Effects of Acute Dietary Polyphenols and Post-Meal Physical Activity on Postprandial Metabolism in Adults with Features of the Metabolic Syndrome
Abstract
:1. Introduction
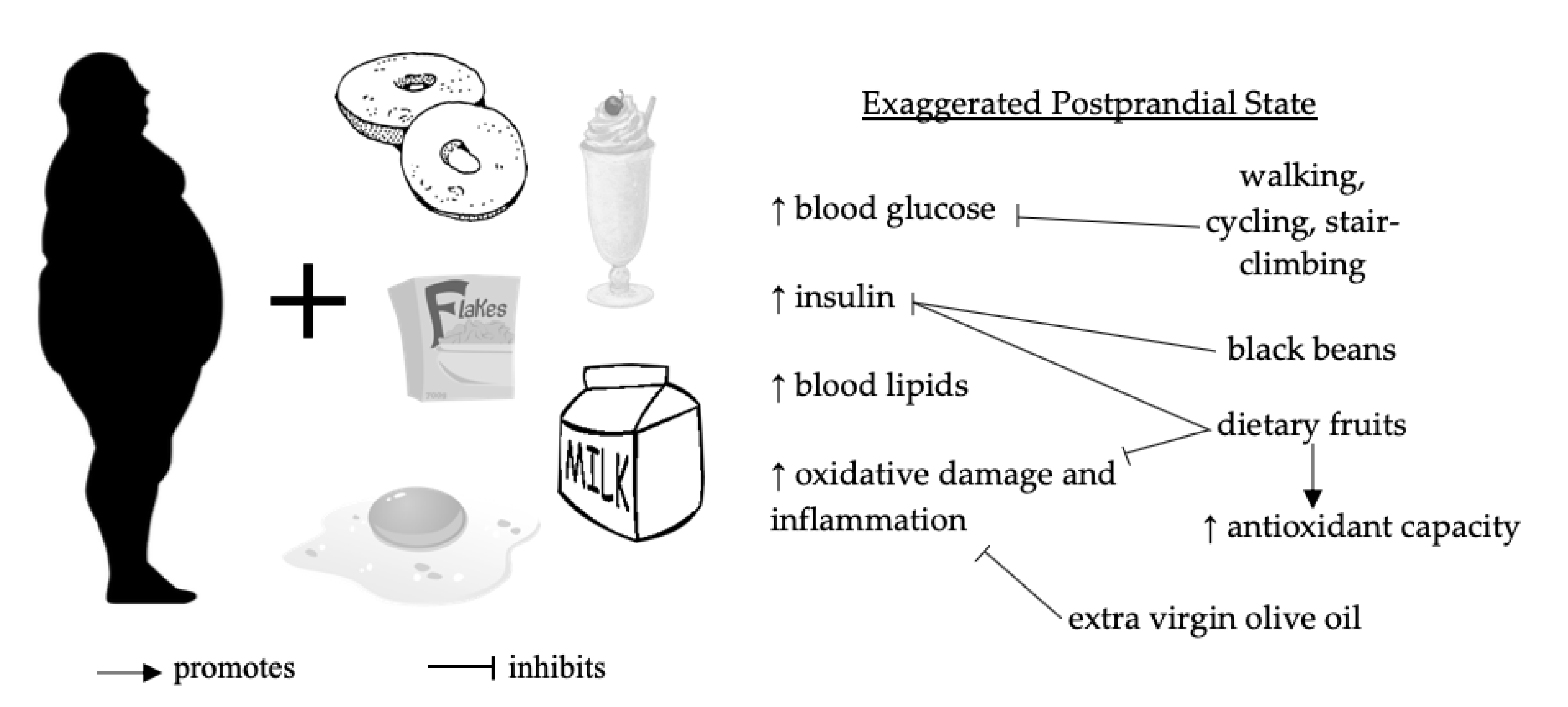
1.1. Postprandial Dysmetabolism
1.2. Preventative Roles of Dietary Polyphenols and Physical Activity
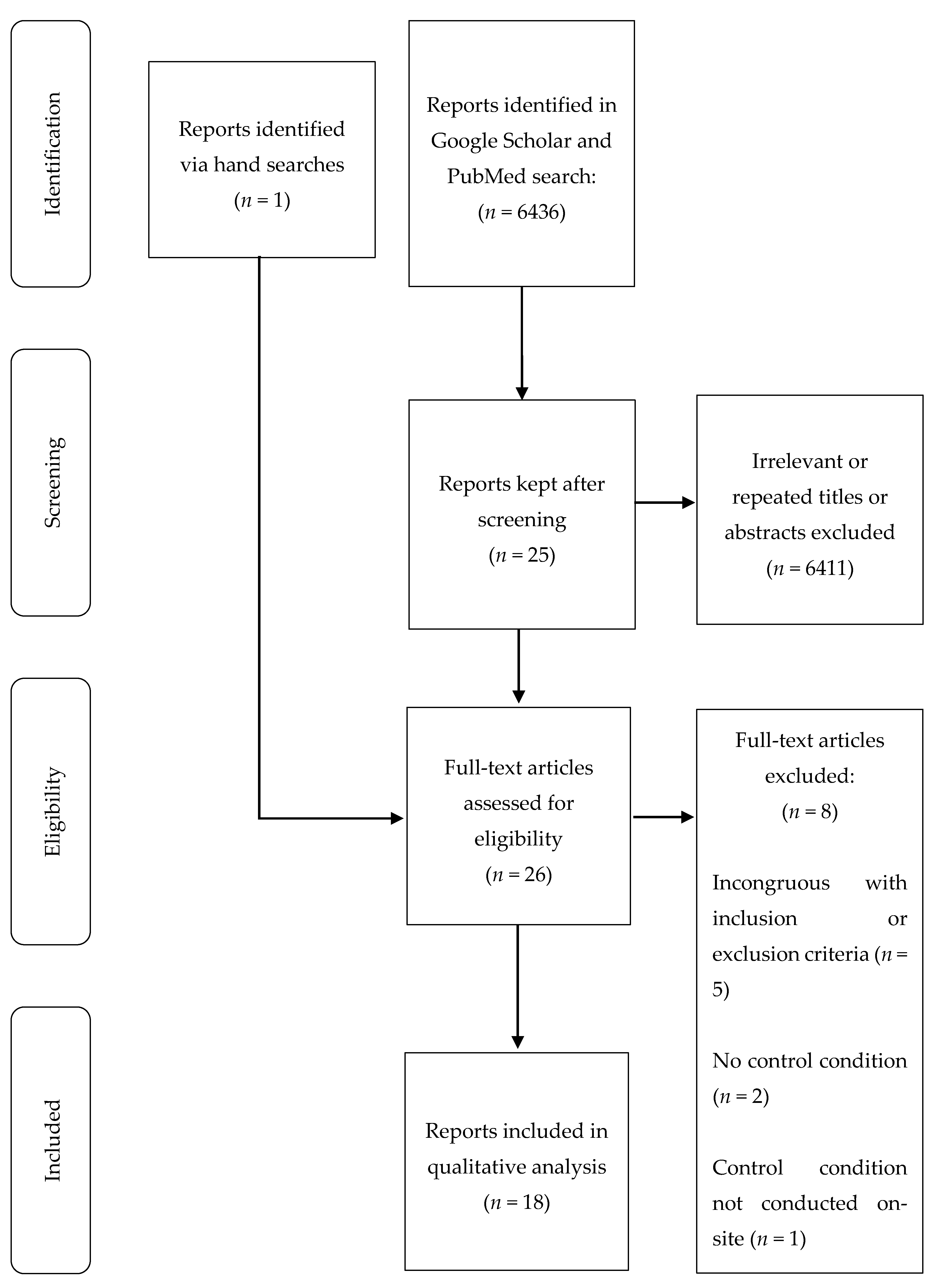
2. Materials and Methods
3. Results
3.1. Demographics
3.2. Effects of Dietary Oils, Fruits, Teas, and Legumes on Postprandial Glucose, Insulin, and Lipids
3.3. Effects of Dietary Oils, Fruits, Teas, and Legumes on Postprandial Markers of Oxidative Damage and Inflammation
3.4. Effects of Physical Activity on Postprandial Glucose, Insulin, and Lipids
3.5. Effects of Physical Activity on Postprandial Markers of Oxidative Damage and Inflammation
4. Discussion
4.1. Dietary Polyphenols from Oils, Fruits, Teas, and Legumes
4.2. Walking, Cycling, and Stair Climbing and Descending
4.3. Recommendations
4.4. Strengths and Weaknesses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Statistics about Diabetes | ADA. Available online: https://www.diabetes.org/resources/statistics/statistics-about-diabetes (accessed on 22 January 2020).
- Moore, J.X. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic. Dis. 2017, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.; Cleeman, J.; Daniels, S.; Donato, K.; Eckel, R.; Franklin, B.; Gordon, D.; Krauss, R.; Savage, P.; Smith, S.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Yearb. Endocrinol. 2006, 2005, 2735–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef] [Green Version]
- Lakka, H.-M. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002, 288, 2709–2716. [Google Scholar] [CrossRef]
- Diminia, L.; Mariotti, F. The postprandial appearance of features of cardiometabolic risk: Acute induction and prevention by nutrients and other dietary substances. Nutrients 2019, 11, 1963. [Google Scholar] [CrossRef] [Green Version]
- Pappas, C.; Kandaraki, E.A.; Tsirona, S.; Kountouras, D.; Kassi, G.; Diamanti-Kandarakis, E. Postprandial dysmetabolism: Too early or too late? HORMONES 2016, 15, 321–344. [Google Scholar] [CrossRef] [Green Version]
- Kohan, A.B. ApoC-III: A potent modulator of hypertriglyceridemia and cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 22, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Pruneta-Deloche, V.; Sassolas, A.; Dallinga-Thie, G.M.; Berthezène, F.; Ponsin, G.; Moulin, P. Alteration in lipoprotein lipase activity bound to triglyceride-rich lipoproteins in the postprandial state in type 2 diabetes. J. Lipid Res. 2004, 45, 859–865. [Google Scholar] [CrossRef] [Green Version]
- Pratley, R.E.; Weyer, C. The role of impaired early insulin secretion in the pathogenesis of type II diabetes mellitus. Diabetologia 2001, 44, 929–945. [Google Scholar] [CrossRef] [Green Version]
- Pratley, R.E.; Weyer, C. Progression from IGT to type 2 diabetes mellitus: The central role of impaired early insulin secretion. Curr. Diab. Rep. 2002, 2, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Pastromas, S.; Terzi, A.-B.; Tousoulis, D.; Koulouris, S. Postprandial lipemia: An under-recognized atherogenic factor in patients with diabetes mellitus. Int. J. Cardiol. 2008, 126, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Sottero, B.; Gargiulo, S.; Russo, I.; Barale, C.; Poli, G.; Cavalot, F. Postprandial dysmetabolism and oxidative stress in type 2 diabetes: Pathogenetic mechanisms and therapeutic strategies: Postprandial dysmetabolism, oxidative stress in T2D. Med. Res. Rev. 2015, 35, 968–1031. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Pang, J.; Romic, G.; Watts, G.F. Postprandial hypertriglyceridemia and cardiovascular disease: Current and future therapies. Curr. Atheroscler. Rep. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Bell, D.S.H. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef]
