Abstract
Diseases, including cardiovascular disease, type II diabetes, and metabolic syndrome, are leading causes of morbidity and mortality worldwide. Non-alcoholic fatty liver disease (NAFLD) is commonly associated with these conditions through shared pathophysiological mechanisms such as insulin resistance, chronic inflammation, and dyslipidemia. Emerging evidence suggests that probiotic formulations containing Bifidobacterium species may support cardiometabolic health by modulating gut microbiota composition. This meta-analysis aimed to assess the efficacy of Bifidobacterium-containing probiotic combinations in improving key cardiometabolic parameters, including lipid profiles, blood pressure, glycemic indices, and inflammatory biomarkers among individuals with NAFLD. A systematic literature search was conducted across PubMed, Embase, the Cochrane Library, and Web of Science databases to identify relevant randomized controlled trials (RCTs) published up to December 2024. A total of 24 RCTs involving 1611 participants met the inclusion criteria. The pooled results demonstrated significant reductions in total cholesterol, triglycerides, and low-density lipoprotein cholesterol following probiotic intervention. Improvements were also observed in fasting glucose levels and inflammatory markers, including high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6). Although modest improvements were noted in NAFLD severity, the effects on liver injury markers were relatively limited. These findings suggest that Bifidobacterium-based probiotic combinations may provide cardiometabolic benefits, particularly in lipid regulation, glucose metabolism, and inflammatory control. Further large-scale, well-designed RCTs are warranted to validate these results and to determine the most effective probiotic strains, compositions, and treatment durations.
1. Introduction
Cardiometabolic risk factors are widely recognized as predictors of chronic diseases and remain a leading cause of mortality worldwide []. These factors are associated with a cluster of metabolic abnormalities, including glucose intolerance, elevated insulin levels, insulin resistance, hypertriglyceridemia, reduced levels of high-density lipoprotein (HDL), and increased concentrations of low-density lipoprotein (LDL). Such imbalances in metabolic regulation contribute to systemic inflammation, as indicated by elevated levels of high-sensitivity C-reactive protein (hs-CRP) and other pro-inflammatory biomarkers [,]. Among these markers, both CRP and tumor necrosis factor-alpha (TNF-α) have been identified as important indicators of cardiovascular mortality, including in cases of coronary artery disease []. Obesity is another critical component of metabolic syndrome, often reflected in elevated body mass index (BMI), increased waist circumference, and central fat accumulation, all of which significantly raise the risk of developing cardiovascular diseases (CVDs) []. Non-alcoholic fatty liver disease (NAFLD), defined by excessive lipid accumulation in liver cells, is increasingly recognized as a hepatic manifestation of metabolic syndrome. It is closely associated with cardiometabolic disorders and related complications [,]. Epidemiological data indicate that approximately 25 percent of the global population is affected by NAFLD, with particularly high prevalence seen among individuals with obesity and features of metabolic syndrome []. The connection between NAFLD and cardiometabolic conditions stems from shared pathophysiological mechanisms, including insulin resistance, dyslipidemia, systemic inflammation, and cellular stress in the endoplasmic reticulum. These mechanisms collectively promote liver fat accumulation and elevate cardiovascular risk [,]. Evidence also indicates that NAFLD is independently associated with an increased risk of cardiovascular disease, beyond traditional risk factors, through pathways involving oxidative stress and chronic inflammation that impair endothelial function and promote atherogenesis [,]. NAFLD represents a spectrum of liver disorders ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), which may progress to advanced stages such as fibrosis, cirrhosis, or hepatocellular carcinoma []. Despite its increasing prevalence and serious clinical implications, NAFLD lacks approved pharmacological therapies. Current management guidelines emphasize lifestyle modification, including dietary changes and increased physical activity, as the main strategies for disease prevention and control [].
The gut–liver axis plays a central role in the pathogenesis and advancement of non-alcoholic fatty liver disease (NAFLD), highlighting the dynamic interaction between the gut microbiota and liver metabolism. An altered gut microbial environment, known as a state of dysbiosis, compromises the integrity of the intestinal barrier. This impairment facilitates the passage of bacterial endotoxins, particularly lipopolysaccharides (LPS), into the portal vein. Once in the liver, these endotoxins stimulate inflammatory responses and contribute to the development of insulin resistance, both of which are key contributors to the progression of NAFLD [,].
Growing evidence supports the influential role of gut microbiota in regulating both cardiometabolic and liver-related health. Among the beneficial microbial genera, Bifidobacterium has been widely studied for its capacity to restore microbial homeostasis and reinforce the integrity of the intestinal barrier. Several studies have demonstrated that Bifidobacterium species contribute to the regulation of lipid metabolism, glucose homeostasis, and inflammatory pathways, which are closely associated with the development of NAFLD and cardiometabolic disorders [,]. Supplementation with probiotics containing Bifidobacterium has been shown to enhance barrier function in the gut epithelium [], decrease circulating endotoxins, and positively influence lipid metabolism through mechanisms involving the gut–liver axis []. Moreover, Bifidobacterium has been reported to reduce levels of LPS and inhibit the hepatic activation of toll-like receptor 4 (TLR4), thereby attenuating hepatic inflammation and oxidative stress [,]. Additional findings suggest that Bifidobacterium may also support bile acid metabolism, which plays a crucial role in lipid regulation and reducing hepatic fat accumulation, offering therapeutic promise in the context of NAFLD [,].
This meta-analysis was conducted to assess the effectiveness of Bifidobacterium supplementation in improving key cardiometabolic risk parameters, such as lipid profile, blood pressure, glycemic regulation, and inflammatory biomarkers. By synthesizing evidence from randomized controlled trials, the study aimed to determine whether Bifidobacterium could function as a supportive therapeutic option for mitigating cardiometabolic risk, especially in individuals with NAFLD and related metabolic disorders.
2. Methods
This meta-analysis is registered with PROSPERO (registration number: CRD42024605844) and was conducted in accordance with the PRISMA reporting guidelines [].
2.1. Data Sources and Selection Criteria
A comprehensive search was performed across PubMed, Embase, the Cochrane Library, and Web of Science to identify relevant RCTs published up to December 2024. Search terms included combinations such as “Bifidobacterium”, “probiotics”, “cardiometabolic”, “lipid profile”, “fatty liver”, “non-alcoholic fatty liver disease”, “non-alcoholic steatohepatitis”, and “steatohepatitis.” In addition, the reference lists of retrieved articles were manually screened to identify further eligible studies. Exclusion criteria included case reports, technical reports, conference abstracts, review articles, editorials, letters, and in vitro or animal-based research.
2.2. Selection of Studies
Two independent researchers conducted the screening and evaluation of eligible studies, with a third researcher reviewing the process to maintain consistency and accuracy. Hard copies of all relevant publications were obtained and thoroughly reviewed to ensure detailed assessment. The complete study selection process is outlined in the PRISMA flow diagram (Figure 1).
