Abstract
Chronic kidney disease (CKD) is cumulative worldwide and an increasing public health issue. Aside from the widely known protein restriction and medical therapy, less evident is the renal protection of nutrition supplements in CKD patients. This systematic review (SR), using a Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, aims to summarize and quantify evidence about the prevention effects of vitamin D and analogues, omega-3 polyunsaturated fatty acid (omega-3 PUFA), dietary fiber, coenzyme Q10 (CoQ10), and biotics on CKD progression. This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement to examine SRs and/or meta-analysis of clinical controlled trials identified from PubMed, Embase, and the Cochrane Library. Finally, seventeen SRs were included in the qualitative analysis. The beneficial effects of these nutrition supplements in CKD patients mostly seem to be at low to very low evidence on proteinuria, kidney function, and inflammations and did not appear to improve CKD prognosis. The recommendation of nutrition supplements in CKD patients needs to discuss with physicians and consider the benefits over the adverse effects. Longer follow-up of larger randomized trials is necessary to clarify the benefits of nutrition supplements in CKD patients.
1. Introduction
Chronic kidney disease (CKD), a gradual loss of kidney function, is an increasing public health issue. Over the past decades, CKD incidence is cumulative worldwide [1,2,3], paralleling epidemics in diabetes [4], hypertension [5], and metabolic syndrome [6]. Prevalence is estimated to be 8–16% worldwide [7]. Complications of CKD are associated with the risk of all-cause mortality, cardiovascular events, hospitalization, cognitive decline, and fracture [8,9,10,11]. Recently, some prognostic biomarkers are developed and helped to improve risk stratification and anticipate diagnosis of cardiovascular diseases [12]. However, CKD prognosis that is ameliorated by patient awareness and management strategies could be considered as an essential issue.
Dietary management to stop CKD progress has some benefits in patients with heavy proteinuria from some large trials [13,14], which were consistent with some reports from smaller-population trials [15,16,17]. The benefits of dietary protein restriction need to subsequently evaluate the problems of malnutrition and protein wasting syndrome [13,14]. Awareness of the disorder and strategies to reduce medical costs related to CKD need to be included in public policy and receiving increasing attention.
Aside from the widely known protein restriction and medical therapy, nutrition supplements have been reported to have a role in CKD prevention [18,19,20,21,22,23,24,25]. For example, a meta-analysis providing data for 688 patients has displayed that active vitamin D analogs reduced proteinuria (−16% (95% confidence interval (CI), −13% to −18%)) compared with controls (+6% (95% CI, 0% to +12%); p < 0.001) [18]. Additionally, the meta-analysis in diabetes patients indicated that vitamin D analogs provide beneficial effects on proteinuria and inflammation indexes [high-sensitivity C-reactive protein (hs-CRP), interleukin 6 (IL-6) or tumor necrosis factor-alpha (TNF-α)], but not on serum creatinine and estimated glomerular filtration rate (eGFR) [21]. However, other studies on meta-analysis have the contrary results of proteinuria protection [26,27]. Thus, the effects of vitamin D analogs on kidney disease merit to investigate and clarify in CKD patients.
In addition to vitamin D analogs, lower dietary acid loads (e.g., more fruits and vegetables and fewer meats and cheeses) had been reported to prevent CKD progress in clinical studies [19,20,24]. Other nutrition supplements [e.g., Omega-3 polyunsaturated fatty (Omega-3 PUFA), and biotics] had been reported to help maybe improve kidney function [22,23,25]. These nutritional supplements may have been used in a clinical application until now.
The SRs and/or meta-analysis are the level I study designs to answer the intervention question in clinical practice. Nevertheless, the previous SRs on the relationship between nutrition supplements and CKD were inconsistent and the evidence certainty was lacking. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach offers a transparent and structured process for developing and presenting summaries of evidence, including its effect size and certainty, and recommendations in health care. The objective of this systematic review was to determine the effects of current and relevant SRs of nutrition supplements on renal protection in CKD patients through using the GRADE approach.
2. Materials and Methods
2.1. Study Methods
This systematic reviews (SRs) was conducted and reported following the Preferred Reporting Items for SRs and Meta-Analyses (PRISMA) statement [28] to examine the renal protection effect of nutrition supplements in CKD (Table S1).
2.2. Search Strategy
We searched PubMed, Embase, and Cochrane CENTRAL databases for relevant SRs from the inception through October 2020. The strategy and keywords used for the systematic search were (“herbal supplements” OR “herbal supplement” OR “dietary supplements” OR “dietary supplement” OR “nutritional supplements” OR “nutritional supplement” OR supplementation* OR “nutrition supplements” OR “nutrition supplement” OR “health foods” OR “health food”) AND (renal or kidney). We applied a high-sensitivity and high-specificity customized filter [29], a sensitivity of 96.0% and a specificity of 99.96%, for efficiently retrieving SRs. We also performed hand searches from the relevant studies of the included SRs to identify additional studies.
2.3. Selection Criteria
Inclusion criteria included: (i) study design: SRs and/or meta-analysis of clinically controlled trials (whether randomization was applied will be judged in quality assessment) designed to evaluate the influence of nutrition supplementation on renal protection in CKD patients compared to placebo, regardless of language or publish date, (ii) population: any stages of CKD patients should not need dialysis or renal transplantation at baseline, (iii) intervention: nutrition or health supplements, (iv) comparison: placebo or other treatment, (v) renal protection outcomes: a study reported at least one clinical important issue or surrogate outcome data or outcome data can be extracted from subgroup analysis was available. The critical clinical outcomes included kidney function change from baseline and the risk of progression to end-stage renal disease (ESRD); the surrogate outcomes included inflammatory factors, for instances, hs-CRP, CRP, indoxyl sulphate (IS), or p-cresyl sulphate (PCS), and oxidative stress marker, malondialdehyde (MDA). Exclusion criteria: (i) nutrition supplement that has obtained FDA approval of drug license for CKD patients, (ii) Chinese herbal medicine, (iii) studies were presented as conference abstracts, case reports, letters to editors, or in vitro studies.
