Maternal Body Mass Index, Gestational Weight Gain, and Risk of Cancer in Offspring: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Exposure and Outcomes
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Study Quality Assessments
2.6. Statistical Analyses
3. Results
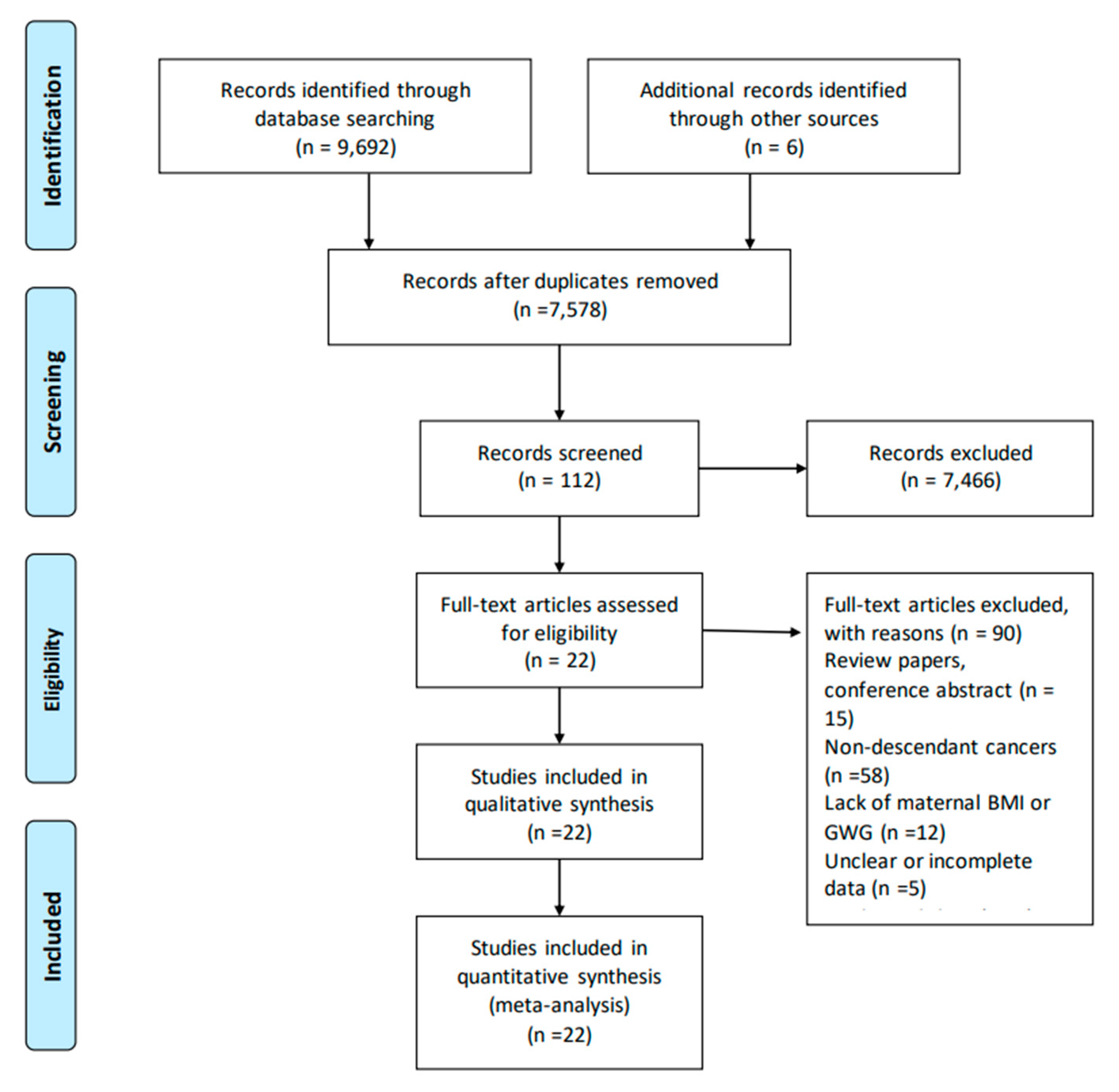
3.1. Identification and Characteristics of Studies
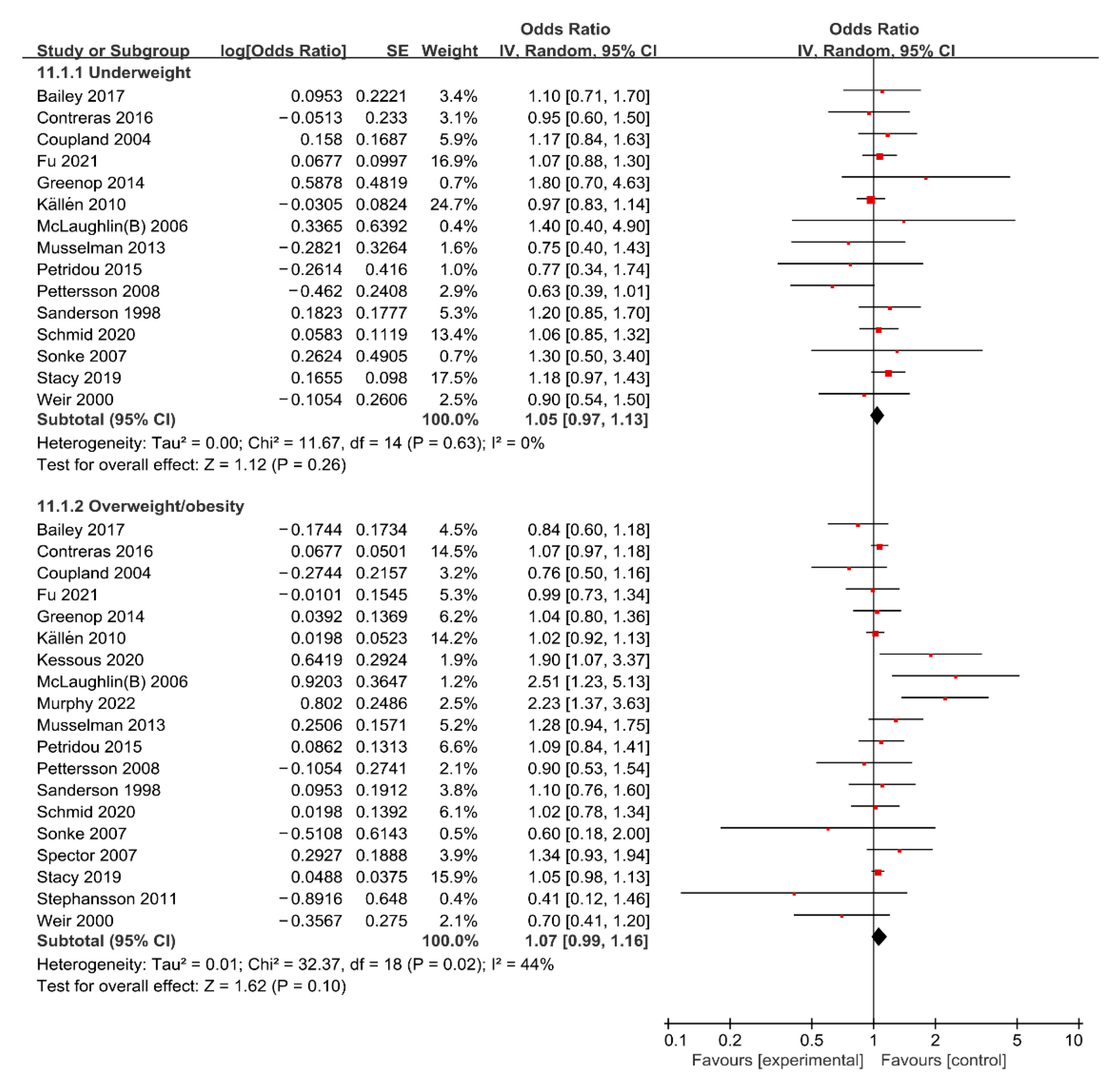
3.2. Meta-Analyses of Maternal BMI and Risk of Cancer in Offspring
Maternal Underweight and Risk of Cancer in Offspring
3.3. Maternal Overweight/Obesity and Risk of Cancer in Offspring
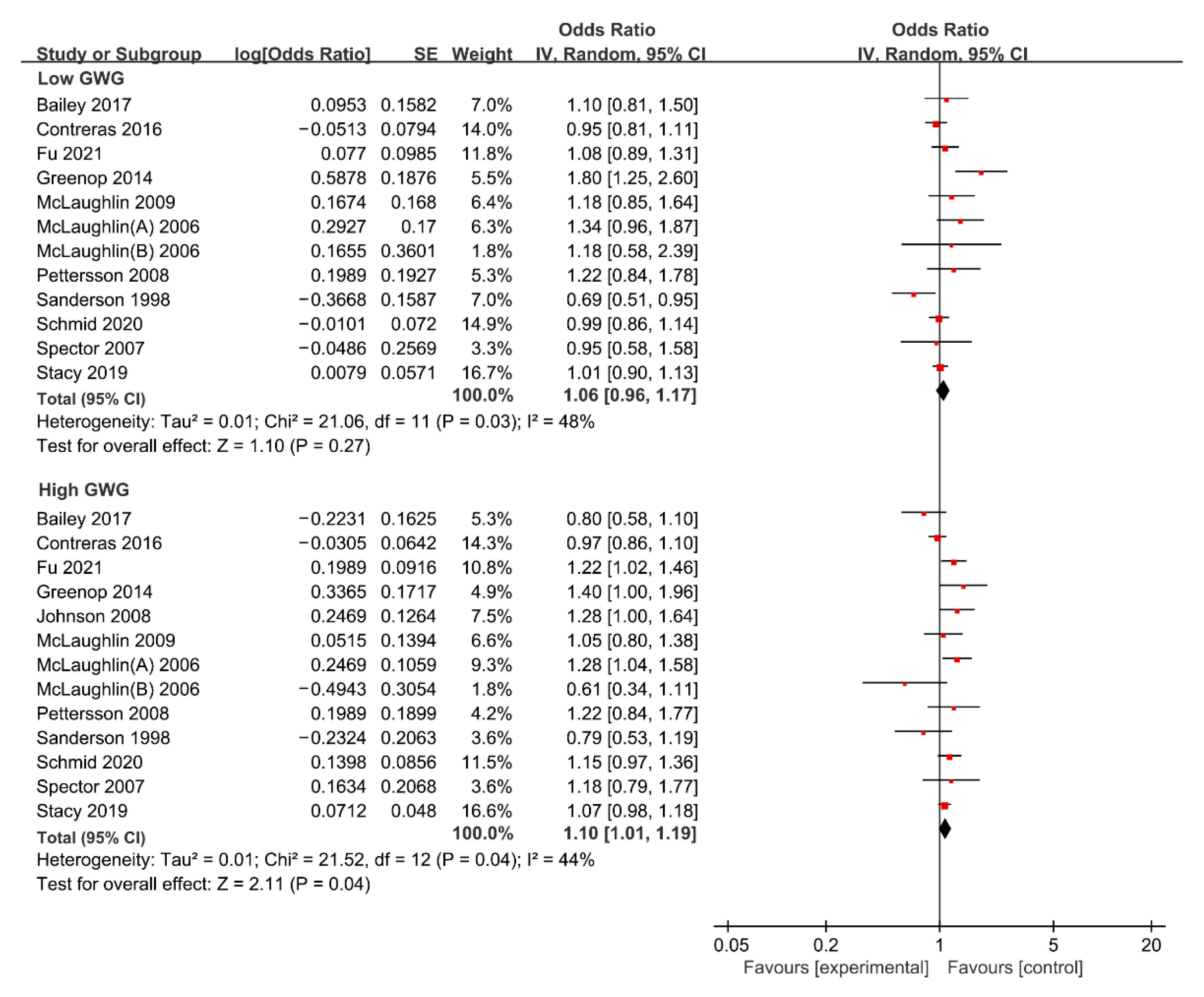
3.4. Meta-Analysis of Gestational Weight Gain and Risk of Cancer in Offspring
3.4.1. Maternal Low GWG and Risk of Cancer in Offspring
3.4.2. Maternal High GWG and Risk of Cancer in Offspring
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Acknowledgments
Conflicts of Interest
References
- Shrestha, G.; Thakur, R.K.; Singh, R.; Mulmi, R.; Shrestha, A.; Pradhan, P.M.S. Cancer burden in Nepal, 1990–2017: An analysis of the Global Burden of Disease study. PLoS ONE 2021, 16, e0255499. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. The global cancer burden: Necessity is the mother of prevention. Nat. Rev. Cancer 2019, 19, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 582–597. [CrossRef] [PubMed]
- Chen, W.; Xia, C.; Zheng, R.; Zhou, M.; Lin, C.; Zeng, H.; Zhang, S.; Wang, L.; Yang, Z.; Sun, K.; et al. Disparities by province, age, and sex in site-specific cancer burden attributable to 23 potentially modifiable risk factors in China: A comparative risk assessment. Lancet Glob. Health 2019, 7, e257–e269. [Google Scholar] [CrossRef]
- Islami, F.; Goding, S.A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Torre, L.A.; Pearson-Stuttard, J.; Islami, F.; Fedewa, S.A.; Sauer, A.G.; Shuval, K.; Gapstur, S.M.; Jacobs, E.J.; et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J. Clin. 2019, 69, 88–112. [Google Scholar] [CrossRef]
- Steele, C.B.; Thomas, C.C.; Henley, S.J.; Massetti, G.M.; Galuska, D.A.; Agurs-Collins, T.; Puckett, M.; Richardson, L.C. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity—United States, 2005–2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1052–1058. [Google Scholar] [CrossRef]
- Ackerman, S.E.; Blackburn, O.A.; Marchildon, F.; Cohen, P. Insights into the Link Between Obesity and Cancer. Curr. Obes. Rep. 2017, 6, 195–203. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Arnold, M.; Leitzmann, M.; Freisling, H.; Bray, F.; Romieu, I.; Renehan, A.; Soerjomataram, I. Obesity and cancer: An update of the global impact. Cancer Epidemiol. 2016, 41, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.C.; Chowdhury-Paulino, I.M.; Giovannucci, E.L.; Mucci, L.A. Prenatal and perinatal factors and risk of cancer in middle and older adulthood among men. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Kessous, R.; Wainstock, T.; Sheiner, E. Pre-pregnancy obesity and childhood malignancies: A population-based cohort study. Pediatr. Blood Cancer 2020, 67, e28269. [Google Scholar] [CrossRef] [PubMed]
