Recent Advances in Recovery of Lycopene from Tomato Waste: A Potent Antioxidant with Endless Benefits
Abstract
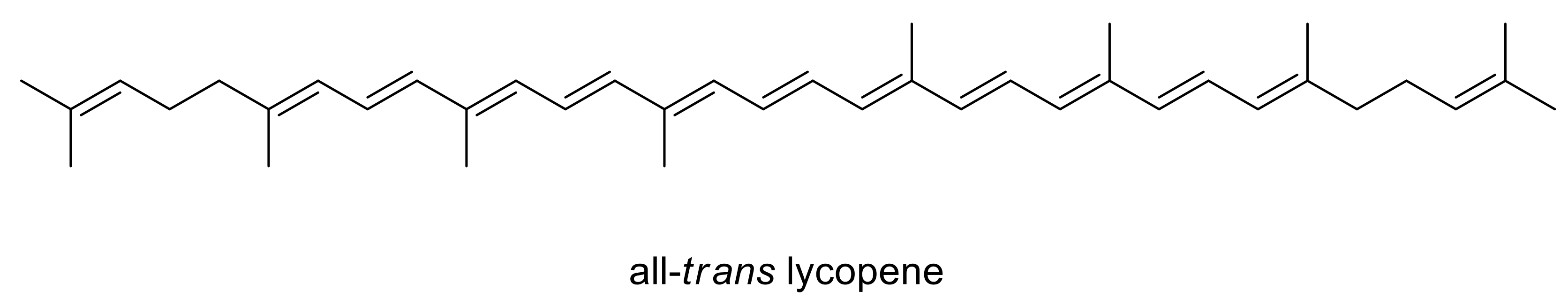
:1. Introduction
2. Extraction of Lycopene from TBPs
3. Pretreatments of TBPs Before Extraction
3.1. Pulsed Electric Fields (PEF) Treatment
3.2. Enzyme-Assisted Extraction (EAE)
4. Supercritical Fluid Extraction (SFE) of TBPs
5. Ultrasonic-Assisted Extraction
6. Microwave-Assisted Extraction
7. Microemulsion Technique
8. Water-Induced Hydrocolloidal Complexation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food waste and byproducts: An opportunity to minimize malnutrition and hunger in developing countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [Green Version]
- Castellana, S.; Ranzino, L.; Beritognolo, I.; Cherubini, M.; Luneia, R.; Villani, F.; Mattioni, C. Genetic characterization and molecular fingerprint of traditional Umbrian tomato (Solanum lycopersicum L.) landraces through SSR markers and application for varietal identification. Genet. Resour. Crop. Evol. 2020, 1–14. [Google Scholar] [CrossRef]
- Tomatoes Statistics. Available online: https://ec.europa.eu/info/food-farming-fisheries/farming/facts-and-figures/markets/overviews/market-observatories/fruit-and-vegetables/tomatoes-statistics_en (accessed on 4 April 2021).
- Szabo, K.; Cătoi, A.-F.; Vodnar, D.C. Bioactive compounds extracted from tomato processing by-products as a source of valuable nutrients. Plant. Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef] [PubMed]
- PA Silva, Y.; Borba, B.C.; Pereira, V.A.; Reis, M.G.; Caliari, M.; Brooks, M.S.-L.; Ferreira, T.A. Characterization of tomato processing by-product for use as a potential functional food ingredient: Nutritional composition, antioxidant activity and bioactive compounds. Int. J. Food Sci. Nutr. 2019, 70, 150–160. [Google Scholar] [CrossRef]
- Fărcaş, A.C.; Socaci, S.A.; Michiu, D.; Biriş, S.; Tofană, M. Tomato waste as a source of biologically active compounds. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2019, 76, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltran, J.C.M.; Stange, C. Apocarotenoids: A new carotenoid-derived pathway. In Carotenoids in Nature, 1st ed.; Stange, C., Ed.; Springer: Cham, Switzerland, 2016; Volume 79, pp. 239–272. [Google Scholar]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Zheng, Z.; Jiang, Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats. Biomed. Pharm. 2019, 109, 2070–2077. [Google Scholar] [CrossRef]
- Das, K.K.; Razzaghi-Asl, N.; Tikare, S.N.; Di Santo, R.; Costi, R.; Messore, A.; Pescatori, L.; Crucitti, G.C.; Jargar, J.G.; Dhundasi, S.A.; et al. Hypoglycemic activity of curcumin synthetic analogues in alloxan-induced diabetic rats. J. Enzym. Inhib. Med. Chem. 2016, 31, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mein, J.R.; Lian, F.; Wang, X.D. Biological activity of lycopene metabolites: Implications for cancer prevention. Nutr. Rev. 2008, 66, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Lin, Y.H.; Yang, C.M.; Hu, M.L. Lycopene inhibits angiogenesis both in vitro and in vivo by inhibiting MMP-2/uPA system through VEGFR2-mediated PI3K-Akt and ERK/p38 signaling pathways. Mol. Nutr. Food Res. 2012, 56, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Tam, C.C.; Kim, J.H.; Escobar, S.; Gong, S.; Liu, M.; Mao, X.Y.; Do, C.; Kuang, I.; Boateng, K.; et al. Anti-parasitic activity of cherry tomato peel powders. Foods 2021, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Saccoliti, F.; Madia, V.N.; Tudino, V.; De Leo, A.; Pescatori, L.; Messore, A.; De