Systematic Review of Nutrition Supplements in Chronic Kidney Diseases: A GRADE Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Methods
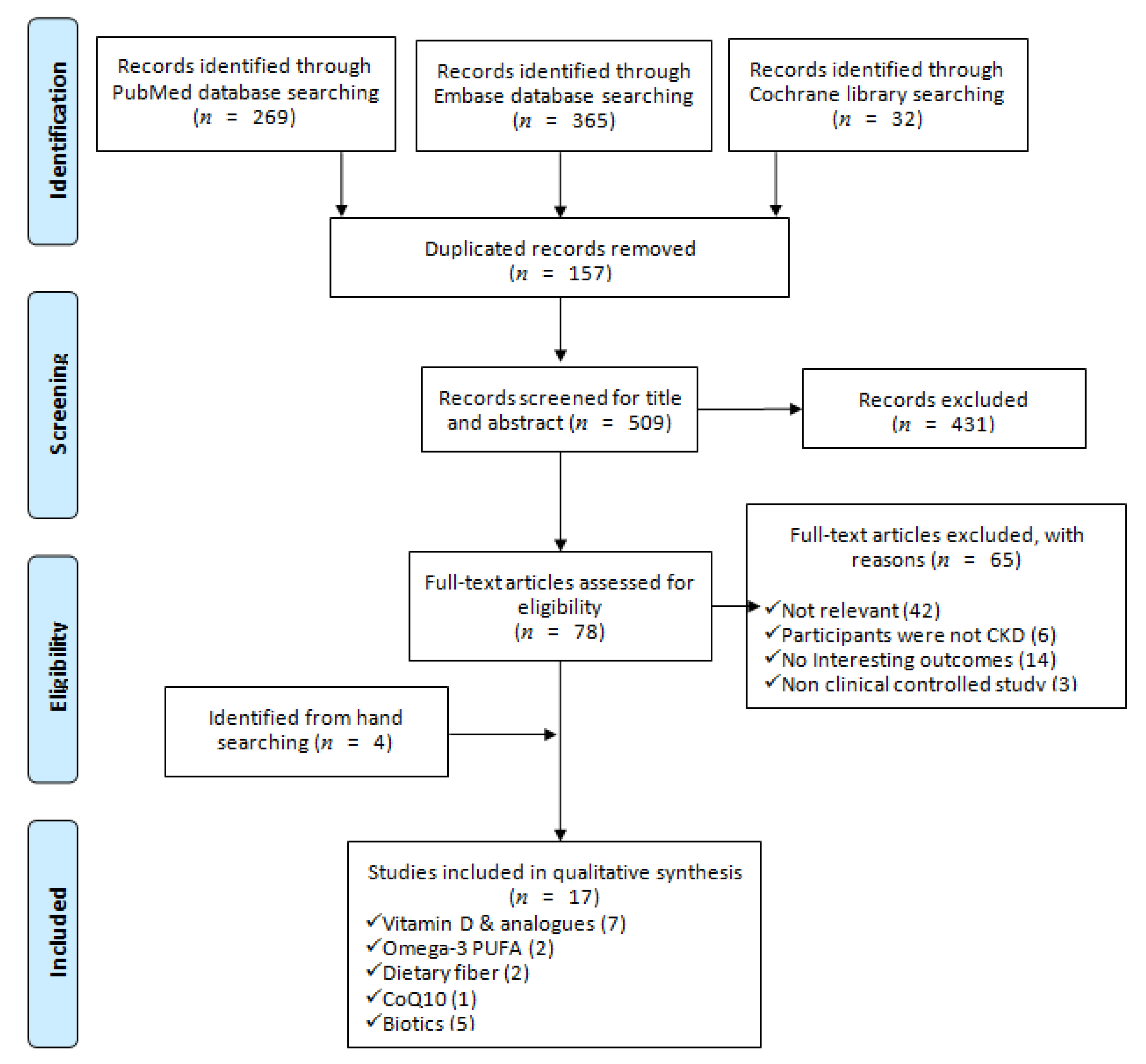
2.2. Search Strategy
2.3. Selection Criteria
2.4. Data Extraction
2.5. Grading of Recommendations Assessment, Development and Evaluation (GRADE) Grading the Evidence
2.6. Data Synthesis and Statistics
3. Results
3.1. Baseline Information of Included SRs
3.2. Grading of Recommendations Assessment, Development and Evaluation (GRADE) Qualifying the Evidence
3.3. Vitamin D and Analogues on Renal Protection
3.4. Omega-3 Polyunsaturated Fatty Acid (PUFA) on Renal Protection
3.5. Dietary Fiber on Renal Protection
3.6. Coenzyme Q10 (CoQ10) on Renal Protection
3.7. Probiotics, Prebiotics and Synbiotics on Renal Protection
4. Discussion
4.1. Findings and Implications of this Systematic Review
4.2. Strengths, Limitations and Further Research Needs
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BUN | blood urea nitrogen |
| CCr | creatinine clearance |
| CI | confidence interval |
| CKD | chronic kidney disease |
| CoQ10 | coenzyme Q10 |
| CRP | C-reactive protein |
| DN | diabetic nephropathy |
| eGFR | estimated glomerular filtration rate |
| ESKD | end stage kidney disease |
| ESRD | end-stage renal disease |
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
| hs-CRP | high-sensitivity C-reactive protein |
| IL-6 | interleukin 6 |
| IS | indoxyl sulphate |
| MD | mean difference |
| MDA | malondialdehyde |
| OIS | optimal information size |
| PCS | p-cresyl sulphate |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PUFA | polyunsaturated fatty acid |
| RCTs | randomized-controlled trials |
| RoB | risk of bias |
| RR | risk ratio |
| SCr | serum creatinine |
| SMD | standardized mean difference |
| SR | systematic review |
| T1DN | type 1 diabetic nephtopathy |
| T2DN | type 2 diabetic nephtopathy |
| TNF-α | tumor necrosis factor-alpha |
| UACR | urine albumin creatinine ratio |
| UAER | urinary albumin excretion rate |
| UPCR | urine protein creatinine ratio |
References
- Van Dijk, P.C.; Jager, K.J.; Stengel, B.; Gronhagen-Riska, C.; Feest, T.G.; Briggs, J.D. Renal replacement therapy for diabetic end-stage renal disease: Data from 10 registries in Europe (1991–2000). Kidney Int. 2005, 67, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.M.; Akizawa, T.; Jager, K.J.; Kerr, P.G.; Saran, R.; Pisoni, R.L. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: Differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 2016, 388, 294–306. [Google Scholar] [CrossRef]
- Caskey, F.J.; Kramer, A.; Elliott, R.F.; Stel, V.S.; Covic, A.; Cusumano, A.; Geue, C.; Macleod, A.M.; Zwinderman, A.H.; Stengel, B.; et al. Global variation in renal replacement therapy for end-stage renal disease. Nephrol. Dial. Transplant. 2011, 26, 2604–2610. [Google Scholar] [CrossRef] [PubMed]
- Ritz, E.; Orth, S.R. Nephropathy in patients with type 2 diabetes mellitus. N. Engl. J. Med. 1999, 341, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.A.; Norris, K.C.; Li, S.; Chang, T.I.; Tamura, M.K.; Jolly, S.E.; Bakris, G.; McCullough, P.A.; Shlipak, M.; KEEP investigators. Blood pressure components and end-stage renal disease in persons with chronic kidney disease: The Kidney Early Evaluation Program (KEEP). Arch. Intern. Med. 2012, 172, 41–47. [Google Scholar] [CrossRef]
- Stefansson, V.T.N.; Schei, J.; Solbu, M.D.; Jenssen, T.G.; Melsom, T.; Eriksen, B.O. Metabolic syndrome but not obesity measures are risk factors for accelerated age-related glomerular filtration rate decline in the general population. Kidney Int. 2018, 93, 1183–1190. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.M.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Matsushita, K.; Coresh, J.; Sang, Y.; Chalmers, J.; Fox, C.; Guallar, E.; Jafar, T.; Jassal, S.K.; Landman, G.W.D.; Muntner, P.; et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015, 3, 514–525. [Google Scholar] [CrossRef]
- Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; van der Velde, M.; Woodward, M.; Levey, A.S.; Jong, P.E.; Coresh, J.; Astor, B.C.; Matsushita, K. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011, 79, 1331–1340. [Google Scholar] [CrossRef]
