Abstract
Exercise-based cardiac rehabilitation is a highly recommended intervention towards the advancement of the cardiovascular disease (CVD) patients’ health profile; though with low participation rates. Although home-based cardiac rehabilitation (HBCR) with the use of wearable sensors is proposed as a feasible alternative rehabilitation model, further investigation is needed. This systematic review and meta-analysis aimed to evaluate the effectiveness of wearable sensors-assisted HBCR in improving the CVD patients’ cardiorespiratory fitness (CRF) and health profile. PubMed, Scopus, Cinahl, Cochrane Library, and PsycINFO were searched from 2010 to January 2022, using relevant keywords. A total of 14 randomized controlled trials, written in English, comparing wearable sensors-assisted HBCR to center-based cardiac rehabilitation (CBCR) or usual care (UC), were included. Wearable sensors-assisted HBCR significantly improved CRF when compared to CBCR (Hedges’ g = 0.22, 95% CI 0.06, 0.39; I2 = 0%; p = 0.01), whilst comparison of HBCR to UC revealed a nonsignificant effect (Hedges’ g = 0.87, 95% CI −0.87, 1.85; I2 = 96.41%; p = 0.08). Effects on physical activity, quality of life, depression levels, modification of cardiovascular risk factors/laboratory parameters, and adherence were synthesized narratively. No significant differences were noted. Technology tools are growing fast in the cardiac rehabilitation era and promote exercise-based interventions into a more home-based setting. Wearable-assisted HBCR presents the potential to act as an adjunct or an alternative to CBCR.
1. Introduction
Cardiovascular diseases (CVD) are a major cause of morbidity and mortality worldwide, thus adding a significant economic burden on national health care systems. Coronary heart disease (CHD) is the most common type of CVD and accounts for a high proportion of all CVD deaths and has more disability-adjusted lifetime than other diseases such as cancer and diabetes [1]. Therefore, secondary prevention interventions that support CVD management are critical in reducing disease burden and health care expenditure. Exercise-based cardiac rehabilitation (EBCR) is highly recommended as the key multicomponent intervention for the prevention of cardiac-induced mortality, the reduction of hospital readmissions, and the improvement of quality of life (QoL) [2]. CR is a safe, efficient, and cost-effective intervention that reduces overall health service expenditure [3]. CR programs are designed to improve physical, psychological, and social functioning by combining medical evaluation, individualized exercise prescription, cardiac risk factor modification, education, and counseling [4,5,6,7,8,9].
Despite the well-documented benefits of CR implementation in the cardiovascular population, attendance rates remain low and suboptimal [10,11]. Accessibility-related factors, including limited availability of programs, unwillingness to participate in group programs, inconvenient timing of programs, career responsibilities, transportation and parking costs, lack of time, and disbelief in their ability to control their CHD, are prominent barriers to CR enrollment and adherence [12,13,14]. Moreover, during the COVID-19 pandemic era, safe distancing measures were adopted to curb the spreading of the virus, thus leading to the temporary cessation of many CR programs, the discontinuation of CR provision, and thus the further deterioration of CVD patients’ cardiovascular function [15,16,17]. Furthermore, a recent systematic review found that CVD patients affected by COVID-19 presented worse outcomes and increased risk of morbidity, whereas COVID-19 itself also induced myocardial injury, arrhythmia, acute coronary syndrome, and venous thromboembolism [18].
The home-based model of CR (HBCR) may act as a sufficient alternative for dealing with some of these barriers and improving cardiac patients’ cardiorespiratory fitness (CRF), QoL, CVD risk factors, mortality, and accessibility/participation rates [19,20,21]. Moreover, the rapid proliferation and widespread use of affordable information and communication technologies (ICTs) in the area of telehealth allow their engagement in the CR procedures, enabling the sufficient provision of feedback, coaching, and specialist consultancy to the CVD population [22]. The significant growth in the use of technology among older adults [23] and the widespread accessibility of the internet also contribute to the implementation of sophisticated telemedicine and mobile CR programs, aiming to better accessibility, individualization, and utilization by cardiac patients. Several systematic reviews have demonstrated the efficacy and feasibility of digital CR interventions in improving cardiac patients’ physical activity (PA) and QoL [24]. Furthermore, patients’ adherence to medical therapy, ability to meet blood pressure and exercise targets, and increased awareness of diet and exercise significance display positive effects in the mobile health CR group [25]. Additionally, a systematic review and meta-analysis has demonstrated that CR telehealth interventions are significantly associated with lower rehospitalization or cardiac events rates and advanced lipid and smoking profiles [22].
Recently, the integration of remote technologies and wearable sensors has enabled the almost real-time monitoring of “at home” patients’ performance data for physical activity features (such as intensity, time, distance traveled, steps taken, and sedentary time), heart rate and blood pressure levels, and cardiac electrical potential waveforms (electrocardiography) can be retrieved through wearable sensors. Subsequently, these recorded data can be assessed almost instantly by the medical staff via remote technology applications, thus allowing constant surveillance and immediate feedback between patients and CR providers. However appealing the concept is, the comprehensiveness of remote CR programs using wearable sensors still lacks proper study investigation. A recent review by Batalik et al. proposes remotely, via wearable sensors, monitored cardiac telerehabilitation, as a feasible, efficient, and safe intervention [26]. Furthermore, cardiac telerehabilitation was demonstrated to be similar in training intensities to conventional outpatient CR in CVD patients with low to moderate cardiovascular risk [27].
Though more thorough and systematic search is needed since the integration of wearable sensors in CR procedures is at its early stages and everyday new and more complicated technology is being used, CRF is a powerful and independent predictor of CVD patients’ cardiac and all-cause mortality [7]. Therefore, based on the significance of the CRF, this systematic review aims primarily to explore and examine the effectiveness of wearable sensors-assisted CR in improving CVD patients’ CRF. The secondary aim is to analyze the impact on physical activity (PA), QoL, adherence, and cardiac risk factors compared with center-based CR (CBCR) or usual care.
2. Materials and Methods
2.1. Study Design
This is a systematic review of randomized controlled trials (RCTs) and is written following the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA). The protocol was registered in PROSPERO (International Prospective Register of Systematic Reviews) (registration number: CRD42021265665) before screening search results, and was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (Supplementary Table S2).