2.4. Data Extraction
A piloted form of data collection was created in excel, and two authors (Lin, Chou) extracted data from the included SRs independently. Collected variables included the first author, publish year, search databases, search duration, included study design and numbers, critical appraisal tool, population, intervention (dose and frequency if available), control group, outcomes related to kidney function, and nutrition supplement duration. Data were collected from publishing papers or online supplements, from study groups or subgroups. If the author provided the original data of included controlled trials and only used the text to describe the significance of effect size rather than presented the statistical data, we further calculated the effect size by author-reported statistical methods. Similarly, if the author only describes overall appraisal results rather than the present individual outcomes-related risk of bias, we further appraise the quality of the subgroup based on the data provided in the SR. Any discrepancies on data extraction or quality assessment were discussed and reached an agreement by consulting with the third author (Chen).
2.5. Grading of Recommendations Assessment, Development and Evaluation (GRADE) Grading the Evidence
We rate the certainty of included SRs using the GRADE approach as adopted for the “summary of findings” table. GRADE specified the quality of evidence to four categories—high, moderate, low, and very low. If the context of an SR comes from randomized-controlled trials, the evidence category begins as high-quality. There are five reasons to possibly rate down 1–2 grade of evidence, including the risk of bias (most information comes from studies at moderate or high risk of bias), imprecision (the sample size or “optimal information size” [OIS] criterion is not met or the 95% CI overlap no effect), inconsistency (substantial heterogeneity between studies and unexplained, I2 more than 50% was set as substantial heterogeneity) [30], indirectness (depend on the extent of differences between our interests and the SR on patient populations, interventions, measurements of the outcome, and the methods of the trials of the candidate interventions) and publication bias (asymmetric funnel plots presented, Deeks’ test or the trim and fill method of non-significance, or included studies come from several small studies and most of which were commercially funded) [31,32,33,34,35,36,37].
2.6. Data Synthesis and Statistics
Since the included SRs, aim to evaluate the renal protection effect on the same nutrition supplement, may consist of duplicated RCTs and thus become independent, we abandoned to summarize the data by a statistical method and present the result as an SR. If standardized mean difference (SMD) was used in an SR to show the pooled outcome measure; we considered a SMD of 0.2, 0.5, and 0.8 a small, moderate, and large effect, respectively [38]. We used GRADEpro GDP software (GRADEpro GDT: GRADEpro Guideline Development Tool (Software). McMaster University, 2020 (developed by Evidence Prime, Inc.: Hamilton, ON, Canada). Available from gradepro.org.) to synthesis and present the certainty and summary of findings for the included SRs.
3. Results
3.1. Baseline Information of Included SRs
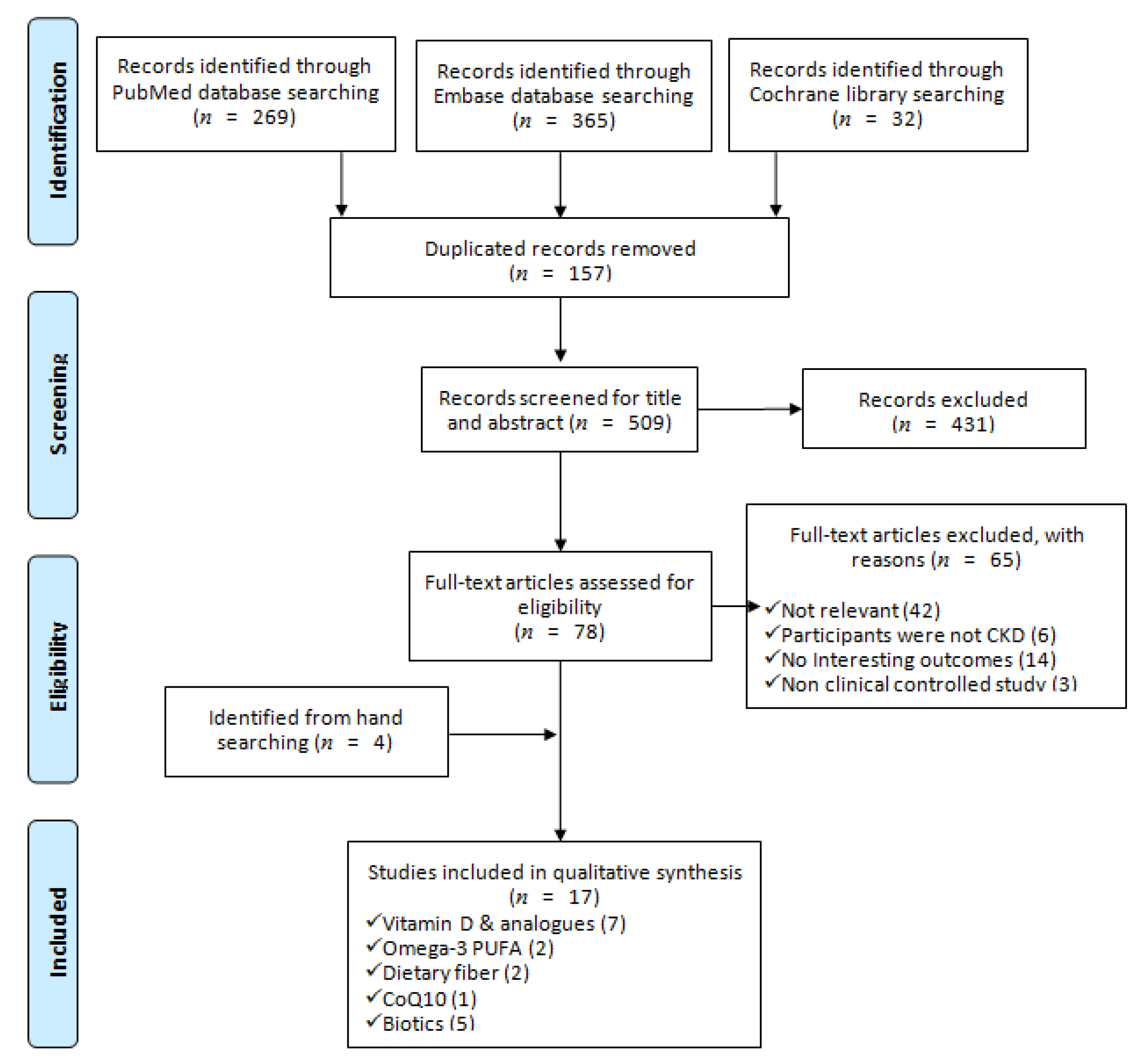
We identified 666 articles from the electronic databases searching, excluding duplicates, irrelevant, and not fulfilled the inclusion criteria and left 13 SRs. Furthermore, we retrieved 4 SRs by hand searching from the reference lists of relevant studies, eventually included 17 SRs in this SR. Figure 1 outlined the process of systematic search and adding a PRISMA flow diagram. PRISMA checklist was provided as supplementary material (Table S1).
