Abstract
The field of probiotic has been exponentially expanding over the recent decades with a more therapeutic-centered research. Probiotics mediated microbiota modulation within the microbiota–gut–brain axis (MGBA) have been proven to be beneficial in various health domains through pre-clinical and clinical studies. In the context of mental health, although probiotic research is still in its infancy stage, the promising role and potential of probiotics in various mental disorders demonstrated via in-vivo and in-vitro studies have laid a strong foundation for translating preclinical models to humans. The exploration of the therapeutic role and potential of probiotics in major depressive disorder (MDD) is an extremely noteworthy field of research. The possible etio-pathological mechanisms of depression involving inflammation, neurotransmitters, the hypothalamic–pituitary–adrenal (HPA) axis and epigenetic mechanisms potentially benefit from probiotic intervention. Probiotics, both as an adjunct to antidepressants or a stand-alone intervention, have a beneficial role and potential in mitigating anti-depressive effects, and confers some advantages compared to conventional treatments of depression using anti-depressants.
1. Introduction
The foundation of a revolutionary medicine begins when the birth of theoretical knowledge is dynamically unified with its empirical application. The bidirectional communication within the microbiome–gut–brain axis (MGBA) is one such conceptualization and possibly a major breakthrough in existing medicine. The two-way interaction between the gut and the brain may not necessarily be an entirely alien concept previously, but the enormous attention this field of science has garnered in recent years is noteworthy. This school of thought dates back to around 400 BC when The Father of Medicine, Hippocrates, coined the idea “let food be thy medicine, and medicine be thy food” [1]. The bidirectional communication between the gut and the brain is mediated by trillions of microbes residing in the human gut through several mechanisms encompassing neural, immunologic and humoral pathways. This conceptualization has paved the way for scientists to explore and link various health conditions to the MGBA in an attempt to explain the possible etiopathologies involved in their occurrence. The number of studies involving some of the common disorders in the gastrointestinal, psychiatric, cardiometabolic and neurological domains, as well as cancers, in relation to the MGBA has been exponentially growing over recent decades and steadily moving towards a promising therapeutic direction in the context of human health [2,3,4,5,6,7,8].
The microbial pattern in every human is largely individualized and highly evolving across the lifespan. The first colonization of microbes in humans is thought to occur during birth although there are studies suggesting that this could occur as early as in utero via placental colonization [9,10,11,12]. Various factors influence the composition of microbiota in an individual which can be classified into extrinsic and intrinsic factors [2,6]. The influence of these factors largely manifests as the result of a dynamic and complex interplay between the environment and the human microbiome in a way yet to be fully understood. The emerging field of epigenetics provides one of the far-reaching theoretical explanations that describes the influence of environment on the human genome in the context of MGBA. The microbiota, which is a part of the environmental component, is capable of modifying the human genome expression in the gut–brain axis without altering the deoxyribonucleic acid (DNA) sequence through an epigenetic mechanism, thus resulting in changes which may possibly manifest as a health disorder. This epigenetic modification is long-lasting and heritable; however, it is reversible. Therefore, any disturbance in the intestinal microbial equilibrium, also referred as gut dysbiosis, induces a long-lasting yet reversible effect through the epigenetic modification of the human genome within the gut–brain axis pathway [13]. On the positive side, the reversibility of the microbial-induced changes in human biology allows scientists to explore various methods of microbiota modulation with the aim of reversing intestinal dysbiosis as part of a preventive and therapeutic approach [11,14].
Probiotics are among the most promising microbiota modulators that have led to a tremendous expansion of both animal and clinical studies over recent decades. The therapeutic potential of probiotics has been explored in a vast array of health conditions including type 2 diabetes, obesity, irritable bowel syndrome, asthma, cancers, arthritis, and mental health disorders [15,16,17,18,19,20,21,22]. The study of gut microbiome and microbial modulation using probiotics has extended to some of the most debilitating and severe mental disorders, including but not limited to major depressive disorder, bipolar disorder, and schizophrenia [23,24,25]. The immense potential of probiotics, particularly in treating mental disorders, has drawn a great deal of interest among researchers and clinicians. The number of research projects and clinical trials involving probiotics in the mental health domain has greatly accelerated within the last decade. There are indisputable advantages of probiotics over the conventional psychiatric treatment modalities, which highly favor its use in the treatment of psychiatric disorders. Clinical trials have demonstrated the efficacy of probiotic supplementation in ameliorating mental illness. The clinical efficacy of probiotics coupled with their lack of detrimental side effects and stigmatization make them a promising therapeutic intervention in mental disorders [26,27,28,29]. Although existing clinical trials are limited and more substantial research outcomes are needed, probiotics are a noteworthy therapeutic intervention as far as the mental health domain is concerned.
In this review, we aim to explore the role and potential of probiotics in the field of mental health with a particular focus on major depressive disorder (MDD). Recent meta-analyses and systematic reviews have substantiated the efficacy of probiotic supplementation in the treatment of depressive disorder in human subjects. Depression scores based on validated clinical scales and certain biomarkers associated with depression including cortisol, proinflammatory cytokines and brain-derived neurotrophic factor (BDNF) levels were used to demonstrate the anti-depressive effect of probiotic intervention [25,27,30,31]. Clinical trials of probiotics in patients with depression, although limited compared to pre-clinical studies, have demonstrated comparable outcome in terms of mediating anti-depressive effects, especially studies involving the measurement of biomarkers [18,32,33,34,35]. The basic understanding of the anti-depressive mechanism of probiotics has been largely gathered from animal studies [36,37,38,39,40,41,42]. Although research outcomes generally favor the utilization of probiotics in the treatment of depression, there are gaps when it comes to translating preclinical studies to human studies, due to the heterogenous nature of depressive disorder and the dynamicity of individual microbes [43,44]. Nevertheless, the exploration of MGBA in depressive disorder has certainly laid the foundation for the expansion of a clinical approach in the management of this debilitating disorder utilizing probiotics, despite the shortcomings, as this is an emerging, yet a rapidly growing field of research [23,43,45]. Therefore, we recognize the importance of understanding the basic mechanism of probiotics by specifically relating this to the possible etiopathologies of depression. Evidence is largely gathered from preclinical followed by clinical models. The integrative exploration of the mechanistic pathways of depression-associated etiopathology and probiotics in depression models is able to provide a more insightful and organized perspective in understanding the role and potential of probiotics in depression, while identifying possible gaps in the existing research knowledge. There are four domains which have been reviewed in this context including inflammation, neurotransmitters (serotonin (5-HT), dopamine (DA), noradrenaline (NE), gamma-aminobutyric acid (GABA)), the hypothalamic–pituitary–adrenal (HPA) axis and epigenetic mechanism. As healthcare professionals have an important intermediary role between the stakeholders of probiotics and consumers as the field of probiotics rapidly progresses in a therapeutic direction, we discuss probiotics from both scientific and clinical aspects to facilitate the practice of an evidence-based medicine, as well as to encourage the growth of this promising field.
2. Revisiting the Term “Probiotic”
The word probiotic is an etymological hybrid with both Latin (pro) and Greek (bios) origins which translate into “for life”. The use of fermented dairy products since time immemorial ascertains the deep-rooted existence and use of probiotics in human history. Over the years, there has been a widespread commercialization of probiotic-containing food products and probiotics as dietary supplements in the form of capsules, tablet, liquid and powder [46,47]. In 1905, a Russian scientist, Elie Metchnikoff, was the first to deduce the possible influence of microbes on human health by attributing the enhanced longevity of the rural Bulgarians to the regular consumption of fermented dairy products which contain Lactobacilli [48,49]. He considered Lactobacilli as probiotics and put forward the probiotic concept through his description of intestinal microbes which are dependent on food consumed, hence susceptible to compositional modifications. His hypothesis greatly contributed to the establishment and development of the modern dairy industry in France, the first in Europe [48]. The scientific community was first acquainted with the term probiotic in 1953 when a German scientist, Werner Kollath, referred to the active substances which are necessary for a healthy development of life as probiotics [48]. In 1989, Fuller provided a more specific definition of probiotics as live microorganisms which benefit the host animal by promoting intestinal microbial balance [50].
From the 1950s to the 1980s, probiotic research focused on identifying strains of probiotics and understanding the underlying mechanism of action. In 2000s, clinical trials using probiotics in human subjects started to emerge. However, the diverse range of probiotic strains used in various clinical trials made it difficult to derive disease-specific strains, as the therapeutic potential of probiotics is highly strain-dependent [49]. Over the years, the probiotic field continued to expand tremendously involving a huge array of probiotic stakeholders and consumers which, in a negative sense, gives rise to the exploitation and misuse of the term probiotic. This prompted scientists to revisit the concept of probiotics in 2013, when the International Scientific Association for Probiotics and Prebiotics (ISAPP) convened a panel of experts to examine and discuss the field. The widely adopted definition of probiotics published by the Food and Agriculture Organization of the United Nations and the World Health Organization (FAO/WHO) in 2001 was revisited and accepted with improved grammatical precision. A general consensus was reached by defining probiotics as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [51]. In the context of mental health, a more specific probiotic terminology known as psycho-biotics was introduced by Dinan et al. in 2013. Psycho-biotics are probiotics which exert a beneficial effect on patients suffering from mental health issues [52]. However, probiotics remains the preferred term due to the lack of independent criteria and mode of action of pyscho-biotics [47].
