Gut Microbiota and Liver Health: Meta-Analysis of Bifidobacterium-Containing Probiotics in NAFLD Management
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Selection Criteria
2.2. Selection of Studies
2.3. Data Extraction
2.4. Outcomes
2.5. Assessment of Methodological Quality
2.6. Data Analysis
3. Results
3.1. Quality Assessment
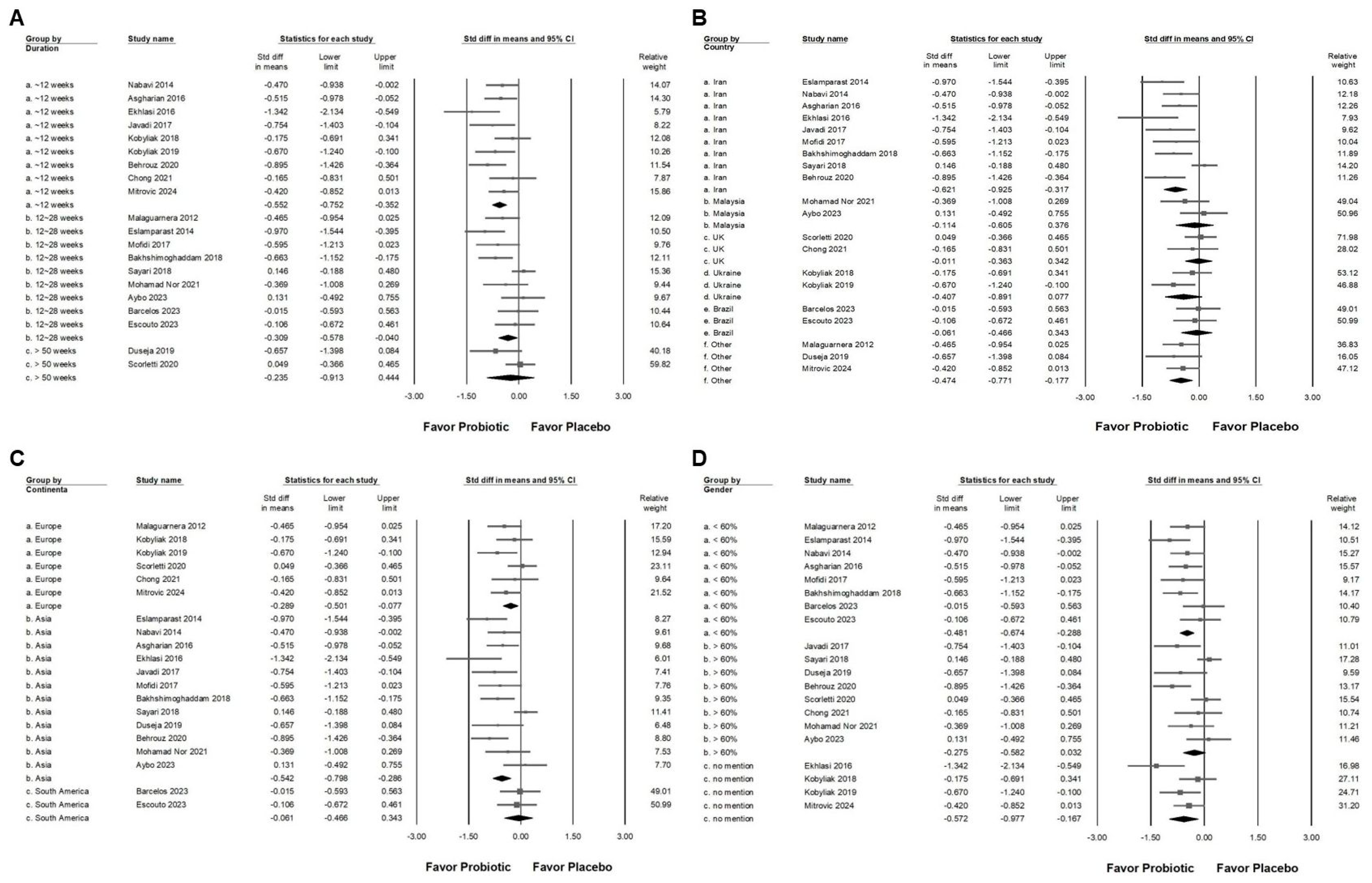
3.2. Effect of Combinations with Bifidobacterium on Alanine Aminotransferase Expression
3.3. Effect of Combinations with Bifidobacterium on Liver Injury Markers and Liver Fat
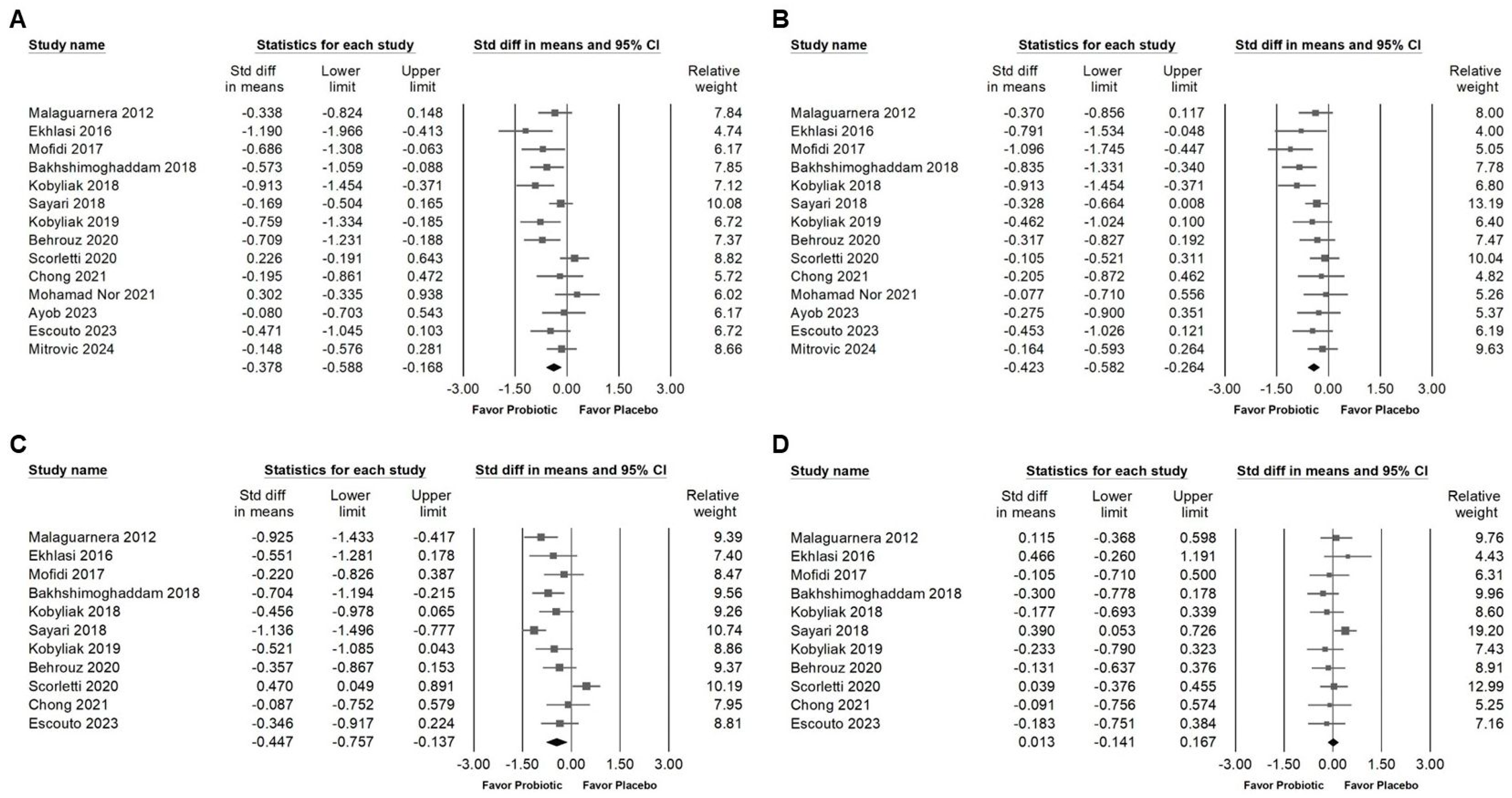
3.4. Influence of Combinations with Bifidobacterium on Blood Lipids and Lipoproteins
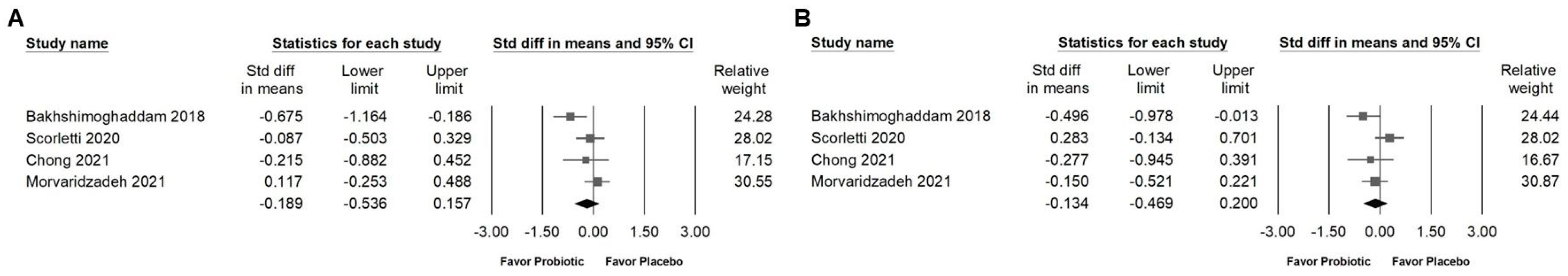
3.5. Effects of Combinations with Bifidobacterium on Fasting Plasma Glucose, Serum Insulin, HbA1C, and Homeostasis Model Assessment for Insulin Resistance (HOMA-IR)
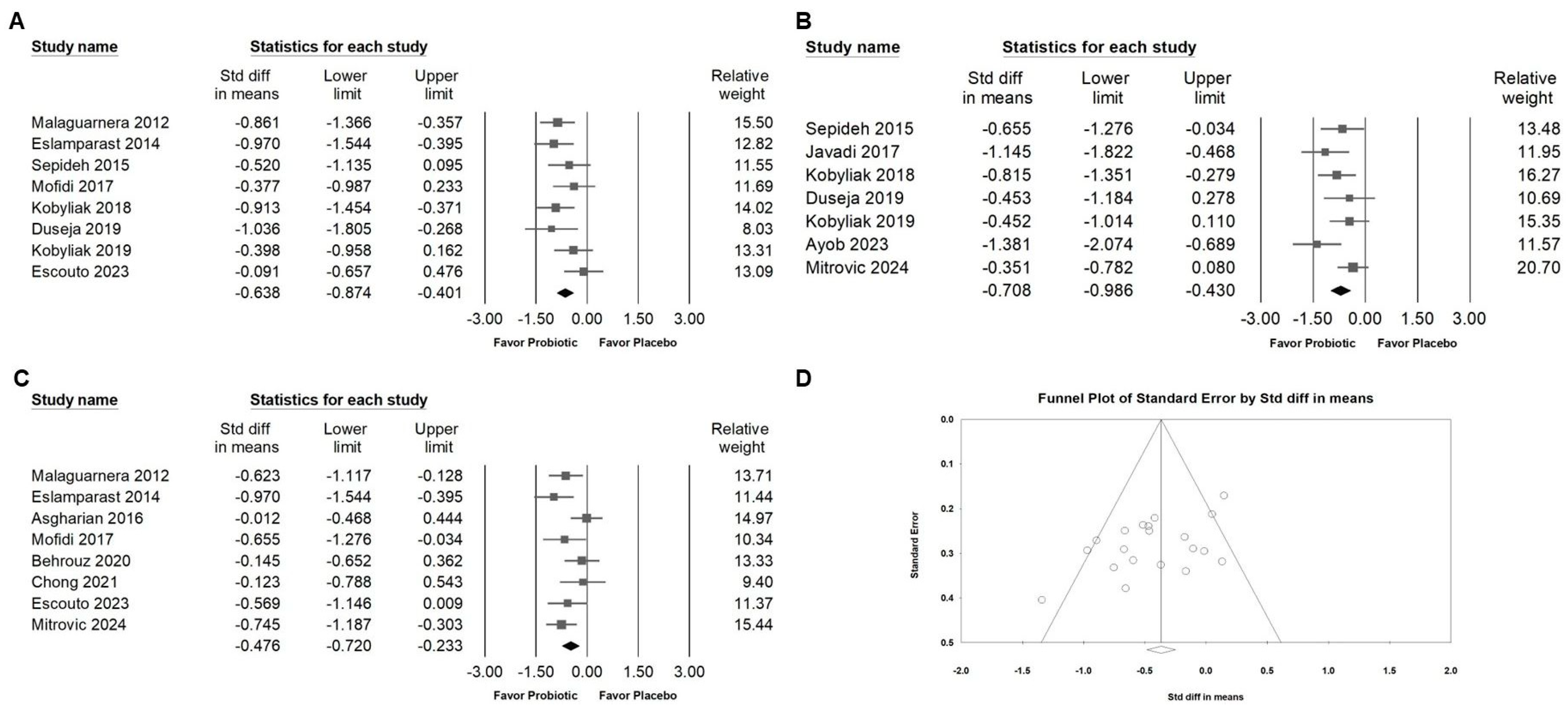
3.6. Effect of Combinations with Bifidobacterium on Blood Pressure and Inflammatory Cytokines
3.7. Publishing Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joshi, R.; Agrawal, T.; Fathima, F.; Usha, T.; Thomas, T.; Misquith, D.; Kalantri, S.; Chidambaram, N.; Raj, T.; Singamani, A.; et al. Cardiovascular risk factor reduction by community health workers in rural India: A cluster randomized trial. Am. Heart J. 2019, 216, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: Implications for longevity. Nutr. Rev. 2007, 65, S253–S259. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Targher, G.; Day, C.P.; Bonora, E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010, 363, 1341–1350. [Google Scholar] [CrossRef]
- Ezpeleta, M.; Gabel, K.; Cienfuegos, S.; Kalam, F.; Lin, S.; Pavlou, V.; Song, Z.; Haus, J.M.; Koppe, S.; Alexandria, S.J.; et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: A randomized controlled trial. Cell Metab. 2023, 35, 56–70.e53. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, S.T.A.; Silva-Sperb, A.S.; Moraes, H.A.; Longo, L.; de Moura, B.C.; Michalczuk, M.T.; Uribe-Cruz, C.; Cerski, C.T.S.; da Silveira, T.R.; Dall’Alba, V.; et al. Oral 24-week probiotics supplementation did not decrease cardiovascular risk markers in patients with biopsy proven NASH: A double-blind placebo-controlled randomized study. Ann. Hepatol. 2023, 28, 100769. [Google Scholar] [CrossRef] [PubMed]
- Escouto, G.S.; Port, G.Z.; Tovo, C.V.; Fernandes, S.A.; Peres, A.; Dorneles, G.P.; Houde, V.P.; Varin, T.V.; Pilon, G.; Marette, A.; et al. Probiotic Supplementation, Hepatic Fibrosis, and the Microbiota Profile in Patients with Nonalcoholic Steatohepatitis: A Randomized Controlled Trial. J. Nutr. 2023, 153, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhao, D.; Liu, X. Nutritional supplements improve cardiovascular risk factors in overweight and obese patients: A Bayesian network meta-analysis. Front. Nutr. 2023, 10, 1140019. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Q.; Chang, R.; Zhou, X.; Xu, C. Intestinal Barrier Function–Non-alcoholic Fatty Liver Disease Interactions and Possible Role of Gut Microbiota. J. Agric. Food Chem. 2019, 67, 2754–2762. [Google Scholar] [CrossRef]
- Zafari, N.; Velayati, M.; Fahim, M.; Maftouh, M.; Pourali, G.; Khazaei, M.; Nassiri, M.; Hassanian, S.M.; Ghayour-Mobarhan, M.; Ferns, G.A.; et al. Role of gut bacterial and non-bacterial microbiota in alcohol-associated liver disease: Molecular mechanisms, biomarkers, and therapeutic prospective. Life Sci. 2022, 305, 120760. [Google Scholar] [CrossRef]
- Madsen, K.L. The Use of Probiotics in Gastrointestinal Disease. Can. J. Gastroenterol. Hepatol. 2001, 15, 690741. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Dongiovanni, P. The Role of Probiotics in Nonalcoholic Fatty Liver Disease: A New Insight into Therapeutic Strategies. Nutrients 2019, 11, 2642. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; p. xxviii. 694p. [Google Scholar]
- Famouri, F.; Shariat, Z.; Hashemipour, M.; Keikha, M.; Kelishadi, R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 413–417. [Google Scholar] [CrossRef]
- Scorletti, E.; Afolabi, P.R.; Miles, E.A.; Smith, D.E.; Almehmadi, A.; Alshathry, A.; Moyses, H.E.; Clough, G.F.; Wright, M.; Patel, J.; et al. Design and rationale of the INSYTE study: A randomised, placebo controlled study to test the efficacy of a synbiotic on liver fat, disease biomarkers and intestinal microbiota in non-alcoholic fatty liver disease. Contemp. Clin. Trials 2018, 71, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Mousavi, S.; Alavian, S.M.; Sohrabpour, A.A.; Dashti, F.; Djafarian, K.; Esmaillzadeh, A. The effect of daily consumption of probiotic yogurt on liver enzymes, steatosis and fibrosis in patients with nonalcoholic fatty liver disease (NAFLD): Study protocol for a randomized clinical trial. BMC Gastroenterol. 2022, 22, 102. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Won, G.L.; Chim, A.M.; Chu, W.C.; Yeung, D.K.; Li, K.C.; Chan, H.L. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann. Hepatol. 2013, 12, 256–262. [Google Scholar] [CrossRef]
- Hsieh, R.H.; Chien, Y.J.; Lan, W.Y.; Lin, Y.K.; Lin, Y.H.; Chiang, C.F.; Yang, M.T. Bacillus coagulans TCI711 Supplementation Improved Nonalcoholic Fatty Liver by Modulating Gut Microbiota: A Randomized, Placebo-Controlled, Clinical Trial. Curr. Dev. Nutr. 2024, 8, 102083. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.S.; Su, H.; Zhang, J. Protective effect of probiotics in patients with non-alcoholic fatty liver disease. Medicine (Baltimore) 2020, 99, e21464. [Google Scholar] [CrossRef]