2.2. Study Inclusion Criteria
Studies were included if they addressed the implementation of HBCR and encompassed at least two exercise sessions a week. HBCR should be compared to either a usual care group or a CBCR or both. Selected studies should involve interventions among adults (aged ≥18 years and with no restrictions regarding sex, ethnicity, and socioeconomic background), with diagnosed CVD (heart failure, MI, angina, and coronary revascularization), and eligible for phase III of CR. Eligible studies had to involve the assessment of CRF as the primary outcome. Additional inclusion criteria referred to the reporting of at least one more additional outcome measure: PA, QoL, adherence, cardiovascular risk factors, lipid profile, and depression/anxiety levels. The intervention duration should be of at least 8 weeks. The available publications had to be written in English and had to be in full-text version.
Narrative reviews, preclinical studies, duplicate studies, editorial or opinion articles, grey literature, and conference papers were excluded. Systematic reviews and study protocols were not eligible for inclusion; however, relevant systematic reviews were assessed as a guide and cited where appropriate, and results articles were sought to identify additional RCTs study protocols.
HBCR interventions were defined as those with at least 50% of the program delivered via ICT, including wireless devices such as sensors, any mobile phone (i.e., feature phone or smartphone), and/or web-based platforms. CBCR is referred to as face-to-face center-based or community-based CR. Usual care was defined as any routine care for CHD, excluding telehealth intervention, without significant ongoing input from a research team.
2.3. Search Strategy
A systematic electronic literature search was performed across five electronic databases: PubMed, Scopus, Cinahl, Cochrane Library, and PsycINFO, from 2010 up to January 2022. Systematic searches were conducted by combining the search terms from the four categories of the relevant keywords (i.e., heart disease, program/intervention, mode of delivery, wearable sensors). Keywords are presented in Supplementary Material Table S1. Only full-text articles were included and their reference lists were checked to identify any more potentially eligible studies.
2.4. Study Selection Process
Search results were exported to Endnote X9, where, after the exclusion of duplicates, two reviewers (AV, PG) independently screened the titles and abstracts of studies. Those not meeting the eligibility criteria were removed. The full texts of all relevant studies were sought, downloaded, and further evaluated for compliance with the eligibility criteria. Any disagreements between the two reviewers regarding inclusion were resolved by consultation with a third independent reviewer (KE), thus ensuring the minimization of bias, when deciding whether or not to include certain studies. The two reviewers (AV, PG) independently conducted the data extraction from each study. The disagreements were resolved by consulting the previous third independent reviewer (KE).
2.5. Data Extraction
Data extraction was performed on the selected studies, including the following domains: author, year, country, sample size, age, and gender of the participants, design, sampling method, description of interventions (mode of delivery, frequency, and duration, and key component), comparator, wearable sensors, outcome measures and time points, results, attrition rate, and handling of the missing data.
2.6. Effect Size Measurement
The outcome of interest was the mean difference between the HBCR interventions (CBCR or usual care or both) and the control group from the baseline assessment endpoint in CRF; data were retrieved and recorded by AV, PG that worked independently. Any disagreements were resolved by consensus, or by consultation of a third reviewer (MK). Manuscripts were included in the meta-analysis only if the CRF was adequately reported.
2.7. Data Synthesis
Pooled values of weighted mean differences between the HBCR and the CBCR or usual care group, and 95% confidence intervals (CΙ), were calculated using the Der Simonian–Laird random effects, as well as fixed effects models (depending on heterogeneity), using STATA software (version 17, College Station, TX, USA). Estimates of effect size measures were weighted by the inverse of their variances; thus, effect sizes of standardized mean differences were estimated using Hedges’ g statistic and the corresponding 95% CI. The magnitude of Hedges’ g was interpreted as small (g = 0.3), medium (g = 0.5), and large (g = 0.8). Heterogeneity assessed the null hypothesis that all studies evaluated the same effect using the chi-squared test. Inconsistency index (i.e., I2) was used to quantify the total variation consistent with inter-study heterogeneity, ranging from 0% to 100%. p-values of <0.10 for the chi-square test and I2 > 50% were considered to reflect significant heterogeneity [28].
Possible publication bias was assessed using a contour-enhanced funnel plot of each trial’s effect size against the standard error. Funnel plot asymmetry was evaluated by means of the regression-based Egger test for small-study effects. Finally, in the case of multiple assessment time points, the longest one was chosen for inclusion in the meta-analysis.
2.8. Risk of Bias (Quality) Assessment
The Cochrane Risk of Bias tool [29] for randomized trials was used to guide the quality assessment of each included study and consists of the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias (e.g., whether study groups were comparable at baseline). Two independent authors (AV, PG) conducted the quality appraisal individually. Any discrepancies were resolved by a third reviewer.
3. Results
3.1. Study Selection
The initial search from the five electronic databases identified 245 records, of which 52 duplicates were removed. After screening the title and abstracts of 193 records, 144 were excluded for not meeting the inclusion criteria. Forty-nine remaining records were eligible for further full-text review for compliance with the eligibility criteria. The exclusion of 35 records with reasons is documented in Figure 1. Finally, 14 studies were included in this systematic review [30,31,32,33,34,35,36,37,38,39,40,41,42,43].
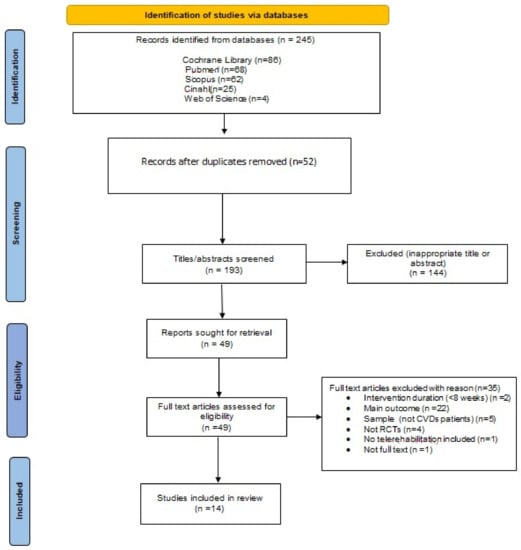
Figure 1.
Flowchart of the study.
3.2. Risk of Bias of Included Studies
Selection bias related to the generation of the random allocation sequence was considered as low risk, with all trials adequately describing random sequence generation. Five trials reported details [30,31,32,33,40] concerning the sample’s allocation concealment and thus were assessed as low risk. The rest lacked either a detailed description of the procedure or some clarified information and were subsequently classified as unclear.
Considering performance bias, the nature of these trials made the participants or rehabilitation providers’ blinding to group allocation impossible. Nevertheless, in such study designs, the outcome assessors’ blinding to the knowledge of trial allocation can be considered of greater importance. However, only seven studies reported having taken measures to blind outcome assessment [30,31,32,35,36,37,38]. Both attrition and reporting bias domains were mostly rated as having low risk. Only one study reported increased attrition rates and was evaluated as high risk [38]. A summary and a graph of the risk of bias are provided in Figure 2 and Figure 3, respectively.