- Coutinho, M.; Gerstein, H.C.; Wang, Y.; Yusuf, S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999, 22, 233–240. [Google Scholar] [CrossRef]
- Balkau, B.; Shipley, M.; Jarrett, R.J.; Pyorala, K.; Pyorala, M.; Forhan, A.; Eschwege, E. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men: 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care 1998, 21, 360–367. [Google Scholar] [CrossRef]
- Chiasson, J.-L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M.; STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: The STOP-NIDDM Trial. JAMA 2003, 290, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Hanefeld, M.; Chiasson, J.L.; Koehler, C.; Henkel, E.; Schaper, F.; Temelkova-Kurktschiev, T. Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke 2004, 35, 1073–1078. [Google Scholar] [CrossRef]
- Sasso, F.C. Glucose metabolism and coronary heart disease in patients with normal glucose tolerance. JAMA 2004, 291, 1857–1863. [Google Scholar] [CrossRef] [Green Version]
- Borch-Johnsen, K.; Neil, A.; Balkau, B.; Larsen, S.; Nissinen, A.; Pekkanen, J.; Tuomilehto, J.; Jousilahti, P.; Lindstrom, J.; Pyorala, M.; et al. Glucose tolerance and cardiovascular mortality—Comparison of fasting and 2-hour diagnostic criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar] [CrossRef]
- Temelkova-Kurktschiev, T.S.; Koehler, C.; Henkel, E.; Leonhardt, W.; Fuecker, K.; Hanefeld, M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 2000, 23, 1830–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, M.J.; Ginsberg, H.N.; Amarenco, P.; Andreotti, F.; Borén, J.; Catapano, A.L.; Descamps, O.S.; Fisher, E.; Kovanen, P.T.; Kuivenhoven, J.A.; et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur. Heart J. 2011, 32, 1345–1361. [Google Scholar] [CrossRef] [Green Version]
- Nordestgaard, B.G.; Varbo, A. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [CrossRef] [PubMed]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Annuzzi, G.; Bozzetto, L.; Costabile, G.; Giacco, R.; Mangione, A.; Anniballi, G.; Vitale, M.; Vetrani, C.; Cipriano, P.; Corte, G.D.; et al. Diets naturally rich in polyphenols improve fasting and postprandial dyslipidemia and reduce oxidative stress: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 463–471. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, Y.; Tani, M.; Kondo, K. Pleiotropic preventive effects of dietary polyphenols in cardiovascular diseases. Eur. J. Clin. Nutr. 2013, 67, 532–535. [Google Scholar] [CrossRef] [Green Version]
- Edirisinghe, I.; Burton-Freeman, B. Anti-diabetic actions of Berry polyphenols—Review on proposed mechanisms of action. J. Berry Res. 2016, 6, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Bozzetto, L.; Annuzzi, G.; Pacini, G.; Costabile, G.; Vetrani, C.; Vitale, M.; Griffo, E.; Giacco, A.; De Natale, C.; Cocozza, S.; et al. Polyphenol-rich diets improve glucose metabolism in people at high cardiometabolic risk: A controlled randomised intervention trial. Diabetologia 2015, 58, 1551–1560. [Google Scholar] [CrossRef] [Green Version]
- Bogani, P.; Galli, C.; Villa, M.; Visioli, F. Postprandial anti-inflammatory and antioxidant effects of extra virgin olive oil. Atherosclerosis 2007, 190, 181–186. [Google Scholar] [CrossRef]