- Asgharian, A.; Mohammadi, V.; Gholi, Z.; Esmaillzade, A.; Feizi, A.; Askari, G. The Effect of Synbiotic Supplementation on Body Composition and Lipid Profile in Patients with NAFLD: A Randomized, Double Blind, Placebo-Controlled Clinical Trial Study. Iranian Red. Crescent Med. J. 2017, 19, 1–7. [Google Scholar] [CrossRef]
- Nabavi, S.; Rafraf, M.; Somi, M.H.; Homayouni-Rad, A.; Asghari-Jafarabadi, M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J. Dairy. Sci. 2014, 97, 7386–7393. [Google Scholar] [CrossRef]
- Javadi, L.; Ghavami, M.; Khoshbaten, M.; Safaiyan, A.; Barzegari, A.; Gargari, B.P. The Potential Role of Probiotics or/and Prebiotic on Serum Lipid Profile and Insulin Resistance in Alcoholic Fatty Liver Disease: A Double Blind Randomized Clinical Trial. Crescent J. Med. Biol. Sci. 2017, 4, 131–138. [Google Scholar]
- Ekhlasi, G.; Zarrati, M.; Agah, S.; Hosseini, A.F.; Hosseini, S.; Shidfar, S.; Soltani Aarbshahi, S.S.; Razmpoosh, E.; Shidfar, F. Effects of symbiotic and vitamin E supplementation on blood pressure, nitric oxide and inflammatory factors in non-alcoholic fatty liver disease. EXCLI J. 2017, 16, 278–290. [Google Scholar] [CrossRef]
- Wang, W.; Shi, L.P.; Shi, L.; Xu, L. Efficacy of probiotics on the treatment of non-alcoholic fatty liver disease. Zhonghua Nei Ke Za Zhi 2018, 57, 101–106. [Google Scholar] [CrossRef]
- Eslamparast, T.; Poustchi, H.; Zamani, F.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study. Am. J. Clin. Nutr. 2014, 99, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Li Volti, G.; et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.; Rafraf, M.; Somi, M.-h.; Homayouni-Rad, A.; Asghari-Jafarabadi, M. Probiotic yogurt improves body mass index and fasting insulin levels without affecting serum leptin and adiponectin levels in non-alcoholic fatty liver disease (NAFLD). J. Funct. Foods 2015, 18, 684–691. [Google Scholar] [CrossRef]
- Sepideh, A.; Karim, P.; Hossein, A.; Leila, R.; Hamdollah, M.; Mohammad, E.G.; Mojtaba, S.; Mohammad, S.; Ghader, G.; Seyed Moayed, A. Effects of Multistrain Probiotic Supplementation on Glycemic and Inflammatory Indices in Patients with Nonalcoholic Fatty Liver Disease: A Double-Blind Randomized Clinical Trial. J. Am. Coll. Nutr. 2015, 35, 500–505. [Google Scholar] [CrossRef]
- Asgharian, A.; Askari, G.; Esmailzade, A.; Feizi, A.; Mohammadi, V. The Effect of Symbiotic Supplementation on Liver Enzymes, C-reactive Protein and Ultrasound Findings in Patients with Non-alcoholic Fatty Liver Disease: A Clinical Trial. Int. J. Prev. Med. 2016, 7, 59. [Google Scholar] [CrossRef]
- Behrouz, V.; Jazayeri, S.; Aryaeian, N.; Zahedi, M.J.; Hosseini, F. Effects of Probiotic and Prebiotic Supplementation on Leptin, Adiponectin, and Glycemic Parameters in Non-alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Middle East. J. Dig. Dis. 2017, 9, 150–157. [Google Scholar] [CrossRef]
- Ekhlasi, G.; Kolahdouz Mohammadi, R.; Agah, S.; Zarrati, M.; Hosseini, A.F.; Arabshahi, S.S.; Shidfar, F. Do symbiotic and Vitamin E supplementation have favorite effects in nonalcoholic fatty liver disease? A randomized, double-blind, placebo-controlled trial. J. Res. Med. Sci. 2016, 21, 106. [Google Scholar] [CrossRef]
- Bakhshimoghaddam, F.; Shateri, K.; Sina, M.; Hashemian, M.; Alizadeh, M. Daily Consumption of Synbiotic Yogurt Decreases Liver Steatosis in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. J. Nutr. 2018, 148, 1276–1284. [Google Scholar] [CrossRef]
- Javadi, L.; Ghavami, M.; Khoshbaten, M.; Safaiyan, A.; Barzegari, A.; Pourghassem Gargari, B. The Effect of Probiotic and/or Prebiotic on Liver Function Tests in Patients with Nonalcoholic Fatty Liver Disease: A Double Blind Randomized Clinical Trial. Iran. Red Crescent Med. J. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Manzhalii, E.; Virchenko, O.; Falalyeyeva, T.; Beregova, T.; Stremmel, W. Treatment efficacy of a probiotic preparation for non-alcoholic steatohepatitis: A pilot trial. J. Dig. Dis. 2017, 18, 698–703. [Google Scholar] [CrossRef]
- Mofidi, F.; Poustchi, H.; Yari, Z.; Nourinayyer, B.; Merat, S.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: A pilot, randomised, double-blind, placebo-controlled, clinical trial. Br. J. Nutr. 2017, 117, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Abenavoli, L.; Mykhalchyshyn, G.; Kononenko, L.; Boccuto, L.; Kyriienko, D.; Dynnyk, O. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J. Gastrointestin Liver Dis. 2018, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Sayari, S.; Neishaboori, H.; Jameshorani, M. Combined effects of synbiotic and sitagliptin versus sitagliptin alone in patients with nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2018, 24, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Duseja, A.; Acharya, S.K.; Mehta, M.; Chhabra, S.; Rana, S.; Das, A.; Dattagupta, S.; Dhiman, R.K.; Chawla, Y.K. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): A randomised, double-blind, proof of concept study. BMJ Open Gastroenterol. 2019, 6, e000315. [Google Scholar] [CrossRef]
- Behrouz, V.; Aryaeian, N.; Zahedi, M.J.; Jazayeri, S. Effects of probiotic and prebiotic supplementation on metabolic parameters, liver aminotransferases, and systemic inflammation in nonalcoholic fatty liver disease: A randomized clinical trial. J. Food Sci. 2020, 85, 3611–3617. [Google Scholar] [CrossRef]
