Abstract
The ethyl acetate extract of the roots of Moutabea guianensis gave 1,6-dihydroxy-4,7,8-trimethoxy-9H-xanthen-9-one (1), a new xanthone. The isolation was accomplished by column chromatography on silica gel and the structural elucidation of this compound was established by spectroscopic analyses including 1D and 2D NMR and HRESIMS.
1. Introduction
Polygalaceae plants have been the source of many xanthones [,], in addition to coumarins, phenols, triterpenes, steroids, pyrones derivatives and alkaloids [,,]. These species contain chemical compounds with a large spectrum of biological activities [], including anti-depressant [] and anti-angiogenic []. Moutabea guianensis Aubl is a Polygalaceae plant of the Amazon area and recently, we carried out a chemical and phytotoxic investigation with its roots. The chemical investigation afforded three known steroids spinasterol, spinasterone and glucopyranosylspinasterol [].
As a part of ongoing research to characterize the chemical components in the roots of M. guianensis, we now report the isolation and structural elucidation of a new xanthone. It was identified as 1,6-dihydroxy-4,7,8-trimethoxy-9H-xanthen-9-one (1), and named moutabeone (Figure 1).
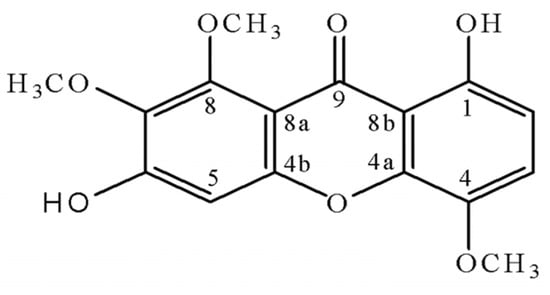
Figure 1.
Structure of compound 1.
2. Results and Discussion
The 1H-NMR spectrum of compound 1 showed eight signals, three singlets between δ 3.94–4.03 indicative of three methoxyl groups; three aromatic hydrogens at δ 6.68 and 7.18 (two doublets with J = 9.0 Hz) and δ 6.90 (s) and one singlet at δ 12.52 assigned to one chelated OH group placed at C1. The 13C-NMR spectrum further reveals signals of three methoxyl carbons (δ 57.5–61.9), three methine carbons (δ 99.3, 108.9, 119.8), ten non hydrogenated carbons, including one carbonyl group at δ 181.3 characteristic for a monochelated carbonyl carbon [], two which have no oxygen substituent (both at δ 109.8) and seven of which have an oxygen substituent (137.6–155.9). The unambiguous attribution was established by means of two-dimensional NMR spectroscopic techniques. The chemical assignments of methine carbons C2, C3, C4 and methoxyl carbons in the 13C-NMR were achieved by HETCOR experiment [1J(CH)]. The other chemical shifts were assigned by long-range [2J(CH), 3J(CH) and 4J(CH)] correlation observed in the HMBC spectrum (Table 1). Its molecular formula (C16H14O7) was determined based on a peak in the HRESIMS data at m/z 319.0817 [M+H]+ (Calculated for C16H15O7), suggesting 10 degrees of unsaturation. These data were sufficient to consider the possibility of a pentasubstituted xanthone structure with two hydroxyl groups at C1 and C6. Thus, the structure of 1 was fully elucidated, and it was named moutabeone.

Table 1.
1H-NMR (300 MHz) and 13C-NMR (75 MHz) spectral data for compound 1 in CDCl3.
| Positions | δH ( J in Hz) | δC | DEPT | HMBC (H→C) | |
|---|---|---|---|---|---|
| 1 | - | 154.9 | C | ||
| 2 | 6.68 (d, 9.0) | 108.9 | CH | 8b, 1, 4 | |
| 3 | 7.18 (d, 9.0) | 119.8 | CH | 1, 4a, 4 | |
| 4 | - | 139.4 | C | ||
| 4a | - | 145.1 | C | ||
| 4b | - | 154.5 | C | ||
| 5 | 6.90 (s) | 99.3 | CH | 8a, 9, 4b, 6, 7 | |
| 6 | - | 155.9 | C | ||
| 7 | - | 137.6 | C | ||
| 8 | - | 152.2 | C | ||
| 8a | - | 109.3 | C | ||
| 8b | - | 109.3 | C | ||
| 9 | - | 181.3 | C | ||
| 4-OCH3 | 3.93 (s) | 57.5 | CH3 | 4 | |
| 7-OCH3 | 4.02 (s) | 61.7 | CH3 | 7 | |
| 8-OCH3 | 4.00 (s) | 61.9 | CH3 | 8 | |
| 1-OH | 12.52 (s) | - | - | 2, 1, 8b |
3. Experimental
3.1. General Information
NMR spectra were recorded on a Varian 300 MHz NMR spectrometer (300 MHz and 75 MHz for 1H and 13C, respectively) using TMS as internal standard. HRESIMS was carried out on a Waters Xevo G2-S QTof/Tof spectrometer. IR was carried out on a Shimadzu Prestige 21. Column chromatography was performed on silica gel (70–230 mesh, MACHEREY-NAGEL; Düren, Germany). TLC was performed on silica gel 60 F254 (Sorbent Technologies, Sorbent Technologies; Norcross, GA, USA) plates. Spots were visualized by spraying with aqueous H2SO4 (15%) saturated with CeSO4 solution, followed by heating.