- Nyambe-Silavwe, H.; Williamson, G. Polyphenol- and fibre-rich dried fruits with green tea attenuate starch-derived postprandial blood glucose and insulin: A randomised, controlled, single-blind, cross-over intervention. Br. J. Nutr. 2016, 116, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Miyashita, M.; Suzuki, K.; Bae, S.; Kim, H.-K.; Wakisaka, T.; Matsui, Y.; Takeshita, M.; Yasunaga, K. Acute ingestion of catechin-rich green tea improves postprandial glucose status and increases serum thioredoxin concentrations in postmenopausal women. Br. J. Nutr. 2014, 112, 1542–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmori, R.; Iwamoto, T.; Tago, M.; Takeo, T.; Unno, T.; Itakura, H.; Kondo, K. Antioxidant activity of various teas against free radicals and LDL oxidation. Lipids 2005, 40, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, S.; Kanner, J.; Schurr, D.; Kohen, R. A rational approach to prevent postprandial modification of LDL by dietary polyphenols. J. Funct. Foods 2013, 5, 163–169. [Google Scholar] [CrossRef]
- Josic, J.; Olsson, A.T.; Wickeberg, J.; Lindstedt, S.; Hlebowicz, J. Does green tea affect postprandial glucose, insulin and satiety in healthy subjects: A randomized controlled trial. Nutr. J. 2010, 9. [Google Scholar] [CrossRef] [Green Version]
- Minuk, H.L.; Vranic, M.; Marliss, E.B.; Hanna, A.K.; Albisser, A.M.; Zinman, B. Glucoregulatory and metabolic response to exercise in obese noninsulin-dependent diabetes. Am. J. Physiol.-Endocrinol. Metab. 1981, 240, E458–E464. [Google Scholar] [CrossRef]
- Kennedy, J.W.; Hirshman, M.F.; Gervino, E.V.; Ocel, J.V.; Forse, R.A.; Hoenig, S.J.; Aronson, D.; Goodyear, L.J.; Horton, E.S. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 1999, 48, 1192–1197. [Google Scholar] [CrossRef]
- Jessen, N.; Goodyear, L.J. Contraction signaling to glucose transport in skeletal muscle. J. Appl. Physiol. 2005, 99, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, T.; Wojtaszewski, J.F.P.; Goodyear, L.J. Exercise regulation of glucose transport in skeletal muscle. Am. J. Physiol.-Endocrinol. Metab. 1997, 273, E1039–E1051. [Google Scholar] [CrossRef]
- Ahlborg, G.; Björkman, O. Carbohydrate utilization by exercising muscle following pre-exercise glucose ingestion. Clin. Physiol. 1987, 7, 181–195. [Google Scholar] [CrossRef]
- Sylow, L.; Kleinert, M.; Richter, E.A.; Jensen, T.E. Exercise-stimulated glucose uptake—Regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017, 13, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Ballard, K.D.; Berry, C.W.; Varty, C.J.; Arslain, K.B.; Timmerman, K.L. Aerobic or resistance exercise performed the previous day does not attenuate postprandial hyperglycemia-induced endothelial dysfunction in overweight/obese adults. Eur. J. Appl. Physiol. 2019, 119, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; McClean, C.M.; Davison, G.W.; Brown, J.C.W.; Murphy, M.H. Preceding exercise and postprandial hypertriglyceridemia: Effects on lymphocyte cell DNA damage and vascular inflammation. Lipids Health Dis. 2019, 18, 125. [Google Scholar] [CrossRef] [Green Version]
- Paul, D.J.; Nassis, G.P.; Kerouani, A.C.; Bangsbo, J. Postprandial lipaemia 10 and 34 hours after playing football: Does playing frequency affect the response? PLoS ONE 2019, 14, e0218043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gay, J.L.; Buchner, D.M.; Erickson, M.L.; Lauture, A. Effect of short bouts of high intensity activity on glucose among adults with prediabetes: A pilot randomized crossover study. Diabetes Res. Clin. Pract. 2018, 141, 168–174. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, A.T.; Tolves, T.; Lenzi, T.L.; Signori, L.U.; da Silva, A.M.V. High-intensity interval training versus continuous training on physiological and metabolic variables in prediabetes and type 2 diabetes: A meta-analysis. Diabetes Res. Clin. Pract. 2018, 137, 149–159. [Google Scholar] [CrossRef]
- Mestek, M.L.; Plaisance, E.P.; Ratcliff, L.A.; Taylor, J.K.; Wee, S.-O.; Grandjean, P.W. Aerobic exercise and postprandial lipemia in men with the metabolic syndrome. Med. Sci. Sports Exerc. 2008, 40, 2105–2111. [Google Scholar] [CrossRef]