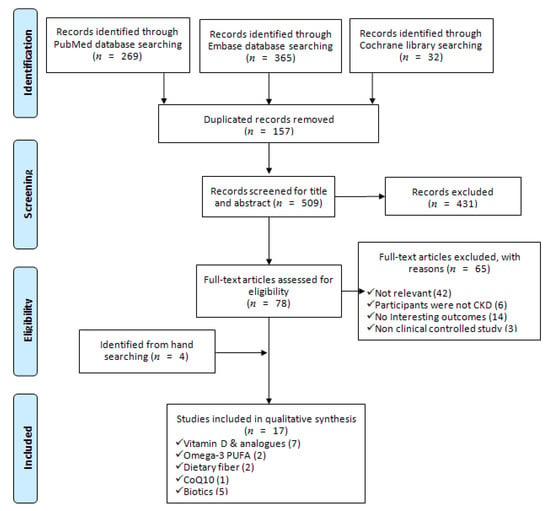
Figure 1.
PRISMA flow diagram of including systematic reviews in this study [28].
Among the included SRs, 7, 2, 2, 1 and 5 studies aim to evaluate the renal protective effects of vitamin D and analogs [21,26,27,39,40,41,42], Omega-3 PUFA [22,43], dietary fiber [24,44], coenzyme Q10 (CoQ10) [45], and biotics [23,25,46,47,48], respectively. All of the included SRs performed the search on at least three databases, and the majority of searching, except for Zhang et al. study [48], had no restriction on publication date. Five SRs evaluated the effects of nutrition supplements in diabetic nephropathy patients only, and the others in any stages and any etiologies of CKDs. The most used appraisal tools for included RCRs was a Cochrane risk of bias (RoB), 10 SRs were using this checklist. Additionally, there were 2, 1 and 1 studies using the Jadad Scale, Newcastle-Ottawa Scale, and Heyland Methodological Quality Score to appraise the quality of included RCTs, respectively. Moreover, one study assessed the quality by RoB and GRADE simultaneously. In the SRs of vitamin D and analogues, 4 assessed the effects of established vitamin D compounds (vitamin D2, eregocalcifefol, ercalcidiol, ercalcitriol, vitamin D3, cholecalciferol, carcidiol, calcitriol) or the newer analogues (paricalcitol and doxercalcigerol) [26,40,41,42], the others only focused on established compounds. In the biotics SRs, 2 focused on the effect of probiotics [46,47], 1 on probiotics or prebiotics [25], and 2 on probiotics, prebiotics or synbiotics [23,48]. The included clinically controlled trials in all SRs received nutrition supplements at least 1 week. All detailed information of the included SRs was presented in Table 1.

Table 1.
Baseline information of included systematic reviews.
3.2. Grading of Recommendations Assessment, Development and Evaluation (GRADE) Qualifying the Evidence
Only four clinical important outcomes (urinary albumin excretion rate (UAER), reduced proteinuria, the occurrence of ESRD, serum urea) and one surrogate outcome (CRP) reported in the five individual SRs [27,40,43,47,48] reached the moderate level of certainty, the other evidence rated low to very low (Table 2 and Table 3). The most being downgraded domain was the risk of bias in included RCTs. In this SR, several outcome data were extracted from subgroup analysis in the original SR. Although the overall quality of the primary outcome may be presented as low or unclear risk of bias, the other outcome-specific risks of bias were not assessed and reported. Thus, downgrading one point was performed. Furthermore, nine clinical relevant outcome evidence presented a high risk of bias in more than half of RCTs; we downgraded two points to very severe in the domain of risk of bias. In the domain of indirectness, we downgraded all surrogate outcomes one point to severe; on the contrary, the clinically relevant outcomes were not treated the same.

Table 2.
Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach evidence certainty and summary of findings of the clinical important outcomes.

Table 3.
GRADE approach evidence certainty and summary of findings of the surrogate outcomes.
3.3. Vitamin D and Analogues on Renal Protection
Five SRs focused on diabetic nephropathy reported 13 clinical relevant outcome data. In very low to low certainty evidence of vitamin D and analogues (established vitamin D compounds) appeared to reduce urinary albumin excretion rate (UAER) (2 SRs, included two and eight RCTs, respectively, mean difference −0.39, 95% CI −0.71 to −0.07 and −67.36, 95% CI −91.96 to −42.76). The vitamin D and analogue effect on urine albumin creatinine ratio (UACR) varied in the included SRs. Very low to moderate certainty evidence showed no significant impact on SRs only enrolled supplement with established vitamin D compounds. SRs included RCTs with receiving newer vitamin D analogue (paricalcitol or doxercalciferol) rather than established compounds alone suggested a significant decrease in UACR (two SRs, included eight and four RCTs, respectively, mean difference −0.49, 95% CI −0.9 to −0.08 and SMD −0.29, 95% CI −0.48 to −0.1). However, the effect seems small, and the evidence was very low. In very low to low certainty of evidence suggested vitamin D and analogues reduce proteinuria 0.23–0.26 gm per 24 h (2 SRs, included 11 and 14 RCTs, respectively, mean difference −0.26, 95% CI −0.34 to −0.17 and −0.23, 95% CI −0.3 to −0.15). The effect on serum creatinine varied and the certainty of the evidence was very low. Only one SR reported vitamin D and analogues does not affect urine protein creatinine ratio (UPCR) and eGFR, respectively (Table 2 and Table 4).

Table 4.
Summary of clinical important outcomes and certainties on the renal protection effect of vitamin D and analogues compared to placebo.
Two SRs focused on diabetic nephropathy reported four surrogate outcome data. Very low certainty of evidence suggested Vitamin D and analogues decreased the inflammatory markers of hs-CRP, IL-6, and TNF-α (hs-CRP: MD −0.80 to −0.69; IL-6: −0.73; TNF-α: −56.79), as shown in Table 3.