The core definition of probiotics has persistently remained central to the viability and active state of the ingestible substance; however, newer terminology such as parabiotic, postbiotic and para-psycho-biotic, which utilize an inactive, non-viable form of probiotics, are emerging. The health benefits of the inactivated form of probiotics and their metabolites are comparable to probiotics, and potentially a safer alternative to due to their downregulation of the host’s inflammatory response [47,53,54,55]. Another aspect of probiotic definition emphasizes the administration of an adequate amount to confer health benefits. Nonetheless, studies which demonstrate the dose–response relationship between probiotics and health outcomes in humans are insufficient [56]. There is no consensus on the adequate amount of probiotics which are required to elicit optimum health benefits specific to the target health condition of the host. However, a minimum amount of 109 colony forming units (CFU) per daily dose of probiotic has been recommended to impart health benefits in general [46,57]. In the context of mental disorders, based on a systematic review, the doses of probiotics used were between 109 and 1010 CFU over a duration of 2 weeks in animal studies and 4 weeks in clinical studies to demonstrate efficacy [58].
The ability of probiotics to confer health benefits to the host is the most cardinal element in the use of probiotic terminology. The diverse range of health benefits associated with the consumption of probiotics is certainly overwhelming. Ultimately, probiotics work by restoring intestinal dysbiosis and reversing associated adverse effects on gut health which have been identified as the culprit in the occurrence of various diseases. The anti-inflammatory, antipathogenic and antimicrobial properties of probiotics exert positive effects in terms of restoring and maintaining intestinal homeostasis and microbial balance. This contributes to the strengthening of bidirectional communication within the MGBA. The role of probiotics in immune modulation, gut homeostasis and restoration of microbial equilibrium confers both preventive and therapeutic health benefits to the host. Probiotics are, therefore, unapologetically perceived as the ‘fit-for-all’ formula which could potentially eradicate various disorders, especially those rooted in the gastrointestinal, immunologic, and neurological domains [42,59,60,61,62].
3. Mental Disorders: The Enigmatic Malady in the Field of Medicine
Mental disorders or mental illnesses are clinically diagnosable mental health problems with reference to the criteria outlined in the diagnostic manuals (i.e., Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5, International Classification of Diseases, Tenth Revision: ICD-10). Unlike other branches of medicine, the field of psychiatry often deals with ambiguity and clinical heterogeneity which impose major challenges in the management of psychiatric illnesses. There are no identifiable common pathogenic pathways and central disease mechanisms involved in the occurrence of mental disorders. The recognizable clinical patterns and symptoms of mental disorders are exemplified in the diagnostic manuals, which may be subjected to revision and modifications [63]. Whether psychiatric disorders can be classified as disorders of the brain or disorders that affect the brain remains a question. Mental disorders broadly involve mood, cognition, perception and behavioral aspects of the higher cortical function. However, it is impossible to localize the brain region involved and clinically measure the direct etiological factors which contribute to the development of a mental disorder. Mental disorders are, rather, a constellation of symptoms, often self-reported, which require the clinical expertise of a psychiatrist to arrive at a diagnosis [64,65].
Despite ambiguity and enigma, a new horizon and hypothetical perspective has begun to emerge in the field of mental health with the application of MGBA conceptualization. The idea of mental disorders merely contained within the head has been gradually experiencing a paradigm shift and a nudge to look beyond the neckline [64,66]. Landmark studies using germ-free mice models and fecal–microbial transplant (FMT) from patients with a particular mental disorder to healthy mice models have provided an insight into the implication of microbiomes in the development of neuropathological and behavioral symptoms which mimic the mental disorder that is being examined [67,68,69,70]. The consistent finding of gut dysbiosis or altered intestinal microbial composition and reduced diversity of microbial ecosystem within the gut among patients with mental disorders compared to corresponding healthy controls further ascertained the relationship between gut microbiome and its possible etio-pathological role in the occurrence of mental disorders [71,72,73,74].
Probiotics are involved in the modulation of microbiota within the MGBA. To explore the role and potential of probiotics, it is important to understand the target mechanisms in which probiotics exert their beneficial effect. The key point of interest will be in identifying some of the pathological mechanisms implicated in the occurrence of mental disorders and the possible mechanistic role of probiotics in tackling those pitfalls specific to each mental disorder.
4. Major Depressive Disorder (MDD)
MDD is clinically diagnosable when an individual is persistently in a depressed mood and/or experiencing anhedonia for at least 2 weeks along with other symptoms outlined in DSM-5, with a minimum five symptoms from: changes in appetite or weight, sleep disturbance, psychomotor changes, diminished concentration, fatigue, feeling of worthlessness, excessive guilty and suicidal ideation [75]. In this review, the term “depression” refers to the symptoms that depict MDD. Depression is one of the most common disorders affecting more than 350 million people worldwide with an estimated lifetime prevalence of up to 10.8% based on a community survey involving 30 countries between the years 1994 and 2014. The World Health Organization (WHO) predicts that depression may become the second leading burden of global disease, outranking cardiovascular disease, from its current fourth rank by 2030 [45,76]. An estimate of 800,000 deaths by suicide associated with depression yearly has been reported which reflects the debilitating and severe nature of this mental disorder [77].
The gut microbiome profile of depressive patients markedly differs from that of healthy controls, indicating the presence of significant gut dysbiosis in the former. At the phylum level, significant alterations within the main four phyla, Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria, were reported in patients with MDD [72,78]. More specifically at the genus level, a notable decrease in Bifidobacterium, Lactobacillus, Faecalibacterium, Ruminococcus and an increase in Prevotella, Clostridium, Klebsiella, Streptococcus and Oscillibacter were observed in MDD patients [72,79,80]. These findings have prompted further hypothetical exploration of the possible etiopathology of depression from the microbiota–gut–brain angle.
5. Exploring the Role and Potential of Probiotics in Depression
The potential of probiotics in treating mental disorders has been explored using numerous models of a hypothetical mechanism, based largely on findings from studies performed in vitro and in vivo using animal models. The possible mechanistic role of probiotics in depression through their anti-inflammatory effects, restoration of gut permeability, modulation of neurotransmitters, attenuation of HPA axis and epigenetic mechanism will be discussed in more detail in the following subsections. Generally, the anti-inflammatory effects of probiotics have been demonstrated either through direct observation of reduced plasma concentration of proinflammatory cytokines or, indirectly, through suppression within the kynurenine pathway and restoration of gut permeability, which have been related to the etiopathology of depression [32,33,37,40,81,82,83,84]. The administration of probiotics has also been shown to restore and elevate the depleted levels of the neurotransmitters of interest, namely, 5-HT, DA, NE and GABA, which have been implicated in the occurrence of depression. This particular mechanistic attribute of the probiotic has been likened to the mechanism in which certain anti-depressants work, with comparable efficacy [36,85,86,87,88,89,90,91,92]. The role of probiotics in the attenuation of the exaggerated HPA-axis associated with depression has mainly been observed through the suppression of cortisol level, a stress biomarker in human subjects, cortisone level in animal models of depression [34,35,39,93,94,95], and alteration within the neurotransmitter circuitry involving the HPA-axis [87,96,97,98]. There are no current studies which have exclusively explored the epigenetic mechanism of probiotics in depression, nevertheless, the regulation of BDNF expression and histone deacetylase (HDAC) inhibition by butyrate produced by probiotics demonstrates the possible epigenetic potential of probiotics in the context of depression [58,99,100,101].
5.1. Inflammation
Inflammation affecting the central nervous system (CNS) through immune activation is one of the pathogenic mechanisms implicated in the occurrence of depression. Proinflammatory cytokines form the periphery and are the basis of neuroinflammation in depression, capable of disrupting the brain’s regulatory and signaling mechanisms involving behavioral and emotional aspects. Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) have been found to be significantly elevated in patients with MDD [102,103,104]. A compromised intestinal permeability which has been frequently associated with depression could be one of the contributing factors to elevated proinflammatory cytokine levels. This is consistent with the leaky-gut hypothesis, which narrates the vicious cycle of interaction between the gut microbiota, CNS and the periphery in the activation of inflammatory responses [103,105,106,107]. One of the proposed mechanisms in which proinflammatory cytokines induce depressive symptoms is through the activation of the indoleamine 2,3-dioxygenase (IDO) enzyme that facilitates the metabolic breakdown of tryptophan (TRP) into kynurenine (KYN) [108]. Elevated plasma KYN and the KYN/TRP ratio have been positively correlated with the severity of depressive symptoms. Increased plasma KYN has also been associated with suicidal behavior while decreased level has been associated with improved cognitive function in patients with MDD [32,109,110,111,112].