- Stacy, S.L.; Buchanich, J.M.; Ma, Z.-Q.; Mair, C.; Robertson, L.; Sharma, R.K.; Talbott, E.O.; Yuan, J.-M. Maternal Obesity, Birth Size, and Risk of Childhood Cancer Development. Am. J. Epidemiol. 2019, 188, 1503–1511. [Google Scholar] [CrossRef]
- Contreras, Z.A.; Ritz, B.; Virk, J.; Cockburn, M.; Heck, J.E. Maternal pre-pregnancy and gestational diabetes, obesity, gestational weight gain, and risk of cancer in young children: A population-based study in California. Cancer Causes Control 2016, 27, 1273–1285. [Google Scholar] [CrossRef]
- Ratnasiri, A.; Lee, H.C.; Lakshminrusimha, S.; Parry, S.S.; Arief, V.N.; DeLacy, I.H.; Yang, J.-S.; Dilibero, R.J.; Logan, J.; Basford, K.E. Trends in maternal prepregnancy body mass index (BMI) and its association with birth and maternal outcomes in California, 2007–2016: A retrospective cohort study. PLoS ONE 2019, 14, e0222458. [Google Scholar] [CrossRef]
- Chen, C.; Xu, X.; Yan, Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE 2018, 13, e0202183. [Google Scholar] [CrossRef]
- Zhao, R.; Xu, L.; Wu, M.L.; Huang, S.H.; Cao, X.J. Maternal pre-pregnancy body mass index, gestational weight gain influence birth weight. Women Birth. 2018, 31, e20–e25. [Google Scholar] [CrossRef]
- Greenop, K.R.; Blair, E.M.; Bower, C.; Armstrong, B.K.; Milne, E. Factors relating to pregnancy and birth and the risk of childhood brain tumors: Results from an Australian case-control study. Pediatr. Blood Cancer 2014, 61, 493–498. [Google Scholar] [CrossRef]
- Bailey, H.D.; Rios, P.; Lacour, B.; Guerrini-Rousseau, L.; Bertozzi, A.I.; Leblond, P.; Faure-Conter, C.; Pellier, I.; Freycon, C.; Michon, J.; et al. Factors related to pregnancy and birth and the risk of childhood brain tumours: The ESTELLE and ESCALE studies (SFCE, France). Int. J. Cancer 2017, 140, 1757–1769. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.G.; Davies, S.M.; Robison, L.L.; Hilden, J.M.; Roesler, M.; Ross, J.A. Birth characteristics, maternal reproductive history, and the risk of infant leukemia: A report from the Children’s Oncology Group. Cancer Epidemiol. Biomark. Prev. 2007, 16, 128–134. [Google Scholar] [CrossRef]
- Musselman, J.R.; Georgieff, M.K.; Ross, J.A.; Tomlinson, G.E.; Feusner, J.; Krailo, M.; Spector, L.G. Maternal pregnancy events and exposures and risk of hepatoblastoma: A Children’s Oncology Group (COG) study. Cancer Epidemiol. 2013, 37, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.S.; Cantwell, M.M.; Cardwell, C.R.; Cook, M.B.; Murray, L.J. Maternal body mass index and risk of testicular cancer in male offspring: A systematic review and meta-analysis. Cancer Epidemiol. 2010, 34, 509–515. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Yaktine, A.L. (Eds.) Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. In Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Q.; Xu, Y.Y. Medical Statistics, 4th ed.; People’s Medical Publishing House: Beijing, China, 2014. [Google Scholar]