3.4. Omega-3 Polyunsaturated Fatty Acid (PUFA) on Renal Protection
Table 5 showed the effects of omega-3 PUFA on eGFR, progression to ESRD, serum creatinine (SCr), proteinuria and creatinine clearance (CCr) from 2 SRs. In very low to moderate evidence appeared to reduce progression to ESRD significantly and consistently (relative risk, 0.3, 95% CI 0.09 to 0.98 and 0.49, 95% CI 0.24 to 0.99). The effect on reducing proteinuria varied, and the certainty of evidence was low (Table 5).

Table 5.
Summary of clinical important outcomes and certainties on the renal protection effect of Omega-3 polyunsaturated fatty acid compared to placebo.
3.5. Dietary Fiber on Renal Protection
One SR reported two clinical relevant outcome data. Very low certainty evidence suggested that dietary fiber has no effect on Scr or serum urea (Table 2). One SR reported two surrogate outcome data comparing dietary fiber to placebo. Low certainty evidence suggested dietary fiber decreased uremic toxin PCS significantly (1 SR, 7 RCTs, MD –16.16, 95% CI −23.824 to −8.492), but not indoxyl sulphate (IS) (Table 3).
3.6. Coenzyme Q10 (CoQ10) on Renal Protection
3.7. Probiotics, Prebiotics and Synbiotics on Renal Protection
Table 6 shows the effect of biotics on serum urea, BUN, and SCr. Three SRs suggested biotics effects on serum urea varied; however, significant decrease serum urea compared to placebo (low certainty: MD −2.12 mmol/L, 95% CI −3.86 to −0.37; moderate certainty: MD −5.0 mmol/L, 95% CI −9.45 to −0.54) with low to moderate certainty evidence. Very low certainty of evidence suggested biotics did not affect BUN and SCr.

Table 6.
Summary of clinical important outcomes and certainties on the renal protection effect of biotics compared to placebo.
Low to moderate certainty evidence suggested biotics decreased MDA, CRP and PCS significantly (MDA: 1 SR, 4 RCTs, SMD –0.79 SD, 95% CI −1.38 to −0.20; CRP: 1 SR, 3 RCTs, SMD –0.71 SD, 95% CI −1.01 to −0.40; PCS: 1 SR, 2 RCTs, MD −0.70, 95% CI −1.4 to −0.01), but no effect on IL-6 (Table 3).
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
4. Discussion
SRs through the GRADE approach recommended by the Cochrane Collaboration provides rating and strengthening the quality of evidence and has been increasingly adopted by researchers worldwide. The GRADE approach divides the quality of SRs into four evidence levels. For example, the high evidence is reliable without side effects; the moderate or low evidence is needed to consult physicians; and, the very low evidence is not recommended. In this SR and meta-analysis, we adopted the GRADE approach to evaluate the beneficial effects of nutrition supplements on CKD prevention. Our findings provided that evidence of the beneficial effects of those nutrition supplements in CKD patients mostly seems to be low to very low evidence on proteinuria, kidney function, and inflammations. Thus, it is recommended in CKD patients to consult physicians for the prescription of nutrition supplements.
4.1. Findings and Implications of this Systematic Review
Vitamin D, including vitamin D2 and vitamin D3, is available for over-the-counter purchase and responsible for increasing intestinal absorption of calcium and phosphate and multiple biological effects [49]. Vitamin D3 is the type that most experts recommend to be utilized in clinical practice because of the more stable and potent form [50]. A review of observational studies has shown associations between vitamin D deficiency and risk of CKD, cardiovascular diseases, cancer, diabetes, infectious diseases, and death [49,51]. This study pooled the results of seven SRs that addressed the effects of vitamin D and analogues administered to patients with proteinuria and renal dysfunction. Data of two SRs show that vitamin D and analogues supplementation in patients with diabetes could reduce proteinuria but not affect kidney function [21,52]; however, other SR data show the contrary results of proteinuria protection [26,27]. Furthermore, most results from SRs suggested no effects on kidney function after receiving vitamin D and analogues in diabetes patients [21,52] and CKD patients [40]. Moreover, there is very low evidence that vitamin D and analogues supplements maybe decreased the inflammatory markers of hs-CRP, IL-6, and TNF-α. Through GRADE approach, our results showed that the beneficial effects of receiving vitamin D and analogues seem smaller in diabetes patients, and the certainty of the evidence was low to very low on proteinuria and very low on kidney function and inflammations. In CKD patients, the beneficial effects of vitamin D and analogues supplements were moderate evidence of proteinuria and low evidence of kidney function. Thus, consistent evidence showed vitamin D and analogues decreased the risk of proteinuria and/or UAER in diabetic nephropathy and CKD, but the strength of the evidence is mostly limited by the quality of the individual studies. It is recommended to discuss with a physician before deciding to receive vitamin D and analogues.
Omega-3 PUFA, a class of particular fatty acids with many biological functions, has been reported to or used together with diet and exercise to help lower triglyceride levels in the blood [53]. Reviews of clinical and epidemiological studies have shown the beneficial effects of omega-3 PUFAs supplements on a series of illnesses such as coronary artery disease and heart failure [54], stroke [55], metabolic syndrome [56], and neurodegenerative diseases (Parkinson’s and Alzheimer’s diseases) [57]. Apart from these disorders, there have been found lower levels of serum Omega-3 PUFA in patients with advanced CKD compared with the general population probably due to malabsorption, metabolic changes, and Omega-3 PUFA loss during the dialysis process [58,59,60]. Additionally, Omega-3 PUFA deficiency is independently related to cardiovascular disease in advanced CKD patients [61,62]. In CKD patients without receiving dialysis, Omega-3 PUFA intake may lower the risk of developing end-stage kidney disease [22,43] and cardiovascular deaths [22]. The possible causes have been reported from the pleiotropic effects of Omega-3 PUFA, including reducing blood pressure levels, lessening inflammation, and improving endothelial function, and altering platelet function and blood viscosity [63,64,65,66]. Via using GRADE approach, our data showed that the moderate to very low evidence appeared to reduce progression to ESRD (relative risk, 0.3, 95% CI 0.09 to 0.98 and 0.49, 95% CI 0.24 to 0.99). Moreover, the effect on reducing proteinuria varied, and the certainty of the evidence was low. Due to the inconsistency of variable evidence in different studies on the eGFR, SCr, proteinuria, or CCr, the recommendation of Omega-3 PUFA supplements needs to consider the benefits over the adverse effects in CKD patients after discussing with physicians.