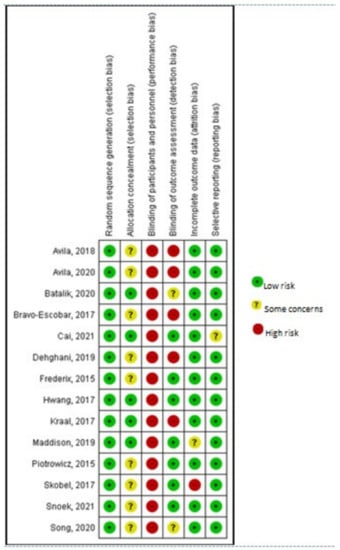
Figure 2.
Risk of bias summary. Avila, 2018 [42]; Avila, 2020 [41]; Batalik, 2020 [40]; Bravo-Escobar, 2017 [38]; Cai, 2021 [30]; Dehghani, 2019 [43]; Frederix, 2015 [34]; Hwang, 2017 [32]; Kraal, 2017 [33]; Maddison, 2019 [31]; Piotrowicz, 2015 [35]; Skobel, 2017 [37]; Snoek, 2021 [36]; Song, 2020 [39].
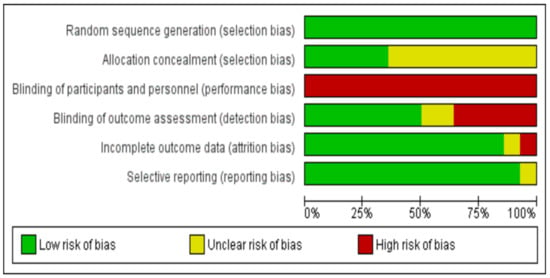
Figure 3.
Risk of bias graph.
3.3. Study Characteristics
A summary of the characteristics of the included studies is presented in Table 1. Eleven studies were two-arm RCTs [30,31,32,33,37], two were three-arm RCTs [36,41,42], and one included four groups (two intervention and two control) [43] involving a total of 1363 participants (sample size ranging between 28 and 179). Three studies were conducted in Belgium [35,41,42], two in China [30,40], one in the Netherlands [33], one in New Zealand [31], one in Spain [39], one in Iran [43], one in Australia [32], one in Czech Republic [40], one in Poland, one in Germany [36,38], and another one was a multi-center study across Europe [37]. Based on the World Bank database, almost all studies were implemented in countries classified as of high-income, according to their gross national income (GNI) per capita [44]. Sole exceptions were China, classified as an upper-middle-income country, and Iran, which presented a lower-income country classification. Eligible participants in this review were diagnosed with the following: angina [31,40], myocardial infarction (MI) [31,40,41,42,43], acute coronary syndrome (ACS) [33], CHD [35,38,40,41,42], or had undergone coronary revascularization [31,40], ischemic cardiomyopathy (ICM) [39], radiofrequency catheter ablation (RFCA) [39], or chronic heart failure [32,37]. The mean age of participants ranged from 51.4 to 72.4 years and 51.5 to 73.6 years for the intervention and control groups, respectively. A total of 226 females participated in the studies, accounting for 16.6% of the overall sample size. Description of the usual care group varied but mainly referred to encouragement to be physically active, but no participation in supervised CR programs, self-initiated access to CR education sessions, and psychosocial support.

Table 1.
Study characteristics.
3.4. Intervention Characteristics
The CR implementation features (frequency, intensity, duration, and type of exercise) differed significantly among the studies. In particular, four studies reported a 6-month duration of technology-assisted interventions [35,36,37,38,40], seven studies reported a 12-week duration [30,31,32,33,40,41,42], and three studies had an 8-week duration [36,39,43]. The frequency of the exercise sessions ranged from two to six sessions per week, and the duration of each exercise session ranged from 30 min to 80 min per session. Most of the studies reported exercise intensity individually set at 70–80% of each participant’s heart rate reserve (HRR) and 11–13 Borg score of perceived exertion. Only two studies reported lower intensity levels (40–65%) [31,36]. Most programs used individually tailored exercise prescriptions, thus making it difficult to quantify the volume of the exercise taken.
Randomization procedures were reported in all studies. The number and the type of the comparators’ groups differed among the studies. Six studies compared one HBCR group to a traditional CBCR group [31,32,33,34,35,39], five studies compared HBCR to a usual care group [30,36,37,38,40], and one study compared three groups: an HBCR and a CBCR versus a usual care group [42]. One study included four comparators: two intervention and two control groups. Two groups (intervention and control) consisted solely of male participants and two groups (intervention and control) consisted solely of female participants [43].
CBCR programs were based on either a supervised treadmill or cycling exercise, whilst all HBCR programs were orientated to aerobic training. Only two studies based on HBCR interventions included strengthening [38,39] and stretching exercises [30]. Supplementary forms of communication, such as text messages, phone calls, video calls, and emails, were utilized between the participants and the intervention team to provide feedback. Feedback was orientated on adjustment of exercise modalities and features and checking the incidence of adverse events and possible barriers preventing the patients’ participation in the intervention procedures [35,42]. Telephone contacts, delivered once/weekly, were the most common modes of providing behavior change education, psychological support, and evaluation of exercise modalities, training adherence, and CR barriers [33,36,37,40,41]. Hwang et al. provided HBCR patients with educational topics delivered as electronic slide presentations with embedded audio files [32]. Direct messaging via short message service (SMS) or emails, once every week, were also utilized for exercise, dietary, and smoking cessation recommendations [31,35,42]. Support systems with artificial intelligence (AI) were utilized to extract and upload monitored data and provide patients with educational material and motivational and training feedback [38,40].
Stress management and psychological support were the most minor addressed issues mentioned in only four studies [33,36,38,39]. Smoking [35] and dietary [30,35,39] recommendations were also provided whilst only Hwang et al. reported an HBCR being implemented in groups of up to five participants [32]. By design, these trials were impossible to achieve and ensure blinding to group allocation for the participants and the CR professional providers. However, all studies reported measures taken for achieving blinding on the outcome assessment. All studies reported sources of trial funding; though none of them reported funding from any agency with a commercial interest in the results of their study.