- Brouwers, L.; van der Meiden-van, R.A.; Savelkoul, C.; Vogelvang, T.E.; Lely, A.T.; Franx, A.; van Rijn, B.B. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: A systematic review and meta-analysis. BJOG 2018, 125, 1642–1654. [Google Scholar] [CrossRef]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Colao, A.; Lenzi, A.; Giugliano, D. Metabolic Syndrome and Risk of Cancer: A systematic review and meta-analysis. Diabetes Care 2012, 35, 2402–2411. [Google Scholar] [CrossRef]
- Schmid, D.; Willett, W.C.; Ding, M.; Michels, K.B. Maternal and Infant Anthropometric Characteristics and Breast Cancer Incidence in the Daughter. Sci. Rep. 2020, 10, 2550. [Google Scholar] [CrossRef] [PubMed]
- Petridou, E.T.; Sergentanis, T.N.; Skalkidou, A.; Antonopoulos, C.N.; Dessypris, N.; Svensson, T.; Stephansson, O.; Helle, K.; Karin, E.S. Maternal and birth anthropometric characteristics in relation to the risk of childhood lymphomas: A Swedish nationwide cohort study. Eur. J. Cancer Prev. 2015, 24, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Stephansson, O.; Wahnström, C.; Pettersson, A.; Sørensen, H.T.; Tretli, S.; Gissler, M.; Troisi, R.; Akre, O.; Grotmol, T. Perinatal risk factors for childhood testicular germ-cell cancer: A Nordic population-based study. Cancer Epidemiol. 2011, 35, e100–e104. [Google Scholar] [CrossRef] [PubMed]
- Källén, B.; Finnström, O.; Lindam, A.; Nilsson, E.; Nygren, K.G.; Olausson, P.O. Cancer risk in children and young adults conceived by in vitro fertilization. Pediatrics 2010, 126, 270–276. [Google Scholar] [CrossRef]
- McLaughlin, C.C.; Baptiste, M.S.; Schymura, M.J.; Zdeb, M.S.; Nasca, P.C. Perinatal risk factors for neuroblastoma. Cancer Causes Control. 2009, 20, 289–301. [Google Scholar] [CrossRef]
- Pettersson, A.; Richiardi, L.; Cnattingius, S.; Kaijser, M.; Akre, O. Gestational hypertension, preeclampsia, and risk of testicular cancer. Cancer Res. 2008, 68, 8832–8836. [Google Scholar] [CrossRef]
- Johnson, K.J.; Soler, J.T.; Puumala, S.E.; Ross, J.A.; Spector, L.G. Parental and infant characteristics and childhood leukemia in Minnesota. BMC Pediatr. 2008, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Sonke, G.S.; Chang, S.; Strom, S.S.; Sweeney, A.M.; Annegers, J.F.; Sigurdson, A.J. Prenatal and perinatal risk factors and testicular cancer: A hospital-based case-control study. Oncol. Res. 2007, 16, 383–387. [Google Scholar] [CrossRef]
- McLaughlin, C.C.; Baptiste, M.S.; Schymura, M.J.; Nasca, P.C.; Zdeb, M.S. Maternal and infant birth characteristics and hepatoblastoma. Am. J. Epidemiol. 2006, 163, 818–828. [Google Scholar] [CrossRef]
- McLaughlin, C.C.; Baptiste, M.S.; Schymura, M.J.; Nasca, P.C.; Zdeb, M.S. Birth weight, maternal weight and childhood leukaemia. Br. J. Cancer 2006, 94, 1738–1744. [Google Scholar] [CrossRef]
- Coupland, C.A.; Forman, D.; Chilvers, C.E.; Davey, G.; Pike, M.C.; Oliver, R.T.D. Maternal risk factors for testicular cancer: A population-based case-control study (UK). Cancer Causes Control. 2004, 15, 277–283. [Google Scholar] [CrossRef]