3.2. Plant Material
The roots of M. guianensis were collected in the experimental field of Embrapa Amazônia Oriental, located in Belém, Pará State, Brazil, on March 2012. A voucher specimen (195862) was kept in the Herbarium MG of the Museu Paraense Emílio Goeldi (MPEG). Roots were dried on forced air circulation on 40 °C for five days and powdered in a knife mill.
3.3. Extraction and Isolation
The roots of M. guianensis (928 g) dried and powdered, were submitted to successive extractions with hexane (3 L), ethyl acetate (3 L) and methanol (3 L) at room temperature for five days. After removal of the solvent in vacuo, the hexane, ethyl acetate and methanol extracts were obtained, respectively. The ethyl acetate extract (2.0 g) was subjected to silica gel column chromatography and eluted with hexane-EtOAc and EtOAc-MeOH, collecting 20 fractions of 125 mL each. The fractions were combined according to TLC to give seven groups (G1–G7). G4 (254.5 mg) was rechromatographed on silica gel with hexane-EtOAc as eluent, collecting 170 fractions of 13 mL each. The fractions 111–117 were combined according to TLC to afford compound 1 (32 mg) as a yellow amourphous solid. Yellow amorphous solid; 1H-NMR and 13C-NMR data see Table 1; UV λmax/nm (acetonitrile-water): 199, 240, 284, 314, 358 (sh). IR (KBr) 3475, 2999, 2939, 2837, 1602, 1485, 1471, 1288, 1228, 1099, 1008, 981, 948, 794, 736, 609 cm−1. HRESIMS m/z: 319.0817 [M+H]+ (calcd for C16H15O7).
4. Conclusions
A new xanthone 1,6-dihydroxy-4,7,8-trimethoxy-9H-xanthen-9-one (1), was isolated from the ethyl acetate extract of the roots of M. guianensis. This compound is expected to be an antioxidant and free radical scavenger like other xanthones and its biological activity will be studied in future.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/7/8885/s1.
Acknowledgments
The authors are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
Author Contributions
HSRF, LCP, ESA, MJCC and LSS designed research; HSRF, LCP and LSS performed research and analyzed the data; GMSPG, HSRF and LSS wrote the paper. All authors read and approved the final manuscript.
Acknowledgments
The authors are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Pró-reitoria de Pesquisa da UFPA (PROPESP) and Fundação de Amparo e Desenvolvimento da Pesquisa (FADESP) for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Klein Junior, L.C.; Gandolfi, R.B.; Santin, J.R.; Lemos, M.; Cechinel Filho, V.; Andrade, S.F. Antiulcerogenic activity of extract, fractions, and some compounds obtained from Polygala cyparissias St. Hillaire e Mouquin (Polygalaceae). N.-S. Arch. Pharmacol. 2010, 381, 121–126. [Google Scholar] [CrossRef]
- Yang, X.; Xu, L.; Yang, S. Xanthones from the stems of Securidaca inappendiculata. Phytochemistry 2001, 58, 1245–1249. [Google Scholar] [CrossRef]
- Bergeron, C.; Marston, A.; Wolfender, J.L.; Mavi, S.; Rogers, C.; Hostettmann, K. Isolation of polyphenols from Polygala gazensis and liquid chromatography-mass spectrometry of related African Polygala species. Phytochem. Anal. 1997, 8, 32–36. [Google Scholar]
- Pinheiro, T.R.; Cechinel, V.; Santos, A.R.S.; Calixto, J.B.; Delle-Monache, F.; Pizzolatti, M.G.; Yunes, R.A. Three xanthones from Polygala cyparissias. Phytochemistry 1998, 48, 725–728. [Google Scholar] [CrossRef]
- Pizzolatti, M.G.; Cunha, A., Jr.; Pereira, W.S.; Delle Monache, F.A. A new styryl-2-pyrone derivative from Polygala sabulosa (Polygalaceae). Biochem. Syst. Ecol. 2004, 32, 603–606. [Google Scholar]
- Rocha, J.L.C.; Pastore, J.F.B.; Brandão, H.N.; Azevedo, A.; Devid, J.P.; Santos, E.O.; David, J.M. Quantificação de salicilato de metila em quatro gêneros de Polygalaceae, POR CLAE-DAD. Quim. Nova 2012, 35, 2263–2266. [Google Scholar] [CrossRef]
- Capra, J.C.; Cunha, M.P.; Machado, D.G.; Zomkowski, A.D.E.; Mendes, B.G.; Santos, A.R.S.; Pizzolatti, M.G.; Rodrigues, A.L.S. Antidepressant-like effect of scopoletin, a coumarin isolated from Polygala sabulosa (Polygalaceae) in mice: Evidence for the involvement of monoaminergic systems. Eur. J. Pharmacol. 2010, 643, 232–238. [Google Scholar] [CrossRef]
- Lepore, L.; Malafronte, N.; Condero, F.B.; Gualtieri, M.J.; Abdo, S.; Piaz, F.D.; Tommasi, N.D. Isolation and structural characterization of glycosides from an anti-angiogenic extract of Monnina obtusifolia H.B.K. Fitoterapia 2007, 82, 178–183. [Google Scholar]
- Ripardo Filho, H.S.; Pacheco, L.C.; Souza Filho, A.P.S.; Guilhon, G.M.S.P.; Arruda, M.S.P.; Santos, L.S. Bioensaios de atividade alelopática dos esteroides espinasterol, espinasterona e glicopiranosil espinasterol. Planta Daninha 2012, 30, 705–712. [Google Scholar]
- Van der Sluis, W.G.; Labadie, R.P. Polyoxygenated xanthones of Centaurium littorale. Phytochemistry 1985, 24, 2601–2605. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compound 1 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).