Vita, D.; Scipione, L.; Brun, R.; Kaiser, M.; et al. Biological evaluation and structure-activity relationships of imidazole-based compounds as antiprotozoal agents. Eur. J. Med. Chem. 2018, 156, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sharma, V.; Kaul, T.; Abdin, M.Z.; Singh, S. Cytotoxic effect of carotenoid phytonutrient lycopene on P. falciparum infected erythrocytes. Mol. Biochem. Parasitol 2014, 197, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Saccoliti, F.; Madia, V.N.; Tudino, V.; De Leo, A.; Pescatori, L.; Messore, A.; De Vita, D.; Scipione, L.; Brun, R.; Kaiser, M.; et al. Design, synthesis, and biological evaluation of new 1-(aryl-1H-pyrrolyl)(phenyl)methyl-1H-imidazole derivatives as antiprotozoal agents. J. Med. Chem. 2019, 62, 1330–1347. [Google Scholar] [CrossRef]
- Seren, S.; Mutchnick, M.; Hutchinson, D.; Harmanci, O.; Bayraktar, Y.; Mutchnick, S.; Sahin, K.; Kucuk, O. Potential role of lycopene in the treatment of hepatitis C and prevention of hepatocellular carcinoma. Nutr. Cancer 2008, 60, 729–735. [Google Scholar] [CrossRef]
- Petyaev, I.M. Lycopene deficiency in ageing and cardiovascular disease. Oxid. Med. Cell Longev. 2016, 2016, 3218605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. Can. Med. Assoc. J. 2000, 163, 739–744. [Google Scholar]
- Tasca, K.I.; Caleffi, J.T.; Correa, C.R.; Gatto, M.; Tavares, F.C.; Camargo, C.C.; Sartori, A.; Biasin, M.; de Souza, L.D.R. Antiretroviral therapy initiation alters the redox system of asymptomatic HIV-infected individuals: A longitudinal study. Oxid. Med. Cell Longev. 2017, 2017, 9834803. [Google Scholar] [CrossRef] [Green Version]
- Cuzzucoli Crucitti, G.; Pescatori, L.; Messore, A.; Madia, V.N.; Pupo, G.; Saccoliti, F.; Scipione, L.; Tortorella, S.; Di Leva, F.S.; Cosconati, S.; et al. Discovery of N-aryl-naphthylamines as in vitro inhibitors of the interaction between HIV integrase and the cofactor LEDGF/p75. Eur. J. Med. Chem. 2015, 101, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Coodley, G.O.; Coodley, M.K.; Nelson, H.D. Micro-nutrients in HIV infected women. J. Womens Health 1995, 4, 303–311. [Google Scholar] [CrossRef]
- Varma, S.; Karwe, M.V.; Lee, T.-C. Effect of high hydrostatic pressure processing on lycopene isomers. Int. J. Food Eng. 2010, 6. [Google Scholar] [CrossRef]
- Lambelet, P.; Richelle, M.; Bortlik, K.; Franceschi, F.; Giori, A.M. Improving the stability of lycopene Z-isomers in isomerised tomato extracts. Food Chem. 2009, 112, 156–161. [Google Scholar] [CrossRef]
- Gómez-Prieto, M.S.; Caja, M.M.; Herraiz, M.; Santa-María, G. Supercritical fluid extraction of all-trans-lycopene from tomato. J. Agric. Food Chem. 2003, 51, 3–7. [Google Scholar] [CrossRef]
- Chen, J.; Shi, J.; Xue, S.J.; Ma, Y. Comparison of lycopene stability in water- and oil-based food model systems under thermal- and light-irradiation treatments. LWT-Food Sci. Technol. 2009, 42, 740–747. [Google Scholar] [CrossRef]
- Shi, J.; Dai, Y.; Kakuda, Y.; Mittal, G.; Xue, S.J. Effect of heating and exposure to light on the stability of lycopene in tomato purée. Food Control. 2008, 19, 514–520. [Google Scholar] [CrossRef]
- Honest, K.N.; Zhang, H.W.; Zhang, L. Lycopene: Isomerization effects on bioavailability and bioactivity properties. Food Rev. Int. 2011, 27, 248–258. [Google Scholar] [CrossRef]
- Arballo, J.; Amengual, J.; Erdman, J.W., Jr. Lycopene: A critical review of digestion, absorption, metabolism, and excretion. Antioxidants 2021, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Le Maguer, M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Biotechnol. 2000, 20, 293–334. [Google Scholar] [CrossRef]