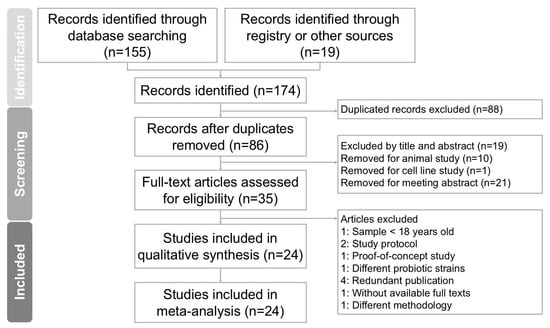
Figure 1.
Diagram outlining study selection process for systematic review and meta-analysis investigating Bifidobacterium combinations interventions for reducing cardiometabolic risk factors in NAFLD. Of 174 records initially identified, 24 studies fulfilled inclusion criteria and were incorporated into final analysis.
2.3. Data Extraction
Data extraction was independently conducted by authors using a standardized form following the Cochrane Handbook guidelines []. Key information collected included author names, publication year and country, participant inclusion criteria, demographic details (such as total sample size and age range), study design, intervention specifics, and reported outcomes, along with the assessment methods used.
2.4. Outcomes
The primary outcomes assessed in this study were liver fat content and liver injury markers, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT). Inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), hs-CRP, and IL-6 were also evaluated. Additionally, BMI and lipid profile parameters including cholesterol, triglycerides, LDL, and HDL were analyzed. The secondary outcomes included fasting plasma glucose, fasting serum insulin, homeostasis model assessment for insulin resistance (HOMA-IR), glycated hemoglobin (HbA1c), and blood pressure.
2.5. Assessment of Methodological Quality
Two independent reviewers systematically evaluated the risk of bias in the included studies using the Cochrane Collaboration’s Risk of Bias tool to assess methodological quality. Any discrepancies between the reviewers were resolved through in-depth discussion with a third reviewer to reach consensus. Studies were deemed to have a high risk of bias if at least one domain was found to raise significant concerns, in accordance with the criteria established by the tool.
2.6. Data Analysis
Data from the selected studies were quantitatively analyzed using SMD with 95% confidence intervals (CIs) to compare outcomes between intervention and control (placebo) groups. SMD values were pooled using a random-effects model to account for interstudy variability. Statistical analyses were performed using Comprehensive Meta-Analysis software, version 3 (Version 3.0; Biostat, Englewood, NJ, USA). Heterogeneity was assessed using the I2 statistic, with values exceeding 50% indicating substantial heterogeneity. Publication bias was evaluated using funnel plots and Egger’s regression test, with significance thresholds set at p < 0.05 for general analyses and p < 0.10 for publication bias assessment. Subgroup analyses were conducted to investigate the possible sources of heterogeneity, and sensitivity analyses were performed by excluding individual studies to evaluate the stability of the overall results.
3. Results
Study search and characteristics of included patients.
The process of screening and selecting trials is illustrated in Figure 1. An initial database search using PubMed, Embase, the Cochrane Library, and Web of Science identified 174 records. After removing duplicate entries, 86 articles remained and were screened based on their titles and abstracts. Of these, 51 articles were excluded due to irrelevance to the research topic. A detailed full-text review was conducted on the remaining 35 articles. Following this review, 11 studies were excluded for the following reasons: one study included participants younger than 18 years [], two articles were study protocols [,], one article was a proof-of-concept study [], one study used different probiotic strains from the target interventions [], and study one applied a different methodology []. Four studies were excluded due to duplicate publication [,,,], and one article was excluded due to the full text being unavailable []. A total of 24 randomized controlled trials met the eligibility criteria and were included in the meta-analysis [,,,,,,,,,,,,,,,,,,,,,,,]. All selected articles were published in English. Table 1 summarizes the characteristics of the included trials, which were conducted between 2012 and 2024. These studies involved a combined total of 1611 participants, with individual trial sample sizes ranging from 17 to 70. Each study evaluated the effects of Bifidobacterium supplementation on body mass index, liver function parameters, and lipid profile outcomes.

Table 1.
Characteristics of included studies.
3.1. Quality Assessment
Figure 2A,B provide a summary of the risk of bias assessments across the studies included. Of the trials analyzed, 21 were assessed as having a low risk of bias, reflecting overall methodological strength and clarity in data reporting. Two studies [,] exhibited high risk in specific domains, particularly in relation to the accuracy of outcome assessment and fidelity to the intervention protocol. Only one study [] demonstrated some concern in the randomization process. In domain 2, most trials were rated as displaying low risk, indicating that the interventions were generally delivered as planned, although both studies [,] were exceptions, with high risk in this area. Domain 3 presented a recurring issue, with several studies [,,,,,,,] rated as displaying low risk, while others showed concerns due to significant missing data. Overall, outcome measurement was consistently and appropriately conducted, with only one trial [] showing high risk in this domain. Additionally, ten studies raised concerns regarding selective reporting due to the lack of a pre-registered protocol or predefined statistical analysis plan [,,,,,,,,,]. As part of our quality assessment, we also conducted a sensitivity analysis excluding studies with a high risk of bias. The results remained consistent, indicating that the primary outcomes were robust and not significantly influenced by lower-quality studies. As part of our quality assessment, we also conducted a sensitivity analysis, excluding studies with a high risk of bias. The results remained consistent, indicating that the primary outcomes were robust and not significantly influenced by lower-quality studies (Figure 3A,B).
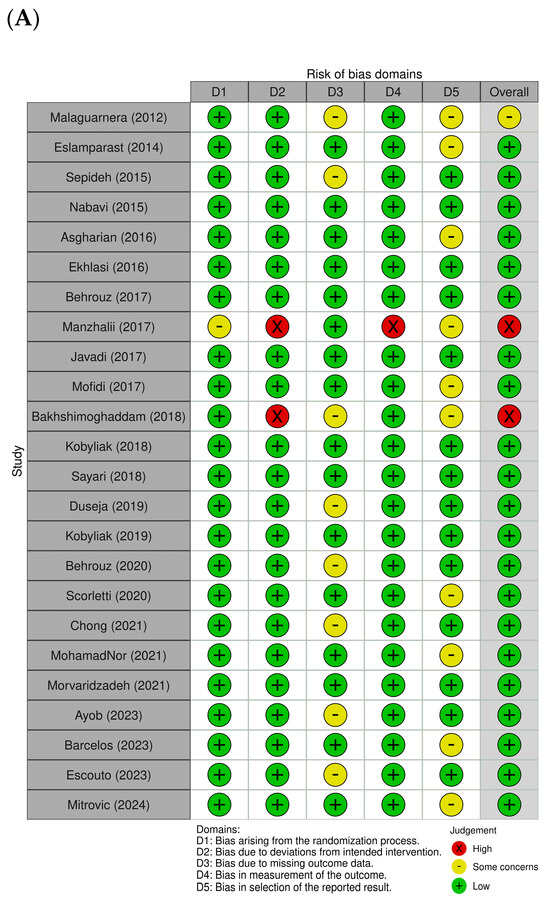
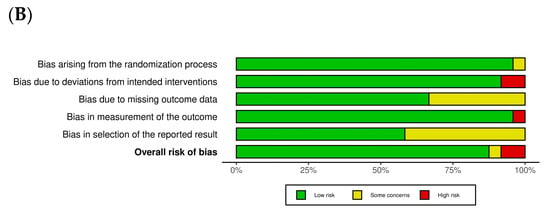
Figure 2.