Higher dietary fiber and lower dietary acid loads (e.g., more fruits and vegetables and fewer meats and cheeses) have been reported to reduce the risk of CKD progression [19,20,24]. A meta-analysis of 14 trials involving 143 CKD patients showed the association of higher dietary fiber intake and lower serum urea and SCr levels (MD −1.76 mmol/l, 95% CI −3.00 to −0.51 and MD −22.83 mmol/l, 95% CI −42.63 to −3.02, respectively) [24]; however, the certainty of evidence is at the very low level because of risk of bias and imprecision form analysis of the GRADE approach. Another meta-analysis of 203 CKD patients, showed that dietary fiber decreased uremic toxin PCS levels (MD –16.16, 95% CI −23.824 to −8.492) [44], which also was low certainty of evidence due to small sample size and risks from some data bias and indirectness. In this study, the data showed very low certainty evidence that dietary fiber has no effect on Scr or serum urea. Furthermore, low certainty evidence suggested dietary fiber decreased uremic toxin PCS levels significantly, but did not affect IS levels. Thus, limited evidence showed that dietary fiber intake might reduce the uremic toxins such as serum urea, SCr, and PCS levels, but there is not found any effect on clinically important outcomes. The evidence is insufficient to recommend dietary fiber for kidney protection in CKD patients.
CoQ10, first identified in 1940, is mostly found in meat, fish, and whole grains [67]. CoQ10 generate adenosine triphosphate (ATP) energy to cell [67] and is most commonly used for an antioxidant that helps improve cardiovascular diseases [68,69], heart failure [70], diabetes [71], hypercholesterolemia [72], migraine headache [73], and many other conditions related to lower CoQ10 levels. Furthermore, CoQ10 has been reported to play an essential role in blood metabolic profiles in diabetic kidney disease [45], which indicates lower blood sugar, blood lipid, and MDA levels, but no effect on serum urea and SCr. By using the GRADE approach, this study further showed the very low certainty of evidence on blood metabolic profiles and no impact on kidney function. Thus, CoQ10 intake is insufficiently evident to recommend in CKD patients.
Microbiota dysbiosis is closely associated with many diseases related to chronic inflammations, including obesity, diabetes, cardiovascular diseases, non-alcoholic fatty liver disease, and obesity-induced CKD [74,75,76]. So far, the prescribed prebiotics, probiotics, and synbiotics may show an impact on the amelioration of these diseases [77,78]. However, the safety issue on these biotics supplements is most important, due to the trend of the broad use of these biotics under different clinical circumstances. There have been reported in a few situations of bacteremia, sepsis, fungal infection, or endocarditis following biotics intake, especially for some immunocompromised patients, malnutrition, or suffering from cancer [79,80,81]. In this study, we investigated the effect of biotics on serum urea, BUN, and SCr, and some metabolic profiles such as MDA, CRP, and PCS in CKD patients. Four SRs showed biotics effects on serum urea were inconsistent results and at very low to moderate certainty of evidence due to the risk of data bias and imprecision [23,25,46,47]. Additionally, the biotics intake appeared to decrease MDA, CRP, and PCS levels significantly [46,48]. However, biotics intake did not affect SCr levels in CKD patients [23,46], which was at the very low certainty of the evidence. Thus, biotics supplements seemed only to reduce serum urea and some metabolic profiles in CKD patients. We suggest CKD patients discuss with a physician before considering biotics supplements.
4.2. Strengths, Limitations and Further Research Needs
In summary, this study was the first and composed of seven SRs of vitamin D and analogues, two SRs of omega-3 PUFA, two SRs of dietary fiber, one SR of CoQ10, and five SRs of biotics for effect on proteinuria, kidney function, and inflammation in CKD patients. According to the GRADE approach assessment, the strength of evidence was low to very low levels for the benefits of these nutrition supplements on proteinuria and kidney function and did not appear to improve CKD prognosis. The recommendation of these nutrition supplements in CKD patients needs to discuss with physicians and consider the benefits over the adverse effects. Multicenter and large samples of RCTs with more than ten years of follow-up merit to be conducted in the future and to verify the benefits of nutrition supplements in CKD patients.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/13/2/469/s1. Table S1: PRISMA checklist of the systematic review.
Author Contributions
Conceptualization, P.-C.L. and J.-S.C.; methodology, P.-C.L. and C.-L.C.; validation, P.-C.L., C.-L.C., S.-H.O., T.-C.F. and J.-S.C.; formal analysis, P.-C.L., S.-H.O. and J.-S.C.; investigation, P.-C.L. and C.-L.C.; resources, J.-S.C.; data curation, P.-C.L., C.-L.C. and T.-C.F.; writing—original draft preparation, P.-C.L., C.-L.C. and S.-H.O.; writing—review and editing, T.-C.F. and J.-S.C.; visualization, P.-C.L.; supervision, T.-C.F. and J.-S.C.; project administration, T.-C.F. and J.-S.C.; funding acquisition, P.-C.L. and J.-S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received the grants from Kaohsiung Veterans General Hospital and the Ministry of Science and Technology of Taiwan [grant numbers: VGHKS109-D03-2, KSVGH110-D02-2; MOST 107-2314-B-075B-010-MY3, MOST 109-2511-H-075B-001-MY2].