- Inker, L.A.; Grams, M.E.; Levey, A.S.; Coresh, J.; Cirillo, M.; Gansevoort, R.T.; Gutierriz, O.M.; Hamano, T.; Heine, G.H.; Ishikawa, S.; et al. Relationship of Estimated GFR and Albuminuria to Concurrent Laboratory Abnormalities: An Individual Participant Data Meta-analysis in a Global Consortium. Am. J. Kidney Dis. 2019, 73, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Andreucci, M.; De Nicola, L.; Garofalo, C.; Battaglia, Y.; Borrelli, S.; Gagliardi, I.; Faga, T.; Michael, A.; Mastroroberto, P.; et al. The role of prognostic and predictive biomarkers for assessing cardiovascular risk in chronic kidney disease patients. BioMed Res. Int. 2020, 2314128. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S.; Levey, A.S.; Beck, G.J.; Caggiula, A.W.; Hunsicker, L.; Kusek, J.W.; Striker, G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N. Engl. J. Med. 1994, 330, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Kopple, J.D.; Wang, X.; Beck, G.J.; Collins, A.J.; Kusek, J.W.; Greene, T.; Levey, A.S.; Sarnak, M.J. Effect of a very low-protein diet on outcomes: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 2009, 53, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Rosman, J.B.; ter Wee, P.M.; Meijer, S.; Piers-Becht, T.P.; Sluiter, W.J.; Donker, A.J. Prospective randomised trial of early dietary protein restriction in chronic renal failure. Lancet 1984, 2, 1291–1296. [Google Scholar] [CrossRef]
- Hansen, H.P.; Christensen, P.K.; Tauber-Lassen, E.; Klausen, A.; Jensen, B.R.; Parving, H.H. Low-protein diet and kidney function in insulin-dependent diabetic patients with diabetic nephropathy. Kidney Int. 1999, 55, 621–628. [Google Scholar] [CrossRef]
- Hansen, H.P.; Tauber-Lassen, E.; Jensen, B.R.; Parving, H.H. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int. 2002, 62, 220–228. [Google Scholar] [CrossRef]
- De Borst, M.H.; Hajhosseiny, R.; Tamez, H.; Wenger, J.; Thadhani, R.; Goldsmith, D.J.A. Active vitamin D treatment for reduction of residual proteinuria: A systematic review. J. Am. Soc. Nephrol. 2013, 24, 1863–1871. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.; Wesson, D.E. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012, 81, 86–93. [Google Scholar] [CrossRef]
- Banerjee, T.; Crews, D.C.; Wesson, D.E.; Tilea, A.M.; Saran, R.; Ríos-Burrows, N.; Williams, D.E.; Powe, N.R.; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. High Dietary Acid Load Predicts ESRD among Adults with CKD. J. Am. Soc. Nephrol. 2015, 26, 1693–1700. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Zhou, Q.; Zhang, H.; Yi, B. Effects of Vitamin D Supplementation on Renal Function, Inflammation and Glycemic Control in Patients with Diabetic Nephropathy, a Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2019, 44, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Saglimbene, V.M.; Wong, G.; van Zwieten, A.; Palmer, S.C.; Ruospo, M.; Natale, P.; Campbell, K.; Teixeira-Pinto, A.; Craig, J.C.; Strippoli, G.F.M. Effects of omega-3 polyunsaturated fatty acid intake in patients with chronic kidney disease: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2020, 39, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Pisano, A.; D’Arrigo, G.; Coppolino, G.; Bolignano, D. Biotic Supplements for Renal Patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1224. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.; Darling, P.B. Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2015, 69, 761–768. [Google Scholar] [CrossRef]
- McFarlane, C.; Ramos, C.I.; Johnson, D.W.; Campbell, K.L. Prebiotic, Probiotic, and Synbiotic Supplementation in Chronic Kidney Disease: A Systematic Review and Meta-analysis. J. Ren. Nutr. 2019, 29, 209–220. [Google Scholar] [CrossRef]
- Gupta, S.; Goyal, P.; Feinn, R.S.; Mattana, J. Role of Vitamin D and Its Analogues in Diabetic Nephropathy: A Meta-analysis. Am. J. Med Sci. 2019, 357, 223–229. [Google Scholar] [CrossRef]
- Derakhshanian, H.; Shab-Bidar, S.; Speakman, J.R.; Nadimi, H.; Djafarian, K. Vitamin D and diabetic nephropathy: A systematic review and meta-analysis. Nutrition 2015, 31, 1189–1194. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Lin, P.C.S.Y.; Hung, P.L.; Tu, S.C. Precision searching: An innovative search strategy for retrieving the newest and optimal systematic reviews from PubMed. Advances in Evidence Synthesis: Special issue. Cochrane Database Syst. Rev. 2020, 9 (Suppl. 1), 311. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.0; Updated July 2019; Cochrane: London, UK, 2019. [Google Scholar]