Assessment of methodological quality of included trials. (A) Risk of bias for each included study. (B) Overall summary of bias of 24 studies [,,,,,,,,,,,,,,,,,,,,,,,].
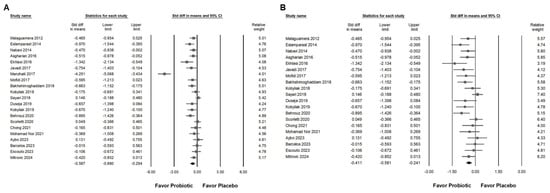
Figure 3.
Forest plots show the effects of Bifidobacterium combination supplementation on serum ALT levels. Panel (A) illustrates the direct impact of the intervention, while Panel (B) presents a sensitivity analysis to validate the findings in Panel (A). Squares represent standardized mean differences, favoring Bifidobacterium (shifted to the left), with horizontal lines indicating 95% confidence intervals. The diamond at the bottom of each plot represents the overall combined effect size for the analysis [,,,,,,,,,,,,,,].
3.2. Effect of Combinations with Bifidobacterium on Alanine Aminotransferase Expression
Probiotic combinations that included Bifidobacterium demonstrated a moderate effect in reducing serum ALT levels, as presented in Figure 3A (SMD: −0.587, 95% CI: −0.880 to −0.294; I2 = 83.757%, p < 0.001). One particular study [] reported a substantially greater effect than the others, which influenced its exclusion during sensitivity analysis. After removing this outlier, the updated results (Figure 3B) still demonstrated a statistically significant but smaller effect of Bifidobacterium combinations on ALT reduction (SMD: −0.411, 95% CI: −0.581 to −0.241; I2 = 50.111%, p = 0.006). Subgroup analysis based on intervention duration (Figure 4A) indicated that supplementation for 12 weeks resulted in a moderate decrease in ALT (SMD: −0.552, 95% CI: −0.752 to −0.352; I2 = 18.343%, p = 0.280). This effect size was greater compared to that observed in studies with durations of 12 to 28 weeks (SMD: −0.309, 95% CI: −0.578 to −0.040; I2 = 56.876%, p = 0.017) or more than 50 weeks (SMD: −0.235, 95% CI: −0.913 to 0.444; I2 = 62.344%, p = 0.103), both of which showed only a minimal effect. Of the included studies, 42.9 percent were conducted in Iran, raising the possibility that regional or ethnic differences may influence the observed outcomes. Figure 4B illustrates that the impact of Bifidobacterium combinations was more pronounced in Iranian participants (SMD: −0.621, 95% CI: −0.925 to −0.317; I2 = 67.364%, p = 0.002) compared to participants from other countries such as Malaysia (SMD: −0.114, 95% CI: −0.605 to 0.376; I2 = 17.282%, p = 0.272), the United Kingdom (SMD: −0.011, 95% CI: −0.363 to 0.342; I2 = 0%, p = 0.593), Ukraine (SMD: −0.407, 95% CI: −0.891 to 0.077; I2 = 37.186%, p = 0.207), and Brazil (SMD: −0.061, 95% CI: −0.466 to 0.343; I2 = 0%, p = 0.827). Further subgroup analysis based on continent of origin (Figure 4C) found that studies conducted in Asia reported a moderate reduction in ALT (SMD: −0.542, 95% CI: −0.796 to −0.286; I2 = 61.386%, p = 0.003). In contrast, studies from Europe demonstrated a smaller effect (SMD: −0.289, 95% CI: −0.501 to −0.077; I2 = 7.837%, p = 0.366), while those from South America showed no significant change (SMD: −0.061, 95% CI: −0.466 to 0.343; I2 = 0%, p = 0.827). Finally, the influence of sex distribution in the intervention groups was assessed (Figure 4D). The results showed no significant difference in terms of the effect of Bifidobacterium combinations between groups composed of less than 60 percent males (SMD: −0.481, 95% CI: −0.674 to 0.288; I2 = 8.492%, p = 0.365) and those with more than 60 percent males (SMD: −0.275, 95% CI: −0.582 to 0.032; I2 = 60.401%, p = 0.014), suggesting that the efficacy of the intervention is not substantially influenced by gender composition.
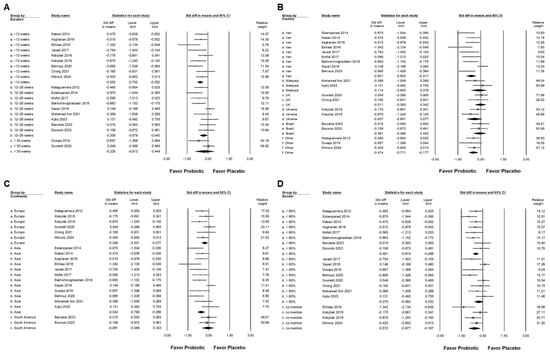
Figure 4.
Forest plots summarizing subgroup analyses of Bifidobacterium combination supplementation on serum ALT levels. Panel (A) evaluates effects by intervention duration, Panel (B) by country, and Panel (C) by continent. Panel (D) examines the influence of proportion of males in intervention groups. Squares indicate standardized mean differences favoring Bifidobacterium (left), with horizontal lines showing 95% confidence intervals. Diamonds represent overall combined effect sizes for each analysis [,,,,,,,,,,,,,,,,,,].
3.3. Effect of Combinations with Bifidobacterium on Liver Injury Markers and Liver Fat
Bifidobacterium combination supplementation demonstrated a moderate effect in reducing AST levels, as shown in Figure 5A (SMD = −0.563, 95% CI: −0.726 to −0.400; I2 = 36.3%, p = 0.063). A smaller yet significant effect was observed for GGT (Figure 5B; SMD = −0.442, 95% CI: −0.664 to −0.220; I2 = 50.1%, p = 0.024). Additionally, a moderate reduction in liver fat content was observed (SMD = −0.555, 95% CI: −0.728 to −0.383; I2 = 20.6%, p = 0.241; Figure 5C). To further explore the influence of intervention duration, we conducted subgroup analysis (Figure 5D). Moderate effects were maintained in both the <12-week group (SMD = −0.611, 95% CI: −0.860 to −0.361; I2 < 0.001%, p = 0.735) and the 12–28-week group (SMD = −0.545, 95% CI: −0.843 to −0.247; I2 = 40.0%, p = 0.139). In contrast, no significant effect was observed in studies lasting over 50 weeks (SMD = −0.556, 95% CI: −1.298 to 0.185; I2 = 72.4%, p = 0.057). These findings suggest that the beneficial effects of Bifidobacterium combinations on liver fat may be more pronounced during short- to medium-term interventions.