- Weir, H.K.; Marrett, L.D.; Kreiger, N.; Darlington, G.A.; Sugar, L. Pre-natal and peri-natal exposures and risk of testicular germ-cell cancer. Int. J. Cancer 2000, 87, 438–443. [Google Scholar] [CrossRef]
- Sanderson, M.; Williams, M.A.; Daling, J.R.; Holt, V.L.; Malone, K.E.; Self, S.G.; Moore, D.E. Maternal factors and breast cancer risk among young women. Paediatr. Perinat. Epidemiol. 1998, 12, 397–407. [Google Scholar] [CrossRef]
- Murphy, C.C.; Cirillo, P.M.; Krigbaum, N.Y.; Singal, A.G.; Lee, M.; Zaki, T.; Burstein, E.; Cohn, B.A. Maternal obesity, pregnancy weight gain, and birth weight and risk of colorectal cancer. Gut 2022, 71, 1332–1339. [Google Scholar] [CrossRef]
- Marley, A.R.; Domingues, A.; Ghosh, T.; Turcotte, L.M.; Spector, L.G. Maternal Body Mass Index, Diabetes, and Gestational Weight Gain and Risk for Pediatric Cancer in Offspring: A Systematic Review and Meta-Analysis. JNCI Cancer Spectr. 2022, 6, pkac020. [Google Scholar] [CrossRef]
- Voerman, E.; Santos, S.; Patro, G.B.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef]
- Rogozińska, E.; Zamora, J.; Marlin, N.; Betrán, A.P.; Astrup, A.; Bogaerts, A.; Cecatti, J.G.; Dodd, J.M.; Facchinetti, F.; International Weight Management in Pregnancy (i-WIP) Collaborative Group; et al. Gestational weight gain outside the Institute of Medicine recommendations and adverse pregnancy outcomes: Analysis using individual participant data from randomised trials. BMC Pregnancy Childbirth 2019, 19, 322. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.L.; Boyle, J.A.; Harrison, C.L.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; et al. Gestational weight gain across continents and ethnicity: Systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018, 16, 153. [Google Scholar] [CrossRef]
- Simmons, D.; Devlieger, R.; van Assche, A.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Desoye, G.; Kautzky-Willer, A.; Damm, P.; et al. Association between Gestational Weight Gain, Gestational Diabetes Risk, and Obstetric Outcomes: A Randomized Controlled Trial Post Hoc Analysis. Nutrients 2018, 10, 1568. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef]
- Rong, K.; Yu, K.; Han, X.; Szeto, I.M.; Qin, X.; Wang, J.; Ning, Y.; Wang, P.; Ma, D. Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: A meta-analysis of observational studies. Public Health Nutr. 2015, 18, 2172–2182. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; Yao, H.; Dai, H.; Zheng, R.; Zhang, W. Prepregnancy BMI, gestational weight gain and risk of childhood atopic dermatitis: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2021, 32, 892–904. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, B.; Wang, Y.; Wang, K.; Zhang, Z.; Niu, W. Pre-pregnancy Maternal Weight and Gestational Weight Gain Increase the Risk for Childhood Asthma and Wheeze: An Updated Meta-Analysis. Front Pediatr. 2020, 8, 134. [Google Scholar] [CrossRef]
- Eitmann, S.; Németh, D.; Hegyi, P.; Szakács, Z.; Garami, A.; Balaskó, M.; Solymár, M.; Erőss, B.; Kovács, E.; Pétervári, E. Maternal overnutrition impairs offspring’s insulin sensitivity: A systematic review and meta-analysis. Matern Child Nutr. 2020, 16, e13031. [Google Scholar] [CrossRef]