- Emerson, S.R.; Kurti, S.P.; Snyder, B.S.; Sitaraman, K.; Haub, M.D.; Rosenkranz, S.K. Effects of thirty and sixty minutes of moderate-intensity aerobic exercise on postprandial lipemia and inflammation in overweight men: A randomized cross-over study. J. Int. Soc. Sports Nutr. 2016, 13, 26. [Google Scholar] [CrossRef] [Green Version]
- Borror, A.; Zieff, G.; Battaglini, C.; Stoner, L. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: A systematic review. Sports Med. 2018, 48, 1479–1491. [Google Scholar] [CrossRef]
- Carnevale, R.; Pastori, D.; Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Novo, M.; Del Ben, M.; Farcomeni, A.; Angelico, F.; Violi, F. Gut-derived lipopolysaccharides increase post-prandial oxidative stress via Nox2 activation in patients with impaired fasting glucose tolerance: Effect of extra-virgin olive oil. Eur. J. Nutr. 2019, 58, 843–851. [Google Scholar] [CrossRef]
- Bardagjy, A.S.; Hu, Q.; Giebler, K.A.; Ford, A.; Steinberg, F.M. Effects of grape consumption on biomarkers of inflammation, endothelial function, and PBMC gene expression in obese subjects. Arch. Biochem. Biophys. 2018, 646, 145–152. [Google Scholar] [CrossRef]
- Vors, C.; Couillard, C.; Paradis, M.-E.; Gigleux, I.; Marin, J.; Vohl, M.-C.; Couture, P.; Lamarche, B. Supplementation with resveratrol and curcumin does not affect the inflammatory response to a high-fat meal in older adults with abdominal obesity: A randomized, placebo-controlled crossover trial. J. Nutr. 2018, 148, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Reverri, E.J.; Randolph, J.M.; Kappagoda, C.T.; Park, E.; Edirisinghe, I.; Burton-Freeman, B.M. Assessing beans as a source of intrinsic fiber on satiety in men and women with metabolic syndrome. Appetite 2017, 118, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Edirisinghe, I.; Wei, H.; Vijayakumar, L.P.; Banaszewski, K.; Cappozzo, J.C.; Burton-Freeman, B. A dose-response evaluation of freeze-dried strawberries independent of fiber content on metabolic indices in abdominally obese individuals with insulin resistance in a randomized, single-blinded, diet-controlled crossover trial. Mol. Nutr. Food Res. 2016, 60, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Reverri, E.; Randolph, J.; Steinberg, F.; Kappagoda, C.; Edirisinghe, I.; Burton-Freeman, B. Black beans, fiber, and antioxidant capacity pilot study: Examination of whole foods vs. functional components on postprandial metabolic, oxidative stress, and inflammation in adults with metabolic syndrome. Nutrients 2015, 7, 6139–6154. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Randolph, J.; Cheema, M.; Tadapaneni, R.; Park, E.; Burton-Freeman, B.; Kappagoda, T. Effect of grape seed extract on postprandial oxidative status and metabolic responses in men and women with the metabolic syndrome—Randomized, cross-over, placebo-controlled study. Funct. Foods Health Dis. 2012, 2, 508–521. [Google Scholar] [CrossRef]
- Huebbe, P.; Giller, K.; de Pascual-Teresa, S.; Arkenau, A.; Adolphi, B.; Portius, S.; Arkenau, C.N.; Rimbach, G. Effects of blackcurrant-based juice on atherosclerosis-related biomarkers in cultured macrophages and in human subjects after consumption of a high-energy meal. Br. J. Nutr. 2012, 108, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Diekmann, C.; Huber, H.; Preuß, M.; Preuß, P.; Predel, H.-G.; Stoffel-Wagner, B.; Fimmers, R.; Stehle, P.; Egert, S. Moderate postmeal walking has no beneficial effects over resting on postprandial lipemia, glycemia, insulinemia, and selected oxidative and inflammatory parameters in older adults with a cardiovascular disease risk phenotype: A randomized crossover trial. J. Nutr. 2019, 149, 1930–1941. [Google Scholar] [CrossRef]