- Hussein, L.; el-Tohamy, M. Vitamin A potency of carrot and spinach carotenes in human metabolic studies. Int. J. Vitam. Nutr. Res. 1990, 60, 229–235. [Google Scholar] [PubMed]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Mai, A.A.; Al Dajah, S.; Murad, A.A.; El-Salem, A.M.; Khafajah, A.M. Extraction, purification, and characterization of lycopene from jordanian vine tomato cultivar, and study of its potential natural antioxidant effect on Samen Baladi. Curr. Res. Nutr. Food Sci. 2019, 7, 532. [Google Scholar] [CrossRef] [Green Version]
- Pandya, D.; Akbari, S.; Bhatt, H.; Joshi, D.; Darji, V. Standardization of solvent extraction process for Lycopene extraction from tomato pomace. J. Appl. Biotechnol. Bioeng. 2017, 2, 00019. [Google Scholar] [CrossRef] [Green Version]
- Strati, I.F.; Oreopoulou, V. Recovery of carotenoids from tomato processing by-products—A review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- Zuorro, A. Enhanced lycopene extraction from tomato peels by optimized mixed-polarity solvent mixtures. Molecules 2020, 25, 2038. [Google Scholar] [CrossRef] [PubMed]
- Lynge, E.; Anttila, A.; Hemminki, K. Organic solvents and cancer. Cancer Causes Control. 1997, 8, 406–419. [Google Scholar] [CrossRef]
- McLaughlin, J.K.; Lipworth, L. Epidemiologic aspects of renal cell cancer. Semin. Oncol. 2000, 27, 115–123. [Google Scholar]
- Dick, F.D. Solvent neurotoxicity. Occup. Environ. Med. 2006, 63, 221–226. [Google Scholar] [CrossRef]
- Buchmann, L.; Mathys, A. perspective on pulsed electric field treatment in the bio-based industry. Front. Bioeng. Biotechnol. 2019, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Luengo, E.; Condón-Abanto, S.; Condón, S.; Álvarez, I.; Raso, J. Improving the extraction of carotenoids from tomato waste by application of ultrasound under pressure. Sep. Purif. Technol. 2014, 136, 130–136. [Google Scholar] [CrossRef]
- Shree, T.J.; Sree, V.G.; Nagaraj, S. Effect of pulsed electric field on lycopene extraction. Int. J. Circuit Theory Appl. 2016, 9, 7345–7350. [Google Scholar]
- Pataro, G.; Carullo, D.; Ferrari, G. Effect of PEF pre-treatment and extraction temperature on the recovery of carotenoids from tomato wastes. Chem. Eng. Trans. 2019, 75. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 102369. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Application of pulsed electric fields to improve product yield and waste valorization in industrial tomato processing. J. Food Eng. 2020, 270, 109778. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, S.M.; Ananthanarayan, L. Enzyme aided extraction of lycopene from tomato tissues. Food Chem. 2007, 102, 77–81. [Google Scholar] [CrossRef]
- Ranveer, R.C.; Patil, S.N.; Sahoo, A.K. Effect of different parameters on enzyme-assisted extraction of lycopene from tomato processing waste. Food Bioprod. Process. 2013, 91, 370–375. [Google Scholar] [CrossRef]
- Munde, P.J.; Muley, A.B.; Ladole, M.R.; Pawar, A.V.; Talib, M.I.; Parate, V.R. Optimization of pectinase-assisted and tri-solvent-mediated extraction and recovery of lycopene from waste tomato peels. 3 Biotech 2017, 7, 206. [Google Scholar] [CrossRef]
- Prokopov, T.; Nikolova, M.; Dobrev, G.; Taneva, D. Enzyme-assisted extraction of carotenoids from Bulgarian tomato peels. Acta Aliment. 2017, 46, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Catalkaya, G.; Kahveci, D. Optimization of enzyme assisted extraction of lycopene from industrial tomato waste. Sep. Purif. Technol. 2019, 2019, 55–63. [Google Scholar] [CrossRef]