3.5. Wearable Sensors
Severable wearable sensors were used to assess, monitor, and record vital signs related to the safety of the exercise sessions and the volume of the participants’ PA. Electrocardiographic (ECG) monitoring was used in four studies [30,36,38,39]. Accelerometer data were collected and recorded in four studies toward objectively monitored PA levels [33,35,41,43]. Kraal et al. estimated accelerometer data (ActiGraph wGT3Xþ monitor, Acti-Graph Corp, USA) to determine the HBCR participants’ physical activity energy expenditure (PAEE) and physical activity level (PAL) [33]. HR devices, either chest belts [37,40] or wrist-worn [42], were used to record the exercise data and evaluate training duration and intensity. Additionally, an automatic sphygmomanometer and a finger pulse oximeter were provided to HBCR participants for self-monitoring and verbally reporting their blood pressure, HR, and oxygen saturation levels at the start of each exercise session [32]. A combination of information regarding heart and respiratory rates, single-lead ECG, and accelerometry were provided by a chest-worn wearable sensor (BioHarness 3, Zephyr Technology, Annapolis, MD, USA) [31].
4. Primary Outcome
Cardiorespiratory Fitness
CRF was evaluated as a primary outcome in all the selected studies of this review (Table 2). Almost all included studies determined CRF as the peak oxygen consumption (VO2peak) assessed during a maximal cardiopulmonary exercise test (CPET) with respiratory gas analysis on a cycle ergometer (Lode Corrival, Groningen, The Netherlands). In four studies, additional CPET parameters were recorded and used for further assessment, such as the ventilatory anaerobic threshold (VAT) using the V-slope peak heart rate, peak respiratory exchange ratio, both ventilatory thresholds (VT1 (ventilatory anaerobic threshold), VT2 (respiratory compensation point)), HR reserve, oxygen pulse (O2/HR, ml/beat), and aerobic work rate dO2/dW (mL/min/W), VE/VCO2 slope, and VE/VCO2 [38,40,41,42].

Table 2.
Results reported in studies.
Two studies [39,43] carried out an exertion test on a treadmill with continual monitoring with a 12-derivation ECG, using the Bruce protocol. The metabolic equivalent of task (MET), VO2max, the total exercise times, and the distance traveled on the treadmill during the exertion test were recorded and used for the evaluation of the participants’ CRF. Additional parameters recorded and used for the evaluation of the physical capacity were the maximum HR reached in the stress test, the HR recovery during the first minute, and the perceived exertion level according to the Borg scale. Only one study performed a six-minute walk test (6MWT) to evaluate the possible effects of its intervention on the physical fitness of its participants [32].
5. Secondary Outcomes
5.1. Physical Activity
PA was determined as physical activity energy expenditure (PAEE) estimated from data of triaxial accelerometers (Table 2). Steps, sedentary time (duration of sedentary activity at an intensity of ≤1.5 metabolic equivalents of task (METs)), active energy expenditure (PA at an intensity of ≥3 METs), duration of moderate, vigorous PA (≥3 METs), and PA level (PAEE/resting metabolic rate) were parameters recorded and used in the analyses. Cain et al. included the International Physical Activity Questionnaire (IPAQ) for the self-reported PA assessment by their study participants [30], whilst Frederix et al. used both accelerometry data and the IPAQ for PA assessment reasons [35]. On the other hand, Snoek et al. used two questions for the assessment of self-reported physical activity: “How many days per week do you perform moderate to vigorous PA (physical activity)?” and “How many minutes per day do you perform moderate to vigorous PA?” Self-reported habitual physical activity was considered the total number of days per week in which a minimum of 30 min of self-reported moderate to vigorous physical activity was registered [37].
5.2. Quality of Life
The Medical Outcome Survey Short Form 36 (SF-36) questionnaire was used to assess the participants’ QoL in four studies, using the corresponding translated version of the SF-36 according to their native language [36,39,41,42]. Kraal et al. used the results from the SF-36 questionnaire to calculate the health utility scores for the cost–utility analyses in their study; whilst the MacNew questionnaire was used for the assessment of the participants’ QoL [33]. Two studies [31,38] used solely the EuroQol five-dimensional (EQ-5D) questionnaire for the evaluation of the QoL, whilst one study [32] combined it with the Minnesota Living with Heart Failure Questionnaire (MLWHFQ).
5.3. Training Adherence
Attendance rates were defined either as the number of sessions attended by each participant or as the percentage counted from the total number of accomplished training sessions of an individual participant [30,31,32,33,36,41]. Adherence in the intervention groups was calculated according to the records provided by the wearable sensors (HR zones, accelerometers, ECG recording devices), whilst for the CBCR group, adherence was determined as the number of attended training sessions at the outpatient clinic or patients’ exercise diaries. No adherent was defined that completed <20% of the prescribed number of training sessions.
5.4. Cardiovascular Risk Factors/Laboratory Parameters
The evaluation of the main cardiovascular risk factors referred to anthropometric measures such as body mass index (BMI), waist and hip circumference, and biochemical parameters of a fasting blood sample (glucose, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides and glycated hemoglobin (HbA1c)). Evaluation of cardiac risk biomarkers such as CRP and ntBNP were also included in one study [38]. Furthermore, Avila et al. calculated the homeostasis assessment model (HOMA) index using the following formula: fasting plasma glucose (mmol/L) times fasting serum insulin (mU/L) divided by 22.5 [41].
5.5. Stress/Patient Satisfaction
Patient satisfaction was measured by the Client Satisfaction Questionnaire (CSQ-8) [32] and the Consumer Quality Index [33]. Psychological status was assessed using the Hospital Anxiety and Depression Scale (HADS) and the patient health questionnaire (PHQ) [32,33]. Cai et al. also used the Health Beliefs Related to Cardiovascular Disease Scale and the Exercise Self-Efficacy Scale to investigate and evaluate the efficacy of their study intervention procedures [30].
5.6. Muscle Strength/Balance
Muscle strength was evaluated through the sitting–rising test (SRT), a handgrip strength dynamometer, and quadriceps maximal isometric knee extension strength [32,41]. Balance was measured in one study [32] using the Balance Outcome Measure for Elder Rehabilitation (BOOMER).
6. Meta-Analysis
6.1. Cardiorespiratory Fitness
A meta-analysis was conducted on pooled data from 12 studies (out of 14 RCT), which compared the HBCR group and the control group (CBCR or usual care or both), after excluding four studies [32,38,39,41] that provided insufficient data for the meta-analysis. The results are displayed in Figure 4 and Figure 5 and in Supplementary Figures S1 and S2.
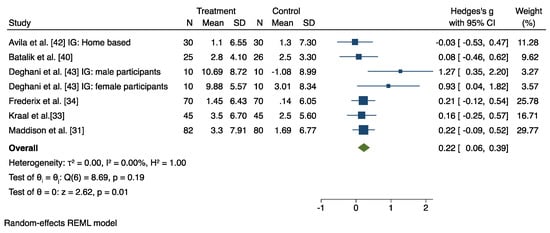
Figure 4.