- Schünemann, H.J.; Mustafa, R.A.; Brozek, J.; Steingart, K.R.; Leeflang, M.; Murad, M.H.; Bossuyt, P.; Glasziou, P.; Jaeschke, R.; Lange, S.; et al. GRADE guidelines: 21 part 2. Test accuracy: Inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2020, 122, 142–152. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Mustafa, R.A.; Brozek, J.; Steingart, K.R.; Leeflang, M.; Murad, M.H.; Bossuyt, P.; Glasziou, P.; Jaeschke, R.; Lange, S.; et al. GRADE guidelines: 21 part 1. Study design, risk of bias, and indirectness in rating the certainty across a body of evidence for test accuracy. J. Clin. Epidemiol. 2020, 122, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Rind, D.; Devereaux, P.J.; Montori, V.M.; Freyschuss, B.; Vist, G.; et al. GRADE guidelines 6. Rating the quality of evidence—Imprecision. J. Clin. Epidemiol. 2011, 64, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Falck-Ytter, Y.; Jaeschke, R.; Vist, G.; et al. GRADE guidelines: 8. Rating the quality of evidence—Indirectness. J. Clin. Epidemiol. 2011, 64, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Montori, V.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Djulbegovic, B.; Atkins, D.; Falck-Ytter, Y.; et al. GRADE guidelines: 5. Rating the quality of evidence—Publication bias. J. Clin. Epidemiol. 2011, 64, 1277–1282. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Montori, V.; Akl, E.A.; Djulbegovic, B.; Falck-Ytter, Y.; et al. GRADE guidelines: 4. Rating the quality of evidence—Study limitations (risk of bias). J. Clin. Epidemiol. 2011, 64, 407–415. [Google Scholar] [CrossRef]
- Ellis, P.D. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results, 1st ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Milajerdi, A.; Ostadmohammadi, V.; Amirjani, S.; Kolahdooz, F.; Asemi, Z. The effects of vitamin D treatment on glycemic control, serum lipid profiles, and C-reactive protein in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Int. Urol. Nephrol. 2019, 51, 1567–1580. [Google Scholar] [CrossRef]
- Xu, L.; Wan, X.; Huang, Z.; Zeng, F.; Wei, G.; Fang, D.; Deng, W.; Li, Y. Impact of vitamin D on chronic kidney diseases in non-dialysis patients: A meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e61387. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Dong, J.J.; Wang, H.P.; Shang, H.X.; Zhang, D.M.; Liao, L. Efficacy and safety of vitamin D3 in patients with diabetic nephropathy: A meta-analysis of randomized controlled trials. Chin. Med. J. 2014, 127, 2837–2843. [Google Scholar]
- Zhang, M.; Liu, T.; Li, W.; Gong, W.; Yang, X.; Xi, J. Efficacy of Vitamin D3 in Patients With Diabetic Nephropathy: An Updated Meta-Analysis. Iran. Red Crescent Med. J. 2017, 19, e64275. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Z.; Zhang, H. Omega-3 fatty acid supplementation as an adjunctive therapy in the treatment of chronic kidney disease: A meta-analysis. Clinics (Sao Paulo) 2017, 72, 58–64. [Google Scholar] [CrossRef]
- Wu, M.; Cai, X.; Lin, J.; Zhang, X.; Scott, E.M.; Li, X. Association between fibre intake and indoxyl sulphate/P-cresyl sulphate in patients with chronic kidney disease: Meta-analysis and systematic review of experimental studies. Clin. Nutr. 2019, 38, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, Z.; Liu, Q.; Quan, H.; Cheng, X. Effects of coenzyme Q10 intervention on diabetic kidney disease: A systematic review and meta-analysis. Medicine (Baltimore) 2019, 98, e15850. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Jia, Q.; Yang, J.; Jia, R.; Zhang, H. Efficacy of Probiotics Supplementation On Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2018, 43, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Tao, S.; Cheng, Y.; Liu, J.; Ma, L.; Fu, P. Effects of probiotic supplements on the progression of chronic kidney disease: A meta-analysis. Nephrology (Carlton) 2019, 24, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.J.; Guo, J.; Wang, Q.; Wang, L.; Wang, Y.; Zhang, F.; Huang, W.J.; Zhang, W.; Liu, W.J.; Wang, Y. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 577–598. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Wolpowitz, D.; Gilchrest, B.A. The vitamin D questions: How much do you need and how should you get it? J. Am. Acad. Dermatol. 2006, 54, 301–317. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, L.J.; Zhou, Y.; Badr, R.; Watson, P.; Ye, A.; Zhou, B.; Zhang, J.; Deng, H.W.; Recker, R.R.; et al. SNP rs11185644 of RXRA gene is identified for dose-response variability to vitamin D3 supplementation: A randomized clinical trial. Sci. Rep. 2017, 7, 40593. [Google Scholar] [CrossRef]
- Sahebkar, A.; Simental-Mendia, L.E.; Mikhailidis, D.P.; Pirro, M.; Banach, M.; Sirtori, C.R.; Reiner, Z. Effect of omega-3 supplements on plasma apolipoprotein C-III concentrations: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. 2018, 50, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Milani, R.V.; Mehra, M.R.; Ventura, H.O. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Saber, H.; Yakoob, M.Y.; Shi, P.; Longstreth, W.T., Jr.; Lemaitre, R.N.; Siscovick, D.; Rexrode, K.M.; Willett, W.C.; Mozaffarian, D. Omega-3 Fatty Acids and Incident Ischemic Stroke and Its Atherothrombotic and Cardioembolic Subtypes in 3 US Cohorts. Stroke 2017, 48, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Park, K. Omega-3 and omega-6 polyunsaturated fatty acids and metabolic syndrome: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 765–773. [Google Scholar] [CrossRef]