Acknowledgments
We thank the librarian, Li-Ping Shih, for assistance in editing and checking the reference citations format.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BUN | blood urea nitrogen |
| CCr | creatinine clearance |
| CI | confidence interval |
| CKD | chronic kidney disease |
| CoQ10 | coenzyme Q10 |
| CRP | C-reactive protein |
| DN | diabetic nephropathy |
| eGFR | estimated glomerular filtration rate |
| ESKD | end stage kidney disease |
| ESRD | end-stage renal disease |
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
| hs-CRP | high-sensitivity C-reactive protein |
| IL-6 | interleukin 6 |
| IS | indoxyl sulphate |
| MD | mean difference |
| MDA | malondialdehyde |
| OIS | optimal information size |
| PCS | p-cresyl sulphate |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PUFA | polyunsaturated fatty acid |
| RCTs | randomized-controlled trials |
| RoB | risk of bias |
| RR | risk ratio |
| SCr | serum creatinine |
| SMD | standardized mean difference |
| SR | systematic review |
| T1DN | type 1 diabetic nephtopathy |
| T2DN | type 2 diabetic nephtopathy |
| TNF-α | tumor necrosis factor-alpha |
| UACR | urine albumin creatinine ratio |
| UAER | urinary albumin excretion rate |
| UPCR | urine protein creatinine ratio |
References
- Van Dijk, P.C.; Jager, K.J.; Stengel, B.; Gronhagen-Riska, C.; Feest, T.G.; Briggs, J.D. Renal replacement therapy for diabetic end-stage renal disease: Data from 10 registries in Europe (1991–2000). Kidney Int. 2005, 67, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.M.; Akizawa, T.; Jager, K.J.; Kerr, P.G.; Saran, R.; Pisoni, R.L. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: Differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 2016, 388, 294–306. [Google Scholar] [CrossRef]
- Caskey, F.J.; Kramer, A.; Elliott, R.F.; Stel, V.S.; Covic, A.; Cusumano, A.; Geue, C.; Macleod, A.M.; Zwinderman, A.H.; Stengel, B.; et al. Global variation in renal replacement therapy for end-stage renal disease. Nephrol. Dial. Transplant. 2011, 26, 2604–2610. [Google Scholar] [CrossRef] [PubMed]
- Ritz, E.; Orth, S.R. Nephropathy in patients with type 2 diabetes mellitus. N. Engl. J. Med. 1999, 341, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.A.; Norris, K.C.; Li, S.; Chang, T.I.; Tamura, M.K.; Jolly, S.E.; Bakris, G.; McCullough, P.A.; Shlipak, M.; KEEP investigators. Blood pressure components and end-stage renal disease in persons with chronic kidney disease: The Kidney Early Evaluation Program (KEEP). Arch. Intern. Med. 2012, 172, 41–47. [Google Scholar] [CrossRef]
- Stefansson, V.T.N.; Schei, J.; Solbu, M.D.; Jenssen, T.G.; Melsom, T.; Eriksen, B.O. Metabolic syndrome but not obesity measures are risk factors for accelerated age-related glomerular filtration rate decline in the general population. Kidney Int. 2018, 93, 1183–1190. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.M.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Matsushita, K.; Coresh, J.; Sang, Y.; Chalmers, J.; Fox, C.; Guallar, E.; Jafar, T.; Jassal, S.K.; Landman, G.W.D.; Muntner, P.; et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015, 3, 514–525. [Google Scholar] [CrossRef]
- Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; van der Velde, M.; Woodward, M.; Levey, A.S.; Jong, P.E.; Coresh, J.; Astor, B.C.; Matsushita, K. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011, 79, 1331–1340. [Google Scholar] [CrossRef]
- Inker, L.A.; Grams, M.E.; Levey, A.S.; Coresh, J.; Cirillo, M.; Gansevoort, R.T.; Gutierriz, O.M.; Hamano, T.; Heine, G.H.; Ishikawa, S.; et al. Relationship of Estimated GFR and Albuminuria to Concurrent Laboratory Abnormalities: An Individual Participant Data Meta-analysis in a Global Consortium. Am. J. Kidney Dis. 2019, 73, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Andreucci, M.; De Nicola, L.; Garofalo, C.; Battaglia, Y.; Borrelli, S.; Gagliardi, I.; Faga, T.; Michael, A.; Mastroroberto, P.; et al. The role of prognostic and predictive biomarkers for assessing cardiovascular risk in chronic kidney disease patients. BioMed Res. Int. 2020, 2314128. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S.; Levey, A.S.; Beck, G.J.; Caggiula, A.W.; Hunsicker, L.; Kusek, J.W.; Striker, G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N. Engl. J. Med. 1994, 330, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Kopple, J.D.; Wang, X.; Beck, G.J.; Collins, A.J.; Kusek, J.W.; Greene, T.; Levey, A.S.; Sarnak, M.J. Effect of a very low-protein diet on outcomes: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 2009, 53, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Rosman, J.B.; ter Wee, P.M.; Meijer, S.; Piers-Becht, T.P.; Sluiter, W.J.; Donker, A.J. Prospective randomised trial of early dietary protein restriction in chronic renal failure. Lancet 1984, 2, 1291–1296. [Google Scholar] [CrossRef]
- Hansen, H.P.; Christensen, P.K.; Tauber-Lassen, E.; Klausen, A.; Jensen, B.R.; Parving, H.H. Low-protein diet and kidney function in insulin-dependent diabetic patients with diabetic nephropathy. Kidney Int. 1999, 55, 621–628. [Google Scholar] [CrossRef]
- Hansen, H.P.; Tauber-Lassen, E.; Jensen, B.R.; Parving, H.H. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int. 2002, 62, 220–228. [Google Scholar] [CrossRef]
- De Borst, M.H.; Hajhosseiny, R.; Tamez, H.; Wenger, J.; Thadhani, R.; Goldsmith, D.J.A. Active vitamin D treatment for reduction of residual proteinuria: A systematic review. J. Am. Soc. Nephrol. 2013, 24, 1863–1871. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.; Wesson, D.E. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012, 81, 86–93. [Google Scholar] [CrossRef]