Probiotics mediate their anti-inflammatory effects via the modulation of proinflammatory cytokines, regulation of IDO activity and restoration of gut permeability. Lactobacillus reuteri, Bifidobacterium infantis and Bifidobacterium adolescentis are examples of probiotics which exert anti-inflammatory effects that could potentially ameliorate depressive symptoms [41,103]. The administration of L. reuteri improved depressive symptoms in mice models by restoring the Lactobacillus population and reversing stress-induced alteration of microbial hydrogen peroxide (H2O2), involved in the inhibition of IDO activity, and plasma KYN levels [82]. L. reuteri also mediates the anti-inflammatory effect by promoting the secretion of microbial histamine which suppresses the production of proinflammatory cytokines involved in IDO activation [81,83,113]. Mice treated with B. infantis were found to have reduced plasma concentration of TRP and suppressed IDO activity [40]. Both B. infantis and B. adolescentis have been associated with reduced plasma IL-6 concentration [37,40]. Probiotic Lactobacillus plantarum has demonstrated an anti-depressive effect in mice models and adults with depression [33,87] and is involved in the mitigation of inflammation through downregulation of IL-6 and TNF-α and significant restoration of intestinal permeability and Lactobacillus population [33,84]. In a randomized, placebo-controlled trial, the administration of L. plantarum significantly reduced plasma KYN level which has been correlated to improved cognitive function in patients with MDD [32]. L. plantarum also strengthens the gut barrier and modulates gut microbiota through the production of butyrate and butyrate-producing bacteria (i.e., Lactobacillus, Bacteroidetes and Roseburia) [84,87]. The probiotic Faecalibacterium prausnitzii is known to confer an anti-depressive effect through its ability to produce an abundance of butyrate and reduce IL-6 level [38]. Butyrate, a short-chain fatty acid (SCFA) produced by gut microbiota, confers a protective role on the gut permeability and exerts anti-inflammatory effects on various organs including the brain [114]. Butyrate has been demonstrated to ameliorate depressive symptoms and likened to an anti-depressant in mice models [58].
5.2. Neurotransmitters (Serotonin (5-HT), Dopamine (DA), Noradrenaline (NE), Gamma-Aminobutyric Acid (GABA))
The neurotransmitters 5-HT, DA, NE and GABA have been implicated in the pathophysiology of depression. These neurotransmitters, which are either gut or brain-derived, have imperative roles in the systemic homeostasis and regulation of neural circuits’ development and plasticity. Depletion in either of these neurotransmitters contributes to the development of various neuropsychiatric disorders including depression. Most antidepressants work by restoring the levels of monoamine neurotransmitters (i.e., 5-HT, DA and NE) [115,116,117,118]. The gut microbiota is involved in the modulation of neurotransmitters via either neural, humoral or immune-mediated pathways. Dysregulation and alteration in the gut microbiota have been found to impair the neurotransmitter circuitry [117,119]. The vagus nerve is one of the crucial mediators between the gut and the brain via the neural pathway, which is involved in the regulation of neurotransmitters by the gut microbiota [97,115].
Probiotics of the Lactobacillus and Bifidobacterium species are the most prominent neurotransmitter enhancers which mediate anti-depressive effect by producing various neurotransmitters including 5-HT, DA, NE and GABA [41,90]. The mechanism of some probiotics has been likened to that of antidepressants in the class of selective serotonin reuptake inhibitors (SSRI), which involve the 5-HT and brain-derived neurotrophic factor (BDNF). 5-HT and BDNF coregulate one another in mediating their physiological roles in the regulation of neural circuits’ development and plasticity. 5-HT stimulates BDNF expression while BDNF promotes the neurogenesis and neuronal survival of 5-HT. Impairment in the 5-HT–BDNF signaling mechanism has been implicated in the pathophysiology of depression [120,121]. The administration of Lactobacillus helveticus and citalopram, an antidepressant of the SSRI class, in rodent models demonstrated similar outcomes of increased levels of 5-HT with anti-depressive effects. The same study also demonstrated restored NE level, which has also been correlated to improved cognitive outcome in rodent models [85]. In another study involving mice models with corticosterone-induced depression, Lactobacillus paracasei demonstrated similar outcomes which were comparable to the efficacy of fluoxetine, an SSRI [36]. These probiotics upregulate the 5-HT-BDNF system to mediate the anti-depressive effect via involving different neural and immune-mediated humoral pathways within the MGBA [41]. In terms of NE, the B. infantis probiotic has been shown to elevate NE levels while downregulating IL-6 levels in depressed mice models [86]. B. infantis is capable of influencing the central NE system through the immune-mediated humoral route without involving the vagus nerve, unlike L. helveticus whose anti-depressive effect ceases with vagotomy [122,123]. DA plays a pivotal role in reward-related circuitry and its dysregulation has been associated with anhedonia, which is one of the cardinal symptoms of MDD [124]. The probiotic L. plantarum has been found to mediate the anti-depressive effect by increasing DA levels and downregulating monoamine oxidase A (MAO-A) expression in mice models [87,88]. The inhibition of MAO reduces degradation of DA and normalizes an exaggerated HPA-axis [87]. Selective inhibitors of MAO-A are known to be effective clinical antidepressants [125]. In terms of GABA, Bifidobacterium strains were ascertained as the most efficient GABA-producing gut-derived bacteria in a study involving 135 Lactobacillus and Bifidobacterium strains derived from the human gut [89]. In its sequential study, probiotics Lactobacillus plantarum and Bifidobacterium adolescentis, which were ascertained as the most efficient GABA-producers with anti-depressive potential, improved depressive behavior in mice models with an efficacy comparable to fluoxetine [90]. Studies have shown that the administration of Lactobacillus brevis, one of the main GABA-producers among Lactobacillus species, significantly improved insomnia in mice models and mediated anti-depressive effects, comparable to fluoxetine [89,91,92,116].
5.3. Hypothalamic–Pituitary–Adrenal (HPA) Axis
Stress is one of the major risk factors in the development of MDD. Chronic stress exposure has consequential effects on the gut microbial composition and HPA-axis [126,127]. Stress activates the HPA-axis which eventually stimulates the release of glucocorticoids (i.e., cortisol in humans and corticosterone in animals) from the adrenal cortex in response to stimulation by the adrenocorticotrophic hormone (ACTH) from the anterior pituitary gland. Increased circulatory glucocorticoids inhibit the hypothalamus secretion of corticotrophin-releasing hormone (CRH) and vasopressin, establishing a negative feedback circuit. On the other hand, stress-induced gut dysbiosis exacerbates gut inflammation and permeability, and stimulate the release of proinflammatory cytokines which further activate the HPA-axis. This vicious cycle of inflammation and HPA-axis activation involving the MGBA is prolonged by the persistent presence of stimulus in the form of chronic stress, rendering the immune system insensitive to the inhibitory signals of glucocorticoids and disrupting the negative feedback circuit. Persistently elevated circulatory glucocorticoids and proinflammatory cytokines also desensitize and downregulate the central neurotransmitter circuits and disrupt the inhibitory mechanism of the neurotransmitters, primarily GABA, on the CRH neurons of the hypothalamic paraventricular nucleus, rendering adverse effects on the brain neurotransmitter systems, although the exact mechanism via which neurotransmitters affect the HPA-axis remains unclear. An exaggerated HPA-axis response with a dysfunctional negative feedback system and increased inflammatory responses have been closely associated with the biological etiology of depression. This echoes the frequently reported elevated levels of cortisol, CRH and ACTH and proinflammatory cytokines in MDD patients [41,115,128,129,130,131].