- Zhuang, Z.; Wang, Z.H.; Huang, Y.Y.; Zheng, Q.; Pan, X.D. Protective effect and possible mechanisms of ligustrazine isolated from Ligusticum wallichii on nephropathy in rats with diabetes: A preclinical systematic review and meta-analysis. J. Ethnopharmacol. 2020, 252, 112568. [Google Scholar] [CrossRef]
- Friedman, A.N.; Moe, S.M.; Perkins, S.M.; Li, Y.; Watkins, B.A. Fish consumption and omega-3 fatty acid status and determinants in long-term hemodialysis. Am. J. Kidney Dis. 2006, 47, 1064–1071. [Google Scholar] [CrossRef]
- Friedman, A.N.; Yu, Z.; Tabbey, R.; Denski, C.; Tamez, H.; Wenger, J.; Thadhani, R.; Li, Y.; Watkins, B.A. Low blood levels of long-chain n-3 polyunsaturated fatty acids in US hemodialysis patients: Clinical implications. Am. J. Nephrol. 2012, 36, 451–458. [Google Scholar] [CrossRef]
- Saglimbene, V.M.; Wong, G.; Ruospo, M.; Palmer, S.C.; Campbell, K.; Larsen, V.G.; Natale, P.; Teixeira-Pinto, A.; Carrero, J.J.; Stenvinkel, P.; et al. Dietary n-3 polyunsaturated fatty acid intake and all-cause and cardiovascular mortality in adults on hemodialysis: The DIET-HD multinational cohort study. Clin. Nutr. 2019, 38, 429–437. [Google Scholar] [CrossRef]
- Shoji, T.; Kakiya, R.; Hayashi, T.; Tsujimoto, Y.; Sonoda, M.; Shima, H.; Mori, K.; Fukumoto, S.; Tahara, H.; Shioi, A.; et al. Serum n-3 and n-6 polyunsaturated fatty acid profile as an independent predictor of cardiovascular events in hemodialysis patients. Am. J. Kidney Dis. 2013, 62, 568–576. [Google Scholar] [CrossRef]
- Friedman, A.N.; Yu, Z.; Tabbey, R.; Denski, C.; Tamez, H.; Wenger, J.; Thadhani, R.; Li, Y.; Watkins, B.A. Inverse relationship between long-chain n-3 fatty acids and risk of sudden cardiac death in patients starting hemodialysis. Kidney Int. 2013, 83, 1130–1135. [Google Scholar] [CrossRef]
- Thies, F.; Garry, J.M.; Yaqoob, P.; Rerkasem, K.; Williams, J.; Shearman, C.P.; Gallagher, P.J.; Calder, P.C.; Grimble, R.F. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet 2003, 361, 477–485. [Google Scholar] [CrossRef]
- Miyajima, T.; Tsujino, T.; Saito, K.; Yokoyama, M. Effects of eicosapentaenoic acid on blood pressure, cell membrane fatty acids, and intracellular sodium concentration in essential hypertension. Hypertens Res. 2001, 24, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Beilin, L.J. Omega-3 fatty acids and inflammation. Curr. Atheroscler. Rep. 2004, 6, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Terano, T.; Hirai, A.; Hamazaki, T.; Kobayashi, S.; Fujita, T.; Tamura, Y.; Kumagai, A. Effect of oral administration of highly purified eicosapentaenoic acid on platelet function, blood viscosity and red cell deformability in healthy human subjects. Atherosclerosis 1983, 46, 321–331. [Google Scholar] [CrossRef]
- Saini, R. Coenzyme Q10: The essential nutrient. J. Pharm. Bioallied Sci. 2011, 3, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Ayers, J.; Cook, J.; Koenig, R.A.; Sisson, E.M.; Dixon, D.L. Recent Developments in the Role of Coenzyme Q10 for Coronary Heart Disease: A Systematic Review. Curr. Atheroscler. Rep. 2018, 20, 29. [Google Scholar] [CrossRef]
- Flowers, N.; Hartley, L.; Todkill, D.; Strangers, S.; Rees, K. Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2014, CD010405. [Google Scholar] [CrossRef]
- Sharma, A.; Fonarow, G.C.; Butler, J.; Ezekowitz, J.A.; Felker, G.M. Coenzyme Q10 and Heart Failure: A State-of-the-Art Review. Circ. Heart Fail. 2016, 9, e002639. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Yang, K.L.; Zeng, L.T.; Wu, X.H.; Huang, H.Y. Effectiveness of Coenzyme Q10 Supplementation for Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2018, 2018, 6484839. [Google Scholar] [CrossRef]
- Pirro, M.; Mannarino, M.R.; Bianconi, V.; Simental-Mendia, L.E.; Bagaglia, F.; Mannarino, E.; Sahebkar, A. The effects of a nutraceutical combination on plasma lipids and glucose: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 110, 76–88. [Google Scholar] [CrossRef]
- Parohan, M.; Sarraf, P.; Javanbakht, M.H.; Ranji-Burachaloo, S.; Djalali, M. Effect of coenzyme Q10 supplementation on clinical features of migraine: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr. Neurosci. 2019, 23, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Boulange, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Saez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, T.L.; Tsai, Y.L.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019, 27, 615–622. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Lin, T.L.; Chang, C.J.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S129–S134. [Google Scholar] [CrossRef]