- Kobyliak, N.; Abenavoli, L.; Mykhalchyshyn, G.; Falalyeyeva, T.; Tsyryuk, O.; Kononenko, L.; Kyriienko, D.; Komisarenko, I. Probiotics and smectite absorbent gel formulation reduce liver stiffness, transaminase and cytokine levels in NAFLD associated with type 2 diabetes: A randomized clinical study. Clin. Diabetol. 2019, 8, 205–214. [Google Scholar] [CrossRef]
- Ayob, N.; Muhammad Nawawi, K.N.; Mohamad Nor, M.H.; Raja Ali, R.A.; Ahmad, H.F.; Oon, S.F.; Mohd Mokhtar, N. The Effects of Probiotics on Small Intestinal Microbiota Composition, Inflammatory Cytokines and Intestinal Permeability in Patients with Non-Alcoholic Fatty Liver Disease. Biomedicines 2023, 11, 640. [Google Scholar] [CrossRef]
- Chong, P.L.; Laight, D.; Aspinall, R.J.; Higginson, A.; Cummings, M.H. A randomised placebo controlled trial of VSL#3((R)) probiotic on biomarkers of cardiovascular risk and liver injury in non-alcoholic fatty liver disease. BMC Gastroenterol. 2021, 21, 144. [Google Scholar] [CrossRef]
- Mohamad Nor, M.H.; Ayob, N.; Mokhtar, N.M.; Raja Ali, R.A.; Tan, G.C.; Wong, Z.; Shafiee, N.H.; Wong, Y.P.; Mustangin, M.; Nawawi, K.N.M. The Effect of Probiotics (MCP((R)) BCMC((R)) Strains) on Hepatic Steatosis, Small Intestinal Mucosal Immune Function, and Intestinal Barrier in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 3192. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Nachvak, S.M.; Mohammadi, R.; Moradi, S.; Mostafai, R.; Pizarro, A.B.; Abdollahzad, H. Probiotic Yogurt Fortified with Vitamin D Can Improve Glycemic Status in Non-Alcoholic Fatty Liver Disease Patients: A Randomized Clinical Trial. Clin. Nutr. Res. 2021, 10, 36–47. [Google Scholar] [CrossRef]
- Scorletti, E.; Afolabi, P.R.; Miles, E.A.; Smith, D.E.; Almehmadi, A.; Alshathry, A.; Childs, C.E.; Del Fabbro, S.; Bilson, J.; Moyses, H.E.; et al. Synbiotics Alter Fecal Microbiomes, But Not Liver Fat or Fibrosis, in a Randomized Trial of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 158, 1597–1610.e7. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, M.; Dobrosavljevic, A.; Odanovic, O.; Knezevic-Ivanovski, T.; Kralj, D.; Erceg, S.; Perucica, A.; Svorcan, P.; Stankovic-Popovic, V. The effects of synbiotics on the liver steatosis, inflammation, and gut microbiome of metabolic dysfunction-associated liver disease patients-randomized trial. Rom. J. Intern. Med. 2024, 62, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Horackova, S.; Plockova, M.; Demnerova, K. Importance of microbial defence systems to bile salts and mechanisms of serum cholesterol reduction. Biotechnol. Adv. 2018, 36, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pasten, A.; Fernandez-Martinez, E.; Perez-Hernandez, N.; Soria-Jasso, L.E.; Carino-Cortes, R. Prebiotics and Probiotics: Effects on Dyslipidemia and NAFLD/NASH and the Associated Mechanisms of Action. Curr. Pharm. Biotechnol. 2023, 24, 633–646. [Google Scholar] [CrossRef]
- Jadhav, P.A.; Thomas, A.B.; Nanda, R.K.; Chitlange, S.S. Correlation of non-alcoholic fatty liver disease and gut microflora: Clinical reports and treatment options. Egypt. Liver J. 2024, 14, 21. [Google Scholar] [CrossRef]
- Hizo, G.H.; Rampelotto, P.H. The Impact of Probiotic Bifidobacterium on Liver Diseases and the Microbiota. Life 2024, 14, 239. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Wu, J.; Hu, J.; Wan, M.; Bie, J.; Li, J.; Pan, D.; Sun, G.; Yang, C. Optimal probiotic combinations for treating nonalcoholic fatty liver disease: A systematic review and network meta-analysis. Clin. Nutr. 2024, 43, 1224–1239. [Google Scholar] [CrossRef]
- Rittiphairoj, T.; Pongpirul, K.; Janchot, K.; Mueller, N.T.; Li, T. Probiotics Contribute to Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 722–734. [Google Scholar] [CrossRef]
- Kazeminasab, F.; Miraghajani, M.; Mokhtari, K.; Karimi, B.; Rosenkranz, S.K.; Santos, H.O. The effects of probiotic supplementation and exercise training on liver enzymes and cardiometabolic markers in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized clinical trials. Nutr. Metab. 2024, 21, 59. [Google Scholar] [CrossRef]
- Musazadeh, V.; Roshanravan, N.; Dehghan, P.; Ahrabi, S.S. Effect of Probiotics on Liver Enzymes in Patients With Non-alcoholic Fatty Liver Disease: An Umbrella of Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 844242. [Google Scholar] [CrossRef]
- Koliada, A.; Moseiko, V.; Romanenko, M.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Vaiserman, A. Sex differences in the phylum-level human gut microbiota composition. BMC Microbiol. 2021, 21, 131. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, H.; Yu, Z.; Zhang, F.; Liang, S.; Liu, H.; Chen, H.; Lu, M. The Gut Microbiome and Sex Hormone-Related Diseases. Front. Microbiol. 2021, 12, 711137. [Google Scholar] [CrossRef] [PubMed]
- Fransen, F.; van Beek, A.A.; Borghuis, T.; Meijer, B.; Hugenholtz, F.; van der Gaast-de Jongh, C.; Savelkoul, H.F.; de Jonge, M.I.; Faas, M.M.; Boekschoten, M.V.; et al. The Impact of Gut Microbiota on Gender-Specific Differences in Immunity. Front. Immunol. 2017, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Rangel-Zuniga, O.A.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Perez-Martinez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortes, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef]
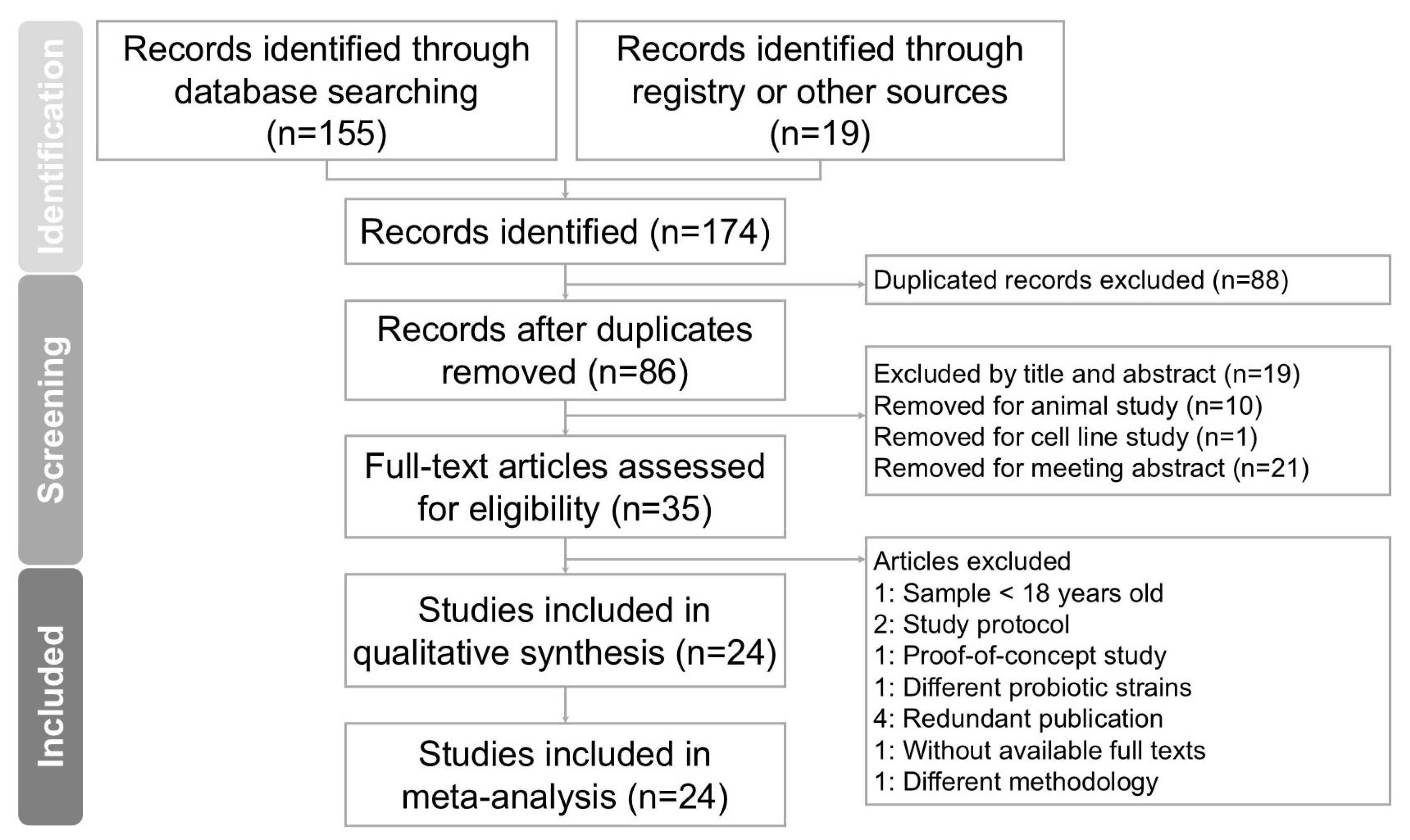
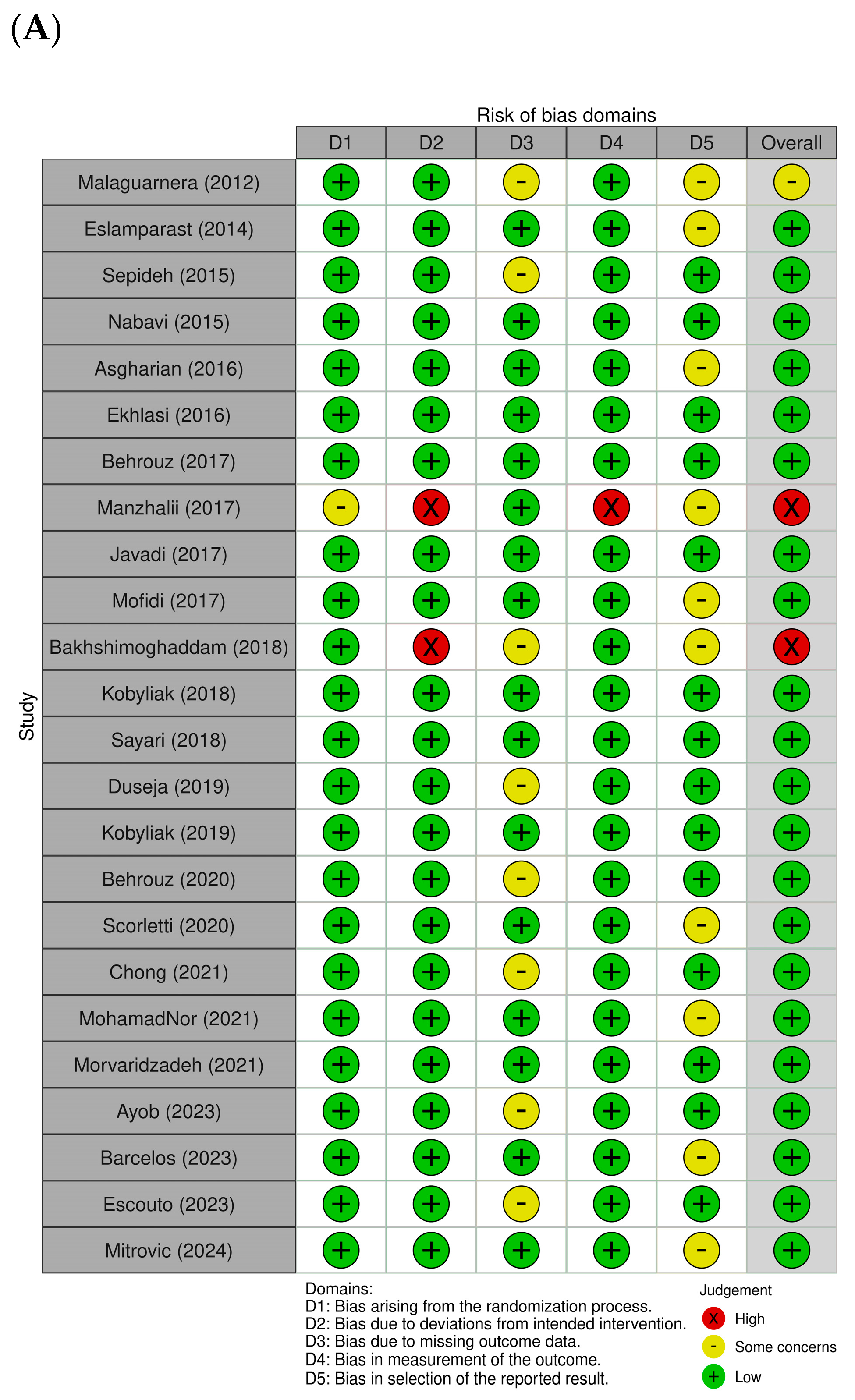
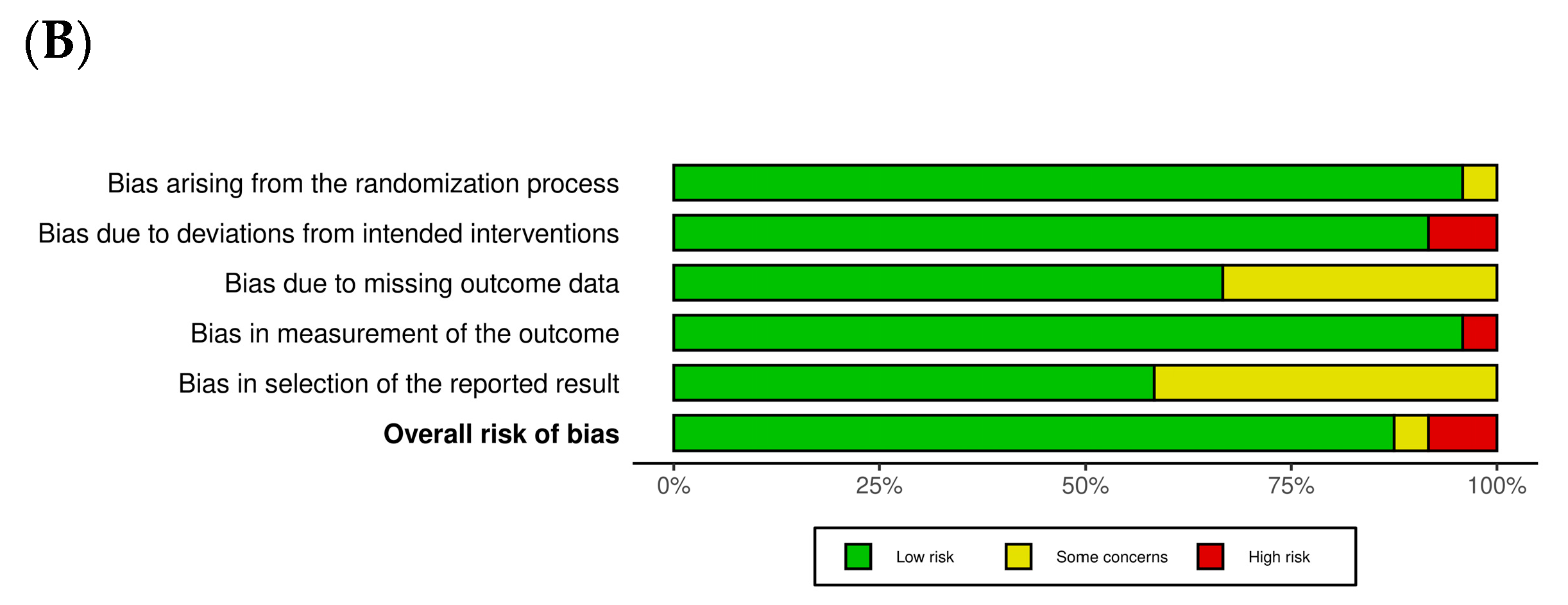
