Abstract
Fish of the tribe Thunnini represent a significant proportion of the stock caught by the fishing industry, with many of these fishes being collectively called tuna. However, only certain species can be used legally as an ingredient in canned tuna products, depending on regional food regulations. In Taiwan, only Thunnus species or Katsuwonus pelamis can be used as canned tuna. Here, we authenticated 90 canned tuna products, including 25 cat food samples, by sequencing two mitochondrial regions, 16S rRNA (16S) and the control region (CR). BLAST analysis revealed that Sarda orientalis, Euthynnus affinis, Auxis rochei, and Auxis thazard are all used as substitutes for legitimate tuna products. We found that 63.33% of investigated samples are true canned tuna, i.e., contain Thunnus species or skipjack tuna. We advocate that the Taiwanese government publishes an official standardized list of fishes, especially so that scientific, Chinese and vernacular names can be assigned unambiguously based on a “one species-one name policy”, thereby clarifying which species can be used in seafood products such as tuna. Furthermore, we feel that the large-scale and long-term monitoring of canned tuna products is warranted to fully assess the extent of tuna product adulteration in Taiwan.
1. Introduction
Approximately 17% of the global human population’s intake of animal protein in 2017 constituted fish []. Although aquaculture satisfied about half of that consumption, wild-capture from oceans, lakes and rivers remains a mainstay of the global fishing industry. Among these wild-caught fishes, scombrids are particularly important fishery resources, especially species in the tribe Thunnini that constitute ~10% of the international seafood market [,]. In 2018, the global catch of Thunnini species represented ~7.9 million tons, 58% of which can be attributed to skipjack tuna (Katsuwonus pelamis) (in Chinese: 正鰹) and yellowfin tuna (Thunnus albacares) (in Chinese: 黃鰭鮪) []. A large proportion of the Thunnini catch is destined for the canning industry [,].
The tribe Thunnini comprises five genera: Thunnus (in Chinese: 鮪屬), Katsuwonus (in Chinese: 正鰹屬), Auxis (in Chinese: 花鰹屬), Euthynnus (in Chinese: 巴鰹屬), and Allothunnus (in Chinese: 細鰹屬). Fishes of this tribe can be generally termed “tuna”. For example, Auxis rochei is called bullet tuna (in Chinese: 圓花鰹), Euthynnus alleteratus is little tunny (in Chinese: 小巴鰹), and Allothunnus fallai is slender tuna (in Chinese: 細鰹). However, the Chinese translation of tuna is 鮪 in Taiwan or 金槍魚 in Mainland China, which specifically refers solely to Thunnus spp. Previously, many different scombrids were used as an ingredient in “canned tuna”, even if they did not belong to the tribe Thunnini. For instance, Sarda (in Chinese: 齒鰆屬) spp. were once widely used in canned tuna because they possess a similar taste and texture to it []. Importantly, a species of the tribe Thunnini may not always be used legally as a canned tuna product ingredient. Various legislative bodies have developed regulations that clearly define which species can be used in canned tuna products (Table 1). The Food and Agriculture Organization (FAO) and the federal government of the United States allow spotted tuna to be used as canned tuna, but that species is prohibited by Taiwanese and Japanese regulations. In general, fishes of the genus Thunnus and skipjack tuna are widely recognized as legal canned tuna species. To align with international standards, the Taiwan Food and Drug Administration allows skipjack tuna to be used in 鮪 (for tuna)-labeled canned tuna products, even though it does not belong to the genus Thunnus, but other “pseudo-tunas” can no longer be used legally as a canned tuna ingredient.

Table 1.
Scientific, English common, Chinese common and vernacular names of scombrid fishes permitted by various legislative bodies as canned tuna or bonito products.
Seafood mislabeling is profuse worldwide [,,,,,,,,,]. Such mislabeling can be categorized into two types, i.e., deliberate or unintentional. Deliberate mislabeling primarily involves the substitution of high-priced fishes with low-priced ones for financial reasons, though the reverse scenario also arises occasionally, perhaps due to illegal fishing. Unintentional mislabeling occurs when morphologically similar fishes are misidentified, when the usage of vernacular names is not unified, or when product information is lost along the supply chain. Whatever the form of mislabeling, it ultimately entails consumer deception, public health risk, problems for fisheries management, and has religious implications (reviewed in Chang et al. []).
Traditional morphology-based identification is rarely applied to seafood because many products undergo physical (e.g., filleting) or chemical (e.g., smoking) processing before being sold. These aspects of food processing typically eliminate diagnostic morphological characters needed for species authentication. Fortunately, molecular authentication based on nucleic acid sequence similarity can overcome this limitation. DNA can be obtained from a tiny piece of tissue and it is more resistant to degradation and food processing. Therefore, DNA-based authentication is being widely employed to identify species in seafood [,,,,,,,,,].