- Tian, Z.-X.; Wan, M.; Gao, Y.-L.; Wu, B.-F.; Xie, Y.; Liu, J.; Su, R.-Z.; Tian, L.-L.; Hu, Y.-Q. Gestational weight gain and risk of autism spectrum disorders in offspring: A systematic review and meta-analysis. J. Obstet. Gynaecol. 2020, 40, 953–960. [Google Scholar] [CrossRef]
- Su, L.; Chen, C.; Lu, L.; Xiang, A.H.; Dodds, L.; He, K. Association Between Gestational Weight Gain and Autism Spectrum Disorder in Offspring: A Meta-Analysis. Obesity (Silver Spring) 2020, 28, 2224–2231. [Google Scholar] [CrossRef]
- Martínez-Hortelano, J.A.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Garrido-Miguel, M.; Soriano-Cano, A.; Martínez-Vizcaíno, V. Monitoring gestational weight gain and prepregnancy BMI using the 2009 IOM guidelines in the global population: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2020, 20, 649. [Google Scholar] [CrossRef]
- Shieh, C.; Cullen, D.L.; Pike, C.; Pressler, S.J. Intervention strategies for preventing excessive gestational weight gain: Systematic review and meta-analysis. Obes. Rev. 2018, 19, 1093–1109. [Google Scholar] [CrossRef]
| First Author and Year | Study Design | Geographic Region | Sample Size | Study Population (Age) | Ascertainment of Maternal Weight | Maternal Weight Categories | Outcomes Reported | Confounds Adjusted | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|
| Fu [13] 2021 | cohort study | America | 5845 | male adults (59.8 ± 6.6 y) | self-reported | maternal pre-pregnancy BMI; GWG | overall cancer | age, time period, race, family history of cancer, maternal education, and paternal education | 8 |
| Schmid [33] 2020 | cohort study | America | 35,133 | female adults (25–42 y) | self-reported | maternal pre-pregnancy BMI; GWG | breast cancer | age, race, family history of breast cancer, smoking during pregnancy, weight gain during pregnancy, pre-pregnancy BMI, adult caloric intake, adult alcohol intake, adult smoking, and adult BMI | 7 |
| Kessous [14] 2020 | cohort study | Israel | 241,273 | children (<18 y) | medical record | maternal pre-pregnancy BMI | overall cancer, lymphoma, leukemia, brain cancer | maternal age, diabetes mellitus, hypertensive disorders, preterm delivery, type of delivery, and fetuses that are large for gestational age | 7 |
| Stacy [15] 2019 | cohort study | America | 1,827,875 | children (<14 y) | medical record | maternal pre-pregnancy BMI; GWG | overall cancer, leukemia | maternal age and race | 8 |
| Bailey [21] 2017 | case-control study | France | 3612 | children (<15 y) | self-reported | maternal pre-pregnancy BMI; GWG | brain cencer | sex and age | 8 |
| Contreras [16] 2016 | case-control study | America | 281,296 | children (<6 y) | medical record | maternal pre-pregnancy BMI; GWG | leukemia; astrocytomas; intracranial and intraspinal embryonal brain tumors; germ cell tumors; hepatoblastoma; neuroblastoma; retinoblastoma; rhabdomyosarcoma; Wilms’ tumor | year of birth, maternal/paternal race/ethnicity, and maternal age | 9 |