- Lavecchia, R.; Zuorro, A. Improved lycopene extraction from tomato peels using cell-wall degrading enzymes. Eur. Food Res. Technol. 2008, 228, 153. [Google Scholar] [CrossRef]
- Zuorro, A.; Fidaleo, M.; Lavecchia, R. Enzyme-assisted extraction of lycopene from tomato processing waste. Enzym. Microb. Technol. 2011, 6, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Lombardelli, C.; Liburdi, K.; Benucci, I.; Esti, M. Tailored and synergistic enzyme-assisted extraction of carotenoid-containing chromoplasts from tomatoes. Food Bioprod. Process. 2020, 121, 43–53. [Google Scholar] [CrossRef]
- Cuccolini, S.; Aldini, A.; Visai, L.; Daglia, M.; Ferrari, D. Environmentally friendly lycopene purification from tomato peel waste: Enzymatic assisted aqueous extraction. J. Agric. Food Chem. 2013, 61, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Jun Xue, S.; Jiang, Y.; Ye, X. 20-Supercritical-fluid extraction of lycopene from tomatoes. Sep. Extr. Conc. Process. Food Beverage Nutraceutical Ind. Woodhead Publ. 2013, 619–645.e646. [Google Scholar] [CrossRef]
- Pellicano, T.M.; Sicari, V.; Loizzo, M.R.; Leporini, M.; Falco, T.; Poiana., M. Optimizing the supercritical fluid extraction process of bioactive compounds from processed tomato skin by-products. Food Sci. Technol. 2020, 40, 692–697. [Google Scholar] [CrossRef] [Green Version]
- Madia, V.N.; De Angelis, M.; De Vita, D.; Messore, A.; De Leo, A.; Ialongo, D.; Tudino, V.; Saccoliti, F.; De Chiara, G.; Garzoli, S.; et al. Investigation of Commiphora myrrha (Nees) Engl. Oil and Its Main Components for Antiviral Activity. Pharmaceuticals 2021, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- De Vita, D.; Messore, A.; Toniolo, C.; Frezza, C.; Scipione, L.; Bertea, C.M.; Micera, M.; Di Sarno, V.; Madia, V.N.; Pindinello, I.; et al. Towards a new application of amaranth seed oil as an agent against Candida albicans. Nat. Prod. Res. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Kehili, M.; Kammlott, M.; Choura, S.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Supercritical CO2 extraction and antioxidant activity of lycopene and β-carotene-enriched oleoresin from tomato (Lycopersicum esculentum L.) peels by-product of a Tunisian industry. Food Bioprod. Process. 2017, 102, 340–349. [Google Scholar] [CrossRef]
- Rozzi, N.L.; Singh, R.K.; Vierling, R.A.; Watkins, B.A. Supercritical fluid extraction of lycopene from tomato processing by-products. J. Agric. Food Chem. 2002, 50, 2638–2643. [Google Scholar] [CrossRef]
- Topal, U.; Sasaki, M.; Goto, M.; Hayakawa, K. Extraction of lycopene from tomato skin with supercritical carbon dioxide: Effect of operating conditions and solubility analysis. J. Agric. Food Chem. 2006, 54, 5604–5610. [Google Scholar] [CrossRef] [PubMed]
- Mayeaux, M.; Xu, Z.; King, J.M.; Prinyawiwatkul, W. Effects of cooking conditions on the lycopene content in tomatoes. J. Food Sci. 2006, 71, 461–464. [Google Scholar] [CrossRef]
- Vági, E.; Simándi, B.; Vásárhelyiné, K.; Daood, H.; Kéry, Á.; Doleschall, F.; Nagy, B. Supercritical carbon dioxide extraction of carotenoids, tocopherols and sitosterols from industrial tomato by-products. J. Supercrit. Fluids 2007, 40, 218–226. [Google Scholar] [CrossRef]
- Zhang, K.S.; Jiang, H.; Ren, Y.X. The effect of technical parameters on lycopene extraction in supercritical fluid extraction from freeze-dried tomato pomace (peels and seeds). Adv. Mater. Res. 2011, 236, 2868–2871. [Google Scholar] [CrossRef]