Considering the multiple pitfalls within the MGBA involving the HPA-axis, probiotics have both direct and indirect roles in attenuating or normalizing HPA-axis hyperactivity through the restoration of gut dysbiosis and permeability and modulation of neurotransmitters [116]. The restoration of elevated cortisol levels is one of the main parameters of normalization of the HPA-axis. The administration of probiotic L. rhamnosus mitigated depressive behaviors in mice models by reducing stress-induced plasma corticosterone levels [39]. L. rhamnosus has been found to alter the expression of central GABA receptors and elevate GABA levels, which can be linked to its possible influence on the downregulation of the HPA-axis via the neural route (i.e., the vagus nerve) to exert an anti-depressive effect [97,98]. The administration of L. plantarum in depressed mice models showed reduction in MAO levels and increased levels of DA and its metabolites, with anti-depressive effects [87,96]. Excessive glucocorticoids hyper-stimulate enzymatic activity of MAO which is involved in the degradation of monoamine neurotransmitters; thus, it is proposed that L. plantarum regulates DA circuitry and the HPA-axis by modulating glucocorticoid-induced activation of MAO [41,132]. In mice models subjected to chronic stress, combination probiotics L. Helveticus and Bifidobacterium longum significantly improved depressive-like behaviors, reduced corticosterone levels, prevented stress-induced reduction of hippocampal neurogenesis of noradrenaline, and restored the gut barrier. Reduced cortisol levels have been linked to the attenuation of HPA-axis hyperactivity [95]. Consistently, in clinical trials of combination probiotics L. Helveticus and B. longum, clinical depression and mood outcomes were significantly improved in MDD patients and healthy human volunteers respectively, with reduced urinary cortisol levels at the end of probiotic trials [34,93]. In human subjects, the administration of Lactobacillus casei in stressed individuals reduced salivary cortisol levels and alleviated stress, as well as abdominal dysfunction [35].
5.4. Epigenetic Mechanism
Epigenetic mechanisms which involve the integration of environmental signals to modify gene expression, independent of changes in the actual DNA sequence, reveal another dimension of hypothetical understanding of the dynamic interaction between the gut microbiota and the host’s genome within the MGBA. Widely studied epigenetic mechanisms including DNA methylation, histone tail modifications and non-coding ribonucleic acids (RNAs) induce heritable changes in the host’s gene expression. The modifications in DNA and histone are enzyme-mediated which may either inhibit or promote gene expression [13,133,134]. One way in which the gut microbiota is postulated to regulate these epigenetic modifications is through its metabolites (SCFAs; butyrate, acetate, propionate), either via direct inhibition of the enzymes or alteration in the availability of substrates required for the enzymatic activity [135].
In the context of the mental health domain, it is understood that the gut microbiota primarily exerts epigenetic effects within the MGBA involving key genes in the CNS through their metabolites (SCFAs, mainly butyrate) that ultimately influence behavioral outcomes [133]. Any significant gut dysbiosis negatively impacts epigenetic activity either directly or indirectly at the host genome level, which is known to be long-lasting, yet reversible. Probiotics are, therefore, utilized to modulate gut microbiota and restore epigenetic changes in a similar manner to that of the gut microbiota, which ultimately produces beneficial behavioral outcomes [133,136]. The regulation of BDNF expression and HDAC inhibition by butyrate are possible epigenetic mechanisms of probiotics in mediating anti-depressive effect. HDAC-mediated epigenetic alteration on histone tails downregulates histone acetylation and chromatin accessibility. The altered levels of histone acetylation and HDAC have been observed in the hippocampus of animal models of stress-induced depression [137,138,139]. Conversely, the administration of HDAC-inhibitors attenuated depressive-like behaviors and normalized epigenetic changes by promoting upregulation of histone acetylation [99,140,141,142,143]. Butyrate is one of the potent inhibitors of HDAC. The administration of butyrate has not only been shown to mitigate anti-depressive effects, but also promote hippocampal histone acetylation and BDNF expression as well as reducing gut inflammation [58,99,100,101]. Probiotics L. plantarum, B. infantis, Clostridium butyricum and F. prausnitzii are butyrate-producing probiotics which have been shown to ameliorate depressive behaviors in mice models [38,41,87,144,145]. L. plantarum and B. infantis also augment the production of butyrate-producing bacteria, while increased BDNF expression was observed with treatment with B. infantis and C. butyricum [87,144,145]. Treatment wjth F. prausnitzii in depressed mice models increased butyrate levels and reduced IL-6 levels, which have been associated with its ability to restore gut barrier function and mediate anti-inflammatory effects [38]. The anti-inflammatory effects of F. prausnitzii have been particularly attributed to the butyrate it produces, which downregulates proinflammatory cytokines by inhibiting HDAC1 in rats with induced colitis [146]. Although the epigenetic mechanism of probiotics seems indirect and remains largely unexplored, the butyrate-producing bacterial strains which target HDAC inhibition either at the hippocampal level or the intestinal epithelial, modulating BDNF expression and proinflammatory cytokines with anti-depressive effects, may serve as a suitable probiotic candidate to facilitate a better understanding of the epigenetic role and potential of probiotics in treating depression. An overview of the anti-depressive mechanisms of probiotics has been illustrated in Figure 1.
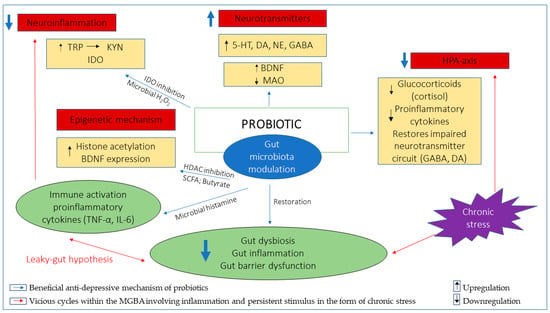
Figure 1.
Illustration of potential anti-depressive mechanisms of probiotics, fundamentally involving gut microbiota modulation. The restoration of gut dysbiosis, inflammation and gut barrier dysfunction downregulates inflammation involving the TRP/KYN pathway which is implicated in depression and breaks the vicious cycles involving inflammation, the HPA-axis and persistent stimulus in the form of chronic stress. Probiotics promote synthesis of neurotransmitters either directly or indirectly by increasing BDNF levels and/or decreasing MAO levels to mediate anti-depressive effects. Probiotics attenuate exaggerated HPA-axis implicated in depression through downregulation of cortisol, proinflammatory cytokines and restoration of neurotransmitter circuits involving GABA and DA neurotransmitters. The epigenetic mechanism mainly involves butyrate-producing probiotics which inhibit HDAC and promote histone acetylation while upregulating BDNF expression. Tryptophan (TRP); Kynurenine (KYN); Indoleamine 2,3-dioxygenase (IDO); Serotonin (5-HT); Dopamine (DA); Noradrenaline (NE); Gamma-Aminobutyric acid (GABA); Brain-derived neurotrophic factor (BDNF); monoamine oxidase (MAO); Hydrogen peroxide (H2O2); Short-chain fatty acid (SCFA); Histone deacetylase (HDAC); Interleukin-6 (IL-6); Tumor necrosis factor-α (TNF-α).
6. Discussion
The complex and heterogenous nature of a mental disorder with multiple plausible etiopathologies certainly imposes challenges from both scientific and clinical perspectives. The heterogeneity observed in mental disorders could possibly be attributed to the diverse and highly individualized microbial patterns across the human population [43]. The dynamic nature of the human microbiota profile which evolves across an individual’s life span due to various environmental and biological factors evokes questions pertaining to the potential of probiotics to offset such influences in exerting its long-term benefits [44]. Paradoxically, the same flexible nature of the human microbiota has catered to the development of therapeutic modulation using probiotics.
In the context of depression, the potential of probiotics has been explored by mechanistic relation to the possible etiopathologies associated with depression. Although, clinical studies are largely limited and discrepancies may arise when tested in human models, the available pre-clinical studies have produced substantial background to justify further expansion of probiotic research in human subjects. Inflammation, neurotransmitters, HPA-axis and epigenome involvement in the development of depression allows the utilization of probiotics to exert their therapeutic effect via modulation of gut microbiota within the MGBA. However, it is worth pinpointing that the causal link of depression to these possible pathologies is still under discussion and the heterogeneity of depressive symptoms could not possibly be explained using a single model or hypothetical mechanism [147]. Therefore, the multiple therapeutic effects produced by either a single probiotic strain (i.e., B. infantis, L. helveticus, L. rhamnosus) or a combination of Lactobacillus and Bifidobacterium probiotic strains may confer significant benefits in the treatment of depressive disorder [31,41]. The epigenetic mechanism gives a hopeful insight into the possible long-term probiotic-induced benefits; however, there is a dearth of studies available to arrive at a conclusion on the epigenetic potential of probiotics in depression.
Some of the challenges and concerns pertaining to the clinical management of depression are important aspects that cannot be overlooked in order to highlight the advantages of probiotics over the conventional treatment of depression. Clinically depressed patients are mostly managed in an outpatient setting, particularly those in the mild and moderate categories of MDD. Antidepressants are the most commonly prescribed class of drugs, with SSRIs as the first line of preferred antidepressants [148]. The mechanism via which antidepressants work has laid the foundation for the development of the monoamine hypothesis. For decades, the monoamine hypothesis, suggesting insufficiency within the neurotransmission circuit involving monoamine neurotransmitters, has predominantly ruled as the plausible mechanism in the occurrence of depression, hence the monopolization of antidepressants in its management [149]. However, antidepressants appear to be effective only in 46%–60% of patients [131]. Studies have found that patients who have shown improvement using antidepressants mainly resulted from placebo effects, and the antidepressant effects were unspecific. Further, the suicide rate and risks were similar between patients assigned antidepressants and placebo [150]. Tapering of anti-depressants is the norm via which antidepressants are discontinued [148]. However, the lack of guidelines on the discontinuation of antidepressants and the withdrawal effects associated with either abrupt or tapered discontinuation have been another concerning factor [151,152]. A systematic review of the withdrawal effects of antidepressants revealed that a weighted average of 56% of patients experience withdrawal effects with either abrupt or tapered discontinuation of antidepressants over varying durations from 6 weeks to several months. The side effects associated with prolonged use of antidepressants including weight gain, increased dependency, relapse rates and mortality risk, have also been highlighted as major concerns [152]. The use of SSRIs has also been associated with detrimental side effects in 46%–60% of patients, which include diminished sexual function, suicidality, apathy and addiction [153]. The stigma associated with the use of antidepressants also impacts adherence to the treatment [154].