- Banerjee, T.; Crews, D.C.; Wesson, D.E.; Tilea, A.M.; Saran, R.; Ríos-Burrows, N.; Williams, D.E.; Powe, N.R.; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. High Dietary Acid Load Predicts ESRD among Adults with CKD. J. Am. Soc. Nephrol. 2015, 26, 1693–1700. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Zhou, Q.; Zhang, H.; Yi, B. Effects of Vitamin D Supplementation on Renal Function, Inflammation and Glycemic Control in Patients with Diabetic Nephropathy, a Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2019, 44, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Saglimbene, V.M.; Wong, G.; van Zwieten, A.; Palmer, S.C.; Ruospo, M.; Natale, P.; Campbell, K.; Teixeira-Pinto, A.; Craig, J.C.; Strippoli, G.F.M. Effects of omega-3 polyunsaturated fatty acid intake in patients with chronic kidney disease: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2020, 39, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Pisano, A.; D’Arrigo, G.; Coppolino, G.; Bolignano, D. Biotic Supplements for Renal Patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1224. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.; Darling, P.B. Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2015, 69, 761–768. [Google Scholar] [CrossRef]
- McFarlane, C.; Ramos, C.I.; Johnson, D.W.; Campbell, K.L. Prebiotic, Probiotic, and Synbiotic Supplementation in Chronic Kidney Disease: A Systematic Review and Meta-analysis. J. Ren. Nutr. 2019, 29, 209–220. [Google Scholar] [CrossRef]
- Gupta, S.; Goyal, P.; Feinn, R.S.; Mattana, J. Role of Vitamin D and Its Analogues in Diabetic Nephropathy: A Meta-analysis. Am. J. Med Sci. 2019, 357, 223–229. [Google Scholar] [CrossRef]
- Derakhshanian, H.; Shab-Bidar, S.; Speakman, J.R.; Nadimi, H.; Djafarian, K. Vitamin D and diabetic nephropathy: A systematic review and meta-analysis. Nutrition 2015, 31, 1189–1194. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Lin, P.C.S.Y.; Hung, P.L.; Tu, S.C. Precision searching: An innovative search strategy for retrieving the newest and optimal systematic reviews from PubMed. Advances in Evidence Synthesis: Special issue. Cochrane Database Syst. Rev. 2020, 9 (Suppl. 1), 311. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.0; Updated July 2019; Cochrane: London, UK, 2019. [Google Scholar]
- Schünemann, H.J.; Mustafa, R.A.; Brozek, J.; Steingart, K.R.; Leeflang, M.; Murad, M.H.; Bossuyt, P.; Glasziou, P.; Jaeschke, R.; Lange, S.; et al. GRADE guidelines: 21 part 2. Test accuracy: Inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2020, 122, 142–152. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Mustafa, R.A.; Brozek, J.; Steingart, K.R.; Leeflang, M.; Murad, M.H.; Bossuyt, P.; Glasziou, P.; Jaeschke, R.; Lange, S.; et al. GRADE guidelines: 21 part 1. Study design, risk of bias, and indirectness in rating the certainty across a body of evidence for test accuracy. J. Clin. Epidemiol. 2020, 122, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Rind, D.; Devereaux, P.J.; Montori, V.M.; Freyschuss, B.; Vist, G.; et al. GRADE guidelines 6. Rating the quality of evidence—Imprecision. J. Clin. Epidemiol. 2011, 64, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Falck-Ytter, Y.; Jaeschke, R.; Vist, G.; et al. GRADE guidelines: 8. Rating the quality of evidence—Indirectness. J. Clin. Epidemiol. 2011, 64, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Montori, V.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Djulbegovic, B.; Atkins, D.; Falck-Ytter, Y.; et al. GRADE guidelines: 5. Rating the quality of evidence—Publication bias. J. Clin. Epidemiol. 2011, 64, 1277–1282. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Montori, V.; Akl, E.A.; Djulbegovic, B.; Falck-Ytter, Y.; et al. GRADE guidelines: 4. Rating the quality of evidence—Study limitations (risk of bias). J. Clin. Epidemiol. 2011, 64, 407–415. [Google Scholar] [CrossRef]
- Ellis, P.D. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results, 1st ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Milajerdi, A.; Ostadmohammadi, V.; Amirjani, S.; Kolahdooz, F.; Asemi, Z. The effects of vitamin D treatment on glycemic control, serum lipid profiles, and C-reactive protein in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Int. Urol. Nephrol. 2019, 51, 1567–1580. [Google Scholar] [CrossRef]
- Xu, L.; Wan, X.; Huang, Z.; Zeng, F.; Wei, G.; Fang, D.; Deng, W.; Li, Y. Impact of vitamin D on chronic kidney diseases in non-dialysis patients: A meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e61387. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Dong, J.J.; Wang, H.P.; Shang, H.X.; Zhang, D.M.; Liao, L. Efficacy and safety of vitamin D3 in patients with diabetic nephropathy: A meta-analysis of randomized controlled trials. Chin. Med. J. 2014, 127, 2837–2843. [Google Scholar]
- Zhang, M.; Liu, T.; Li, W.; Gong, W.; Yang, X.; Xi, J. Efficacy of Vitamin D3 in Patients With Diabetic Nephropathy: An Updated Meta-Analysis. Iran. Red Crescent Med. J. 2017, 19, e64275. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Z.; Zhang, H. Omega-3 fatty acid supplementation as an adjunctive therapy in the treatment of chronic kidney disease: A meta-analysis. Clinics (Sao Paulo) 2017, 72, 58–64. [Google Scholar] [CrossRef]
- Wu, M.; Cai, X.; Lin, J.; Zhang, X.; Scott, E.M.; Li, X. Association between fibre intake and indoxyl sulphate/P-cresyl sulphate in patients with chronic kidney disease: Meta-analysis and systematic review of experimental studies. Clin. Nutr. 2019, 38, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, Z.; Liu, Q.; Quan, H.; Cheng, X. Effects of coenzyme Q10 intervention on diabetic kidney disease: A systematic review and meta-analysis. Medicine (Baltimore) 2019, 98, e15850. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Jia, Q.; Yang, J.; Jia, R.; Zhang, H. Efficacy of Probiotics Supplementation On Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2018, 43, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Tao, S.; Cheng, Y.; Liu, J.; Ma, L.; Fu, P. Effects of probiotic supplements on the progression of chronic kidney disease: A meta-analysis. Nephrology (Carlton) 2019, 24, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.J.; Guo, J.; Wang, Q.; Wang, L.; Wang, Y.; Zhang, F.; Huang, W.J.; Zhang, W.; Liu, W.J.; Wang, Y. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 577–598. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Wolpowitz, D.; Gilchrest, B.A. The vitamin D questions: How much do you need and how should you get it? J. Am. Acad. Dermatol. 2006, 54, 301–317. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, L.J.; Zhou, Y.; Badr, R.; Watson, P.; Ye, A.; Zhou, B.; Zhang, J.; Deng, H.W.; Recker, R.R.; et al. SNP rs11185644 of RXRA gene is identified for dose-response variability to vitamin D3 supplementation: A randomized clinical trial. Sci. Rep. 2017, 7, 40593. [Google Scholar] [CrossRef]