- Baysal, T.; Ersus, S.; Starmans, J.D.A. Supercritical CO2 extraction of b-carotene and lycopene from tomato paste waste. J. Agric. Food Chem. 2000, 48, 5507–5511. [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Niu, H.; Li, D.; Zhang, J. Optimization of operating parameters for supercritical carbon dioxide extraction of lycopene by response surface methodology. J. Food Eng. 2008, 89, 298–302. [Google Scholar] [CrossRef]
- Shi, J.; Yi, C.; Xue, J.; Jiang, Y.; Ma, Y.; Li, D. Effects of modifier on lycopene extract profile from tomato skin using supercritical-CO2 fluid. J. Food Eng 2009, 93, 431–436. [Google Scholar] [CrossRef]
- Yi, C.; Shi, J.; Xue, J.; Jiang, Y.; Li, D. Effects of supercritical fluid extraction parameters on lycopene yield and antioxidant activity. Food Chem. 2009, 113, 1088–1094. [Google Scholar] [CrossRef]
- Margotta, M.; Simone, M.C.D. Supercritical Fluid Extraction of Lycopene and Omega-3. New Technol., Dev. Appl. III 2020, 128, 750–758. [Google Scholar] [CrossRef]
- Perretti, G.; Troilo, A.; Bravi, E.; Marconi, O.; Galgano, F.; Fantozzi, P. Production of a lycopene-enriched fraction from tomato pomace using supercritical carbon dioxide. J. Supercrit. Fluids 2013, 82, 177–182. [Google Scholar] [CrossRef]
- Machmudah, S.; Winardi, S.; Sasaki, M.; Goto, M.; Kusumoto, N.; Hayakawa, K. Lycopene extraction from tomato peel by-product containing tomato seed using supercritical carbon dioxide. J. Food Eng. 2012, 108, 290–296. [Google Scholar] [CrossRef]
- Mayer-Miebach, E.; Behsnilian, D.; Regier, M.; Schuchmann, H.P. Thermal processing of carrots: Lycopene stability and isomerisation with regard to antioxidant potential. Food Res. Int. 2005, 38, 1103–1108. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Applied Sonochemistry: Uses of Power Ultrasound in Chemistry and Processing, 1st ed.; Wiley-VCH: Weeinheim, Germany, 2002; pp. 1–24. [Google Scholar] [CrossRef]
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial product. Innov. Food Sci. Emerg. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties, bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Khan, M.K.; Vian, M.A.; Tixier, S.F.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Sivakumar, V.; Anna, J.L.; Vijayeeswarri, J.; Swaminathan, G. Ultrasound assisted enhancement in natural dye extraction from beetroot for industrial applications and natural dyeing of leather. Ultrason. Sonochem. 2009, 16, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z. Comparison of extraction methods for quantifying vitamin E from animal tissues. Bioresour. Technol. 2008, 99, 8705–8709. [Google Scholar] [CrossRef]
- Yilmaz, T.; Kumcuoglu, S.; Tavman, S. Ultrasound assisted extraction of lycopene and β–carotene from tomato processing wastes. Ital. J. Food Sci. 2017, 29, 186–194. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, D.; Chen, J.; Ye, X.; Yu, D. Effects of different factors of ultrasound treatment on the extraction yield of the all-trans-β-carotene from citrus peels. Ultrason. Sonochem. 2011, 18, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Kumcuoglu, S.; Yilmaz, T.; Tavman, S. Ultrasound assisted extraction of lycopene from tomato processing wastes. J. Food Sci. Technol. 2014, 51, 4102–4107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Pan, S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf. ). Ultrason. Sonochem. 2013, 20, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Jerman, T.; Trebše, P.; Mozetič Vodopivec, B. Ultrasound-assisted solid liquid extraction (USLE) of olive fruit (Olea europaea) phenolic compounds. Food Chem. 2010, 123, 175–182. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, G.; Ye, X.; Kakuda, Y.; Meng, R. Stability of all-trans-beta-carotene under ultrasound treatment in a model system: Effects of different factors, kinetics and newly formed compounds. Ultrason. Sonochem. 2010, 17, 654–661. [Google Scholar] [CrossRef]