Although most of pre-clinical studies and some clinical studies have demonstrated the efficacy of probiotics as a stand-alone treatment in depressive disorders, one of the recent meta-analyses of probiotic intervention in clinical depression concluded that probiotics are best used as an adjunct to antidepressants rather than as stand-alone treatment [25]. As an adjunct to antidepressants, probiotics could be utilized to tackle specific symptoms of depression. For example, GABA-producing probiotics have been associated with improved insomnia [155]. Probiotics L. plantarum, L. paracasei, B. infantis and B. breve, which are involved in the modulation of the DA system, may be effective in treating anhedonia [41]. In terms of duration of probiotic intervention, significant beneficial effects of probiotics in human subjects were observable after 4 weeks of administration [156,157]. Discontinuation of probiotic intervention requires no tapering and no withdrawal effects have been reported in any study to date. Most importantly, probiotics are generally safe with no harmful side effects reported thus far. Further, no stigma is associated with the use of probiotics and their consumption may also contribute to improvement in general health [26,157,158,159].
Last but not least, relating to the COVID-19 pandemic, the increased prevalence of depression in the community has become a huge concern in recent times. One of the recent meta-analyses of twelve large community-based studies published between January 2020 and May 2020 revealed a seven-fold increase in the pooled prevalence of depression amongst the general population during the COVID-19 outbreak, compared to the latest estimated global prevalence of depression based on Global Burden of Disease data in 2017 [160]. The impact of the pandemic on the mental health of the general population is immeasurable and imposes a huge burden on healthcare globally. In such circumstances, a non-prescription intervention such as probiotics becomes more relevant and valuable, especially for stress-induced depression [30,158].
7. Conclusions
It is beyond any reasonable doubt that probiotics have a beneficial role and potential in the mental health domain. The various mechanisms of probiotics overlap with the possible pathophysiological routes in depression, which may benefit from the probiotic modulation of microbiota within the MGBA. The mechanistic role and potential of probiotics, which are largely based on hypothetical connections based on findings from pre-clinical studies, require more intense research input based on human studies to justify the plausibility and validity of their proposed beneficial actions. Although probiotics appear to be a promising intervention in depression, the reproducibility of pre-clinical results in clinical studies remains a gap that needs to be addressed.
Author Contributions
D.J. performed the literature search, data analysis and review writing. S.T., V.L., K.-G.C. and L.-H.L. provided vital technical support and proofreading. L.-H.L. set up this review writing project. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Jeffrey Cheah School of Medicine and Health Sciences (JCSMHS) SEED Fund 2020, PVC Award Grant (Project No. PVC-ECR-2016), External Industry Grant (Biotek Abadi–Vote No. GBA-808138 and GBA-808813) awarded to L-H.L., and University of Malaya Research Grant (FRGS Grant No: FP022-2018A) awarded to K-G.C.
Acknowledgments
The authors would like to acknowledge Professor Shajahan Yasin from Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wegener, G. ‘Let food be thy medicine, and medicine be thy food’: Hippocrates revisited. Acta Neuropsychiatr. 2014, 26, 1–3. [Google Scholar] [CrossRef]
- Integrative, H.; Proctor, L.M.; Creasy, H.H.; Fettweis, J.M.; Lloyd-Price, J.; Mahurkar, A.; Zhou, W.; Buck, G.A.; Snyder, M.P.; Strauss, J.F., III. The integrative human microbiome project. Nature 2019, 569, 641–648. [Google Scholar]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.W.Y.; Tan, L.T.-H.; Ab Mutalib, N.-S.; Wong, S.H.; Letchumanan, V.; Lee, L.-H. The chemistry of gut microbiome in health and diseases. Prog. Microbes Mol. Biol. 2021, 4, 1–40. [Google Scholar] [CrossRef]
- Du Toit, A. The gut microbiome and mental health. Nat. Rev. Microbiol. 2019, 17, 196. [Google Scholar] [CrossRef]
- Johnson, D.; Letchumanan, V.; Thurairajasingam, S.; Lee, L.-H. A revolutionizing approach to autism spectrum disorder using the microbiome. Nutrients 2020, 12, 1983. [Google Scholar] [CrossRef]
- Lee, L.-H.; Letchumanan, V.; Tan, L.T.-H.; Ser, H.-L.; Law, J.W.-F. Gut-skin axis: Decoding the link between the gut microbiome and hives. Gut 2020, 9, A17–A18. [Google Scholar]
- Lee, L.-H.; Law, J.W.-F.; Tan, L.T.-H.; Ser, H.-L.; Letchumanan, V. Budding association between gut microbiome in the development of Myasthenia Gravis. Gut 2020, 69, A17–A18. [Google Scholar]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Jiménez, E.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Is meconium from healthy newborns actually sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef]
- Lee, L.-H.; Wong, S.H.; Chin, S.-F.; Singh, V.; Ab Mutalib, N.-S. Human Microbiome: Symbiosis to Pathogenesis. Front. Microbiol. 2021, 12, 605783. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.-F.; Hern Tan, L.T.; Ramadas, A.; Ab Mutalib, N.-S.; Lee, L.-H. Exploring the Role of Gut Bacteria in Health and Disease in Preterm Neonates. Int. J. Environ. Res. Public Health 2020, 17, 6963. [Google Scholar] [CrossRef]
- Louwies, T.; Johnson, A.C.; Orock, A.; Yuan, T.; Greenwood-Van Meerveld, B. The microbiota-gut-brain axis: An emerging role for the epigenome. Exp. Biol. Med. 2020, 245, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ser, H.-L.; Letchumanan, V.; Goh, B.-H.; Wong, S.H.; Lee, L.-H. The Use of Fecal Microbiome Transplant in Treating Human Diseases: Too Early for Poop? Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Azad, M.; Kalam, A.; Sarker, M.; Li, T.; Yin, J. Probiotic species in the modulation of gut microbiota: An overview. BioMed Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Lin, Y.L.; Jan, R.L.; Chen, H.H.; Wang, J.Y. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr. Pulmonol. 2010, 45, 1111–1120. [Google Scholar] [CrossRef]
- Ishaque, S.M.; Khosruzzaman, S.; Ahmed, D.S.; Sah, M.P. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult®) in the management of diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2018, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Falalyeyeva, T.; Mykhalchyshyn, G.; Kyriienko, D.; Komissarenko, I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 617–624. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Chu, S.-H.; Jeon, J.Y.; Lee, M.-K.; Park, J.-H.; Lee, D.-C.; Lee, J.-W.; Kim, N.-K. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: A double-blind, randomized, placebo-controlled trial. Dig. Liver Dis. 2014, 46, 1126–1132. [Google Scholar] [CrossRef]
- Lei, M.; Guo, C.; Wang, D.; Zhang, C.; Hua, L. The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: A randomised double-blind, placebo-controlled clinical trial. Benef. Microbes 2017, 8, 697–703. [Google Scholar] [CrossRef]
- Szulińska, M.; Łoniewski, I.; Van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-dependent effects of multispecies probiotic supplementation on the lipopolysaccharide (LPS) level and cardiometabolic profile in obese postmenopausal women: A 12-week randomized clinical trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Ngo, A.L.; Simopoulos, T.; Kaye, A.D.; Colontonio, M.M. Gut microbiome dysbiosis and depression: A comprehensive review. Curr. Pain Headache Rep. 2020, 24, 1–14. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kosciolek, T.; Eyler, L.T.; Knight, R.; Jeste, D.V. Overview and systematic review of studies of microbiome in schizophrenia and bipolar disorder. J. Psychiatr. Res. 2018, 99, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.L.; Cleare, A.J.; Young, A.H.; Stone, J.M. Updated Review and Meta-Analysis of Probiotics for the Treatment of Clinical Depression: Adjunctive vs. Stand-Alone Treatment. J. Clin. Med. 2021, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.J.; Foster, J.A.; Soares, C.N.; Milev, R.V. The effects of probiotics on symptoms of depression: Protocol for a double-blind randomized placebo-controlled trial. Neuropsychobiology 2020, 79, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Amirani, E.; Milajerdi, A.; Mirzaei, H.; Jamilian, H.; Mansournia, M.A.; Hallajzadeh, J.; Ghaderi, A. The effects of probiotic supplementation on mental health, biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 49, 102361. [Google Scholar] [CrossRef] [PubMed]