- DiPietro, L.; Gribok, A.; Stevens, M.S.; Hamm, L.F.; Rumpler, W. Three 15-min bouts of moderate postmeal walking significantly improves 24-h glycemic control in older people at risk for impaired glucose tolerance. Diabetes Care 2013, 36, 3262–3268. [Google Scholar] [CrossRef] [Green Version]
- Lunde, M.S.H.; Hjellset, V.T.; Høstmark, A.T. Slow post meal walking reduces the blood glucose response: An exploratory study in female pakistani immigrants. J. Immigr. Minor. Health 2012, 14, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Derave, W.; Mertens, A.; Muls, E.; Pardaens, K.; Hespel, P. Effects of post-absorptive and postprandial exercise on glucoregulation in metabolic syndrome. Obesity 2007, 15, 704–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butacnum, A.; Chongsuwat, R.; Bumrungpert, A. Black tea consumption improves postprandial glycemic control in normal and pre-diabetic subjects: A randomized, double-blind, placebo-controlled crossover study. Asia Pac. J. Clin. Nutr. 2017, 26, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Bartholomae, E.; Johnson, Z.; Moore, J.; Ward, K.; Kressler, J. Reducing glycemic indicators with moderate intensity stepping of varied, short durations in people with pre-diabetes. J. Sports Sci. Med. 2018, 17, 680–685. [Google Scholar] [PubMed]
- Takaishi, T.; Hayashi, T. Stair ascending–descending exercise accelerates the decrease in postprandial hyperglycemia more efficiently than bicycle exercise. BMJ Open Diabetes Res. Care 2017, 5. [Google Scholar] [CrossRef]
- Takaishi, T.; Imaeda, K.; Tanaka, T.; Moritani, T.; Hayashi, T. A short bout of stair climbing–descending exercise attenuates postprandial hyperglycemia in middle-aged males with impaired glucose tolerance. Appl. Physiol. Nutr. Metab. 2012, 37, 193–196. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Linares, A.; Hyson, D.; Kappagoda, T. Strawberry modulates ldl oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J. Am. Coll. Nutr. 2010, 29, 46–54. [Google Scholar] [CrossRef]
- Vergès, B. Pathophysiology of diabetic dyslipidaemia: Where are we? Diabetologia 2015, 58, 886–899. [Google Scholar] [CrossRef] [Green Version]
- Pang, J.; Chan, D.C.; Barrett, P.H.R.; Watts, G.F. Postprandial dyslipidaemia and diabetes: Mechanistic and therapeutic aspects. Curr. Opin. Lipidol. 2012, 23, 303–309. [Google Scholar] [CrossRef]
- Harte, A.L.; Varma, M.C.; Tripathi, G.; McGee, K.C.; Al-Daghri, N.M.; Al-Attas, O.S.; Sabico, S.; O’Hare, J.P.; Ceriello, A.; Saravanan, P.; et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care 2012, 35, 375–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witztum, J.L. The oxidation hypothesis of atherosclerosis. Lancet 1994, 344, 793–795. [Google Scholar]
- Rajkovic, N.; Zamaklar, M.; Lalic, K.; Lalic, N.M.; Popovic, L.; Draskovic-Radojkovic, D.; Singh, S.; Stosic, L.; Jotic, A.; Lukic, L.; et al. OP3: Oxidized LDL as residual lipid risk marker in type 2 diabetes. Diabetes Metab. 2012, 38, S98–S99. [Google Scholar] [CrossRef]
- Corder, R.; Douthwaite, J.A.; Lees, D.M.; Khan, N.Q.; dos Santos, A.C.V.; Wood, E.G.; Carrier, M.J. Endothelin-1 synthesis reduced by red wine. Nature 2001, 414, 863–864. [Google Scholar] [CrossRef] [PubMed]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [Green Version]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Peetz, D.; Hafner, G.; Tiret, L.; Meyer, J.; The AtheroGene Investigators. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 2001, 104, 1336–1342. [Google Scholar] [CrossRef] [Green Version]
- Strawberry, Raw. Available online: http://phenol-explorer.eu/contents/food/69 (accessed on 28 January 2020).