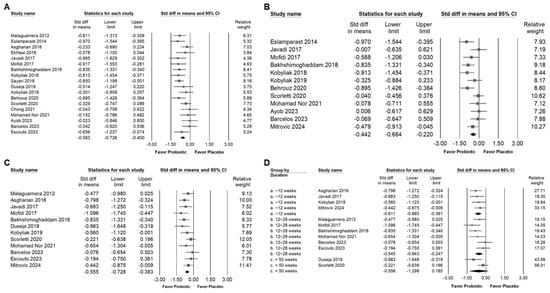
Figure 5.
Forest plots illustrate the effects of Bifidobacterium combination supplementation on liver enzymes and liver fat. Panel (A) presents results for AST levels, Panel (B) presents results for GGT levels, and Panel (C) presents results for liver fat content; Panel (D) shows a subgroup analysis of liver fat content based on intervention duration. Squares represent effect estimates with horizontal lines showing 95% confidence intervals. Diamonds at the bottom of each panel indicate the overall pooled effect size for each outcome [,,,,,,,,,,,].
3.4. Influence of Combinations with Bifidobacterium on Blood Lipids and Lipoproteins
Supplementation with Bifidobacterium combinations demonstrated a small but statistically significant reduction in serum total cholesterol levels, as indicated in Figure 6A (SMD: −0.378, 95% CI: −0.588 to −0.168; I2 = 54.782%, p = 0.007). Similar effects were observed for triglycerides (Figure 6B; SMD: −0.423, 95% CI: −0.582 to −0.264; I2 = 22.693%, p = 0.208) and LDL cholesterol (Figure 6C; SMD: −0.447, 95% CI: −0.757 to −0.137; I2 = 74.125%, p = 0). In contrast, no significant change was detected in HDL cholesterol levels (Figure 6D; SMD: 0.013, 95% CI: −0.141 to 0.167; I2 = 3.937%, p = 0.405). These results suggest that Bifidobacterium combinations may contribute to improved lipid metabolism, particularly through reductions in atherogenic lipid components such as total cholesterol, triglycerides, and LDL.
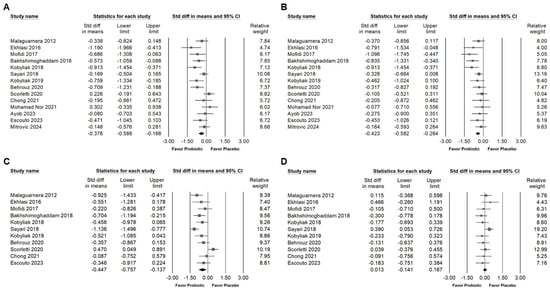
Figure 6.
Forest plots illustrating effects of Bifidobacterium combination supplementation on lipid profile parameters. Panel (A) shows total cholesterol, Panel (B) shows triglycerides, Panel (C) shows LDL, and Panel (D) shows HDL levels. Squares represent standardized mean differences with 95% confidence intervals, while diamonds summarize the overall pooled effect size for each outcome, highlighting the intervention’s impact on lipid metabolism [,,,,,,,,,,,,].
3.5. Effects of Combinations with Bifidobacterium on Fasting Plasma Glucose, Serum Insulin, HbA1C, and Homeostasis Model Assessment for Insulin Resistance (HOMA-IR)
Supplementation with probiotic combinations containing Bifidobacterium was associated with a small but significant reduction in fasting blood glucose levels (Figure 7A; SMD: −0.364, 95% CI: −0.581 to −0.147; I2 = 54.496%, p = 0.012), serum insulin concentrations (Figure 7B; SMD: −0.343, 95% CI: −0.589 to −0.097; I2 = 49.413%, p = 0.045), and HOMA-IR values (Figure 7C; SMD: −0.403, 95% CI: −0.623 to −0.182; I2 = 44.094%, p = 0.057). In contrast, no significant change was found in glycated hemoglobin (HbA1c) levels (Figure 7D; SMD: −0.070, 95% CI: −0.340 to 0.199; I2 = 0%, p = 0.931). These results suggest that Bifidobacterium combinations may contribute to short-term improvements in glycemic regulation, particularly by lowering fasting glucose and enhancing insulin sensitivity, while their influence on long-term glycemic markers remains limited.
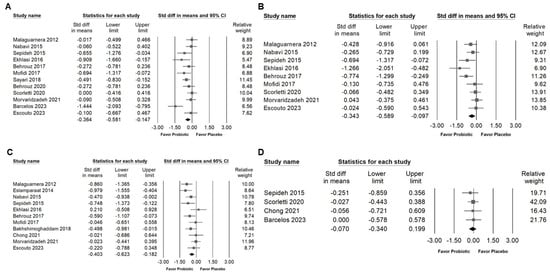
Figure 7.
Forest plots summarizing the effects of Bifidobacterium combination supplementation on glycemic control markers. Panel (A) shows fasting blood glucose, Panel (B) shows fasting serum insulin, Panel (C) shows HOMA-IR, and Panel (D) shows HbA1C levels. Squares indicate effect estimates with 95% confidence intervals, and diamonds at the bottom of each panel represent the overall pooled effect size, illustrating the intervention’s impact on glycemic markers [,,,,,,,,,,,].
3.6. Effect of Combinations with Bifidobacterium on Blood Pressure and Inflammatory Cytokines
The meta-analysis indicated that Bifidobacterium combinations did not significantly influence systolic blood pressure (Figure 8A; SMD: −0.189, 95% CI: −0.536 to 0.157; I2 = 54.257%, p = 0.087) or diastolic blood pressure (Figure 8B; SMD: −0.134, 95% CI: −0.469 to 0.200; I2 = 51.150%, p = 0.105). In contrast, supplementation with Bifidobacterium combinations was associated with a significant reduction in pro-inflammatory cytokines, including TNF-α (Figure 9A; SMD: −0.638, 95% CI: −0.874 to −0.401; I2 = 23.801%, p = 0.240), IL-6 (Figure 9B; SMD: −0.708, 95% CI: −0.986 to −0.430; I2 = 35.094%, p = 0.160), and hs-CRP (Figure 9C; SMD: −0.476, 95% CI: −0.720 to −0.233; I2 = 40.249%, p = 0.110), suggesting a potential anti-inflammatory benefit despite the limited impact on blood pressure parameters.

Figure 8.