- Genedi, M.; Janmaat, I.E.; Haarman, B.B.C.; Sommer, I.E. Dysregulation of the gut–brain axis in schizophrenia and bipolar disorder: Probiotic supplementation as a supportive treatment in psychiatric disorders. Curr. Opin. Psychiatry 2019, 32, 185–195. [Google Scholar] [CrossRef]
- Zagórska, A.; Marcinkowska, M.; Jamrozik, M.; Wiśniowska, B.; Paśko, P. From probiotics to psychobiotics–the gut-brain axis in psychiatric disorders. Benef. Microbes 2020, 11, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Liu, C.; Sutthawongwadee, S.; Li, Y.; Lv, W.; Chen, W.; Yu, L.; Zhou, J.; Guo, A.; Li, Z. Effects of probiotics on depressive or anxiety variables in healthy participants under stress conditions or with a depressive or anxiety diagnosis: A meta-analysis of randomized controlled trials. Front. Neurol. 2020, 11, 421. [Google Scholar] [CrossRef]
- Goh, K.K.; Liu, Y.-W.; Kuo, P.-H.; Chung, Y.-C.E.; Lu, M.-L.; Chen, C.-H. Effect of probiotics on depressive symptoms: A meta-analysis of human studies. Psychiatry Res. 2019, 282, 112568. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Lew, L.-C.; Hor, Y.-Y.; Yusoff, N.A.A.; Choi, S.-B.; Yusoff, M.S.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.; Abdullah, M.F.I.; Zakaria, N. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Djafarian, K. Effect of prebiotic and probiotic supplementation on circulating pro-inflammatory cytokines and urinary cortisol levels in patients with major depressive disorder: A double-blind, placebo-controlled randomized clinical trial. J. Funct. Foods 2019, 52, 596–602. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Kawai, M.; Kikuchi-Hayakawa, H.; Suda, K.; Ishikawa, H.; Gondo, Y.; Shimizu, K.; Matsuki, T. Fermented milk containing Lactobacillus casei strain Shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl. Environ. Microbiol. 2016, 82, 3649–3658. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-L.; Wang, S.; Yen, J.-T.; Cheng, Y.-F.; Liao, C.-L.; Hsu, C.-C.; Wu, C.-C.; Tsai, Y.-C. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. 2019, 1711, 202–213. [Google Scholar] [CrossRef]
- Jang, H.-M.; Lee, K.-E.; Kim, D.-H. The preventive and curative effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on immobilization stress-induced anxiety/depression and colitis in mice. Nutrients 2019, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Wang, W.; Guo, R.; Liu, H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology 2019, 104, 132–142. [Google Scholar] [CrossRef] [PubMed]
- McVey Neufeld, K.-A.; Kay, S.; Bienenstock, J. Mouse strain affects behavioral and neuroendocrine stress responses following administration of probiotic Lactobacillus rhamnosus JB-1 or traditional antidepressant fluoxetine. Front. Neurosci. 2018, 12, 294. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Yong, S.J.; Tong, T.; Chew, J.; Lim, W.L. Antidepressive mechanisms of probiotics and their therapeutic potential. Front. Neurosci. 2020, 13, 1361. [Google Scholar] [CrossRef]
- Halloran, K.; Underwood, M.A. Probiotic mechanisms of action. Early Hum. Dev. 2019, 135, 58–65. [Google Scholar] [CrossRef]
- Hayes, C.L.; Peters, B.J.; Foster, J.A. Microbes and mental health: Can the microbiome help explain clinical heterogeneity in psychiatry? Front. Neuroendocrinol. 2020, 58, 100849. [Google Scholar] [CrossRef] [PubMed]
- Gerber, G.K. The dynamic microbiome. FEBS Lett. 2014, 588, 4131–4139. [Google Scholar] [CrossRef]
- McEwen, B.; Fenasse, R. Probiotics and depression: ‘The link between the microbiome-gut-brain axis and digestive and mental health’. J. Aust. Tradit. -Med. Soc. 2019, 25, 127. [Google Scholar]
- Zucko, J.; Starcevic, A.; Diminic, J.; Oros, D.; Mortazavian, A.M.; Putnik, P. Probiotic–friend or foe? Curr. Opin. Food Sci. 2020, 32, 45–49. [Google Scholar] [CrossRef]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics history. J. Clin. Gastroenterol. 2016, 50, S116–S119. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. From yaks to yogurt: The history, development, and current use of probiotics. Clin. Infect. Dis. 2015, 60, S85–S90. [Google Scholar] [CrossRef] [PubMed]
- AFRC, R.F. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Caicedo, R.; Neu, J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-α–induced interleukin-8 production in caco-2 cells. J. Nutr. 2005, 135, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Li, N.; Kataria, J.; Russell, M.; Neu, J. Live and ultraviolet-inactivated Lactobacillus rhamnosus GG decrease flagellin-induced interleukin-8 production in Caco-2 cells. J. Nutr. 2008, 138, 2264–2268. [Google Scholar] [CrossRef] [PubMed]
- Ostad, S.; Salarian, A.; Ghahramani, M.; Fazeli, M.; Samadi, N.; Jamalifar, H. Live and heat-inactivated lactobacilli from feces inhibit Salmonella typhi and Escherichia coli adherence to Caco-2 cells. Folia Microbiol. 2009, 54, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A. A review of dose-responses of probiotics in human studies. Benef. Microbes 2017, 8, 143–151. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Hong, G.; Pang, M.; Xu, H.; Li, H.; Tian, F.; Fang, R.; Yao, Y.; Liu, J. Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci. Lett. 2016, 618, 159–166. [Google Scholar] [CrossRef]
- Rogers, G.; Keating, D.; Young, R.; Wong, M.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef]
- Principi, N.; Cozzali, R.; Farinelli, E.; Brusaferro, A.; Esposito, S. Gut dysbiosis and irritable bowel syndrome: The potential role of probiotics. J. Infect. 2018, 76, 111–120. [Google Scholar] [CrossRef]
- de Oliveira, G.L.V.; Leite, A.Z.; Higuchi, B.S.; Gonzaga, M.I.; Mariano, V.S. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology 2017, 152, 1–12. [Google Scholar] [CrossRef]
- Oelschlaeger, T.A. Mechanisms of probiotic actions–A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Borsboom, D. A network theory of mental disorders. World Psychiatry 2017, 16, 5–13. [Google Scholar] [CrossRef]
- Lucas, G. Gut thinking: The gut microbiome and mental health beyond the head. Microb. Ecol. Health Dis. 2018, 29, 1548250. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.F.; Geschwind, D.H. Defining the genetic, genomic, cellular, and diagnostic architectures of psychiatric disorders. Cell 2019, 177, 162–183. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Logan, A.; Millstein, R.; Katszman, M. Biodiversity, the human microbiome and mental health: Moving toward a new clinical ecology for the 21st Century. Int. J. Biodivers. 2016, 2016, 1–18. [Google Scholar] [CrossRef]
- Uzbay, T. Germ-free animal experiments in the gut microbiota studies. Curr. Opin. Pharmacol. 2019, 49, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Liu, M.; Chen, J.; Pan, J.; Han, Y.; Liu, Y.; Cheng, K.; Zhou, C.; Wang, H. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 2019, 5, eaau8317. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Sublette, M.E.; Cheung, S.; Lieberman, E.; Hu, S.; Mann, J.J.; Uhlemann, A.C.; Miller, J.M. Bipolar disorder and the gut microbiome: A systematic review. Bipolar Disord. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Xie, X.-H.; Zhao, J.; Lai, W.-T.; Wang, M.-B.; Xu, D.; Liu, Y.-H.; Guo, Y.-Y.; Xu, S.-X.; Deng, W.-F. Similarly in depression, nuances of gut microbiota: Evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J. Psychiatr. Res. 2019, 113, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.I.; Mörkl, S.; Sandhu, K.V.; Cryan, J.F.; Dinan, T.G. The Gut Microbiome and Mental Health: What Should We Tell Our Patients?: Le microbiote Intestinal et la Santé Mentale: Que Devrions-Nous dire à nos Patients? Can. J. Psychiatry 2019, 64, 747–760. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kosciolek, T.; Maldonado, Y.; Daly, R.E.; Martin, A.S.; McDonald, D.; Knight, R.; Jeste, D.V. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr. Res. 2019, 204, 23–29. [Google Scholar] [CrossRef]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Lim, G.Y.; Tam, W.W.; Lu, Y.; Ho, C.S.; Zhang, M.W.; Ho, R.C. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z. Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611.e5. [Google Scholar] [CrossRef]