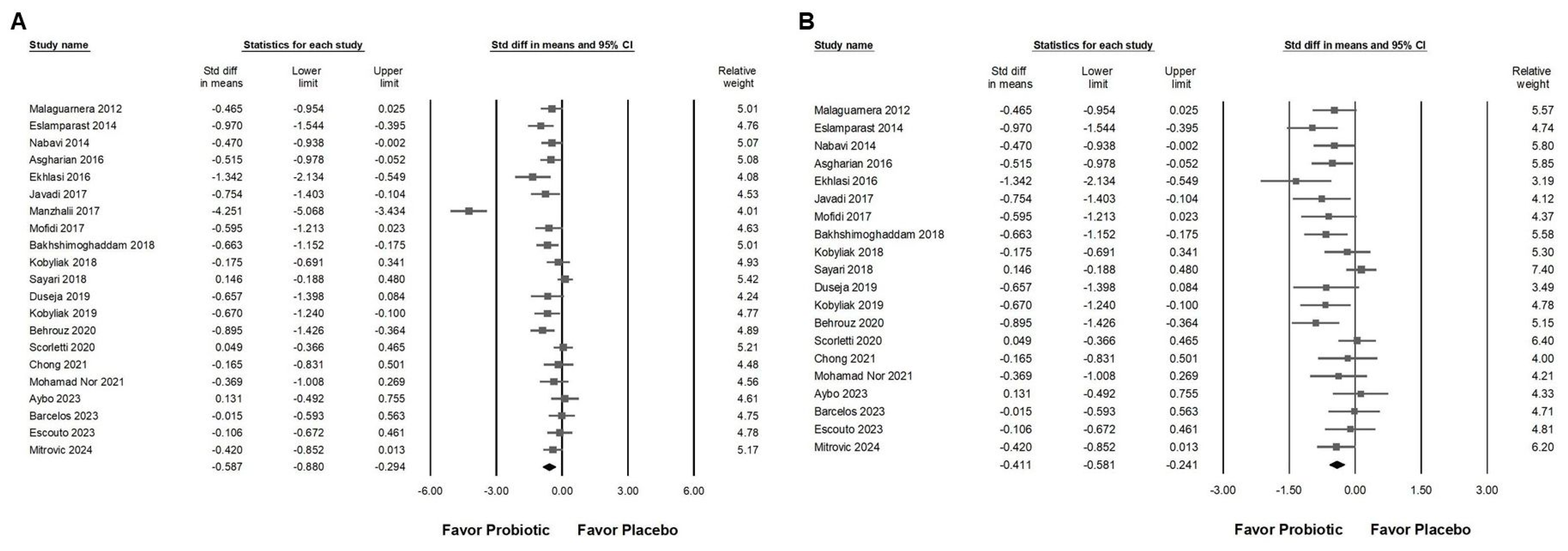


| Author (Year)/Country | Inclusion Criteria | Exclusion Criteria | Sample Size (% of Male)/Age | Study Design | Placebo Using | Intervention/Duration | Main Results | Secondary Results |
|---|---|---|---|---|---|---|---|---|
| 8 Weeks | ||||||||
| Nabavi (2015) [34]/Iran | With NAFLD, aged 23–63, BMI between 25–40 kg/m2, and ultrasound-confirmed NAFLD. | Kidney disease, other liver diseases (e.g., hepatitis B, C), thyroid disorders, inflammatory bowel disease, immune deficiency diseases, pregnancy, breastfeeding, use of specific medications (cholesterol-lowering drugs, estrogen, progesterone, or diuretics), recent use of probiotics or probiotic yogurt within the last two months. | P: 36 (50) I: 36 (48.2)/ P: 44.05 ± 8.14 I: 42.75 ± 8.72 | RCT/double-blind/placebo | Conventional yogurt as control (no probiotic strains added). | 300 g/day of probiotic yogurt (containing Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12). | Reductions in BMI and fasting insulin levels. No differences observed in serum leptin or adiponectin levels. | No change in waist circumference, insulin resistance (HOMA-IR), or leptin-to-adiponectin ratio. |
| Sepideh (2015) [35]/Iran | Adults diagnosed with NAFLD by ultrasound, aged 18–65. | Alcohol use, thyroid/kidney disease, viral hepatitis, pregnancy, lactation, recent use of probiotics, NSAIDs, or antibiotics within two months prior. | P: 21 (71.4) I: 21 (61.9)/ P: 47.33 ± 2.53 I: 42.10 ± 1.99 | RCT/double-blind/placebo | Capsules similar in appearance to probiotic capsules. | Probiotic containing multiple strains (Lactobacillus acidophilus, Bifidobacterium breve) two capsules/day. | Reduction in FBS, insulin, HOMA-IR, and TNF-α and IL-6. | No changes in HbA1C levels between groups. |
| Asgharian (2016) [36]/Iran | 1. Aged between 18 and 60 years and diagnosed with NAFLD by ultrasound. 2. Have no other liver diseases, such as hepatitis B or C. 3. No use of corticosteroids or specific medications that influence liver function. | 1. Pregnancy or lactation. 2. Use of vitamin-mineral, antioxidant, or omega-3 supplementation. 3. Inability to comply with the study’s protocol (<90% compliance). 4. Use of antibiotics during the study. | P: 36 (35.3) I: 38 (17.5)/ P: 47.78 ± 1.7 I: 46.57.05 ± 1.7 | RCT/double-blind/placebo | 500 mg capsules containing 120 mg starch | 500 mg symbiotic capsule containing: Lactobacillus casei, L. acidophilus, L. rhamnosus, L. bulgaricus, Bifidobacterium breve, B. longum, Streptococcus thermophilus and fructooligosaccharides. | Improved the ultrasound grade of hepatic steatosis. Significant weight reduction. | No differences in physical activity or dietary intake were observed between the groups. No change in ALT and AST within the symbiotic group, but the placebo group showed a significant increase in both ALT and AST levels. CRP levels remained unchanged in both groups. |
| Ekhlasi (2016) [38]/Iran | Diagnosed with NAFLD via ultrasound (Grade 1–3) and elevated ALT levels (>30 mg/dL) for at least six months before and at the time of randomization, ages 25–64, BMI 25–35 kg/m2. | Liver pathologies like viral hepatitis, alcohol consumption, hypothyroidism, autoimmune diseases, diabetes, cancer history, and certain medication use (NSAIDs, antibiotics, probiotics, supplements). | Placebo: 15 Probiotic: 15 Vitamin E: 15 Probiotic + Vitamin E: 15/ no mention | RCT/double-blind/placebo/four-arm study | Identical placebo capsules (corn starch) for both symbiotic and vitamin E groups. | Symbiotic: Twice daily (Lactobacillus casei, L. rhamnosus, S. thermophilus, B. breve, etc.). Vitamin E: 400 IU per day. | Combination of symbiotic and vitamin E significantly reduced ALT, AST, ALP, and leptin levels. Significant decreases in TG, TC, and LDL were observed in both the symbiotic and symbiotic + vitamin E groups. | Significant reductions in FBS and insulin levels were observed in the symbiotic + vitamin E group. No significant changes were seen in HDL or HOMA across all groups. |
| Kobyliak (2018) [43]/Ukraine | Adult participants aged 18–65 years with a BMI > 25 kg/m2, diagnosed with NAFLD based on clinical examination, laboratory values, liver enzyme activities (ALT, AST), and ultrasound examination. | Alcohol abuse, chronic viral hepatitis, drug-induced liver disease, Wilson’s disease, hereditary deficiency of antitrypsin-1, idiopathic hemochromatosis, history of decompensated liver disease, regular use of probiotics or prebiotics within 3 months prior to enrollment, antibiotic use within 3 months prior to enrollment, uncontrolled cardiovascular or respiratory disease, active malignancy, chronic infections, use of medications that affect NAFLD, active infection, pregnancy, or lactation. | P: 28 I: 30/ P: 57.29 ± 10.45 I: 53.4 ± 9.55 | RCT/double-blind/placebo | A placebo sachet identical in appearance, texture, and weight to the probiotic sachet. | 1 sachet (10 g) of multiprobiotic “Symbiter” containing 14 live probiotic strains (Lactobacillus, Lactococcus, Bifidobacterium, Propionibacterium, Acetobacter) or placebo daily. | Probiotic intake significantly decreased the FLI, serum levels of AST and GGT, and the levels of inflammatory cytokines TNF-α and IL-6 in NAFLD patients. | Significant reduction in TG, total cholesterol, LDL-C, and VLDL-C levels was observed in the probiotic group compared to the placebo group. |