| Petridou [34] 2015 | cohort study | Sweden | 3,444,136 | children (<14 y) | medical record | maternal BMI | Hodgkin lymphoma; non-Hodgkin lymphoma | sex, maternal education and age, gestational age, and birth order of the index infant | 9 |
| Greenop [20] 2014 | case-control study | Australia | 1398 | children (<14 y) | self-reported | maternal pre-pregnancy BMI; GWG | brain tumors | matching variables, child’s year of birth group, maternal age group, child sethnicity, and maternal pre-pregnancy folate supplementation | 8 |
| Musselman [23] 2013 | case-control study | America | 770 | children (<6 y) | self-reported | maternal pre-pregnancy BMI | hepatoblastoma | NA | 6 |
| Stephansson [35] 2011 | case-control study | Nordic countries (Norway, Sweden, Finland, and Denmark) | 1672 | children (<15 y) | medical record | maternal BMI | testicular germ-cell cancer | birth weight, gestational age, parity, and maternal age | 9 |
| Källén [36] 2010 | case-control study | Sweden | 2,424,336 | children and young adults | medical record | maternal BMI | overall cancer | birth, number of previous miscarriages, and years of unwanted childlessness | 9 |
| McLaughlin [37] 2009 | cohort study | America | 12,539 | children (1 month–14 y) | medical record | GWG | neuroblastoma | NA | 6 |
| Johnson [39] 2008 | cohort study | America | 9397 | Children (28 day–14 y) | medical record | GWG | leukemia | birth year | 7 |
| Pettersson [38] 2008 | case-control study | Sweden | 1154 | young adults or adults (age ≥ 15) | medical record | maternal pre-pregnancy BMI; GWG | testicular germ-cell cancer | maternal age at pregnancy and birth order | 8 |
| Spector [22] 2007 | case-control study | America | 495 | children (<1 y) | self-reported | maternal pre-pregnancy BMI; GWG | leukemia | sex, race, and maternal education | 7 |
| Sonke [40] 2007 | case-control study | America | 230 | adults (18–50 y) | self-reported | maternal pre-pregnancy BMI | testicular germ-cell cancer | mother’s race, education, and body mass index; son’s birth weight, age, and history of cryptorchidism; nausea during pregnancy, and length of pregnancy | 7 |
| McLaughlin [41](A) 2006 | cohort study | America | 10,756 | children (<10 y) | medical record | GWG | leukemia | birth year, gender, race and ethnicity, maternal age, gestational age, and birth weight | 8 |
| McLaughlin [42](B) 2006 | cohort study | America | 6114 | children (1 month–5 y) | medical record | maternal pre-pregnancy BMI; GWG | hepatoblastoma | birth year and birth weight | 7 |
| Coupland [43] 2004 | case-control study | The United Kingdom | 851 | young adults or adults (15–49 y) | self-reported | maternal BMI at index pregnancy | testicular germ-cell cancer | age, region, son’s social class, undescended testis or inguinal hernia before 15 years of age, and maternal age at index pregnancy | 7 |
| Weir [44] 2000 | case-control study | Canada | 867 | young adults or adults (16–59 y) | self-reported | maternal BMI at index pregnancy | testicular germ-cell cancer | age | 7 |