Forest plots summarizing the effects of Bifidobacterium combination supplementation on blood pressure. Panel (A) shows changes in systolic blood pressure, and Panel (B) in diastolic blood pressure. Squares represent effect estimates with 95% confidence intervals, and diamonds indicate the overall pooled effect size, highlighting the intervention’s impact on blood pressure [,,,].
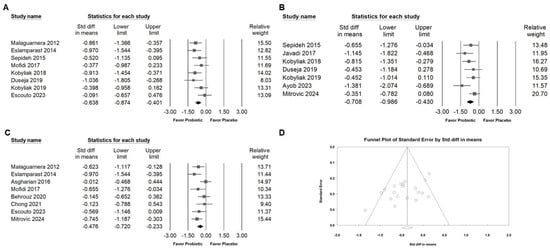
Figure 9.
Forest plots summarizing the effects of Bifidobacterium combination supplementation on inflammatory markers. Panel (A) examines TNF-α, Panel (B) shows IL-6, and Panel (C) shows hs-CRP levels. Squares represent effect estimates with 95% confidence intervals, and diamonds indicate the overall pooled effect size. Panel (D) shows a funnel plot for the studies in Figure 3B; the lines represent the confidence intervals around the effect estimates, indicating the range within which the true effect size is likely to fall. Each circle corresponds to an individual study included in the meta-analysis, with the size of the circle potentially reflecting the study’s weight or sample size. Larger circles denote studies with greater weight or larger sample sizes. The diamond symbol signifies the overall effect estimate from the meta-analysis. The center of the diamond marks the pooled effect size, and the width of the diamond indicates the confidence interval for this estimate [,,,,,,,,,,,].
3.7. Publishing Bias
4. Discussion
This meta-analysis examined the impact of Bifidobacterium-containing probiotic combinations on metabolic and hepatic outcomes, including lipid profiles, glycemic control, inflammatory cytokines, and liver injury markers. Significant improvements were observed, particularly in lipid modulation and inflammation attenuation. However, the effect sizes varied according to supplementation duration and population characteristics.
Our findings indicated that probiotic combinations containing Bifidobacterium produced a moderate reduction in liver fat content and exerted a notable influence on lipid metabolism, particularly by decreasing levels of total cholesterol, triglycerides, and LDL. These outcomes are consistent with earlier research suggesting that probiotics may regulate lipid metabolism through mechanisms such as bile salt deconjugation and cholesterol assimilation by gut microbiota []. In contrast, HDL levels exhibited minimal change in response to Bifidobacterium supplementation. This disparity suggests that probiotics may be more effective in lowering harmful lipid fractions than in promoting beneficial ones. The relatively stable HDL concentrations may stem from the unique physiological role of HDL in reverse cholesterol transport—a process less susceptible to modulation by dietary and microbial interventions compared to LDL and triglyceride pathways []. Probiotic action, particularly from Bifidobacterium, appears to target cholesterol metabolism by altering bile acid composition, decreasing intestinal cholesterol absorption, and increasing fecal lipid excretion. These mechanisms predominantly influence LDL and triglyceride levels. Consequently, Bifidobacterium combinations may offer a promising adjunctive approach for managing dyslipidemia. The concurrent reduction in BMI observed across studies is clinically relevant, given the strong association between obesity and NAFLD. Weight reduction remains a primary strategy in NAFLD management, and even modest decreases in BMI have been linked to reductions in hepatic fat accumulation and improvements in systemic metabolic markers, thereby lowering the risk of disease progression [,].
The intervention was associated with modest improvements in glycemic regulation, as evidenced by reductions in fasting blood glucose, serum insulin, and the homeostatic model assessment for insulin resistance (HOMA-IR). However, no significant changes were detected in HbA1c levels—a finding that aligns with previous studies [,]. The lack of improvement in HbA1c, a marker of long-term glycemic status, suggests that these probiotic interventions may not sustain glucose-lowering effects over extended periods. This disparity may reflect the time-limited nature of probiotic influence on insulin signaling pathways or the requirement for prolonged intervention to achieve durable metabolic benefits. As such, while Bifidobacterium combinations appear to offer supplementary benefits in early glycemic control, particularly for individuals with insulin resistance or prediabetes, future research involving longer intervention durations and longitudinal monitoring is warranted to confirm their long-term efficacy.
An important observation from this meta-analysis was the moderate reduction in hepatic enzyme levels, particularly ALT and AST, following supplementation with Bifidobacterium combinations. In contrast, reductions in GGT were less pronounced. Notably, ALT levels declined more substantially in trials with shorter intervention periods of around 12 weeks, suggesting that the hepatoprotective effects may reach a therapeutic plateau over time []. This finding is clinically relevant, as elevated ALT and AST are well-established biomarkers of hepatocellular injury, especially in individuals with NAFLD. Their reduction implies a decrease in hepatic inflammation and injury, indicating improved liver health []. The beneficial effect on liver enzymes may be attributable to the ability of Bifidobacterium to modulate the gut–liver axis by restoring microbial balance, reinforcing intestinal barrier integrity, and reducing the translocation of endotoxins such as lipopolysaccharides. These actions help dampen systemic inflammation and alleviate hepatic stress. Moreover, Bifidobacterium has been shown to regulate bile acid metabolism and mitigate oxidative stress, mechanisms that are critical in hepatocyte injury and regeneration []. These multifaceted pathways likely contribute to the greater responsiveness of ALT and AST compared to systemic markers like HbA1c or HDL, which may require longer durations to exhibit significant changes due to their association with chronic metabolic regulation.
Subgroup analysis indicated that Bifidobacterium combination supplementation significantly reduced ALT levels in studies where the proportion of males in the intervention group was less than 60%. This outcome suggests that the intervention may have a more pronounced effect in female participants, potentially reflecting gender-specific sensitivity to probiotic therapy. One possible explanation lies in sex-based differences in gut microbiota composition. Women have been reported to exhibit a higher Firmicutes-to-Bacteroidetes (F/B) ratio compared to men, a microbial profile that may influence how probiotics like Bifidobacterium interact with the host’s intestinal ecosystem []. Additionally, sex hormones such as estrogen play a modulatory role in shaping the gut microbiota, potentially affecting how females respond to probiotic supplementation []. Despite these insights, few studies have specifically explored the relationship between gender and changes in ALT levels following probiotic use. Some evidence suggests gender-dependent differences in microbial responses to metabolic stimuli. For instance, one study showed that sex-specific gut microbiota patterns are associated with variations in metabolic risk and liver enzyme levels []. Another investigation highlighted how age and gender may influence the clinical efficacy of probiotics, especially regarding metabolic outcomes []. However, a systematic evaluation of sex as a modifying factor in probiotic trials is still lacking. Moreover, differences in probiotic strain composition, dosage, and duration across the included studies may further confound subgroup analyses. Therefore, future research should aim to incorporate standardized intervention protocols and stratify outcomes by gender to clarify the extent to which probiotic efficacy differs between sexes and to inform precision-based therapeutic strategies.