- Boyle, R.J.; Tang, M.L. The role of probiotics in the management of allergic disease. Clin. Exp. Allergy 2006, 36, 568–576. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Therap. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
| Author Year | Search Databases | Search Duration (Through) | Included Study Design | Study No. | Critical Appraise Tool | Population | Intervention | Control | Outcomes Related to Kidney Function | Treatment Duration |
|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D and Analogues | ||||||||||
| Gupta 2019 [26] | PubMed, Scopus, and Google scholar | January 2018 | RCTs | 9 T2DN (7) T1DN (1) NA (1) | RoB | DN | calcitriol 0.25–0.5 mcg QD-BIW or 50,000IU QW paricalcitol 1–2 mcg QD cholecalciferol 50,000IU QW vitamin D3 50,000IU QM | placebo | UACR, UPCR, 24-h urine protein excretion, UAER, SCr, | 8–24 weeks |
| Milajerdi 2019 [39] | EMBASE, Scopus, PubMed, Cochrane Library, and Web of Science | November 2018 | clinical trials | 17 non-dialysis (6)dialysis (11) | Cochrane RoB | CKD with or without dialysis | Calcitriol 0.03 mc/kg BIW−0.5 mcg/day Vitamin D3 4662–350000IU/week (QD QW Q2W QM) ergocalciferol 50,000 IU QW-QM | placebo | CRP | 3 and 52 weeks |
| Wang 2019 [21] | Pubmed, Embase, Cochrane Library, CNKI, WANGFANG and VIP | September 2007~July 2018 | RCTs | 20 T2DN (8) T1DN+T2DN (12) | Cochrane RoB | DN | calcitriol 0.14–1 mcg/day (QD, BIW) alfacalcidol 0.25–0.5 mcg/day cholecalciferol 800 IU/day Vitamin D3 50,000 IU/day | placebo or blank treatment | 24-h urine protein; UAER; SCr; eGFR; hs-CRP; TNF-α; IL-6 | 8–24 weeks |
| Zhang 2017 [42] | PubMed, Embase, Scopus, Index Copernicus, DOAJ, CNKI, and Wanfang | January 2017 | RCTs | 24 T2DN (17) T1DN (1) NA (6) | Newcastle–Ottawa scale | DN DN (20) DN+Vit.D deficient (4) | alfacalcidol calcitriol cholecalciferol paricalcitol | placebo | 24-h proteinuria, UACR, hs-CRP, SCr | 6–24 weeks |
| Derakhshanian 2015 [27] | PubMed, SCOPUS, and Google Scholar | September 2014 | RCTs (4), cross-sectional studies (6) | 10 | RCTs: Jadad score Cross-sectional studies: Newcastle—Ottawa Scale | DN | RCTs cholecalciferol 5600–50,000 IU/week (QD, QW) calcitriol 20 IU/day | placebo | UACR | RCTs 6–24 weeks |
| Zhao 2014 [41] | Pubmed, Embase, Sinomed, CNKI, Wanfang and clinical trial register centers | 1 January 2014 | RCTs | 20 | RoB | DN | Vitamin D3, paricalcitol, cholecalciferol, calcitrio, alfacalcidol | placebo or blank control | Change of 24-h proteinuria from baseline, UACR, urine microalbumin | F/U: 4–48 weeks |
| Xu 2013 [40] | PubMed, EMBASE and OvidSP | September 2012 | RCTs | 18 | Cochrane risk of bias | CKD without dialysis or renal transplantation | cholecalciferol 7000–75,000 IU/week (QD, QW, QM) calcitriol 0.25–0.5 mcg QD-BIW paricalcitol 0.86–4 mcg/day, QDTIW doxercalcigerol 1 mcg/day alfacalcidol 0.25–1 mcg QD | placebo or no medication | 24 hr-urine protein, UACR, eGFR, CCr | study duration: 1–24 months |
| Omega-3 polyunsaturated fatty acid (n-3 PUFA) | ||||||||||
| Saglimbene 2020 [22] | MEDLINE, Embase, and CENTRAL | 12 January 2018 | RCTs or quasi RCTs | 60 CKD stage 1–5 (20) dialysis (24) transplant (16) | GRADE | adults and children with CKD across all stages | Fish or n-3 PUFA supplementation (0.4–12 g/day) | placebo, standard care, or any other treatment | ESKD, acute transplant rejection, and allograft loss | treatment and F/U: 1–48 months |
| Hu 2017 [43] | PubMed, Embase, and Cochrane Library | October 2014 | RCTs | 9 CKD IgA nephropathy (7) ADPKD (1) | Jadad score | CKD (not on ESRD) | EPA 1.8–10 g/d, fish oil 6 g/day omega-3 capsules 4 g/day PUFAs 3.0 g/day | placebo, ACEI/ARB, low dose EPA and DHA, RASB, corn oil, olive oil | proteinuria, CCR, eGFR, occurrence of ESRD | F/U: 2–76.8 months |
| Dietary fiber | ||||||||||
| Wu 2019 [44] | PubMed, Web of Science and Cochrane Library | 1 September 2017 | clinical controlled trials; RCTs (6) pre-post trials (6) | 12 RCTs (6) pre-post trials (6) | Heyland Methodological Quality Score * | stage 3–5 of CKD with or without dialysis | dietary fibre intake 7.5–25 g/day | placebo | IS, PCS | mean 5 weks 2.1–10 weeks |
| Chiavaroli 2015 [24] | MEDLINE, EMBASE, CINHAL and Cochrane Library | 1 September 2014 | controlled feeding trials | 14 non-dialysis(10) HD (4) | Heyland Methodological Quality Score * | CKD with or without dialysis | dietary fiber intake (median fiber dose 26.9 g/day (range 3.1–50.0 g/day)) | non-fiber supplemented diets or low-fiber diets | serum urea, SCr | F/U median 4.5 weeks (range:1.4–20 weeks) |