| Sanderson [45] 1998 | case-control study | America | 946 | female adults (<45 y) | self-reported | maternal pre-pregnancy BMI; GWG | breast cancer | NA | 7 |
| Murphy [46] 2022 | cohort study | America | 18,751 | adults (18–56 y) | self-reported | maternal BMI | colorectal cancer | race/ethnicity, gestational age, and maternal BMI (rate of early weight gain); race/ethnicity, gestational age, maternal BMI, and rate of early weight gain (total weight gain); and race/ethnicity, gestational age, maternal BMI, rate of early weight gain, and total weight gain (birth weight) | 8 |
| Variable | No. of Studies | RR (95% CI) | I2 (%) | p Value for Heterogeneity | Test for Subgroup Differences | ||
|---|---|---|---|---|---|---|---|
| χ2 | p | I2 (%) | |||||
| Maternal underweight and offspring cancer | |||||||
| Geographic region | 1.53 | 0.220 | 35 | ||||
| America | 8 | 1.10 (0.99, 1.22) | 0 | 0.890 | |||
| Non-America | 7 | 0.98 (0.84, 1.14) | 12 | 0.340 | |||
| Study design | 1.43 | 0.230 | 30 | ||||
| Cohort study | 5 | 1.10 (0.98, 1.23) | 0 | 0.810 | |||
| Case-control study | 10 | 1.00 (0.89, 1.12) | 0 | 0.470 | |||
| Study population | 0.01 | 0.910 | 0 | ||||
| Children | 8 | 1.04 (0.93, 1.16) | 0 | 0.590 | |||
| Adults | 7 | 1.05 (0.93, 1.18) | 1 | 0.420 | |||
| Ascertainment of maternal weight | 1.67 | 0.200 | 40 | ||||
| Self-reported | 9 | 1.10 (0.98, 1.23) | 0 | 0.960 | |||
| Medical record | 6 | 0.96 (0.81, 1.14) | 36 | 0.160 | |||
| Whether confounding factors were adjusted | 0.00 | 0.950 | 0 | ||||
| Yes | 13 | 1.04 (0.96, 1.14) | 0 | 0.610 | |||
| No | 2 | 1.03 (0.67, 1.58) | 36 | 0.210 | |||
| Maternal BMI | 0.10 | 0.750 | 0 | ||||
| Overweight | 9 | 1.08 (0.98, 1.19) | 39 | 0.110 | |||
| Obese | 10 | 1.05 (0.94, 1.18) | 29 | 0.180 | |||
| Maternal Overweight/obesity and offspring cancer | |||||||
| Geographic region | 2.85 | 0.090 | 65 | ||||
| America | 10 | 1.14 (1.02, 1.28) | 51 | 0.030 | |||
| Non-America | 9 | 0.98 (0.86, 1.12) | 32 | 0.160 | |||
| Study design | 2.94 | 0.090 | 66 | ||||
| Cohort study | 7 | 1.24 (1.02, 1.51) | 50 | 0.080 | |||
| Case-control study | 12 | 1.03 (0.95, 1.12) | 15 | 0.290 | |||
| Study population | 0.33 | 0.570 | 0 | ||||
| Children | 11 | 1.08 (1.00, 1.18) | 42 | 0.070 | |||
| Adults | 8 | 1.01 (0.81, 1.26) | 53 | 0.040 | |||
| Ascertainment of maternal weight | 0.02 | 0.900 | 0 | ||||
| Self-reported | 11 | 1.06 (0.90, 1.24) | 49 | 0.030 | |||
| Medical record | 8 | 1.07 (0.98, 1.17) | 45 | 0.080 | |||
| Whether confounding factors were adjusted | 1.01 | 0.310 | 1.2 | ||||
| Yes | 17 | 1.06 (0.97, 1.16) | 48 | 0.010 | |||
| No | 2 | 1.21 (0.95, 1.53) | 0 | 0.530 | |||
| Variable | No. of Studies | OR (95% CI) | I2 (%) | p Value for Heterogeneity | Test for Subgroup Differences | ||
|---|---|---|---|---|---|---|---|
| χ2 | p | I2 (%) | |||||
| Maternal low GWG and offspring cancer | |||||||
| Geographic region | 3.20 | 0.070 | 69 | ||||
| America | 9 | 1.00 (0.92, 1.09) | 25 | 0.220 | |||
| Non-America | 3 | 1.33 (0.99, 1.78) | 53 | 0.120 | |||
| Study design | 0.02 | 0.880 | 0 | ||||