- Wojdyło, A.; Figiel, A.; Oszmiański, J. Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. J. Agric. Food Chem. 2009, 57, 1337–1343. [Google Scholar] [CrossRef]
- Domitrovic, R. The molecular basis for the pharmacological activity of anthocyans. Curr. Med. Chem. 2011, 18, 4454–4469. [Google Scholar] [CrossRef]
- Parrinello, C.M.; Lutsey, P.L.; Ballantyne, C.M.; Folsom, A.R.; Pankow, J.S.; Selvin, E. Six-year change in high-sensitivity C-reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am. Heart J. 2015, 170, 380–389.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, M.; Kobayashi, Y.; Suzuki, M.; Satsu, H.; Miyamoto, Y. Regulation of intestinal glucose transport by tea catechins. BioFactors 2000, 13, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Furne, J.K.; Levitt, M.D. An extract of black, green, and mulberry teas causes malabsorption of carbohydrate but not of triacylglycerol in healthy volunteers. Am. J. Clin. Nutr. 2006, 84, 551–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryans, J.A.; Judd, P.A.; Ellis, P.R. The effect of consuming instant black tea on postprandial plasma glucose and insulin concentrations in healthy humans. J. Am. Coll. Nutr. 2007, 26, 471–477. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Jennings, A.; Welch, A.A.; Spector, T.; Macgregor, A.; Cassidy, A. Intakes of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J. Nutr. 2014, 144, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.V.; Winham, D.M.; Hutchins, A.M. Bean and rice meals reduce postprandial glycemic response in adults with type 2 diabetes: A cross-over study. Nutr. J. 2012, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Spadafranca, A.; Rinelli, S.; Riva, A.; Morazzoni, P.; Magni, P.; Bertoli, S.; Battezzati, A. Phaseolus vulgaris extract affects glycometabolic and appetite control in healthy human subjects. Br. J. Nutr. 2013, 109, 1789–1795. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, J.F. Fatty acid mobilization from adipose tissue during exercise. Trends Endocrinol. Metab. 2003, 14, 386–392. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Bonen, A.; Luiken, J.J.F.P. Exercise and insulin increase muscle fatty acid uptake by recruiting putative fatty acid transporters to the sarcolemma. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Klip, A.; Ramlal, T.; Bilan, P.J.; Cartee, G.D.; Gulve, E.A.; Holloszy, J.O. Recruitment of GLUT-4 glucose transporters by insulin in diabetic rat skeletal muscle. Biochem. Biophys. Res. Commun. 1990, 172, 728–736. [Google Scholar] [CrossRef]
- King, P.A.; Horton, E.D.; Hirshman, M.F.; Horton, E.S. Insulin resistance in obese Zucker rat (fa/fa) skeletal muscle is associated with a failure of glucose transporter translocation. J. Clin. Invest. 1992, 90, 1568–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napoli, R.; Hirshman, M.F.; Horton, E.S. Mechanisms and time course of impaired skeletal muscle glucose transport activity in streptozocin diabetic rats. J. Clin. Invest. 1995, 96, 427–437. [Google Scholar] [CrossRef]
- Hirshman, M.F.; Wallberg-Henriksson, H.; Wardzala, L.J.; Horton, E.D.; Horton, E.S. Acute exercise increases the number of plasma membrane glucose transporters in rat skeletal muscle. FEBS Lett. 1988, 238, 235–239. [Google Scholar] [CrossRef] [Green Version]
- Douen, A.G.; Ramlal, T.; Rastogi, S.; Bilan, P.J.; Cartee, G.D.; Vranic, M.; Holloszy, J.O.; Klip, A. Exercise induces recruitment of the “insulin-responsive glucose transporter”. Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J. Biol. Chem. 1990, 265, 13427–13430. [Google Scholar]
- Goodyear, L.J.; Hirshman, M.F.; Horton, E.S. Exercise-induced translocation of skeletal muscle glucose transporters. Am. J. Physiol. 1991, 261, E795–E799. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2017; ISBN 978-1-4963-3906-5. [Google Scholar]
- Office of Disease Prevention and Health Promotion Physical Activity Guidelines for Americans. Available online: https://health.gov/our-work/physical-activity/current-guidelines (accessed on 28 January 2020).
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Price, K.D.; Price, C.S.C.; Reynolds, R.D. Hyperglycemia-induced ascorbic acid deficiency promotes endothelial dysfunction and the development of atherosclerosis. Atherosclerosis 2001, 158, 1–12. [Google Scholar] [CrossRef]
- Blaak, E.E.; Antoine, J.-M.; Benton, D.; Björck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshof, T.; Holst, J.J.; et al. Impact of postprandial glycaemia on health and prevention of disease. Obes. Rev. 2012, 13, 923–984. [Google Scholar] [CrossRef]
- Zorica, Č.; Nada, K.; Vera, Ć.; Zoran, Ć.; Đorđe, M.; Sanja, I.; Biljana, P. Effects of acute exercise on atherogenic lipids in untreated mild hypertensive patients. Vojnosanit. Pregl. 2009, 66, 313–318. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, S.F.; Terada, T.; Chahal, B.S.; Boulé, N.G. Exercise lowers postprandial glucose but not fasting glucose in type 2 diabetes: A meta-analysis of studies using continuous glucose monitoring: Effects of Exercise in Type 2 Diabetes. Diabetes Metab. Res. Rev. 2013, 29, 593–603. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture Dietary Guidelines for Americans 2015–2020. Available online: https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/ (accessed on 28 January 2020).