| Kobyliak (2019) [47]/Ukraine | Adults with type 2 diabetes (T2D), BMI ≥ 25 kg/m2, and NAFLD diagnosed via ultrasound. Patients on stable anti-diabetic medications for at least four weeks prior. | Decompensated liver disease, viral hepatitis, alcohol abuse, recent probiotic, prebiotic, or antibiotic use, recent vitamin E or omega-3 fatty acid supplementation, and active cardiovascular or respiratory disease. | P: 24 I: 26/ P: 57.38 ± 9.92 I: 53.23 ± 10.09 | RCT/double-blind/placebo | Sachets with similar taste and appearance as the intervention. | Symbiter Forte sachets (probiotic with smectite gel), containing multiple probiotic strains, administered daily for 8 weeks. | Reductions in LS and ALT levels in the probiotic-smectite group compared to placebo. Reductions in total cholesterol and cytokines (IL-1b and TNF-a) in the intervention group. | Insignificant changes in FLI and some liver enzymes (e.g., AST) compared to placebo. The intervention group showed improvements in systemic inflammation markers. |
| 8~12 weeks | ||||||||
| Javadi (2017) [40]/Iran | Adults aged 20–60 with NAFLD (diagnosed via ultrasound and elevated ALT/AST levels). | Cardiovascular, thyroid, kidney, autoimmune diseases, hepatitis A, B, or C, hemochromatosis, Wilson’s disease, alcohol consumption, pregnancy, and lactation. | Placebo: 19 (68.4) Probiotic: 20 (85) Prebiotic: 19 (84.2) Probiotic + Prebiotic: 17 (82.4)/ Placebo: 42.21 ± 9.11 Probiotic: 43.90 ± 9.02 Prebiotic: 38.68 ± 10 Probiotic + Prebiotic: 43.24 ± 6.95 | RCT/double-blind/placebo/four-arm study | Maltodextrin powder for prebiotic placebo and lactose-free milk powder for probiotic placebo. | Probiotic: Bifidobacterium longum and Lactobacillus acidophilus (2 × 10^7 CFU daily) Prebiotic: Inulin (10 g/day) | Reductions in LDL and increases in HDL in the probiotic and combined groups compared to placebo. Weight and BMI decreased in the intervention groups. | Insulin resistance markers (HOMA-IR, fasting insulin) significantly improved within the combined probiotic and prebiotic group compared to baseline, but no notable changes in glucose levels across groups. |
| Manzhalii (2017) [41]/Ukraine | 1. Age 30–60 years. 2. Diagnosed with NASH based on ultrasonography, elevated ALT and GGT levels, and increased liver stiffness as measured by Fibroscan. | 1. Chronic liver diseases (e.g., viral hepatitis, alcoholic steatohepatitis, autoimmune hepatitis). 2. Obesity (BMI > 30 kg/m2). 3. Diabetes mellitus (fasting blood glucose > 5.6 mmol/L). 4. Hypertriglyceridemia (>1.7 mmol/L). 5. Severe comorbidities. 6. Pregnant or lactating women. | P: 37 (43.2) I: 38 (28.9)/ P: 43.5 ± 1.3 I: 44.3 ± 1.5 | RCT/double-blind/placebo | No placebo was used. Control group received only a low-fat/low-calorie diet without probiotics. | Probiotic cocktail containing: Lactobacillus casei, L. rhamnosus, L. bulgaricus, Bifidobacterium longum, Streptococcus thermophilus, and fructooligosaccharides. | Improvements in BMI and total cholesterol levels in the probiotic group. Liver stiffness (measured by Fibroscan) improved significantly in the probiotic group (p < 0.05). | Reduction in ALT and AST levels in the probiotic group (p < 0.05). No significant reduction in GGT levels. Changes in the fecal microbial composition showed an increase in beneficial bacteria (Bifidobacteria, Lactobacilli) and a reduction in pathogenic bacteria (e.g., Klebsiella, Candida) in the probiotic group. |
| Behrouz (2017) [37]/Iran | Adults aged 20–60 with NAFLD, BMI ≥ 25 kg/m2, ALT > 1.5 times the upper limit of normal, and liver steatosis greater than grade II on ultrasound. | Alcohol use, pregnancy, lactation, liver conditions (hepatitis B/C, cirrhosis), celiac disease, cardiovascular, kidney, lung diseases, recent use of antibiotics, supplements, NSAIDs, and corticosteroids. | Prebiotic: 29 (69) Placebo: 30 (70) Probiotic: 30 (73.3)/Prebiotic: 38.41 ± 9.21 Placebo: 38.43 ± 10.09 Probiotic: 38.46 ± 7.11 | RCT/double-blind/placebo/three-arm study | Maltodextrin capsules. | Probiotic: Lactobacillus casei, L. rhamnosus, L. acidophilus, Bifidobacterium longum, and B. breve (5 billion CFU/day). Prebiotic: Oligofructose powder (16 g/day). | Reductions in serum leptin, insulin, FBS, and HOMA. | QUICKI (insulin sensitivity) improved in probiotic and prebiotic groups. |
| Behrouz (2020) [46]/Iran | Diagnosed with NAFLD (elevated ALT >1.5 × ULN, steatosis grade > II on ultrasound), age 20–60, BMI 25–40 kg/m2. | Severe cardiac/renal impairment, other liver disorders, diabetes, hypertension, inflammatory diseases, recent weight-loss diets, regular corticosteroids/NSAIDs, recent antibiotic use, pregnancy, and alcohol abuse. | Prebiotic: 29 (69) Placebo: 30 (70) Probiotic: 30 (73.3)/ no mention | RCT/double-blind/placebo/three-arm study | Maltodextrin capsules. | Probiotic: 5 billion CFU mix (Lactobacillus casei, L. rhamnosus, L. acidophilus, Bifidobacterium longum, B. breve) daily. Prebiotic: Oligofructose 8 g twice/day. | No change in adiponectin levels. | hs-CRP decreased in all groups, without significant differences between groups. No significant changes in HDL, LDL, HDL ratios, or glucose levels across groups. |