- Lin, P.; Ding, B.; Feng, C.; Yin, S.; Zhang, T.; Qi, X.; Lv, H.; Guo, X.; Dong, K.; Zhu, Y. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J. Affect. Disord. 2017, 207, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.P.; Hall, A.; Ayyaswamy, S.; Nelson, J.W.; Fultz, R.; Major, A.; Haag, A.; Esparza, M.; Lugo, M.; Venable, S. Diacylglycerol kinase synthesized by commensal Lactobacillus reuteri diminishes protein kinase C phosphorylation and histamine-mediated signaling in the mammalian intestinal epithelium. Mucosal. Immunol. 2018, 11, 380–393. [Google Scholar] [CrossRef]
- Marin, I.A.; Goertz, J.E.; Ren, T.; Rich, S.S.; Onengut-Gumuscu, S.; Farber, E.; Wu, M.; Overall, C.C.; Kipnis, J.; Gaultier, A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 2017, 7, 1–10. [Google Scholar]
- Gao, C.; Major, A.; Rendon, D.; Lugo, M.; Jackson, V.; Shi, Z.; Mori-Akiyama, Y.; Versalovic, J. Histamine H2 receptor-mediated suppression of intestinal inflammation by probiotic Lact. Reuteri. MBio 2015, 6. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Dhaliwal, J.; Singh, D.; Singh, S.; Pinnaka, A.K.; Boparai, R.; Bishnoi, M.; Kondepudi, K.; Chopra, K. Lactobacillus plantarum MTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation-induced behaviour, biochemical and selected gut microbial aberrations in mice. J. Appl. Microbiol. 2018, 125, 257–269. [Google Scholar] [CrossRef]
- Liu, W.-H.; Chuang, H.-L.; Huang, Y.-T.; Wu, C.-C.; Chou, G.-T.; Wang, S.; Tsai, Y.-C. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 2016, 298, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Yunes, R.; Poluektova, E.; Dyachkova, M.; Klimina, K.; Kovtun, A.; Averina, O.; Orlova, V.; Danilenko, V. GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 2016, 42, 197–204. [Google Scholar] [CrossRef]
- Yunes, R.; Poluektova, E.; Vasileva, E.; Odorskaya, M.; Marsova, M.; Kovalev, G.; Danilenko, V. A multi-strain potential probiotic formulation of GABA-producing Lactobacillus plantarum 90sk and bifidobacterium adolescentis 150 with antidepressant effects. Probiotics Antimicrob. Proteins 2020, 12, 973–979. [Google Scholar] [CrossRef]
- Ko, C.Y.; Lin, H.-T.V.; Tsai, G.J. Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process. Biochem. 2013, 48, 559–568. [Google Scholar] [CrossRef]
- Han, S.H.; Hong, K.B.; Suh, H.J. Biotransformation of monosodium glutamate to gamma-aminobutyric acid by isolated strain Lactobacillus brevis L-32 for potentiation of pentobarbital-induced sleep in mice. Food Biotechnol. 2017, 31, 80–93. [Google Scholar] [CrossRef]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Takada, M.; Nishida, K.; Kataoka Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Liu, W.-H.; Wu, C.-C.; Juan, Y.-C.; Wu, Y.-C.; Tsai, H.-P.; Wang, S.; Tsai, Y.-C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Janik, R.; Thomason, L.A.; Stanisz, A.M.; Forsythe, P.; Bienenstock, J.; Stanisz, G.J. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage 2016, 125, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Yamagata, H.; Seki, T.; Watanabe, Y. Epigenetic mechanisms of major depression: Targeting neuronal plasticity. Psychiatry Clin. Neurosci. 2018, 72, 212–227. [Google Scholar] [CrossRef]
- Schroeder, F.A.; Lin, C.L.; Crusio, W.E.; Akbarian, S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry 2007, 62, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.R.; Gonçalves, P.; Magro, F.; Martel, F. Microbiota-derived butyrate regulates intestinal inflammation: Focus on inflammatory bowel disease. Pharmacol. Res. 2020, 104947. [Google Scholar] [CrossRef]
- Komori, T. The significance of proinflammatory cytokines and Th1/Th2 balance in depression and action of antidepressants. Neuropsychiatry 2017, 7, 57–60. [Google Scholar] [CrossRef]
- Carlessi, A.S.; Borba, L.A.; Zugno, A.I.; Quevedo, J.; Réus, G.Z. Gut microbiota–brain axis in depression: The role of neuroinflammation. Eur. J. Neurosci. 2021, 53, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.W.; Kim, Y.-K. Inflammation-induced depression: Its pathophysiology and therapeutic implications. J. Neuroimmunol. 2017, 313, 92–98. [Google Scholar] [CrossRef]
- Calarge, C.A.; Devaraj, S.; Shulman, R.J. Gut permeability and depressive symptom severity in unmedicated adolescents. J. Affect. Disord. 2019, 246, 586–594. [Google Scholar] [CrossRef]
- Ohlsson, L.; Gustafsson, A.; Lavant, E.; Suneson, K.; Brundin, L.; Westrin, Å.; Ljunggren, L.; Lindqvist, D. Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatr. Scand. 2019, 139, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Evrensel, A.; Ünsalver, B.Ö.; Ceylan, M.E. Neuroinflammation, gut-brain axis and depression. Psychiatry Investig. 2020, 17, 2. [Google Scholar] [CrossRef]
- Hunt, B.C.; e Cordeiro, T.M.; Robert, S.; de Dios, C.; Leal, V.A.C.; Soares, J.C.; Robert, D.; Antonio, T.; Sudhakar, S.M. Effect of mmune Activation on the Kynurenine Pathway and Depression Symptoms–A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 118, 514–523. [Google Scholar] [CrossRef]
- Sublette, M.E.; Galfalvy, H.C.; Fuchs, D.; Lapidus, M.; Grunebaum, M.F.; Oquendo, M.A.; Mann, J.J.; Postolache, T.T. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain. Behav. Immun. 2011, 25, 1272–1278. [Google Scholar] [CrossRef]
- Umehara, H.; Numata, S.; Watanabe, S.-Y.; Hatakeyama, Y.; Kinoshita, M.; Tomioka, Y.; Nakahara, K.; Nikawa, T.; Ohmori, T. Altered KYN/TRP, Gln/Glu, and Met/methionine sulfoxide ratios in the blood plasma of medication-free patients with major depressive disorder. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Bradley, K.A.; Case, J.A.; Khan, O.; Ricart, T.; Hanna, A.; Alonso, C.M.; Gabbay, V. The role of the kynurenine pathway in suicidality in adolescent major depressive disorder. Psychiatry Res. 2015, 227, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, W.; Liu, W.; Wang, C.; Zhan, Y.; Li, H.; Chen, L.; Ning, Y. Cross-sectional relationship between kynurenine pathway metabolites and cognitive function in major depressive disorder. Psychoneuroendocrinology 2019, 101, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Jansen, K.; Titus, S.; Carvalho, A.F.; Gabbay, V.; Quevedo, J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. J. Psychiatr. Res. 2015, 68, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. Mitochondria could be a potential key mediator linking the intestinal microbiota to depression. J. Cell. Biochem. 2020, 121, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, G. Gut–brain axis and mood disorder. Front. Psychiatry 2018, 9, 223. [Google Scholar] [CrossRef]
- Yu, L.; Han, X.; Cen, S.; Duan, H.; Feng, S.; Xue, Y.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q. Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol. Res. 2020, 233, 126409. [Google Scholar] [CrossRef]
- Huang, F.; Wu, X. Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression. Front. Cell Dev. Biol. 2021, 9, 472. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.h.; Mittal, J.; Yan, D.; Eshraghi, A.A. Neurotransmitters: The critical modulators regulating gut–brain axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef]
- Pan, J.-X.; Deng, F.-L.; Zeng, B.-H.; Zheng, P.; Liang, W.-W.; Yin, B.-M.; Wu, J.; Dong, M.-X.; Luo, Y.-Y.; Wang, H.-Y. Absence of gut microbiota during early life affects anxiolytic Behaviors and monoamine neurotransmitters system in the hippocampal of mice. J. Neurol. Sci. 2019, 400, 160–168. [Google Scholar] [CrossRef]
- Yang, W.S.; Shi, Z.G.; Dong, X.Z.; Liu, P.; Chen, M.l.; Hu, Y. Involvement of 5-HT-BDNF signaling axis in mediating synergistic antidepressant-like effects after combined administration of two oligosaccharide esters. Food Sci. Nutr. 2021, 9, 1180–1191. [Google Scholar] [CrossRef]