| Cohort study | 6 | 1.04 (0.96, 1.12) | 0 | 0.570 | |||
| Case-cohort study | 6 | 1.06 (0.84, 1.34) | 71 | 0.004 | |||
| Study population | 1.31 | 0.250 | 23 | ||||
| Children | 8 | 1.11 (0.98, 1.27) | 47 | 0.070 | |||
| Adults | 4 | 0.98 (0.82, 1.17) | 57 | 0.070 | |||
| Ascertainment of maternal weight | 0.02 | 0.900 | 0 | ||||
| Self-reported | 6 | 1.05 (0.86, 1.27) | 69 | 0.007 | |||
| Medical record | 6 | 1.03 (0.95, 1.13) | 3 | 0.400 | |||
| Whether confounding factors were adjusted | 0.40 | 0.530 | 0 | ||||
| Yes | 10 | 1.07 (0.98, 1.18) | 36 | 0.120 | |||
| No | 2 | 0.90 (0.53, 1.52) | 81 | 0.020 | |||
| Whether GWG was classified according to 2009 IOM guidelines | 0.67 | 0.410 | 0 | ||||
| Yes | 3 | 1.20 (0.84,1.70) | 80 | 0.007 | |||
| No | 9 | 1.03 (0.93,1.13) | 27 | 0.210 | |||
| Maternal high GWG and offspring cancer | |||||||
| Geographic region | 0.00 | 0.970 | 0 | ||||
| America | 10 | 1.10 (1.01, 1.19) | 42 | 0.080 | |||
| Non-America | 3 | 1.10 (0.78, 1.56) | 67 | 0.050 | |||
| Study design | 1.32 | 0.250 | 24 | ||||
| Cohort study | 7 | 1.14 (1.04, 1.25) | 30 | 0.200 | |||
| Case-control study | 6 | 1.02 (0.87, 1.21) | 44 | 0.110 | |||
| Study population | 0.39 | 0.530 | 0 | ||||
| Children | 9 | 1.08 (0.97, 1.20) | 51 | 0.040 | |||
| Adults | 4 | 1.14 (1.00, 1.30) | 20 | 0.290 | |||
| Ascertainment of maternal weight | 0.00 | 0.950 | 0 | ||||
| Self-reported | 6 | 1.10 (0.94, 1.28) | 49 | 0.080 | |||
| Medical record | 7 | 1.09 (0.98, 1.21) | 46 | 0.090 | |||
| Whether confounding factors were adjusted | 1.19 | 0.270 | 16 | ||||
| Yes | 11 | 1.11 (1.02, 1.22) | 48 | 0.040 | |||
| No | 2 | 0.95 (0.73, 1.24) | 23 | 0.250 | |||
| Whether GWG was classified according to 2009 IOM guidelines | 0.64 | 0.420 | 0 | ||||
| Yes | 3 | 1.01 (0.79,1.30) | 66 | 0.050 | |||
| No | 10 | 1.13 (1.04,1.23) | 23 | 0.230 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, J.; Chen, Y.; Liu, X.; Ye, C.; Zhou, X.; Yang, Z.; Gong, Z.; Chen, L.; Wang, T. Maternal Body Mass Index, Gestational Weight Gain, and Risk of Cancer in Offspring: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 1601. https://doi.org/10.3390/nu15071601
Miao J, Chen Y, Liu X, Ye C, Zhou X, Yang Z, Gong Z, Chen L, Wang T. Maternal Body Mass Index, Gestational Weight Gain, and Risk of Cancer in Offspring: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(7):1601. https://doi.org/10.3390/nu15071601
Chicago/Turabian StyleMiao, Junxiang, Yan Chen, Xiaoling Liu, Changxiang Ye, Xuan Zhou, Ziqi Yang, Ziqiang Gong, Lizhang Chen, and Tingting Wang. 2023. "Maternal Body Mass Index, Gestational Weight Gain, and Risk of Cancer in Offspring: A Systematic Review and Meta-Analysis" Nutrients 15, no. 7: 1601. https://doi.org/10.3390/nu15071601
APA StyleMiao, J., Chen, Y., Liu, X., Ye, C., Zhou, X., Yang, Z., Gong, Z., Chen, L., & Wang, T. (2023). Maternal Body Mass Index, Gestational Weight Gain, and Risk of Cancer in Offspring: A Systematic Review and Meta-Analysis. Nutrients, 15(7), 1601. https://doi.org/10.3390/nu15071601