| Morvaridzadeh (2021) [51]/Iran | Diagnosed NAFLD patients aged 25–55 years with mild to moderate fatty liver confirmed by ultrasonography. | Use of supplements or medications within six weeks before or during the study; sensitivity to yogurt; and presence of other chronic diseases, including heart, lung, kidney disease, or cancer. | P: 52 (54.5) I: 52 (52.2)/ P: 39.91 ± 7.16 I: 40.39 ± 6.22 | RCT/double-blind/placebo | Yogurt without Vitamin D fortification | Daily consumption of 100 g of fortified yogurt (intervention) or regular yogurt (control) over 12 weeks. Multi-strain probiotic with Vitamin D fortification. | Significant increase in serum 25(OH)D3 levels. Significant decrease in insulin levels in the intervention group. No significant change in fasting blood sugar levels between groups. | No significant changes in anthropometric measures, such as weight, BMI, waist circumference, or lean body mass. |
| Chong (2021) [49]/UK | Patients aged 25–70 years with confirmed NAFLD (via biopsy or imaging) and at least a 20% risk of a cardiovascular event over the next 10 years (using an adjusted QRisk2 score). | Established cardiovascular disease, decompensated liver cirrhosis, allergy/intolerance to VSL#3®, chronic alcohol intake above set limits, recent or repeated antibiotic use, solid organ or bone marrow transplant, and oral corticosteroid therapy. | P: 16 (81.2) I: 19 (78.9)/ P: 58 ± 7 I: 57 ± 8 | RCT/double-blind/placebo | non-identifiable sachets that matched the VSL#3® sachets in appearance, containing an inert substance. | VSL#3® probiotic (8 different strains including Lactobacillus and Bifidobacterium) | No improvement in insulin resistance, endothelial function, oxidative stress, inflammation, or liver injury markers with VSL#3® compared to placebo. | Positive correlations noted between markers of inflammation and endothelial dysfunction at baseline, but no significant change in NAFLD fibrosis score or other markers post-treatment. |
| Mitrović (2024) [53]/Serbia | Patients diagnosed with MASLD, defined by an elastometric attenuation coefficient (ATT) greater than 0.63 dB/cm/MHz with an ALT level above 40 U/L for men and 35 U/L for women. | Unwillingness to provide informed consent, alcohol consumption of more than 20 g per day for the 6 months before enrollment, viral hepatitis, liver cancer, bowel or liver resection, previous IBD, hypothyroidism, or the use of different probiotic or antibiotic therapy in the two weeks before and during the study. | P: 43 I: 41/ P: 68 ± 9 I: 69 ± 8 | RCT/double-blind/placebo | Identical capsules containing 6.8 g of maltodextrin. | Two capsules containing a total of 64 billion CFU of Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium lactis, combined with 6.4 g of inulin. | Synbiotic therapy significantly reduced liver steatosis as measured by ATT and decreased levels of hs-CRP compared to the placebo group. The microbiome analysis showed a significant enrichment in Lactobacillus, Bifidobacterium, Faecalibacterium, and Streptococcus genera, along with a reduction in Ruminococcus and Enterobacterium. | Synbiotics significantly shortened gut transition time and reduced constipation symptoms in MASLD patients. However, the therapy did not show significant effects on liver stiffness (E median), ALT levels, lipid metabolism, HbA1c, or eGFR when compared to the placebo group. |
| 12~24 weeks | ||||||||
| Malaguarnera (2012) [33]/Italy | 1. Aged between 30 and 65 years. 2. Diagnosis of NASH confirmed by liver biopsy. 3. Abnormal aminotransferase levels for at least six months. 4. Sonographic findings compatible with hepatic steatosis. | 1. Chronic liver diseases (e.g., hepatitis B, hepatitis C, autoimmune liver disease). 2. Prior surgical procedures such as jejunoileal bypass. 3. Decompensated liver disease (ascites, bleeding varices, hepatic encephalopathy). 4. Pregnancy. 5. History of alcohol consumption greater than 10 g/day for females or 20 g/day for males. 6. Use of lipid-lowering agents, steroids, methotrexate, or high-dose synthetic estrogens. | P: 32 (46.9) I: 34 (52.9)/ P: 46.7 ± 5.7 I: 46.9 ± 5.4 | RCT/double-blind/placebo | Sachets with placebo (matching Bifidobacterium longum with fructo-oligosaccharides formulation) | Bifidobacterium longum with FOS. | Reductions in liver enzymes (AST and ALT). Reduction in LDL, cholesterol, and CRP (−2.9 vs. −0.7 mg/L). Improvement in insulin resistance. | Significant histological improvement in the NASH activity index and fibrosis scores. |
| Bakhshimoghaddam (2018) [39]/Iran | Patients with grade 1–3 NAFLD and aged > 18 years. | History of alcohol abuse, chronic viral hepatitis, autoimmune hepatitis, primary biliary cirrhosis, Wilson disease, history of diabetes, α1-antitrypsin deficiency, hemochromatosis, uncontrolled thyroid conditions, psychiatric disorders, impaired renal function, use of drugs potentially affecting glucose and lipid metabolism, medications that increase the risk of NAFLD, inability to give informed consent, pregnancy, and lactation. | P: 30 (47.1) C: 28 (50) I: 32 (50)/ P: 39.9 ± 10.8 C: 41.1 ± 8.5 I: 38.8 ± 9.0 | RCT/Open-label/Placebo/three arm study | Conventional Yogurt | 300 g/day of synbiotic yogurt containing Bifidobacterium animalis subsp. lactis (BB-12) and 1.5 g inulin/24 weeks | Synbiotic yogurt consumption significantly reduced liver steatosis grade and serum levels of ALT, AST, ALP, and GGT compared to conventional yogurt and the control group. | Improvements observed in total cholesterol, TG, LDL, cholesterol, FBS, systolic blood pressure (SBP), liver span, HOMA, QUICKI, total antioxidant capacity (TAC), total oxidant status (TOS), C1q/TNF-related protein 5 (CTRP-5), and glucagon-like peptide 2 (GLP-2) in the synbiotic group compared to other groups. |