| Coenzyme Q10 (CoQ10) | ||||||||||
| Zhang 2019 [45] | PubMed, Web of Science, Ovid-Medline, ProQuest, Science Direct, Springer link et al. ** | June 2018 | RCTs (4), experimental studies (4) | 8 | Cochrane risk of bias | type 1 or 2 diabetic kidney disease | CoQ10 (30–1000 mg/day) in combination with western medicine or CoQ10 | western medicine or placebo | eGFR, Serum Urea, BUN, Scr | 12 weeks |
| Probiotics, Prebiotics, Synbiotics | ||||||||||
| Zheng 2020 [48] | PubMed, Cochrane CENTRAL, and the Web of Science | 1 January 2000~15 May 2019 | RCTs | 13 CKD stages 2–4 (2) HD (7) DN (4) | Cochrane ROB | DN | Probiotics alone or associated with prebiotics (synbiotics) | placebo | CRP | 4–12 weeks |
| McFarlane 2019 [25] | MEDLINE, CINAHL, EMBASE, Cochrane Central Register of Controlled Trials, and International Clinical Trials Register and clinicaltrials.gov | July 2017 | RCTs | 16 non-dialysis (8)HD (7) PD (1) | Cochrane RoB | adults and children CKD with or without dialysis | prebiotic 2.3–50 g/day probiotic 11 × 106–2 × 1012 CFU/day | placebo | eGFR, SCr, proteinuria, serum urea, free and protein-bound concentrations of serum IS and PCS, progression to ESKD | 1–24 weeks |
| Tao 2019 [47] | PubMed, Embaseand Cochrane | September 2018 | RCTs | 10 non-dialysis (4) HD (5) PD (1) | Cochrane RoB | CKD with or without dialysis | probiotic supplementation 2 × 109–1.8 × 1011 CFU/day | placebo | urea, uric acid, CRP, SCr, eGFR | 6–24 weeks |
| Jia 2018 [46] | PubMed, EMBASE and Cochrane Library | 31 March 2018 | RCTs | 8 non-dialysis (4) HD (3) PD (1) | Cochrane RoB, GRADE | CKD with or without dialysis | Probiotics: 4 × 109–1.8 × 1011 CFU/day | placebo | BUN, SCr, CRP, IL-6 | 6–24 weeks |
| Pisano 2018 [23] | Ovid-MEDLINE, PubMed and CENTRAL | 5 March 2018 | RCTs | 17 non-dialysis (10) HD (5) PD (1) transplant (1) | Cochrane renal group, risk of bias | CKD or ESKD on chronic renal replacement | prebiotics 20–50 g/day, probiotics 2# tid; 2 x 109–9 × 1010 CFU/day synbiotics: 3–6#/day;1 5 gm powder/day; 1.1 × 107 CFU+inulin 2.31 g/day; 15 g powder+2#/day | placebo or standard therapy | CCr, eGFR, SCr, albuminuria, CRP, serum urea, TNF-α; IL-6 | 4–24 weeks |
| Certainty Assessment | Certainty * | Summary of Findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author year | Outcomes | No of Studies | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall Certainty of Evidence | Relative Effect (95% CI) | Risk Difference with Nutrition Compared to Placebo (95% CI) |
| Vitamin D and Analogues | ||||||||||
| Gupta 2019 [26] | UAER | 2 RCTs | serious a | serious b | not serious | not serious | none | ⨁⨁◯◯ LOW | - | MD 0.39 lower (0.71 lower to 0.07 lower) |
| UACR | 5 RCTs | very serious c | not serious | not serious | serious d | none | ⨁◯◯◯ VERY LOW | - | MD 0.14 lower (0.34 lower to 0.06 higher) | |
| UPCR | 1 RCT | serious e | not serious | not serious | serious d | none | ⨁⨁◯◯ LOW | - | MD 0.19 lower (0.9 lower to 0.51 higher) | |
| Wang 2019 [21] | eGFR | 4 RCTs | serious f | not serious | not serious | serious d | publication bias strongly suspected g | ⨁◯◯◯ VERY LOW | - | MD 2.13 higher (2.06 lower to 6.32 higher) |
| SCr | 9 RCTs | serious f | not serious | not serious | serious d | publication bias strongly suspected g | ⨁◯◯◯ VERY LOW | - | MD 0.83 lower (3.67 lower to 2.02 higher) | |
| Proteinuria (g/24 h) | 11 RCTs | serious f | not serious h | not serious | not serious | publication bias strongly suspected g | ⨁⨁◯◯ LOW | - | MD 0.26 lower (0.34 lower to 0.17 lower) | |
| UAER | 8 RCTs | serious f | very serious i | not serious | not serious | publication bias strongly suspected g | ⨁◯◯◯ VERY LOW | - | MD 67.36 lower (91.96 lower to 42.76 lower) | |
| Zhang 2017 [42] | Proteinuria (g/24 h) | 14 RCTs | serious j | very serious i | not serious | not serious | none | ⨁◯◯◯ VERY LOW | - | MD 0.23 lower (0.3 lower to 0.15 lower) |
| UACR | 8 RCTs | serious j | serious b | not serious | not serious | none | ⨁⨁◯◯ LOW | - | MD 0.49 lower (0.9 lower to 0.08 lower) | |
| SCr | 9 RCTs | serious j | serious b | not serious | serious d | none | ⨁◯◯◯ VERY LOW | SMD 0.16 SD lower (0.42 lower to 0.11 higher) | ||
| Derakhshanian 2015 [27] | UACR | 4 RCTs | not serious | not serious | not serious | serious d | none | ⨁⨁⨁◯ MODERATE | MD 17.99 higher (35.36 lower to 71.33 higher) | |
| Zhao 2014 [41] | Proteinuria (g/24 h) | 9 RCTs | very serious k | not serious | not serious | not serious | publication bias strongly suspected l | ⨁◯◯◯ VERY LOW | - | MD 0.44 lower (0.54 lower to 0.34 lower) |
| UACR | 4 RCTs | very serious k | not serious | not serious | not serious | publication bias strongly suspected l | ⨁◯◯◯ VERY LOW | - | SMD 0.29 SD lower (0.48 lower to 0.1 lower) | |