- Sahebkar, A.; Simental-Mendia, L.E.; Mikhailidis, D.P.; Pirro, M.; Banach, M.; Sirtori, C.R.; Reiner, Z. Effect of omega-3 supplements on plasma apolipoprotein C-III concentrations: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. 2018, 50, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Milani, R.V.; Mehra, M.R.; Ventura, H.O. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Saber, H.; Yakoob, M.Y.; Shi, P.; Longstreth, W.T., Jr.; Lemaitre, R.N.; Siscovick, D.; Rexrode, K.M.; Willett, W.C.; Mozaffarian, D. Omega-3 Fatty Acids and Incident Ischemic Stroke and Its Atherothrombotic and Cardioembolic Subtypes in 3 US Cohorts. Stroke 2017, 48, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Park, K. Omega-3 and omega-6 polyunsaturated fatty acids and metabolic syndrome: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 765–773. [Google Scholar] [CrossRef]
- Zhuang, Z.; Wang, Z.H.; Huang, Y.Y.; Zheng, Q.; Pan, X.D. Protective effect and possible mechanisms of ligustrazine isolated from Ligusticum wallichii on nephropathy in rats with diabetes: A preclinical systematic review and meta-analysis. J. Ethnopharmacol. 2020, 252, 112568. [Google Scholar] [CrossRef]
- Friedman, A.N.; Moe, S.M.; Perkins, S.M.; Li, Y.; Watkins, B.A. Fish consumption and omega-3 fatty acid status and determinants in long-term hemodialysis. Am. J. Kidney Dis. 2006, 47, 1064–1071. [Google Scholar] [CrossRef]
- Friedman, A.N.; Yu, Z.; Tabbey, R.; Denski, C.; Tamez, H.; Wenger, J.; Thadhani, R.; Li, Y.; Watkins, B.A. Low blood levels of long-chain n-3 polyunsaturated fatty acids in US hemodialysis patients: Clinical implications. Am. J. Nephrol. 2012, 36, 451–458. [Google Scholar] [CrossRef]
- Saglimbene, V.M.; Wong, G.; Ruospo, M.; Palmer, S.C.; Campbell, K.; Larsen, V.G.; Natale, P.; Teixeira-Pinto, A.; Carrero, J.J.; Stenvinkel, P.; et al. Dietary n-3 polyunsaturated fatty acid intake and all-cause and cardiovascular mortality in adults on hemodialysis: The DIET-HD multinational cohort study. Clin. Nutr. 2019, 38, 429–437. [Google Scholar] [CrossRef]
- Shoji, T.; Kakiya, R.; Hayashi, T.; Tsujimoto, Y.; Sonoda, M.; Shima, H.; Mori, K.; Fukumoto, S.; Tahara, H.; Shioi, A.; et al. Serum n-3 and n-6 polyunsaturated fatty acid profile as an independent predictor of cardiovascular events in hemodialysis patients. Am. J. Kidney Dis. 2013, 62, 568–576. [Google Scholar] [CrossRef]
- Friedman, A.N.; Yu, Z.; Tabbey, R.; Denski, C.; Tamez, H.; Wenger, J.; Thadhani, R.; Li, Y.; Watkins, B.A. Inverse relationship between long-chain n-3 fatty acids and risk of sudden cardiac death in patients starting hemodialysis. Kidney Int. 2013, 83, 1130–1135. [Google Scholar] [CrossRef]
- Thies, F.; Garry, J.M.; Yaqoob, P.; Rerkasem, K.; Williams, J.; Shearman, C.P.; Gallagher, P.J.; Calder, P.C.; Grimble, R.F. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet 2003, 361, 477–485. [Google Scholar] [CrossRef]
- Miyajima, T.; Tsujino, T.; Saito, K.; Yokoyama, M. Effects of eicosapentaenoic acid on blood pressure, cell membrane fatty acids, and intracellular sodium concentration in essential hypertension. Hypertens Res. 2001, 24, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Beilin, L.J. Omega-3 fatty acids and inflammation. Curr. Atheroscler. Rep. 2004, 6, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Terano, T.; Hirai, A.; Hamazaki, T.; Kobayashi, S.; Fujita, T.; Tamura, Y.; Kumagai, A. Effect of oral administration of highly purified eicosapentaenoic acid on platelet function, blood viscosity and red cell deformability in healthy human subjects. Atherosclerosis 1983, 46, 321–331. [Google Scholar] [CrossRef]
- Saini, R. Coenzyme Q10: The essential nutrient. J. Pharm. Bioallied Sci. 2011, 3, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Ayers, J.; Cook, J.; Koenig, R.A.; Sisson, E.M.; Dixon, D.L. Recent Developments in the Role of Coenzyme Q10 for Coronary Heart Disease: A Systematic Review. Curr. Atheroscler. Rep. 2018, 20, 29. [Google Scholar] [CrossRef]
- Flowers, N.; Hartley, L.; Todkill, D.; Strangers, S.; Rees, K. Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2014, CD010405. [Google Scholar] [CrossRef]
- Sharma, A.; Fonarow, G.C.; Butler, J.; Ezekowitz, J.A.; Felker, G.M. Coenzyme Q10 and Heart Failure: A State-of-the-Art Review. Circ. Heart Fail. 2016, 9, e002639. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Yang, K.L.; Zeng, L.T.; Wu, X.H.; Huang, H.Y. Effectiveness of Coenzyme Q10 Supplementation for Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2018, 2018, 6484839. [Google Scholar] [CrossRef]
- Pirro, M.; Mannarino, M.R.; Bianconi, V.; Simental-Mendia, L.E.; Bagaglia, F.; Mannarino, E.; Sahebkar, A. The effects of a nutraceutical combination on plasma lipids and glucose: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 110, 76–88. [Google Scholar] [CrossRef]
- Parohan, M.; Sarraf, P.; Javanbakht, M.H.; Ranji-Burachaloo, S.; Djalali, M. Effect of coenzyme Q10 supplementation on clinical features of migraine: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr. Neurosci. 2019, 23, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Boulange, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Saez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, T.L.; Tsai, Y.L.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019, 27, 615–622. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Lin, T.L.; Chang, C.J.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S129–S134. [Google Scholar] [CrossRef]
- Boyle, R.J.; Tang, M.L. The role of probiotics in the management of allergic disease. Clin. Exp. Allergy 2006, 36, 568–576. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Therap. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).