This meta-analysis highlights the potential of Bifidobacterium-containing probiotics in addressing NAFLD-related risk factors; however, several limitations warrant consideration. First, significant heterogeneity was observed across the studies included, likely stemming from differences in probiotic strains, combinations with other genera, dosing regimens, and the duration of the interventions. These variations hinder direct comparison and reduce the precision of the overall effect estimates. Although subgroup and sensitivity analyses were conducted, not all sources of heterogeneity could be fully explained. Second, Egger’s regression test indicated potential publication bias (p = 0.01574), suggesting that studies with null or unfavorable outcomes may be underrepresented in the literature. This raises the possibility that the observed benefits could be overstated due to selective reporting. Third, the duration of most included trials was relatively short, typically ranging from 8 to 12 weeks. Although nine studies had follow-up periods between 12 and 28 weeks and demonstrated a sustained, albeit smaller, effect (SMD = −0.309), longer-term outcomes remain uncertain. In particular, key endpoints such as HbA1c, HDL cholesterol, histological liver changes, and progression of NAFLD were either minimally affected or not assessed, limiting the ability to determine the durability and clinical significance of the interventions. Fourth, nearly half of the included trials (12 out of 24) were conducted in Iran. This regional concentration may restrict the generalizability of the findings, as dietary habits, lifestyle factors, and genetic backgrounds can significantly influence gut microbiota composition and responsiveness to probiotics. Moreover, some studies may involve overlapping participant populations from the same institutions, raising concerns about potential data redundancy and inflation of pooled effects. Lastly, none of the included trials systematically accounted for habitual dietary intake or host genetic factors—both of which are known to modulate the gut microbiome and may interact with probiotic efficacy. Without controlling for these variables, the interpretation of the observed benefits across diverse populations remains limited. To enhance future research, well-designed, multicenter randomized controlled trials with longer durations are needed. These studies should incorporate standardized probiotic formulations, stratify factors such as diet and genetics by host, and include clinically meaningful endpoints to better assess the long-term efficacy and applicability of probiotic interventions.
5. Conclusions
In conclusion, while current evidence does not support the reversal of NAFLD by Bifidobacterium combinations, this meta-analysis suggests their potential in improving key metabolic and inflammatory markers, such as LDL, triglycerides, glucose, insulin, TNF-α, IL-6, and hs-CRP. These combinations may serve as adjuncts to lifestyle changes. Larger, standardized RCTs focused on diverse populations are needed to confirm long-term benefits and clinical applicability.
Author Contributions
Data curation and investigation, K.-S.C. Data curation and investigation, W.-H.K. Data curation, M.-H.C. Visualization, Y.H. Conceptualization, data curation, investigation, visualization, writing—original draft, writing—review, and editing, R.-Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Chung Shan Medical University-Yuan-Rung Hospital Research Collaboration Project No. 1140057.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We would like to thank Jhen-You Hu from the Creative Science Experimental Class at Taichung Municipal Taichung Girls’ Senior High School, Taichung, Taiwan, for her valuable assistance in searching for RCTs for this meta-analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Joshi, R.; Agrawal, T.; Fathima, F.; Usha, T.; Thomas, T.; Misquith, D.; Kalantri, S.; Chidambaram, N.; Raj, T.; Singamani, A.; et al. Cardiovascular risk factor reduction by community health workers in rural India: A cluster randomized trial. Am. Heart J. 2019, 216, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: Implications for longevity. Nutr. Rev. 2007, 65, S253–S259. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Targher, G.; Day, C.P.; Bonora, E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010, 363, 1341–1350. [Google Scholar] [CrossRef]
- Ezpeleta, M.; Gabel, K.; Cienfuegos, S.; Kalam, F.; Lin, S.; Pavlou, V.; Song, Z.; Haus, J.M.; Koppe, S.; Alexandria, S.J.; et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: A randomized controlled trial. Cell Metab. 2023, 35, 56–70.e53. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, S.T.A.; Silva-Sperb, A.S.; Moraes, H.A.; Longo, L.; de Moura, B.C.; Michalczuk, M.T.; Uribe-Cruz, C.; Cerski, C.T.S.; da Silveira, T.R.; Dall’Alba, V.; et al. Oral 24-week probiotics supplementation did not decrease cardiovascular risk markers in patients with biopsy proven NASH: A double-blind placebo-controlled randomized study. Ann. Hepatol. 2023, 28, 100769. [Google Scholar] [CrossRef] [PubMed]
- Escouto, G.S.; Port, G.Z.; Tovo, C.V.; Fernandes, S.A.; Peres, A.; Dorneles, G.P.; Houde, V.P.; Varin, T.V.; Pilon, G.; Marette, A.; et al. Probiotic Supplementation, Hepatic Fibrosis, and the Microbiota Profile in Patients with Nonalcoholic Steatohepatitis: A Randomized Controlled Trial. J. Nutr. 2023, 153, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhao, D.; Liu, X. Nutritional supplements improve cardiovascular risk factors in overweight and obese patients: A Bayesian network meta-analysis. Front. Nutr. 2023, 10, 1140019. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Q.; Chang, R.; Zhou, X.; Xu, C. Intestinal Barrier Function–Non-alcoholic Fatty Liver Disease Interactions and Possible Role of Gut Microbiota. J. Agric. Food Chem. 2019, 67, 2754–2762. [Google Scholar] [CrossRef]
- Zafari, N.; Velayati, M.; Fahim, M.; Maftouh, M.; Pourali, G.; Khazaei, M.; Nassiri, M.; Hassanian, S.M.; Ghayour-Mobarhan, M.; Ferns, G.A.; et al. Role of gut bacterial and non-bacterial microbiota in alcohol-associated liver disease: Molecular mechanisms, biomarkers, and therapeutic prospective. Life Sci. 2022, 305, 120760. [Google Scholar] [CrossRef]