| Xu 2013 [40] | eGFR | 12 RCTs | serious f | not serious | not serious | serious d | none | ⨁⨁◯◯ LOW | - | SMD 0.1 SD lower (0.24 lower to 0.03 higher) |
| risk of dialysis initiation | 4 RCTs | serious f | not serious | not serious | serious d | none m | ⨁⨁◯◯ LOW | RR 1.48 (0.54 to 4.03) | ||
| reduced proteinuria | 6 RCTs | serious f | not serious | not serious | not serious | none m | ⨁⨁⨁◯ MODERATE | RR 2.00 (1.42 to 2.81) | ||
| Omega-3 Polyunsaturated Fatty Acid (Omega-3 PUFA) | ||||||||||
| Saglimbene 2020 [22] | GFR | 6 RCTs | serious a | not serious | not serious | serious d | none | ⨁⨁◯◯ LOW | SMD 0.22 SD higher (0.03 lower to 0.48 higher) | |
| progression to ESKD | 3 RCTs | serious a | serious n | not serious | serious n | none m | ⨁◯◯◯ VERY LOW n | RR 0.3 (0.09 to 0.98) | ||
| SCr | 7 RCTs | serious | serious o | not serious | serious d | none m | ⨁◯◯◯ VERY LOW | - | MD 2.20 higher (17.63 lower to 22.03 higher) | |
| Proteinuria (g/24 h) | 6 RCT | serious | not serious | not serious | serious d | none m | ⨁⨁◯◯ LOW | - | MD 0.16 lower (0.48 lower to 0.15 higher) | |
| Hu 2017 [43] | CCr | 6 RCTs | serious p | serious b | not serious | serious d | none | ⨁◯◯◯ VERY LOW | - | SMD 0.22 SD higher (0.40 lower to 0.84 higher) |
| eGFR | 6 RCTs | serious p | not serious | not serious | serious d | none | ⨁⨁◯◯ LOW | SMD 0.14 SD higher (0.13 lower to 0.42 higher) | ||
| the occurrence of end-stage renal disease | 3 RCTs | serious p | not serious | not serious | not serious | none m | ⨁⨁⨁◯ MODERATE | RR 0.49 (0.24 to 0.99) | ||
| Proteinuria (g/24 h) | 7 RCTs | very serious k | not serious | not serious | not serious | none | ⨁⨁◯◯ LOW | - | SMD 0.31 SD lower (0.53 lower to 0.10 lower) | |
| Dietary Fiber | ||||||||||
| Chiavaroli 2015 [24] | SCr | 8 RCTs | very serious k | not serious | not serious | serious d | none m | ⨁◯◯◯ VERY LOW | MD 21.97 lower (52.22 lower to 8.28 higher) | |
| serum urea | 9 RCTs | very serious k | serious b | not serious | serious d | none m | ⨁◯◯◯ VERY LOW | MD 2.35 lower (4.78 lower to 0.08 higher) | ||
| Coenzyme Q10 (CoQ10) | ||||||||||
| Zhang 2019 [45] | serum urea | 2 RCTs | serious q | very serious i | not serious | serious d | none | ⨁◯◯◯ VERY LOW | - | SMD 1.24 SD lower (4.04 lower to 1.55 higher) |
| Probiotics, Prebiotics, Synbiotics | ||||||||||
| McFarlane 2019 [25] | serum urea | 4 RCTs | very serious k | not serious | not serious | not serious | none | ⨁⨁◯◯ LOW | - | MD 2.12 lower (3.86 lower to 0.37 lower) |
| Tao 2019 [47] | serum urea | 2 RCTs | not serious | serious b | not serious | not serious | none | ⨁⨁⨁◯ MODERATE | - | MD 30.01 lower (56.78 lower to 3.25 lower) |
| Jia 2018 [46] | BUN | 1 RCT | very serious r | not serious | not serious | serious d | none | ⨁◯◯◯ VERY LOW | - | MD 5.78 lower (21.42 lower to 9.86 higher) |
| SCr | 3 RCTs | very serious k | not serious | not serious | serious d | none | ⨁◯◯◯ VERY LOW | - | MD 0.10 higher (0.11 lower to 0.31 higher) | |
| Pisano 2018 [23] | SCr | 7 RCTs | very serious k | not serious | not serious | serious d | none | ⨁◯◯◯ VERY LOW | - | MD 0.02 lower (0.09 lower to 0.05 higher) |
| serum urea | 5 RCTs | very serious k | not serious | not serious | serious d | none | ⨁◯◯◯ VERY LOW | - | SMD 0.20 SD lower (0.41 lower to 0.01 higher) | |
| Certainty Assessment | Certainty * | Summary of Findings | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Author Year | Outcomes | No of Studies | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Certainty of Evidence | Risk Difference with Nutrition Supplements Compared to Placebo (95% CI) |
| Vitamin D and Analogues | |||||||||
| Milajerdi 2019 [39] | CRP or hs-CRP | 5 RCTs | serious a | not serious | serious b | serious c | none | ⨁◯◯◯ VERY LOW | MD 0.41 lower (0.41 lower to 0.27 higher) |
| Wang 2019 [21] | hs-CRP | 7 RCTs | serious a | not serious | serious b | not serious | publication bias strongly suspected d | ⨁◯◯◯ VERY LOW | MD 0.69 lower (0.86 lower to 0.53 lower) |
| IL-6 | 3 RCTs | serious a | not serious | serious b | not serious | publication bias strongly suspected d | ⨁◯◯◯ VERY LOW | MD 0.73 lower (1.03 lower to 0.44 lower) | |
| TNF-α | 3 RCTs | serious a | very serious e | serious b | not serious | publication bias strongly suspected d | ⨁◯◯◯ VERY LOW | MD 56.79 lower (77.05 lower to 36.52 lower) | |
| Zhang 2017 [42] | hs-CRP | 7 RCTs | serious f | serious g | serious b | not serious | none | ⨁◯◯◯ VERY LOW | MD 0.80 lower (1.26 lower to 0.34 lower) |
| Dietary Fiber | |||||||||
| Wu 2019 [44] | IS | 5 RCTs | serious h | not serious i | serious b | serious c | none | ⨁◯◯◯ VERY LOW | MD 0.212 lower (2.35 lower to 1.926 higher) |
