.; Zdeb, M.S.; Nasca, P.C. Perinatal risk factors for neuroblastoma. Cancer Causes Control. 2009, 20, 289–301. [Google Scholar] [CrossRef]
- Pettersson, A.; Richiardi, L.; Cnattingius, S.; Kaijser, M.; Akre, O. Gestational hypertension, preeclampsia, and risk of testicular cancer. Cancer Res. 2008, 68, 8832–8836. [Google Scholar] [CrossRef]
- Johnson, K.J.; Soler, J.T.; Puumala, S.E.; Ross, J.A.; Spector, L.G. Parental and infant characteristics and childhood leukemia in Minnesota. BMC Pediatr. 2008, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Sonke, G.S.; Chang, S.; Strom, S.S.; Sweeney, A.M.; Annegers, J.F.; Sigurdson, A.J. Prenatal and perinatal risk factors and testicular cancer: A hospital-based case-control study. Oncol. Res. 2007, 16, 383–387. [Google Scholar] [CrossRef]
- McLaughlin, C.C.; Baptiste, M.S.; Schymura, M.J.; Nasca, P.C.; Zdeb, M.S. Maternal and infant birth characteristics and hepatoblastoma. Am. J. Epidemiol. 2006, 163, 818–828. [Google Scholar] [CrossRef]
- McLaughlin, C.C.; Baptiste, M.S.; Schymura, M.J.; Nasca, P.C.; Zdeb, M.S. Birth weight, maternal weight and childhood leukaemia. Br. J. Cancer 2006, 94, 1738–1744. [Google Scholar] [CrossRef]
- Coupland, C.A.; Forman, D.; Chilvers, C.E.; Davey, G.; Pike, M.C.; Oliver, R.T.D. Maternal risk factors for testicular cancer: A population-based case-control study (UK). Cancer Causes Control. 2004, 15, 277–283. [Google Scholar] [CrossRef]
- Weir, H.K.; Marrett, L.D.; Kreiger, N.; Darlington, G.A.; Sugar, L. Pre-natal and peri-natal exposures and risk of testicular germ-cell cancer. Int. J. Cancer 2000, 87, 438–443. [Google Scholar] [CrossRef]
- Sanderson, M.; Williams, M.A.; Daling, J.R.; Holt, V.L.; Malone, K.E.; Self, S.G.; Moore, D.E. Maternal factors and breast cancer risk among young women. Paediatr. Perinat. Epidemiol. 1998, 12, 397–407. [Google Scholar] [CrossRef]
- Murphy, C.C.; Cirillo, P.M.; Krigbaum, N.Y.; Singal, A.G.; Lee, M.; Zaki, T.; Burstein, E.; Cohn, B.A. Maternal obesity, pregnancy weight gain, and birth weight and risk of colorectal cancer. Gut 2022, 71, 1332–1339. [Google Scholar] [CrossRef]
- Marley, A.R.; Domingues, A.; Ghosh, T.; Turcotte, L.M.; Spector, L.G. Maternal Body Mass Index, Diabetes, and Gestational Weight Gain and Risk for Pediatric Cancer in Offspring: A Systematic Review and Meta-Analysis. JNCI Cancer Spectr. 2022, 6, pkac020. [Google Scholar] [CrossRef]
- Voerman, E.; Santos, S.; Patro, G.B.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef]
- Rogozińska, E.; Zamora, J.; Marlin, N.; Betrán, A.P.; Astrup, A.; Bogaerts, A.; Cecatti, J.G.; Dodd, J.M.; Facchinetti, F.; International Weight Management in Pregnancy (i-WIP) Collaborative Group; et al. Gestational weight gain outside the Institute of Medicine recommendations and adverse pregnancy outcomes: Analysis using individual participant data from randomised trials. BMC Pregnancy Childbirth 2019, 19, 322. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.L.; Boyle, J.A.; Harrison, C.L.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; et al. Gestational weight gain across continents and ethnicity: Systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018, 16, 153. [Google Scholar] [CrossRef]
- Simmons, D.; Devlieger, R.; van Assche, A.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Desoye, G.; Kautzky-Willer, A.; Damm, P.; et al. Association between Gestational Weight Gain, Gestational Diabetes Risk, and Obstetric Outcomes: A Randomized Controlled Trial Post Hoc Analysis. Nutrients 2018, 10, 1568. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef]
- Rong, K.; Yu, K.; Han, X.; Szeto, I.M.; Qin, X.; Wang, J.; Ning, Y.; Wang, P.; Ma, D. Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: A meta-analysis of observational studies. Public Health Nutr. 2015, 18, 2172–2182. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; Yao, H.; Dai, H.; Zheng, R.; Zhang, W. Prepregnancy BMI, gestational weight gain and risk of childhood atopic dermatitis: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2021, 32, 892–904. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, B.; Wang, Y.; Wang, K.; Zhang, Z.; Niu, W. Pre-pregnancy Maternal Weight and Gestational Weight Gain Increase the Risk for Childhood Asthma and Wheeze: An Updated Meta-Analysis. Front Pediatr. 2020, 8, 134. [Google Scholar] [CrossRef]
- Eitmann, S.; Németh, D.; Hegyi, P.; Szakács, Z.; Garami, A.; Balaskó, M.; Solymár, M.; Erőss, B.; Kovács, E.; Pétervári, E. Maternal overnutrition impairs offspring’s insulin sensitivity: A systematic review and meta-analysis. Matern Child Nutr. 2020, 16, e13031. [Google Scholar] [CrossRef]
- Tian, Z.-X.; Wan, M.; Gao, Y.-L.; Wu, B.-F.; Xie, Y.; Liu, J.; Su, R.-Z.; Tian, L.-L.; Hu, Y.-Q. Gestational weight gain and risk of autism spectrum disorders in offspring: A systematic review and meta-analysis. J. Obstet. Gynaecol. 2020, 40, 953–960. [Google Scholar] [CrossRef]
- Su, L.; Chen, C.; Lu, L.; Xiang, A.H.; Dodds, L.; He, K. Association Between Gestational Weight Gain and Autism Spectrum Disorder in Offspring: A Meta-Analysis. Obesity (Silver Spring) 2020, 28, 2224–2231. [Google Scholar] [CrossRef]
- Martínez-Hortelano, J.A.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Garrido-Miguel, M.; Soriano-Cano, A.; Martínez-Vizcaíno, V. Monitoring gestational weight gain and prepregnancy BMI using the 2009 IOM guidelines in the global population: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2020, 20, 649. [Google Scholar] [CrossRef]
- Shieh, C.; Cullen, D.L.; Pike, C.; Pressler, S.J. Intervention strategies for preventing excessive gestational weight gain: Systematic review and meta-analysis. Obes. Rev. 2018, 19, 1093–1109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).