- Madsen, K.L. The Use of Probiotics in Gastrointestinal Disease. Can. J. Gastroenterol. Hepatol. 2001, 15, 690741. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Dongiovanni, P. The Role of Probiotics in Nonalcoholic Fatty Liver Disease: A New Insight into Therapeutic Strategies. Nutrients 2019, 11, 2642. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; p. xxviii. 694p. [Google Scholar]
- Famouri, F.; Shariat, Z.; Hashemipour, M.; Keikha, M.; Kelishadi, R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 413–417. [Google Scholar] [CrossRef]
- Scorletti, E.; Afolabi, P.R.; Miles, E.A.; Smith, D.E.; Almehmadi, A.; Alshathry, A.; Moyses, H.E.; Clough, G.F.; Wright, M.; Patel, J.; et al. Design and rationale of the INSYTE study: A randomised, placebo controlled study to test the efficacy of a synbiotic on liver fat, disease biomarkers and intestinal microbiota in non-alcoholic fatty liver disease. Contemp. Clin. Trials 2018, 71, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Mousavi, S.; Alavian, S.M.; Sohrabpour, A.A.; Dashti, F.; Djafarian, K.; Esmaillzadeh, A. The effect of daily consumption of probiotic yogurt on liver enzymes, steatosis and fibrosis in patients with nonalcoholic fatty liver disease (NAFLD): Study protocol for a randomized clinical trial. BMC Gastroenterol. 2022, 22, 102. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Won, G.L.; Chim, A.M.; Chu, W.C.; Yeung, D.K.; Li, K.C.; Chan, H.L. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann. Hepatol. 2013, 12, 256–262. [Google Scholar] [CrossRef]
- Hsieh, R.H.; Chien, Y.J.; Lan, W.Y.; Lin, Y.K.; Lin, Y.H.; Chiang, C.F.; Yang, M.T. Bacillus coagulans TCI711 Supplementation Improved Nonalcoholic Fatty Liver by Modulating Gut Microbiota: A Randomized, Placebo-Controlled, Clinical Trial. Curr. Dev. Nutr. 2024, 8, 102083. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.S.; Su, H.; Zhang, J. Protective effect of probiotics in patients with non-alcoholic fatty liver disease. Medicine (Baltimore) 2020, 99, e21464. [Google Scholar] [CrossRef]
- Asgharian, A.; Mohammadi, V.; Gholi, Z.; Esmaillzade, A.; Feizi, A.; Askari, G. The Effect of Synbiotic Supplementation on Body Composition and Lipid Profile in Patients with NAFLD: A Randomized, Double Blind, Placebo-Controlled Clinical Trial Study. Iranian Red. Crescent Med. J. 2017, 19, 1–7. [Google Scholar] [CrossRef]
- Nabavi, S.; Rafraf, M.; Somi, M.H.; Homayouni-Rad, A.; Asghari-Jafarabadi, M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J. Dairy. Sci. 2014, 97, 7386–7393. [Google Scholar] [CrossRef]
- Javadi, L.; Ghavami, M.; Khoshbaten, M.; Safaiyan, A.; Barzegari, A.; Gargari, B.P. The Potential Role of Probiotics or/and Prebiotic on Serum Lipid Profile and Insulin Resistance in Alcoholic Fatty Liver Disease: A Double Blind Randomized Clinical Trial. Crescent J. Med. Biol. Sci. 2017, 4, 131–138. [Google Scholar]
- Ekhlasi, G.; Zarrati, M.; Agah, S.; Hosseini, A.F.; Hosseini, S.; Shidfar, S.; Soltani Aarbshahi, S.S.; Razmpoosh, E.; Shidfar, F. Effects of symbiotic and vitamin E supplementation on blood pressure, nitric oxide and inflammatory factors in non-alcoholic fatty liver disease. EXCLI J. 2017, 16, 278–290. [Google Scholar] [CrossRef]
- Wang, W.; Shi, L.P.; Shi, L.; Xu, L. Efficacy of probiotics on the treatment of non-alcoholic fatty liver disease. Zhonghua Nei Ke Za Zhi 2018, 57, 101–106. [Google Scholar] [CrossRef]
- Eslamparast, T.; Poustchi, H.; Zamani, F.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study. Am. J. Clin. Nutr. 2014, 99, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Li Volti, G.; et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.; Rafraf, M.; Somi, M.-h.; Homayouni-Rad, A.; Asghari-Jafarabadi, M. Probiotic yogurt improves body mass index and fasting insulin levels without affecting serum leptin and adiponectin levels in non-alcoholic fatty liver disease (NAFLD). J. Funct. Foods 2015, 18, 684–691. [Google Scholar] [CrossRef]
- Sepideh, A.; Karim, P.; Hossein, A.; Leila, R.; Hamdollah, M.; Mohammad, E.G.; Mojtaba, S.; Mohammad, S.; Ghader, G.; Seyed Moayed, A. Effects of Multistrain Probiotic Supplementation on Glycemic and Inflammatory Indices in Patients with Nonalcoholic Fatty Liver Disease: A Double-Blind Randomized Clinical Trial. J. Am. Coll. Nutr. 2015, 35, 500–505. [Google Scholar] [CrossRef]
- Asgharian, A.; Askari, G.; Esmailzade, A.; Feizi, A.; Mohammadi, V. The Effect of Symbiotic Supplementation on Liver Enzymes, C-reactive Protein and Ultrasound Findings in Patients with Non-alcoholic Fatty Liver Disease: A Clinical Trial. Int. J. Prev. Med. 2016, 7, 59. [Google Scholar] [CrossRef]
- Behrouz, V.; Jazayeri, S.; Aryaeian, N.; Zahedi, M.J.; Hosseini, F. Effects of Probiotic and Prebiotic Supplementation on Leptin, Adiponectin, and Glycemic Parameters in Non-alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Middle East. J. Dig. Dis. 2017, 9, 150–157. [Google Scholar] [CrossRef]
- Ekhlasi, G.; Kolahdouz Mohammadi, R.; Agah, S.; Zarrati, M.; Hosseini, A.F.; Arabshahi, S.S.; Shidfar, F. Do symbiotic and Vitamin E supplementation have favorite effects in nonalcoholic fatty liver disease? A randomized, double-blind, placebo-controlled trial. J. Res. Med. Sci. 2016, 21, 106. [Google Scholar] [CrossRef]
- Bakhshimoghaddam, F.; Shateri, K.; Sina, M.; Hashemian, M.; Alizadeh, M. Daily Consumption of Synbiotic Yogurt Decreases Liver Steatosis in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. J. Nutr. 2018, 148, 1276–1284. [Google Scholar] [CrossRef]
- Javadi, L.; Ghavami, M.; Khoshbaten, M.; Safaiyan, A.; Barzegari, A.; Pourghassem Gargari, B. The Effect of Probiotic and/or Prebiotic on Liver Function Tests in Patients with Nonalcoholic Fatty Liver Disease: A Double Blind Randomized Clinical Trial. Iran. Red Crescent Med. J. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Manzhalii, E.; Virchenko, O.; Falalyeyeva, T.; Beregova, T.; Stremmel, W. Treatment efficacy of a probiotic preparation for non-alcoholic steatohepatitis: A pilot trial. J. Dig. Dis. 2017, 18, 698–703. [Google Scholar] [CrossRef]