- Udina, M.; Navinés, R.; Egmond, E.; Oriolo, G.; Langohr, K.; Gimenez, D.; Valdés, M.; Gómez-Gil, E.; Grande, I.; Gratacós, M. Glucocorticoid receptors, brain-derived neurotrophic factor, serotonin and dopamine neurotransmission are associated with interferon-induced depression. Int. J. Neuropsychopharmacol. 2016, 19, pyv135. [Google Scholar] [CrossRef]
- Malick, M.; Gilbert, K.; Daniel, J.; Arseneault Breard, J.; Tompkins, T.; Godbout, R.; Rousseau, G. Vagotomy prevents the effect of probiotics on caspase activity in a model of postmyocardial infarction depression. Neurogastroenterol. Motil. 2015, 27, 663–671. [Google Scholar] [CrossRef]
- van der Kleij, H.; O’Mahony, C.; Shanahan, F.; O’Mahony, L.; Bienenstock, J. Protective effects of Lactobacillus reuteri and Bifidobacterium infantis in murine models for colitis do not involve the vagus nerve. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2008, 295, R1131–R1137. [Google Scholar] [CrossRef]
- Belujon, P.; Grace, A.A. Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Finberg, J.P.; Rabey, J.M. Inhibitors of MAO-A and MAO-B in psychiatry and neurology. Front. Pharmacol. 2016, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Dinan, T.G.; Clarke, G.; Cryan, J.F. A psychology of the human brain–gut–microbiome axis. Soc. Personal. Psychol. Compass 2017, 11, e12309. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, X.-R.; Peng, L.; Ge, J.-F. Crosstalk between the microbiota-gut-brain axis and depression. Heliyon 2020, 6, e04097. [Google Scholar] [CrossRef] [PubMed]
- Iob, E.; Kirschbaum, C.; Steptoe, A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: The role of cognitive-affective and somatic symptoms. Mol. Psychiatry 2020, 25, 1130–1140. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing depression from the microbiota–gut–brain axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef] [PubMed]
- Nandam, L.S.; Brazel, M.; Zhou, M.; Jhaveri, D.J. Cortisol and major depressive disorder—Translating findings from humans to animal models and back. Front. Psychiatry 2020, 10, 974. [Google Scholar] [CrossRef]
- Bao, A.-M.; Swaab, D.F. The human hypothalamus in mood disorders: The HPA axis in the center. IBRO Rep. 2019, 6, 45–53. [Google Scholar] [CrossRef]
- Grunewald, M.; Johnson, S.; Lu, D.; Wang, Z.; Lomberk, G.; Albert, P.R.; Stockmeier, C.A.; Meyer, J.H.; Urrutia, R.; Miczek, K.A. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J. Biol. Chem. 2012, 287, 24195–24206. [Google Scholar] [CrossRef]
- Bhat, M.I.; Kapila, R. Dietary metabolites derived from gut microbiota: Critical modulators of epigenetic changes in mammals. Nutr. Rev. 2017, 75, 374–389. [Google Scholar] [CrossRef]
- Alam, R.; Abdolmaleky, H.M.; Zhou, J.R. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am. J. Med Genet. Part. B Neuropsychiatr. Genet. 2017, 174, 651–660. [Google Scholar] [CrossRef]
- Miro-Blanch, J.; Yanes, O. Epigenetic regulation at the interplay between gut microbiota and host metabolism. Front. Genet. 2019, 10, 638. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, M.; D’Urso, F.; Piccininni, C.; Montagna, M.L.; Sardone, R.; Resta, E.; Dibello, V.; Daniele, A.; Giannelli, G.; Bellomo, A. The relationship between epigenetics and microbiota in neuropsychiatric diseases. Epigenomics 2020, 12, 1559–1568. [Google Scholar] [CrossRef]
- Covington, H.E., III; Vialou, V.F.; LaPlant, Q.; Ohnishi, Y.N.; Nestler, E.J. Hippocampal-dependent antidepressant-like activity of histone deacetylase inhibition. Neurosci. Lett. 2011, 493, 122–126. [Google Scholar] [CrossRef]
- You, M.-J.; Park, M.-J.; Yeo, H.-L.; Kwon, M.-S. Possible additional antidepressant-like mecha-nism of sodium butyrate: Targeting the hippocampus. Int. J. Neuropsychopharmacol. 2014, 81, 292–302. [Google Scholar]
- Liu, D.; Qiu, H.-M.; Fei, H.-Z.; Hu, X.-Y.; Xia, H.-J.; Wang, L.-J.; Qin, L.-J.; Jiang, X.-H.; Zhou, Q.-X. Histone acetylation and expression of mono-aminergic transmitters synthetases involved in CUS-induced depressive rats. Exp. Biol. Med. 2014, 239, 330–336. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Zhang, H.; Gatta, E.; Glover, E.J.; Pandey, S.C.; Lasek, A.W. The histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) alleviates depression-like behavior and normalizes epigenetic changes in the hippocampus during ethanol withdrawal. Alcohol 2019, 78, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Meylan, E.M.; Halfon, O.; Magistretti, P.J.; Cardinaux, J.-R. The HDAC inhibitor SAHA improves depressive-like behavior of CRTC1-deficient mice: Possible relevance for treatment-resistant depression. Neuropharmacology 2016, 107, 111–121. [Google Scholar] [CrossRef]
- Covington, H.E., III; Maze, I.; Vialou, V.; Nestler, E.J. Antidepressant action of HDAC inhibition in the prefrontal cortex. Neuroscience 2015, 298, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Fuchikami, M.; Yamamoto, S.; Morinobu, S.; Okada, S.; Yamawaki, Y.; Yamawaki, S. The potential use of histone deacetylase inhibitors in the treatment of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, F.; Hu, X.; Yang, C.; Xu, H.; Yao, Y.; Liu, J. Clostridium butyricum attenuates chronic unpredictable mild stress-induced depressive-like behavior in mice via the gut-brain axis. J. Agric. Food Chem. 2018, 66, 8415–8421. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y. Faecalibacterium prausnitzii produces butyrate to maintain Th17/Treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef]
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model-are we there yet? Behav. Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef]
- Gautam, S.; Jain, A.; Gautam, M.; Vahia, V.N.; Grover, S. Clinical practice guidelines for the management of depression. Indian J. Psychiatry 2017, 59, S34. [Google Scholar]
- Cosci, F.; Chouinard, G. The Monoamine Hypothesis of Depression Revisited: Could It Mechanistically Novel Antidepressant Strategies? In Neurobiology of Depression; Elsevier: Amsterdam, The Netherlands, 2019; pp. 63–73. [Google Scholar]
- Bschor, T.; Kilarski, L.L. Are antidepressants effective? A debate on their efficacy for the treatment of major depression in adults. Expert Rev. Neurother. 2016, 16, 367–374. [Google Scholar] [CrossRef]
- Jha, M.K.; Rush, A.J.; Trivedi, M.H. When discontinuing SSRI antidepressants is a challenge: Management tips. Am. J. Psychiatry 2018, 175, 1176–1184. [Google Scholar] [CrossRef]
- Davies, J.; Read, J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: Are guidelines evidence-based? Addict. Behav. 2019, 97, 111–121. [Google Scholar] [CrossRef]
- Read, J.; Williams, J. Adverse effects of antidepressants reported by a large international cohort: Emotional blunting, suicidality, and withdrawal effects. Curr. Drug Saf. 2018, 13, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Xu, S.; Hebl, M. Utilizing education and perspective taking to remediate the stigma of taking antidepressants. Community Ment. Health J. 2018, 54, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis Lucena, L.; Leopoldino de Bortolli, C.; Tufik, S.; Hachul, H. Effects of supplementation with Lactobacillus probiotics on insomnia treatment. Altern. Ther. Health Med. 2021. online ahead of print. [Google Scholar]
- Wang, H.; Lee, I.-S.; Braun, C.; Enck, P. Effect of probiotics on central nervous system functions in animals and humans: A systematic review. J. Neurogastroenterol. Motil. 2016, 22, 589. [Google Scholar] [CrossRef]
- Wallace, C.J.; Milev, R.V. The Efficacy, Safety, and Tolerability of Probiotics on Depression: Clinical Results From an Open-Label Pilot Study. Front. Psychiatry 2021, 12, 132. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D. Psychobiotics: An emerging alternative to ensure mental health amid the COVID-19 outbreak? Trends Food Sci. Technol. 2020, 103, 386–387. [Google Scholar] [CrossRef]
- Cabana, M.D.; Salminen, S.; Sanders, M.E. Probiotic Safety—Reasonable Certainty of No Harm. JAMA Intern. Med. 2019, 179, 276. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Notivol, J.; Gracia-García, P.; Olaya, B.; Lasheras, I.; López-Antón, R.; Santabárbara, J. Prevalence of depression during the COVID-19 outbreak: A meta-analysis of community-based studies. Int. J. Clin. Health Psychol. 2021, 21, 100196. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).