Results from the restricted maximum likelihood (REML) random effects meta-analysis (Hedges’ g criteria), concerning the difference in cardiorespiratory fitness (CRF) change post-intervention, between the home-based cardiac rehabilitation group (HBCR) and the center-based rehabilitation group (CBCR) [31,33,34,40,42,43].
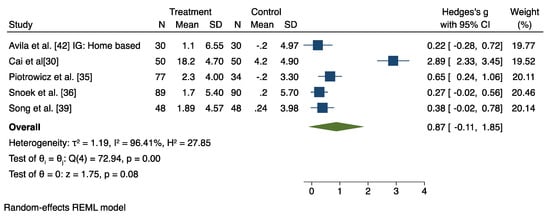
Figure 5.
Results from the restricted maximum likelihood (REML) random effects meta-analysis (Hedges’ criteria), concerning the difference in cardiorespiratory fitness (CRF) change post-intervention, between the home-based cardiac rehabilitation group (HBCR) and the usual care group (UC) [30,35,36,39,42].
6.2. HBCR versus CBCR
Seven studies investigated the effect of HBCR compared to a CBCR group on participants’ CRF levels. The random and fixed effects model revealed a significant post-intervention between-group difference in favor of the HBCR on CRF with a medium effect size (Hedges’ g = 0.22, 95% CI 0.06 to 0.39), and with low heterogeneity (I2 = 0%) (Figure 4). Moreover, the pooled mean difference in favor of the HBCR group on CRF outcome values was 1.27 mL/Kg/min (95% CI 0.24 to 2.30) (Supplementary Figure S1).
Leave-one-out sensitivity analysis showed that, in general, the overall effect in favor of the HBCR ranged from 0.23 to 0.26. However, when the study by Dehghani et al. was removed, the overall effect dropped to 0.19 (male participants) and to 0.20 (female participants) (Supplementary Figure S2).
6.3. HBCR versus Usual Care
The combined analysis of the five studies evaluating the effects of HBCR to UC group on CRF revealed a nonsignificant effect (Hedges’ g = 0.87, 95% CI −0.87 to 1.85), with a large heterogeneity (I2 = 96.41%; z = 1.75, p = 0.08) (Figure 5), although leave-one-out sensitivity analysis showed that with the removal of the Cai et al. study, the overall effect reached significance (Hedges’ g = 0.37, 95% CI 0.18 to 0.56) (Supplementary Figure S3).
7. Other Measurements
7.1. Physical Activity
Physical activity behavior was reported either through the use of accelerometers (Table 2) [33,35,41,42] assessing steps per day, sedentary time, and daily minutes of moderate, vigorous PA, or through self-reported days per week of moderate-vigorous PA, International Physical Activity Questionnaire [30,35], and exercise habits (number of participants reporting 30 min of moderate activity performed 3–5 times/week) [37]. No interaction effect was found for PA levels in all assessment endpoints for studies extracting PA data from accelerometers [33,41,42]. On the contrary, self-reported PA demonstrated improvements in PA levels within telerehabilitation groups compared to control groups [30,35,37].
7.2. Quality of Life
The results of the Medical Outcome Survey Short Form (SF-36) questionnaire, used to measure the QoL, revealed different results (Table 2). In the study of Bravo-Escobar et al., the only difference between the study groups was that the QoL scores were significantly higher in the CBCR group [39]. On the contrary were the results of another study, where the QoL was improved significantly in the intervention group (p < 0.001) [36]. In addition, other studies showed that total QoL improved significantly in both groups (p < 0.01), but no significant difference was found between groups [40] or in the overall score for QoL [41,42]. The Minnesota Living with Heart Failure Questionnaire (MLWHFQ) [32] and the EuroQol five-dimensional (EQ-5D) [31,32,38] did not report any between-group differences regarding their QoL.
7.3. Cardiovascular Risk Factors/Laboratory Parameters
The effects of exercise-based interventions on the CVD patients’ risk profiles reveal controversial results (Table 2). The glycemic control remained stable in all study groups [35] or increased in the control group and remained stable in the intervention group [37]. A tendency towards higher total cholesterol and low-density lipoprotein values was observed in all study participants [31,35,41]. Diastolic blood pressure remained stable in the intervention groups, increasing in the control group [37,41]. Maddison et al. reported smaller waist (p = 0.04) and hip circumferences (p = 0.04) outcomes during the intervention period in the intervention group, though this became absent at the follow up after the 24 weeks (no intervention) period [31].
8. Discussion
This current systematic review and meta-analysis of the available information has identified a positive effect of the wearable-sensors-assisted HBCR with improvements in patients’ CRF, whether the HBCR was used as an adjunct or as an alternative to CBCR. This finding is in accordance with previous systematic reviews that also proclaim the feasibility and effectiveness of digital HBCR in improving the patients’ CRF levels [7,24,45,46,47]. Additionally, the participants’ adherence rates appear to be higher in the intervention HBCR groups (Table 2), thus promoting a more profound aerobic training and probably explaining the more beneficial impact of HBCR on CRF levels when compared to the CBCR group outcomes. Surprisingly, no significant differences in symptom-limited exercising testing between HBCR and UC groups were observed. Similar results were presented in a recent meta-analysis where the HBCR group’s CRF did not differ from the UC in CPET results [46], though when Cai et al.’s study [30] was omitted from the meta-analysis, CRF outcome values differed significantly in favor of the HBCR group. The usual care group was encouraged to participate in an out-of-hospital, unsupervised exercise aerobic training, though with specific intensity prescriptions. Heterogeneity results reveal a contravention of Cai et al.’s study to the rest of the usual care groups, to which only standard counseling to remain physically active was given effect (Supplementary Figure S4).
In this systematic review, objective recording and evaluation of PA activity, via wearable sensors, revealed an inability of HBCR interventions to engage cardiac patients in a more active lifestyle. A previous meta-analysis of eHealth CR interventions, though, showed significant improvement in PA outcomes, in favor of the intervention groups [48], thus leading to inconsistency compared to our study results. This inconsistency may be explained because most of the studies included in this review based their PA evaluation on objective data monitoring and recording via accelerometers. Objective PA monitoring may have prevented a personal, subjective determination of physical status that could have led to a potential measurement recall bias [46,49]. In addition, intervention patients may have presented increased sedentary time levels, due to their engagement in the regular, programmed exercise sessions, thus making them more reluctant to seek additional physical exercise training.