- Lianfu, Z.; Zelong, L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar] [CrossRef]
- Adekunte, A.O.; Tiwari, B.K.; Cullen, P.J.; Scannell, A.G.M.; O’Donnell, C.P. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010, 122, 500–507. [Google Scholar] [CrossRef]
- Amiri-Rigi, A.; Abbasi, S.; Scanlon, M.G. Enhanced lycopene extraction from tomato industrial waste using microemulsion technique: Optimization of enzymatic and ultrasound pre-treatments. Innov. Food Sci. Emerg. Technol. 2016, 35, 160–167. [Google Scholar] [CrossRef]
- Paduano, A.; Caporaso, N.; Santini, A.; Sacchi, R. Microwave and ultrasound-assisted extraction of capsaicinoids from chili peppers (Capsicum annuum L.) in flavored olive oil. J. Food Res. 2014, 3, 51–59. [Google Scholar] [CrossRef] [Green Version]
- De Vita, D.; Madia, V.N.; Tudino, V.; Saccoliti, F.; De Leo, A.; Messore, A.; Roscilli, P.; Botto, A.; Pindinello, I.; Santilli, G.; et al. Comparison of different methods for the extraction of cannabinoids from cannabis. Nat. Prod. Res. 2020, 34, 2952–2958. [Google Scholar] [CrossRef]
- Chaturvedi, A.K. Extraction of nutraceuticals from plants by microwave assisted extraction. Syst. Rev. Pharm. 2018, 9, 31–35. [Google Scholar] [CrossRef]
- Angiolillo, L.; Del Nobile, M.A.; Conte, A. The extraction of bioactive compounds from food residues using microwaves. Curr. Opin. Food Sci. 2015, 5, 93–98. [Google Scholar] [CrossRef]
- Moret, S.; Conchione, C.; Srbinovska, A.; Lucci, P. Microwave-based technique for fast and reliable extraction of organic contaminants from food, with a special focus on hydrocarbon contaminants. Foods 2019, 8, 503. [Google Scholar] [CrossRef] [Green Version]
- Delazar, A.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Microwave-assisted extraction in natural products isolation. Methods Mol. Biol. 2012, 864, 89–115. [Google Scholar] [CrossRef]
- Ganzler, K.; Salgó, A.; Valkó, K. Microwave extraction: A novel sample preparation method for chromatography. J. Chromatogr. A 1986, 371, 299–306. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess. Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave-assisted extraction-an innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Ho, K.K.H.Y.; Ferruzzi, M.G.; Liceaga, A.M.; San Martín-Gonzalez, M.F. Microwave-assisted extraction of lycopene in tomato peels: Effect of extraction conditions on all-trans and cis-isomer yields. LWT-Food Sci. Technol. 2015, 62, 160–168. [Google Scholar] [CrossRef]
- Materna, K.; Szymanowski, J. Separation of phenols from aqueous micellar solutions by cloud point extraction. J. Colloid Interface Sci. 2002, 255, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Radi, M. Food grade microemulsion systems: Canola oil/lecithin: N-propanol/water. Food Chem. 2016, 194, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Radi, M.; Abbasi, S.; Hamidi-Esfahani, Z.; Azizi, M.H. Development of a new method for extraction of canola oil using lecithin based microemulsion systems. Agro Food Ind. Hi-Tech 2013, 24, 70–72. [Google Scholar]
- Ugolini, L.; DeNicola, G.; Palmieri, S. Use of reverse micelles for the simultaneous extraction of oil, proteins, and glucosinolates from Cruciferous oilseeds. J. Agric. Food Chem. 2008, 56, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Rigi, A.; Abbasi, S. Extraction of lycopene using a lecithin-based olive oil microemulsion. Food Chem. 2019, 272, 568–573. [Google Scholar] [CrossRef]