| Sayari (2018) [44]/Iran | With NAFLD, ages 18–60, BMI 25–29.9 kg/m2, impaired fasting glucose or impaired glucose tolerance, elevated ALT levels 1.5–3 times above normal. | Alcohol use >10 g/day (women) or >20 g/day (men), other liver diseases (hepatitis B/C), kidney disease, thyroid disorders, immunodeficiency, cholesterol-lowering medication, pregnancy, or breastfeeding. | P: 68 (55.9) I: 70 (64.3)/ P: 43.42 ± 11.65 I: 42.48 ± 11.41 | RCT/double-blind/placebo | Maltodextrin capsule as a placebo. | Sitagliptin 50 mg with synbiotic (Familakt containing seven probiotic strains and prebiotic) | Significant improvements in FBS, AST, cholesterol, and LDL levels. Decrease in BMI and weight. | N/A |
| Mohamad Nor (2021) [50]/Malaysia | Patients aged 18 years and above with an ultrasound diagnosis of fatty liver, a baseline-controlled attenuation parameter (CAP) score measured by FibroScan of >263 dB/m, and a baseline ALT of more than 35 IU/L for males and 25 IU/L for females. | Evidence of other chronic liver diseases (such as hepatitis B or C), autoimmune hepatitis, alcoholic liver disease, acute disorders affecting the liver, drug-induced liver injury, hepatocellular carcinoma, biliary diseases, liver cirrhosis, pregnancy, and recent use of nutritional supplements or lipid-lowering drugs. | P: 22 (77.2) I:17 (64.7)/ P: 52.47 ± 16.73 I: 54.70 ± 10.19 | RCT/double-blind/placebo | The placebo contained the same excipients as probiotics but without live bacteria. | Probiotic sachets (MCP® BCMC® strains) containing six different Lactobacillus and Bifidobacterium species at a concentration of 30 billion CFU. | No changes in hepatic steatosis (as measured by CAP) and fibrosis levels (as measured by FibroScan) between the probiotics and placebo groups. Similarly, no significant changes were observed in liver enzymes, cholesterol, triglycerides, and fasting glucose levels. | Immunohistochemistry analysis showed no significant expression changes in CD4+ T lymphocytes for both groups. However, there was a significant reduction in the expression of CD8+ T lymphocytes and ZO-1 in the placebo group but not in the probiotics group, suggesting that probiotics might stabilize mucosal immune function and protect against increased intestinal permeability. |
| Barcelos (2023) [12]/Brazil | Adults over 18 years of age with biopsy-proven NASH who had not taken antibiotics or probiotics in the three months prior to enrollment. | Patients with cirrhosis, significant alcohol intake (>15 g ethanol/day), or infections such as HIV, hepatitis B or C. Also excluded were pregnant women, transplant recipients, and patients using immunosuppressants, corticosteroids, valproic acid, tetracycline, amiodarone, or those with other chronic inflammatory diseases or a history of diarrhea. | P: 23 (47.8) I: 23 (34.8)/ P: 51.7 ± 11.9 I: 51.7 ± 11.4 | RCT/Triple-blind/Placebo | consisted of a 1 g sachet containing polydextrose/maltodextrin with an identical appearance to the probiotic sachet. | Probiotic supplementation with a mix of Lactobacillus acidophilus NCFM, Lactobacillus rhamnosus HN001, Lactobacillus paracasei LPC-37, and Bifidobacterium lactis HN019 at 1 × 10 ^ 9 CFU per strain, delivered in 1 g sachets. | No reduce cardiovascular risk markers. Both groups experienced reductions in these markers, but the changes were not significantly different between the probiotic and placebo groups. | No changes in BMI, lipid profiles, or glucose levels in either group. No differences in cardiovascular risk scores. |
| Escouto (2023) [13]/Brazil | Adult patients with histology-proven non-alcoholic steatohepatitis (NASH) confirmed by liver biopsy within six months prior to inclusion. | Hepatitis B, C, or HIV infection, significant alcohol consumption, decompensated liver disease, hepatocellular carcinoma, use of medications like steroids or vitamin E that could cause steatosis, previous surgeries affecting digestion, total parenteral nutrition, pregnancy or breastfeeding, hypothyroidism, Cushing syndrome, type 1 diabetes, and secondary steatosis causes. | P: 25 (28) I: 23 (13)/ P: 57 I: 58 | RCT/Triple-blind/Placebo | Maltodextrin. | Probiotics (Lactobacillus acidophilus and Bifidobacterium lactis, 1 billion CFUs each). | Improvement in fibrosis markers, though no significant changes were observed in liver enzyme levels, inflammatory markers, or gut microbiota composition. | No changes in metabolic markers (BMI, cholesterol levels, glucose, insulin, HOMA) between groups. |
| Ayob (2023) [48]/Malaysia | Aged over 18 yeard, diagnosed with NAFLD via ultrasound, CAP score >263, ALT >35 IU/L for males and >25 IU/L for females. | Presence of chronic liver diseases (hepatitis B/C, autoimmune hepatitis, alcoholic liver disease), drug-induced liver injury, liver cancer, biliary disease, cirrhosis, and recent use of nutritional supplements or lipid-lowering drugs. | P: 22 (77.3) I: 18 (66.7)/ P: 49.95 ± 14.05 I: 55.00 ± 11.07 | RCT/double-blind/placebo | Identical sachet without probiotic strains. | Multi-strain probiotics (HEXBIO® MCP® BCMC® strains, 6 Lactobacillus and Bifidobacterium species). | Reduction in inflammatory cytokines IFN-γ and TNF-α in the probiotic group; no significant biochemical changes in liver enzymes. | No improvements in intestinal permeability markers ZO-1 and zonulin in probiotics versus placebo groups. |
| 28 weeks | ||||||||