Furthermore, wearable sensors with an accelerometer and ECG, combining AI algorithms and continuous monitoring, enabled the more accurate detection and identification of patients’ PA. Using AI, online platforms can facilitate remote communication between patients and clinicians, thus allowing consultations and prescription adjustments. Alternative chatting methods, such as e-mailing within a website, could also promote unlimited communication between patients and rehabilitation teams [49]. AI could improve the efficacy and effectiveness of HBCR by advancing its comprehensiveness; thus, further search on the utilization of AI is highly recommended.
Although the patients’ QoL was assessed in almost all included studies in this review, the majority of them reported no significant between-group differences. Psychological parameters, such as anxiety and depression, were evaluated by only three studies in this review and the implementation of wearable-sensors-assisted CR interventions showed no significant effects [33,37,38]. These findings are supported by a recent meta-analysis that revealed comparable effectiveness in psychological outcomes between technology-assisted HBCR and CBCR [50]. Moreover, in this review, adherence rates were comparable between HBCR and CBCR, with two studies reporting favorable effects in participation percentages for the intervention groups [30,32]. Thus, there is an indication that HBCR may have the potential to act as an alternative to overcome the barriers preventing the patients’ participation. Especially if the HBCR patients receive appropriate monitoring and constant guidance/feedback, their adherence rates appear to be higher than the ones attending CBCR [32,33,40,42].
A systematic review and meta-analysis demonstrated a reduction in cardiovascular risk factors (blood pressure, lipid profile, and smoking status) at medium- to long-term follow-up compared to comparison groups [22]. Contrarily, our review showed no significant differences between intervention and control groups on modifiable cardiac risk factors. Similarly, Chong et al. indicated that technology-assisted HBCR demonstrated comparable results to CBCR [50]. This discrepancy in our findings may be explained by the substantial improvements in cardiac risk profile derived from the major advancements in diagnostic and therapeutic procedures concerning CVDs, the systematic use of cardio-protective pharmacotherapy, and the adoption of the Mediterranean-type diet as a protective tool against recurrent cardiac events [51].
This systematic review provides a deep insight into the feasibility of implementing digital HBCR as an alternative method to widen access to CR for most of the population suffering from heart diseases. Moreover, this review stands out from previous ones since it emphasizes the understanding of the efficiency of digital HBCR interventions, incorporating the use of wearable sensors for the telemonitoring and the tele-guidance of the exercise sessions in the participants’ home environment. The implementation of wearable sensors-assisted HBCR programs can possibly act as a tool to overcome the several barriers that prevent the cardiac patients’ participation in CR interventions. Real time monitoring, through wearable sensors technology, could allow clinicians to implement CR programs even during pandemic eras and address the cardiac population that is lacking economic background or lives in rural, isolated locations. The wearable sensors-assisted HBCR could play the role of an “adjunct” or a “substitute” to conventional CBCR, based on each cardiac patient’s personal needs and current socioeconomic circumstances. Furthermore, there is a profound need for the scientists and the technology engineers to continuously improve and update the standards and the provided functions of the wearable sensors that can assist the implementation of the CR programs in the cardiac patients’ home settings.
9. Limitations
Although this systematic review is probably the first that investigates and evaluates the effectiveness of HBCR interventions incorporating wearable sensors, it displays several limitations. Small sample size and composition are some of them, since many studies had fewer than 100 participants and most of the patients in the included studies were males. Moreover, information about the socioeconomic background, educational level, or place of residence was missing from the participants’ demographic characteristics data, though such information may be necessary for a better understanding and interpretation of the findings of the studies, since they may play a role in the effectiveness and the efficacy of the CR interventions. Furthermore, most of the studies were conducted in high-income countries, thus, limiting the potential to generalize the results in countries with lower socioeconomic profiles. Additionally, the included studies were limited to English-written papers and only original published research articles, which might lead to missing other relevant literature in other languages or information available from the grey literature.
10. Conclusions
Overall, we have demonstrated that HBCR interventions using wearable sensors can be as effective as CBCR. If their implementation is achieved on a larger scale, HBCR with wearable sensors has the potential to increase the accessibility, adherence, and participation rates in CR interventions, by helping to overcome several barriers that prevent CR participation. Rural population, pandemic circumstances (such as the COVID-19 pandemic), and female cardiac patients’ evolvement in CR are aspects that need to be taken under consideration in further studies. Continuous technology advancement of the wearable sensors will help integrate them more successfully into the CR procedures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11133772/s1, Table S1: Search MeSH terms and Keywords; Table S2: PRISMA checklist; Figure S1: Results from REML for CRF between HBCR and CBCR; Figure S2: Results from Leave one out meta-analysis between CBCR and HBCR; Figure S3: Results from Leave one out meta-analysis between HBCR and UC.
Author Contributions
All authors contributed substantially to this article. V.A. and G.P. were responsible for the study conception and design. V.A. and G.P. were responsible for the data acquisition and quality appraisal of the included studies. V.A., G.P. and D.B.P. were responsible for the analysis and interpretation. V.A., G.P., E.K., C.H.D., D.B.P. and L.B. were responsible for drafting and revising the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Health, Czech Republic-conceptual development of research organization (FNBr, 65269705).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Shields, G.E.; Wells, A.; Doherty, P.; Heagerty, A.; Buck, D.; Davies, L.M. Cost-effectiveness of cardiac rehabilitation: A systematic review. Heart 2018, 104, 1403. [Google Scholar] [CrossRef]