- Strawberries, Raw Nutrition Facts & Calories. Available online: https://nutritiondata.self.com/facts/fruits-and-fruit-juices/2064/2 (accessed on 11 February 2020).
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [Green Version]
| Authors, Year (Country) | Trial Design | Participants 1 | Intervention and Dietary Challenge | Glucose and Insulin | Lipids | Markers of Oxidative Damage and Inflammation |
|---|---|---|---|---|---|---|
| Carnevale et al., 2019 (Italy) [52] | Randomized crossover | Obese adults with IFG (n = 30, age = 58 ± 11) | 10 g EVOO Test meal (~725–750 kcal, 28%–30% fat, 53%–54% CHO, 16%–19% PRO) | NR | ↓ ApoB48 at 2 h | ↓ LPS at 1 h and 2 h ↓ OxLDL at 1 h and 2 h ↓ sNox2-dp at 1 h and 2 h |
| Bardagjy et al., 2018 (USA) [53] | Randomized crossover | Obese adults (n = 20, 12/20 with MetS, age = 49 ± 15 years) | 60 g GP Test meal (~1035 kcal, 47% fat, 41% CHO, 12% PRO) | NS 5-h glucose iAUC, 5-h insulin iAUC | NS 5-h TG iAUC | NS IL-6, MCP-1, OxLDL, PAI-1, RBP4, sICAM-1, sVCAM-1, TNF ↓ ET-1 at 5 h |
| Vors et al., 2018 (Canada) [54] | Randomized crossover | Older adults (n = 22, 7/22 with MetS, age = 53–70 years) | Res + Cur (200 mg Res + 100 mg Cur) Homogenized milkshake (~1110 kcal, 75 g fat, 60% fat, 25% CHO, 15% PRO) | NS 6-h glucose iAUC, 6-h insulin iAUC | NS 6-h TG iAUC | NS 6-h iAUC for IL-6, IL-8, MCP-1, CRP, sICAM-1, sE-selectin ↓ 6-h sVCAM-1 iAUC |
| Butacnum et al., 2017 (Thailand) [64] | Randomized crossover | Adults with pre-diabetes (n = 11, age = 45 ± 10 years) | 500 mL black tea with low and high dose of BTPP (110 and 220 g, respectively) 50 g sucrose in 200 mL water | ↓ 1-h and 1.5-h glucose iAUC (110 and 220 mg BTPP) NS insulin | NR | NR |
| Reverri et al., 2017 and Reverri et al., 2015 (USA) [55,57] | Randomized crossover | Adults with MetS (n = 12, age = 49 ± 14 years) | BB, AF, or NF Test meal with BB, AF, or NF (~930 kcal, 25 g fat) | NS glucose ↓ 5-h insulin (BB vs. AF and NF) | NS TG | NS IL-6, OxLDL, sICAM-1, sVCAM-1 |
| Park et al., 2016 (USA) [56] | Randomized crossover | Obese adults with IFG (n = 21, age = 40 ± 14 years) | 0, 10, 20, or 40 g FDS Bagel, cream cheese, margarine, hard-boiled egg, cantaloupe, and whole milk with strawberry beverage (~975 kcal, 25 g fat) | NS glucose ↓ 6-h insulin (40 g FDS vs. 0 g and 10 g FDS) ↓ insulin absolute peak and incremental increase from baseline (40 g FDS) ↓ I:G ratio (40 g vs. 0 g and 10 g FDS) | NS TG | NS IL-6, ORAC ↓ 6-h OxLDL (normalized to fasting; 20 g vs. 40 g, 10 g, and 0 g FDS) |
| Edirisinghe et al., 2012 (USA) [58] | Randomized crossover | Adults with MetS (n = 12, age = 45 ± 15 years) | 300 mg GSE Bagel, cream cheese, margarine, egg, cantaloupe, and whole milk (~670 kcal, 30 g fat) | NS 6-h insulin AUC ↓ 6-h glucose AUC | NS 6-h TG AUC, 6-h cholesterol AUC | NS 6-h IL-6, TNF-α, lipophilic ORAC iAUC ↓ OxLDL at 5 h vs. baseline ↑ 6-h hydrophilic ORAC iAUC |
| Huebbe et al., 2012 (Germany) [59] | Crossover | Adult males with atherosclerosis-prone phenotype (n = 11, age = 37 ± 6 years) | 250 g BC beverage 200 g cream (30% fat) with 75 g sucrose | NS glucose, insulin | NS TG, TC, LDL-C, HDL-C | NS IL-6, IL-1β (ex vivo), OxLDL, α-tocopherol, PON ↑ IL-6 at 4 h compared to baseline ↓ IL-1β and TNF-α (ex vivo) at 4 h vs. baseline (PBO) ↑ ORAC at 1.5 h and 2 h ↑ 2-h and 4-h ORAC AUC ↑ ascorbic acid at 2 h, 2.5 h, 3 h, 3.5 h, and 4 h ↑ 4-h ascorbic acid AUC |
| Edirisinghe et al., 2011, and Burton-Freeman et al., 2010 (USA) [68,69] | Randomized crossover | Overweight, hyperlipidemic adults (n = 24, age = 51 ± 15 years) | 10 g FDS Bagel, cream cheese, margarine, hard-boiled egg, cantaloupe, whole milk, and milk-based strawberry beverage (~960 kcal, 31 g fat) | NS glucose ↓ 6-h insulin and at 1 h and 3 h | ↓ 6-h TG and at 4 h and 5 h ↑ 6-h LDL-C in men | NS PAI-1, TNF-α, IL-1β ↓ 6-h IL-6 and at 6 h ↓ 6-h hs-CRP ↓ 6-h OxLDL (normalized to fasting) in men |