| PCS | 7 RCTs | serious h | not serious | serious b | not serious | none | ⨁⨁◯◯ LOW | MD 16.160 lower (23.824 lower to 8.492 lower) | |
| Coenzyme Q10 (CoQ10) | |||||||||
| Zhang 2019 [45] | MDA | 2 RCTs | serious h | serious g | serious b | not serious | none | ⨁◯◯◯ VERY LOW | SMD 1.29 SD lower (2.32 lower to 0.26 lower) |
| Probiotics, Prebiotics, Synbiotics | |||||||||
| Zheng 2020 [48] | MDA | 4 RCTs | not serious | serious g | serious b | not serious | none | ⨁⨁◯◯ LOW | SMD 0.79 SD lower (1.38 lower to 0.20 lower |
| CRP | 3 RCTs | not serious | not serious | serious b | not serious | none | ⨁⨁⨁◯ MODERATE | SMD 0.71 SD lower (1.01 lower to 0.40 lower | |
| Jia 2018 [46] | IL-6 | 1 RCT | not serious | not serious | serious b | serious c | none | ⨁⨁◯◯ LOW | MD 0.23 lower (0.27 lower to 0.73 higher) |
| PCS | 2 RCTs | not serious | serious g | serious b | not serious | none | ⨁⨁◯◯ LOW | MD 0.70 lower (1.4 lower to 0.01 lower) | |
| Author Year/Clinical Important Outcomes | UAER | UACR | UPCR | eGFR | SCr | Proteinuria | Risk of Dialysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk Difference (95% CI) | Certainty | Risk Difference (95% CI) | Certainty | Risk Difference (95% CI) | Certainty | Risk Difference (95% CI) | Certainty | Risk Difference (95% CI) | Certainty | Risk Difference (95% CI) | Certainty | Relative Risk (95%CI) | Certainty | |
| Diabetic Nephropathy | ||||||||||||||
| Gupta 2019 [26] | MD −0.39 (−0.71 to −0.07) | LOW | MD −0.14 (−0.34 to 0.06) | VERY LOW | MD −0.19 (−0.9 to 0.51) | LOW | - | - | - | - | - | - | - | - |
| Wang 2019 [21] | MD−67.36 (−91.96 to −42.76) | VERY LOW | - | - | - | - | MD 2.13 (−2.06 to 6.32) | VERY LOW | MD −0.83 (−3.67 to 2.02) | VERY LOW | MD −0.26 (−0.34 to −0.17) | LOW | - | - |
| Zhang 2017 [42] | - | - | MD −0.49 (−0.9 to −0.08) | LOW | - | - | - | - | SMD −0.16 SD (−0.42 to 0.11) | VERY LOW | MD −0.23 (−0.3 to −0.15) | VERY LOW | - | - |
| Derakhshanian 2015 [27] | - | - | MD 17.99 (−35.36 to 71.33) | MODERATE | - | - | - | - | - | - | - | - | - | - |
| Zhao 2014 [41] | - | - | SMD −0.29 SD (−0.48 to −0.1) | VERY LOW | - | - | - | - | MD −0.44 (−0.54 to −0.34) | VERY LOW | - | - | - | - |
| Chronic Kidney Disease | ||||||||||||||
| Xu 2013 [40] | - | - | - | - | - | - | SMD −0.1 SD (−0.24 to 0.03 | LOW | - | - | Reduced proteinuria: RR a 2.00 (1.42 to 2.81) | MODERATE | 1.48 (0.54 to 4.03) | LOW |
| Author Year/Clinical Important Outcomes | eGFR | Progression to ESKD | SCr | Proteinuria | CCr | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk Difference (95% CI) | Certainty | Relative Risk (95% CI) | Certainty | Risk Difference (95% CI) | Certainty | Risk Difference (95% CI) | Certainty | Risk Difference (95% CI) | Certainty | |
| Saglimbene 2020 [22] | SMD 0.22 SD (−0.03 to 0.48) | LOW | RR 0.3 (0.09 to 0.98) | VERY LOW | MD 2.20 (−17.63 to 22.03) | VERY LOW | MD −0.16 (−0.48 to 0.15) | LOW | - | - |
| Hu 2017 [43] | SMD 0.14 SD (−0.13 to 0.42) | LOW | RR 0.49 (0.24 to 0.99) | MODERATE | - | - | SMD −0.31 SD (−0.53 to −0.10) | LOW | SMD 0.22 SD (−0.40 to 0.84) | LOW |
| Author Year/Clinical Important Outcomes | Serum Urea | BUN | SCr | |||
|---|---|---|---|---|---|---|
| Risk Difference (95% CI) | Certainty | Risk Difference (95% CI) | Certainty | Risk Difference (95% CI) | Certainty | |
| McFarlane 2019 [25] | MD −2.12 a (−3.86 to −0.37) | LOW | - | - | - | - |
| Tao 2019 [47] | MD −30.01 b (−56.78 to −3.25) | MODERATE | - | - | - | - |
| Jia 2018 [46] | - | - | MD −5.78 (−21.42 to 9.86) | VERY LOW | MD 0.10 (−0.11 to 0.31) | VERY LOW |
| Pisano 2018 [23] | SMD −0.20 SD (−0.41 to 0.01) | VERY LOW | - | - | MD −0.02 (−0.09 to 0.05) | VERY LOW |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.-C.; Chou, C.-L.; Ou, S.-H.; Fang, T.-C.; Chen, J.-S. Systematic Review of Nutrition Supplements in Chronic Kidney Diseases: A GRADE Approach. Nutrients 2021, 13, 469. https://doi.org/10.3390/nu13020469
Lin P-C, Chou C-L, Ou S-H, Fang T-C, Chen J-S. Systematic Review of Nutrition Supplements in Chronic Kidney Diseases: A GRADE Approach. Nutrients. 2021; 13(2):469. https://doi.org/10.3390/nu13020469
Chicago/Turabian StyleLin, Pei-Chin, Chu-Lin Chou, Shih-Hsiang Ou, Te-Chao Fang, and Jin-Shuen Chen. 2021. "Systematic Review of Nutrition Supplements in Chronic Kidney Diseases: A GRADE Approach" Nutrients 13, no. 2: 469. https://doi.org/10.3390/nu13020469
APA StyleLin, P.-C., Chou, C.-L., Ou, S.-H., Fang, T.-C., & Chen, J.-S. (2021). Systematic Review of Nutrition Supplements in Chronic Kidney Diseases: A GRADE Approach. Nutrients, 13(2), 469. https://doi.org/10.3390/nu13020469