- Patel, N.; Schmid, U.; Lawrence, M.J. Phospholipid-based microemulsions suitable for use in foods. J. Agric. Food Chem. 2006, 54, 7817–7824. [Google Scholar] [CrossRef]
- Amiri-Rigi, A.; Abbasi, S. Microemulsion-based lycopene extraction: Effect of surfactants, co-surfactants and pretreatments. Food Chem. 2016, 197 (Pt A), 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, M.B.; Azizi, N.; Chemli, M.; Chevalier, Y.; Boyron, O.; Majdoub, M. Synthesis and emulsifier properties of a new bio-sourced surfactant based on isosorbide. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 1–11. [Google Scholar] [CrossRef]
- Nagarajan, J.; Pui Kay, H.; Krishnamurthy, N.P.; Ramakrishnan, N.R.; Aldawoud, T.M.S.; Galanakis, C.M.; Wei, O.C. Extraction of carotenoids from tomato pomace via water-induced hydrocolloidal complexation. Biomolecules 2020, 10, 1019. [Google Scholar] [CrossRef]
| Extraction Method | Main Features | Advantages | Disadvantages | |
|---|---|---|---|---|
| Classical organic solvent extraction (COSE) | solubilization of the components of interest into organic solvent/s added to the plant matrix |
|
| |
| Pretreatments before COSE | Pulsed electric fields treatment | application of electric field pulsing on plant matrices that induces electropermeabilization |
|
|
| Enzyme-assisted extraction | use of enzymes catalyzing the hydrolytic cleavage of structural components of the cell wall of the waste product |
|
| |
| Supercritical fluid extraction | use of supercritical fluids as the extracting solvents to separate component/s from the matrix |
|
| |
| Ultrasonic-assisted extraction | the ultrasound waves cause a mechanical impact, allowing greater penetration of the solvent into the plant body (“sponge effect”) |
|
| |
| Microwave-assisted extraction | microwaves heat solvents that contain samples, thereby partitioning analytes from the matrix into the solvent |
|
| |
| Microemulsion technique | formation of thermodynamically stable dispersion of two immiscible liquids in the presence of surfactants (microemulsions) that improve the solubilization capacity of both liquids |
|
| |
| Water-induced hydrocolloidal complexation | formation of lycopene and pectin colloidal complexes in an aqueous environment that can be recovered by sedimentation or centrifugation |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madia, V.N.; De Vita, D.; Ialongo, D.; Tudino, V.; De Leo, A.; Scipione, L.; Di Santo, R.; Costi, R.; Messore, A. Recent Advances in Recovery of Lycopene from Tomato Waste: A Potent Antioxidant with Endless Benefits. Molecules 2021, 26, 4495. https://doi.org/10.3390/molecules26154495
Madia VN, De Vita D, Ialongo D, Tudino V, De Leo A, Scipione L, Di Santo R, Costi R, Messore A. Recent Advances in Recovery of Lycopene from Tomato Waste: A Potent Antioxidant with Endless Benefits. Molecules. 2021; 26(15):4495. https://doi.org/10.3390/molecules26154495
Chicago/Turabian StyleMadia, Valentina Noemi, Daniela De Vita, Davide Ialongo, Valeria Tudino, Alessandro De Leo, Luigi Scipione, Roberto Di Santo, Roberta Costi, and Antonella Messore. 2021. "Recent Advances in Recovery of Lycopene from Tomato Waste: A Potent Antioxidant with Endless Benefits" Molecules 26, no. 15: 4495. https://doi.org/10.3390/molecules26154495
APA StyleMadia, V. N., De Vita, D., Ialongo, D., Tudino, V., De Leo, A., Scipione, L., Di Santo, R., Costi, R., & Messore, A. (2021). Recent Advances in Recovery of Lycopene from Tomato Waste: A Potent Antioxidant with Endless Benefits. Molecules, 26(15), 4495. https://doi.org/10.3390/molecules26154495