| Authors, Year (Country) | Trial Design | Participants 1 | Dietary Challenge and Intervention | Glucose and Insulin | Lipids | Markers of Oxidative Damage and Inflammation |
|---|---|---|---|---|---|---|
| Diekmann et al., 2019 (Germany) [60] | Randomized crossover | Older obese adults with dyslipidemia, IFG, or inflammation (n = 26, age = 70 ± 5 years) | Test meal (~1115 kcal, 40–59 g fat) 30 min walking (4.6 ± 0.1 km/h, ~12 RPE) immediately after test meal | NS 4.5-h glucose AUC, 4.5-h insulin AUC ↑ glucose at 1.5 h ↓ insulin at 3 h | NS TG, NEFA AUC | NS OxLDL, sICAM-1, sVCAM-1, sE-selectin, retinol, α-tocopherol, β-carotene ↑ 4.5-h IL-6 AUC ↑ 4.5-h Vitamin C AUC |
| Bartholomae et al., 2018 (USA) [65] | Randomized crossover | Adults with pre-diabetes (n = 30, 26 ± 6 years) | Dietary challenge: 75 g dextrose OGTT 1, 3, or 10 min stair climbing and descending (54%–59% VO2peak/58%–74% HRpeak) at 27, 25, and 18 min, respectively, after OGTT | ↓ peak glucose at 0.5 h (1, 3, and 10 min)) ↓ 1-h glucose AUC (3- and 10-min) | NR | NR |
| Takaishi & Hayashi, 2017 (Japan) [66] | Randomized crossover | Adults with IGT (n = 7, 51 ± 3 years) | Test meal (~660 kcal, 18 g fat) ~8 min stair climbing and descending vs. cycle ergometry (both modalities at 60%–65% HRR), 90 min after starting meal | ↓ glucose at 1.75 h and 2 h (stair climbing and descendingbut not cycling) ↑ glucose clearance between 1.5 h and 1.75 h (stair climbing and descending but not cycling) ↑ net glucose clearance between 1.5 h and 1.75 h (stair climbing and descending vs. cycling) 2 | NR | NR |
| DiPietro et al., 2013 (USA) [61] | Randomized crossover | Older adults with IFG (n = 10, age = 69 ± 6 years) | Three test meals [(~32 kcal/kg body mass) across 3 meals, 31% fat) 15 min walking (4.8 ± 0.6 km/h, 3 METs), 30 min after breakfast, lunch, and dinner (3 total bouts during the day) | ↓ 24-h glucose AUC ↓ 3-h post-dinner glucose AUC | NR | NR |
| Takaishi et al., 2012 (Japan) [67] | Randomized crossover | Adult males with pre-diabetes (n = 8, age = 48 ± 7 years) | Test meal (~660 kcal, 18 g fat) 6 min stair climbing and descending (~60% HRR, 13 RPE), 90 min after starting meal | NS insulin ↓ glucose at 1.75 h and 2 h | NR | NR |
| Lunde et al., 2012 (Norway) [62] | Crossover | Obese adult females (n = 11, 5/11 with IGT) age = 44 ± 9 years) | Corn flakes with milk (50 g available CHO) 20 min or 40 min walking (self-selected pace) immediately after a meal | ↓ peak glucose (40 min walking) ↓ 2-h glucose iAUC (20 and 40 min walking) | NR | NR |
| Derave et al., 2007 (Belgium) [63] | Randomized crossover | Sedentary adult males with MetS (n = 7, age = 45 ± 11 years) | Test meal (~4.8 kcal/kg body mass, 9% fat, 82% CHO, 9% PRO) 45 min cycle ergometer (60% VO2max), 60 min after starting breakfast | ↓ glucose at 0.75 h and 1 h after start of physical activity | NS TG iAUC | NR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, D.W.; Navalta, J.W.; McGinnis, G.R.; Serafica, R.; Izuora, K.; Basu, A. Effects of Acute Dietary Polyphenols and Post-Meal Physical Activity on Postprandial Metabolism in Adults with Features of the Metabolic Syndrome. Nutrients 2020, 12, 1120. https://doi.org/10.3390/nu12041120
Davis DW, Navalta JW, McGinnis GR, Serafica R, Izuora K, Basu A. Effects of Acute Dietary Polyphenols and Post-Meal Physical Activity on Postprandial Metabolism in Adults with Features of the Metabolic Syndrome. Nutrients. 2020; 12(4):1120. https://doi.org/10.3390/nu12041120
Chicago/Turabian StyleDavis, Dustin W, James W Navalta, Graham R McGinnis, Reimund Serafica, Kenneth Izuora, and Arpita Basu. 2020. "Effects of Acute Dietary Polyphenols and Post-Meal Physical Activity on Postprandial Metabolism in Adults with Features of the Metabolic Syndrome" Nutrients 12, no. 4: 1120. https://doi.org/10.3390/nu12041120