| Eslamparast (2014) [32]/Iran | 1. Adult patients aged 18 years or older. 2. Diagnosed with non-alcoholic fatty liver disease (NAFLD) based on ultrasound and elevated ALT levels (>60 U/L) for at least 6 months. | 1. Viral hepatitis, alcohol use, other chronic liver diseases. 2. Diabetes mellitus, untreated hypothyroidism, systemic diseases, psychiatric disorders. 3. Pregnancy, lactation, or ineffective birth control in women of childbearing age. | P: 26 (42.3) I: 26 (53.8)/ P: 45.69 ± 9.5 I: 46.35 ± 8.8 | RCT/double-blind/placebo | Maltodextrin capsules | Synbiotic capsules: Lactobacillus casei, L. rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, L. acidophilus, B. longum, L. bulgaricus and fructooligosacch-aride (prebiotic). | Fibrosis scores measured by transient elastography improved significantly in the synbiotic group. No differences in BMI and waist-to-hip ratio between the groups. | Reduction in inflammatory markers (TNF-α, high-sensitivity C-reactive protein, and NF-kB activity). Reductions in liver enzymes (ALT, AST, GGT). Improvement in insulin resistance (HOMA-IR, fasting glucose, and insulin levels). |
| Mofidi (2017) [42]/Iran | 1. Lean patients (BMI < 25) aged 18 or older. 2. Diagnosed with NAFLD using FibroScan (CAP score > 263). 3. Elevated ALT (>60 U/L) for at least 6 months. | 1. History of alcohol consumption. 2. Other acute or chronic liver diseases (e.g., hepatitis B or C, biliary diseases) 3. Autoimmune diseases or cancer. 4. Recent use of antibiotics, probiotic supplements, or hepatotoxic medications within 6 months. 5. Pregnant. 6. Patients with more than 10% weight loss during the study. | P: 21 (57.1) I: 21 (52.4)/ P: 44.61 ± 10.12 I: 40.09 ± 11.44 | RCT/double-blind/placebo | Maltodextrin capsules | Synbiotic supplementation containing (200 million bacteria): Lactobacillus casei, L. rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, L. acidophilus, B. longum, L. bulgaricus, and fructo-oligosaccharide. | Reductions in hepatic steatosis (FibroScan CAP score) in the synbiotic group compared to the placebo group (p < 0.001). | Reductions in FBS and TG (p < 0.05). Improvement in ALT and AST. Rreductions in inflammatory markers (hs-CRP and NF-κB) (p < 0.05). Improvements in lipid profiles (total cholesterol and LDL cholesterol) were observed. |
| > 50 weeks | ||||||||
| Duseja (2019) [45]/India | With liver biopsy-proven NAFLD, raised liver enzymes (ALT, AST ≥1.5 times normal) for more than three months, BMI ≥ 25 kg/m2, negative for viral and autoimmune liver markers. | Pregnant/lactating women, diabetes mellitus, cirrhosis, recent history of alcohol use above the specified threshold, and use of drugs likely to cause NAFLD (corticosteroids, methotrexate). | P: 20 (75) I: 19 (68)/ P: 33 I: 38 | RCT/double-blind/placebo | Identical in appearance and color to probiotic capsules (contained microcrystalline cellulose) | High-potency multistrain probiotic (675 billion bacteria/day, containing eight strains) | Improvements in liver histology, with reductions in hepatocyte ballooning, lobular inflammation, reductions in inflammatory markers and cytokines (TNF-α, IL-1β, IL-6), and NAFLD activity score (NAS). | No weight changes or impact on metabolic syndrome components in either group. |
| Scorletti (2020) [52]/UK | Adults aged >18 years with proven NAFLD, characterized by liver fat content > 5% as measured by MRS. | History of alcohol consumption exceeding 14 units/week for women and 21 units/week for men, use of antibiotics or probiotic supplements within the last 3 months, history of bariatric surgery, active malignancy, liver disease of other etiologies, or conditions affecting the gut microbiome. | P: 49 (61) I: 55 (69)/ P: 51.6 ± 13.1 I: 50.2 ± 12.4 | RCT/double-blind/placebo | Consisted of 4 g/twice a day of maltodextrin (1 capsule a day plus two sachets a day to stir into a cold drink). | Synbiotic treatment consisting of fructo-oligosaccharide (4 g/twice a day) plus Bifidobacterium animalis subsp. lactis BB-12 (10 billion CFU/day, 1 capsule a day). | Significant decrease in ALT, leptin, TNF-α, and endotoxins in the probiotic group compared to placebo. | Weight loss, rather than the synbiotic treatment, was associated with significant reductions in liver fat content and improvements in fibrosis scores. The synbiotic treatment led to changes in specific microbial species but did not influence overall liver health parameters. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-S.; Kuo, W.-H.; Chang, M.-H.; Hsiao, Y.; Tsai, R.-Y. Gut Microbiota and Liver Health: Meta-Analysis of Bifidobacterium-Containing Probiotics in NAFLD Management. Int. J. Mol. Sci. 2025, 26, 5944. https://doi.org/10.3390/ijms26135944
Chang K-S, Kuo W-H, Chang M-H, Hsiao Y, Tsai R-Y. Gut Microbiota and Liver Health: Meta-Analysis of Bifidobacterium-Containing Probiotics in NAFLD Management. International Journal of Molecular Sciences. 2025; 26(13):5944. https://doi.org/10.3390/ijms26135944
Chicago/Turabian StyleChang, Ko-Shih, Wu-Hsien Kuo, Mu-Hsin Chang, Yao Hsiao, and Ru-Yin Tsai. 2025. "Gut Microbiota and Liver Health: Meta-Analysis of Bifidobacterium-Containing Probiotics in NAFLD Management" International Journal of Molecular Sciences 26, no. 13: 5944. https://doi.org/10.3390/ijms26135944
APA StyleChang, K.-S., Kuo, W.-H., Chang, M.-H., Hsiao, Y., & Tsai, R.-Y. (2025). Gut Microbiota and Liver Health: Meta-Analysis of Bifidobacterium-Containing Probiotics in NAFLD Management. International Journal of Molecular Sciences, 26(13), 5944. https://doi.org/10.3390/ijms26135944