- Piepoli, M.F. Cardiac rehabilitation: What are the latest advances? Dialogues Cardiovasc. Med. 2017, 23, 33–38. [Google Scholar]
- Dibben, G.; Faulkner, J.; Oldridge, N.; Rees, K.; Thompson, D.R.; Zwisler, A.D.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2021, 11, CD001800. [Google Scholar] [CrossRef]
- McGregor, G.; Powell, R.; Kimani, P.; Underwood, M. Does contemporary exercise-based cardiac rehabilitation improve quality of life for people with coronary artery disease? A systematic review and meta-analysis. BMJ Open 2020, 10, e036089. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Oldridge, N.; Thompson, D.R.; Zwisler, A.D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2016, 67, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sandercock, G.R.; Cardoso, F.; Almodhy, M.; Pepera, G. Cardiorespiratory fitness changes in patients receiving comprehensive outpatient cardiac rehabilitation in the UK: A multicentre study. Heart 2013, 99, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Prescott, E.; Eser, P.; Mikkelsen, N.; Holdgaard, A.; Marcin, T.; Wilhelm, M.; Gil, C.P.; González-Juanatey, J.R.; Moatemri, F.; Iliou, M.C.; et al. Cardiac rehabilitation of elderly patients in eight rehabilitation units in western Europe: Outcome data from the EU-CaRE multi-centre observational study. Eur. J. Prev. Cardiol. 2020, 27, 1716–1729. [Google Scholar] [CrossRef]
- Kotseva, K.; De Backer, G.; De Bacquer, D.; Rydén, L.; Hoes, A.; Grobbee, D.; Maggioni, A.; Marques-Vidal, P.; Jennings, C.; Abreu, A.; et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: Results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur. J. Prev. Cardiol. 2019, 26, 824–835. [Google Scholar] [CrossRef]
- Resurrección, D.M.; Moreno-Peral, P.; Gómez-Herranz, M.; Rubio-Valera, M.; Pastor, L.; Caldas de Almeida, J.M.; Motrico, E. Factors associated with non-participation in and dropout from cardiac rehabilitation programmes: A systematic review of prospective cohort studies. Eur. J. Cardiovasc. Nurs. 2019, 18, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Santiago de Araújo Pio, C.; Chaves, G.S.; Davies, P.; Taylor, R.S.; Grace, S.L. Interventions to promote patient utilisation of cardiac rehabilitation. Cochrane Database Syst. Rev. 2019, 2, Cd007131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruano-Ravina, A.; Pena-Gil, C.; Abu-Assi, E.; Raposeiras, S.; van ‘t Hof, A.; Meindersma, E.; Bossano Prescott, E.I.; González-Juanatey, J.R. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int. J. Cardiol. 2016, 223, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Winnige, P.; Filakova, K.; Hnatiak, J.; Dosbaba, F.; Bocek, O.; Pepera, G.; Papathanasiou, J.; Batalik, L.; Grace, S.L. Validity and Reliability of the Cardiac Rehabilitation Barriers Scale in the Czech Republic (CRBS-CZE): Determination of Key Barriers in East-Central Europe. Int. J. Environ. Res. Public Health 2021, 18, 3113. [Google Scholar] [CrossRef]
- Kemps, H.M.C.; Brouwers, R.W.M.; Cramer, M.J.; Jorstad, H.T.; de Kluiver, E.P.; Kraaijenhagen, R.A.; Kuijpers, P.M.J.C.; van der Linde, M.R.; de Melker, E.; Rodrigo, S.F.; et al. Recommendations on how to provide cardiac rehabilitation services during the COVID-19 pandemic. Neth. Heart J. 2020, 28, 387–390. [Google Scholar] [CrossRef]
- Besnier, F.; Gayda, M.; Nigam, A.; Juneau, M.; Bherer, L. Cardiac Rehabilitation During Quarantine in COVID-19 Pandemic: Challenges for Center-Based Programs. Arch. Phys. Med. Rehabil. 2020, 101, 1835–1838. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Ballerini Puviani, M.; Nasi, M.; Farinetti, A. COVID-19 pandemic: The effects of quarantine on cardiovascular risk. Eur. J. Clin. Nutr. 2020, 74, 852–855. [Google Scholar] [CrossRef]
- Pepera, G.; Tribali, M.S.; Batalik, L.; Petrov, I.; Papathanasiou, J. Epidemiology, risk factors and prognosis of cardiovascular disease in the Coronavirus Disease 2019 (COVID-19) pandemic era: A systematic review. Rev. Cardiovasc Med. 2022, 23, 28. [Google Scholar] [CrossRef]
- Dalal, H.M.; Taylor, R.S.; Jolly, K.; Davis, R.C.; Doherty, P.; Miles, J.; van Lingen, R.; Warren, F.C.; Green, C.; Wingham, J.; et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: The REACH-HF multicentre randomized controlled trial. Eur. J. Prev. Cardiol. 2019, 26, 262–272. [Google Scholar] [CrossRef]
- Anderson, L.; Sharp, G.A.; Norton, R.J.; Dalal, H.; Dean, S.G.; Jolly, K.; Cowie, A.; Zawada, A.; Taylor, R.S. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst. Rev. 2017, 6, CD007130. [Google Scholar] [CrossRef]
- Stefanakis, M.; Batalik, L.; Papathanasiou, J.; Dipla, L.; Antoniou, V.; Pepera, G. Exercise-based cardiac rehabilitation programs in the era of COVID-19: A critical review. Rev. Cardiovasc. Med. 2021, 22, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Khonsari, S.; Gallagher, R.; Gallagher, P.; Clark, A.M.; Freedman, B.; Briffa, T.; Bauman, A.; Redfern, J.; Neubeck, L. Telehealth interventions for the secondary prevention of coronary heart disease: A systematic review and meta-analysis. Eur. J. Cardiovasc. Nurs. 2019, 18, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Neubeck, L.; Lowres, N.; Benjamin, E.J.; Freedman, S.B.; Coorey, G.; Redfern, J. The mobile revolution—Using smartphone apps to prevent cardiovascular disease. Nat. Rev. Cardiol. 2015, 12, 350–360. [Google Scholar] [CrossRef]
- Su, J.J.; Yu, D.S.F.; Paguio, J.T. Effect of eHealth cardiac rehabilitation on health outcomes of coronary heart disease patients: A systematic review and meta-analysis. J. Adv. Nurs. 2020, 76, 754–772. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Chen, S.; Hong, L.; Sun, K.; Gong, E.; Li, C.; Yan, L.L.; Schwalm, J.D. Effect of Mobile Health Interventions on the Secondary Prevention of Cardiovascular Disease: Systematic Review and Meta-analysis. Can. J. Cardiol. 2017, 33, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Batalik, L.; Filakova, K.; Batalikova, K.; Dosbaba, F. Remotely monitored telerehabilitation for cardiac patients: A review of the current situation. World J. Clin. Cases 2020, 8, 1818–1831. [Google Scholar] [CrossRef] [PubMed]