- Mofidi, F.; Poustchi, H.; Yari, Z.; Nourinayyer, B.; Merat, S.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: A pilot, randomised, double-blind, placebo-controlled, clinical trial. Br. J. Nutr. 2017, 117, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Abenavoli, L.; Mykhalchyshyn, G.; Kononenko, L.; Boccuto, L.; Kyriienko, D.; Dynnyk, O. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J. Gastrointestin Liver Dis. 2018, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Sayari, S.; Neishaboori, H.; Jameshorani, M. Combined effects of synbiotic and sitagliptin versus sitagliptin alone in patients with nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2018, 24, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Duseja, A.; Acharya, S.K.; Mehta, M.; Chhabra, S.; Rana, S.; Das, A.; Dattagupta, S.; Dhiman, R.K.; Chawla, Y.K. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): A randomised, double-blind, proof of concept study. BMJ Open Gastroenterol. 2019, 6, e000315. [Google Scholar] [CrossRef]
- Behrouz, V.; Aryaeian, N.; Zahedi, M.J.; Jazayeri, S. Effects of probiotic and prebiotic supplementation on metabolic parameters, liver aminotransferases, and systemic inflammation in nonalcoholic fatty liver disease: A randomized clinical trial. J. Food Sci. 2020, 85, 3611–3617. [Google Scholar] [CrossRef]
- Kobyliak, N.; Abenavoli, L.; Mykhalchyshyn, G.; Falalyeyeva, T.; Tsyryuk, O.; Kononenko, L.; Kyriienko, D.; Komisarenko, I. Probiotics and smectite absorbent gel formulation reduce liver stiffness, transaminase and cytokine levels in NAFLD associated with type 2 diabetes: A randomized clinical study. Clin. Diabetol. 2019, 8, 205–214. [Google Scholar] [CrossRef]
- Ayob, N.; Muhammad Nawawi, K.N.; Mohamad Nor, M.H.; Raja Ali, R.A.; Ahmad, H.F.; Oon, S.F.; Mohd Mokhtar, N. The Effects of Probiotics on Small Intestinal Microbiota Composition, Inflammatory Cytokines and Intestinal Permeability in Patients with Non-Alcoholic Fatty Liver Disease. Biomedicines 2023, 11, 640. [Google Scholar] [CrossRef]
- Chong, P.L.; Laight, D.; Aspinall, R.J.; Higginson, A.; Cummings, M.H. A randomised placebo controlled trial of VSL#3((R)) probiotic on biomarkers of cardiovascular risk and liver injury in non-alcoholic fatty liver disease. BMC Gastroenterol. 2021, 21, 144. [Google Scholar] [CrossRef]
- Mohamad Nor, M.H.; Ayob, N.; Mokhtar, N.M.; Raja Ali, R.A.; Tan, G.C.; Wong, Z.; Shafiee, N.H.; Wong, Y.P.; Mustangin, M.; Nawawi, K.N.M. The Effect of Probiotics (MCP((R)) BCMC((R)) Strains) on Hepatic Steatosis, Small Intestinal Mucosal Immune Function, and Intestinal Barrier in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 3192. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Nachvak, S.M.; Mohammadi, R.; Moradi, S.; Mostafai, R.; Pizarro, A.B.; Abdollahzad, H. Probiotic Yogurt Fortified with Vitamin D Can Improve Glycemic Status in Non-Alcoholic Fatty Liver Disease Patients: A Randomized Clinical Trial. Clin. Nutr. Res. 2021, 10, 36–47. [Google Scholar] [CrossRef]
- Scorletti, E.; Afolabi, P.R.; Miles, E.A.; Smith, D.E.; Almehmadi, A.; Alshathry, A.; Childs, C.E.; Del Fabbro, S.; Bilson, J.; Moyses, H.E.; et al. Synbiotics Alter Fecal Microbiomes, But Not Liver Fat or Fibrosis, in a Randomized Trial of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 158, 1597–1610.e7. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, M.; Dobrosavljevic, A.; Odanovic, O.; Knezevic-Ivanovski, T.; Kralj, D.; Erceg, S.; Perucica, A.; Svorcan, P.; Stankovic-Popovic, V. The effects of synbiotics on the liver steatosis, inflammation, and gut microbiome of metabolic dysfunction-associated liver disease patients-randomized trial. Rom. J. Intern. Med. 2024, 62, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Horackova, S.; Plockova, M.; Demnerova, K. Importance of microbial defence systems to bile salts and mechanisms of serum cholesterol reduction. Biotechnol. Adv. 2018, 36, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pasten, A.; Fernandez-Martinez, E.; Perez-Hernandez, N.; Soria-Jasso, L.E.; Carino-Cortes, R. Prebiotics and Probiotics: Effects on Dyslipidemia and NAFLD/NASH and the Associated Mechanisms of Action. Curr. Pharm. Biotechnol. 2023, 24, 633–646. [Google Scholar] [CrossRef]
- Jadhav, P.A.; Thomas, A.B.; Nanda, R.K.; Chitlange, S.S. Correlation of non-alcoholic fatty liver disease and gut microflora: Clinical reports and treatment options. Egypt. Liver J. 2024, 14, 21. [Google Scholar] [CrossRef]
- Hizo, G.H.; Rampelotto, P.H. The Impact of Probiotic Bifidobacterium on Liver Diseases and the Microbiota. Life 2024, 14, 239. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Wu, J.; Hu, J.; Wan, M.; Bie, J.; Li, J.; Pan, D.; Sun, G.; Yang, C. Optimal probiotic combinations for treating nonalcoholic fatty liver disease: A systematic review and network meta-analysis. Clin. Nutr. 2024, 43, 1224–1239. [Google Scholar] [CrossRef]
- Rittiphairoj, T.; Pongpirul, K.; Janchot, K.; Mueller, N.T.; Li, T. Probiotics Contribute to Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 722–734. [Google Scholar] [CrossRef]
- Kazeminasab, F.; Miraghajani, M.; Mokhtari, K.; Karimi, B.; Rosenkranz, S.K.; Santos, H.O. The effects of probiotic supplementation and exercise training on liver enzymes and cardiometabolic markers in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized clinical trials. Nutr. Metab. 2024, 21, 59. [Google Scholar] [CrossRef]
- Musazadeh, V.; Roshanravan, N.; Dehghan, P.; Ahrabi, S.S. Effect of Probiotics on Liver Enzymes in Patients With Non-alcoholic Fatty Liver Disease: An Umbrella of Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 844242. [Google Scholar] [CrossRef]
- Koliada, A.; Moseiko, V.; Romanenko, M.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Vaiserman, A. Sex differences in the phylum-level human gut microbiota composition. BMC Microbiol. 2021, 21, 131. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, H.; Yu, Z.; Zhang, F.; Liang, S.; Liu, H.; Chen, H.; Lu, M. The Gut Microbiome and Sex Hormone-Related Diseases. Front. Microbiol. 2021, 12, 711137. [Google Scholar] [CrossRef] [PubMed]
- Fransen, F.; van Beek, A.A.; Borghuis, T.; Meijer, B.; Hugenholtz, F.; van der Gaast-de Jongh, C.; Savelkoul, H.F.; de Jonge, M.I.; Faas, M.M.; Boekschoten, M.V.; et al. The Impact of Gut Microbiota on Gender-Specific Differences in Immunity. Front. Immunol. 2017, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Rangel-Zuniga, O.A.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Perez-Martinez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortes, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).