- Batalik, L.; Pepera, G.; Papathanasiou, J.; Rutkowski, S.; Líška, D.; Batalikova, K.; Hartman, M.; Felšőci, M.; Dosbaba, F. Is the Training Intensity in Phase Two Cardiovascular Rehabilitation Different in Telehealth versus Outpatient Rehabilitation? J. Clin. Med. 2021, 10, 4069. [Google Scholar] [CrossRef]
- Lau, J.; Ioannidis, J.P.; Schmid, C.H. Quantitative synthesis in systematic reviews. Ann. Intern. Med. 1997, 127, 820–826. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Bao, Z.; Wu, N.; Wu, F.; Sun, G.; Yang, G.; Chen, M. A novel model of home-based, patient-tailored and mobile application-guided cardiac telerehabilitation in patients with atrial fibrillation: A randomised controlled trial. Clin. Rehabil. 2021, 36, 40–50. [Google Scholar] [CrossRef]
- Maddison, R.; Rawstorn, J.C.; Stewart, R.A.H.; Benatar, J.; Whittaker, R.; Rolleston, A.; Jiang, Y.; Gao, L.; Moodie, M.; Warren, I.; et al. Effects and costs of real-time cardiac telerehabilitation: Randomised controlled non-inferiority trial. Heart 2019, 105, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.; Bruning, J.; Morris, N.R.; Mandrusiak, A.; Russell, T. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: A randomised trial. Clin. Rehabil. 2017, 63, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraal, J.J.; Van den Akker-Van Marle, M.E.; Abu-Hanna, A.; Stut, W.; Peek, N.; Kemps, H.M.C. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: Results of the FIT@Home study. Eur. J. Prev. Cardiol. 2017, 24, 1260–1273. [Google Scholar] [CrossRef]
- Frederix, I.; Hansen, D.; Coninx, K.; Vandervoort, P.; Vandijck, D.; Hens, N.; Van Craenenbroeck, E.; Van Driessche, N.; Dendale, P. Medium-Term Effectiveness of a Comprehensive Internet-Based and Patient-Specific Telerehabilitation Program with Text Messaging Support for Cardiac Patients: Randomized Controlled Trial. J. Med. Internet Res. 2015, 17, e185. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, E.; Zieliłski, T.; Bodalski, R.; Rywik, T.; Dobraszkiewicz-Wasilewska, B.; Sobieszczałska-Małek, M.; Stepnowska, M.; Przybylski, A.; Browarek, A.; Szumowski, ł.; et al. Home-based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: A randomised controlled study. Eur. J. Prev. Cardiol. 2015, 22, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Snoek, J.A.; Prescott, E.I.; van der Velde, A.E.; Eijsvogels, T.M.H.; Mikkelsen, N.; Prins, L.F.; Bruins, W.; Meindersma, E.; González-Juanatey, J.R.; Peña-Gil, C.; et al. Effectiveness of Home-Based Mobile Guided Cardiac Rehabilitation as Alternative Strategy for Nonparticipation in Clinic-Based Cardiac Rehabilitation Among Elderly Patients in Europe: A Randomized Clinical Trial. JAMA Cardiol. 2021, 6, 463–468. [Google Scholar] [CrossRef]
- Skobel, E.; Knackstedt, C.; Martinez-Romero, A.; Salvi, D.; Vera-Munoz, C.; Napp, A.; Luprano, J.; Bover, R.; Glöggler, S.; Bjarnason-Wehrens, B.; et al. Internet-based training of coronary artery patients: The Heart Cycle Trial. Heart Vessel. 2017, 32, 408–418. [Google Scholar] [CrossRef]
- Bravo-Escobar, R.; González-Represas, A.; Gómez-González, A.M.; Montiel-Trujillo, A.; Aguilar-Jimenez, R.; Carrasco-Ruíz, R.; Salinas-Sánchez, P. Effectiveness and safety of a home-based cardiac rehabilitation programme of mixed surveillance in patients with ischemic heart disease at moderate cardiovascular risk: A randomised, controlled clinical trial. BMC Cardiovasc. Disord. 2017, 17, 66. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Ren, C.; Liu, P.; Tao, L.; Zhao, W.; Gao, W. Effect of Smartphone-Based Telemonitored Exercise Rehabilitation among Patients with Coronary Heart Disease. J. Cardiovasc. Transl. Res. 2020, 13, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Batalik, L.; Dosbaba, F.; Hartman, M.; Batalikova, K.; Spinar, J. Benefits and effectiveness of using a wrist heart rate monitor as a telerehabilitation device in cardiac patients: A randomized controlled trial. Medicine 2020, 99, e19556. [Google Scholar] [CrossRef]
- Avila, A.; Claes, J.; Buys, R.; Azzawi, M.; Vanhees, L.; Cornelissen, V. Home-based exercise with telemonitoring guidance in patients with coronary artery disease: Does it improve long-term physical fitness? Eur. J. Prev. Cardiol. 2020, 27, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Avila, A.; Claes, J.; Goetschalckx, K.; Buys, R.; Azzawi, M.; Vanhees, L.; Cornelissen, V. Home-Based Rehabilitation with Telemonitoring Guidance for Patients with Coronary Artery Disease (Short-Term Results of the TRiCH Study): Randomized Controlled Trial. J. Med. Internet Res. 2018, 20, e225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghani, M.; Cheraghi, M.; Namdari, M.; Roshan, V.D. Effects of Phase IV Pedometer Feedback Home-Based Cardiac Rehabilitation on Cardiovascular Functional Capacity in Patients With Myocardial Infarction: A Randomized Controlled Trial. Int. J. Basic. Sci. Med. 2019, 4, 75–80. [Google Scholar] [CrossRef] [Green Version]
- World Bank W.D.I. The World by Income and Region. Available online: https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html (accessed on 10 February 2022).
- Zwisler, A.D.; Norton, R.J.; Dean, S.G.; Dalal, H.; Tang, L.H.; Wingham, J.; Taylor, R.S. Home-based cardiac rehabilitation for people with heart failure: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 221, 963–969. [Google Scholar] [CrossRef]
- Ramachandran, H.J.; Jiang, Y.; Tam, W.W.S.; Yeo, T.J.; Wang, W. Effectiveness of home-based cardiac telerehabilitation as an alternative to Phase 2 cardiac rehabilitation of coronary heart disease: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2021, 29, 1017–1043. [Google Scholar] [CrossRef]
- Stefanakis, M.; Batalik, L.; Antoniou, V.; Pepera, G. Safety of home-based cardiac rehabilitation: A systematic review. Heart Lung 2022, 55, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Rawstorn, J.C.; Gant, N.; Direito, A.; Beckmann, C.; Maddison, R. Telehealth exercise-based cardiac rehabilitation: A systematic review and meta-analysis. Heart 2016, 102, 1183. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, Y.; Ke, Q.Q.; Su, J.K.; Yang, Q.H. Mobilizing artificial intelligence to cardiac telerehabilitation. Rev. Cardiovasc. Med. 2022, 23, 45. [Google Scholar] [CrossRef]
- Chong, M.S.; Sit, J.W.H.; Karthikesu, K.; Chair, S.Y. Effectiveness of technology-assisted cardiac rehabilitation: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2021, 124, 104087. [Google Scholar] [CrossRef]
- Panagiotakos, D.; Notara, V.; Kouvari, M.; Pitsavos, C. The Mediterranean and other Dietary Patterns in Secondary Cardiovascular Disease Prevention: A Review. Curr. Vasc. Pharmacol. 2016, 14, 442–451. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).