The increasing global popularity of Japanese cuisine has markedly increased market demand for tuna, since Thunnus fishes are important elements of sashimi and sushi. The development of freezing technology and booming global trade in the early 1970s has transformed the bluefin tunas (T. thynnus (in Chinese: 大西洋黑鮪), T. maccoyii (in Chinese: 南方黑鮪), and T. orientalis (in Chinese: 太平洋黑鮪)) from a cat food into a delicacy served at high-end restaurants []. Bluefin tunas are the most sought after of all Thunnus fishes, attaining the largest size and greatest price. However, increased consumption has also threatened their stocks, which are decreasing and the status of all three species is deemed Critical (IUCN). Today, regional fishery management organizations are responsible for managing and monitoring tuna fishing in order to keep it sustainable [].
The soaring demand for Thunnus fishes, especially bluefin tuna, makes them very vulnerable to mislabeling. Previous molecular authentication studies on sushi reported that many fishes are used as substitutes for Thunnus species, including escolar (Lepidocybium flavobrunneum) (in Chinese: 鱗網帶鰆), salmon (Salmo salar) (in Chinese: 安大略鱒), banded rudderfish (Seriola zonata) (in Chinese: 環帶鰤), great amberjack (Seriola dumerili) (in Chinese: 杜氏鰤), skipjack tuna, little tunny, as well as various shark species [,,,,,,,,]. Furthermore, the value of different Thunnus species varies, prompting high-priced bluefin tuna or bigeye tuna (T. obesus) (in Chinese: 大目鮪) to be substituted for a cheaper species such as yellowfin tuna. Notably, enforcement of fishery management can drive reverse substitution, whereby high-priced bluefin tuna is sold as cheap yellowfin tuna or Thunnus fishes are labeled as skipjack tuna to enable market entry of illegal catch [,,].
Although DNA-based methods are very powerful tools for authenticating fish products, food processing, and especially canning, can limit their applicability. To date, conventional DNA barcoding remains the most widely deployed authentication approach, whereby a ~650-bp region of the mitochondrial gene encoding for cytochrome c subunit I (COI) is sequenced as a bioidentification “barcode” [,]. However, the high heat treatment integral to the canning process largely degrades DNA into small fragments [,], so shorter fragments (or nested polymerase chain reaction, PCR) must be deployed for canned products [,,,,,], but a comprehensive investigation of canned tuna substitution in a particular region has not yet been conducted. In Taiwan, the mislabeling rate of tuna products varies according to the product type. Chang et al. [] documented that all tuna-labeled meals produced at conveyor-belt sushi restaurants appear to truly come from Thunnus fishes, but Xiong et al. [] and Hwang et al. [] detected mislabeled Taiwan canned tuna products. Therefore, the goal of this study is to estimate levels of canned tuna product adulteration and to determine which species of scombrids are being marketed as canned product in the Taiwanese market.
2. Materials and Methods
2.1. Sample Collection
We purchased a total of 90 canned tuna products, belonging to 59 brands, from grocery stores or online, encompassing all major brands in Taiwan. Twenty-five of the collected samples represented canned cat food. Cans were selected if their label showed the Chinese word 鮪 (for tuna), if the company website claimed the product was made from Thunnus fishes, if the ingredients list contained Thunnus spp. or skipjack tuna, or if an image on the label indicated the can harbored Thunnus fishes. We recorded information, typically written in Chinese, on brand, manufacturer or importer, place of manufacture, labeling, and ingredients. If the cans were imported from the USA or Japan, the respective English or Japanese labels were also recorded (Table 2). The sampled cans were first photographed using a smartphone (Supplementary Information S1), and then a small quantity of the contents of each can was removed using autoclaved dissection tools, washed with 95% ethanol, before being preserved in 99.5% ethanol at −20 °C until DNA extraction. Some of the canned cat food products contained more than one type of meat, so potential Thunnus meat was selected based on color and texture.

Table 2.
List of all canned tuna product samples authenticated by 16S rRNA BLAST and the results of neighbor-joining (NJ) analysis based on the mitochondrial control region (CR).
2.2. Molecular Identification
DNA was extracted from each of the 90 tissue samples using DNA Extraction Kit S (Cat No./ID: GS100, Geneaid). PCR amplifications of the mitochondrial 16S rRNA fragment (16S) (85 bp) were performed in a mixture containing 5 ng template DNA, 12.5 μL of 2× Taq PCR MasterMix (GN-PCR201-01, Genomix), and 12.5 μmol of each forward and reverse primer. We used primers designed by Horreo et al. [] and modified them by adding M13 primer to facilitate sequencing: Forward, M13F(-20)16S-HF (5′-GTA AAA CGA CGG CCA GTA TAA CAC GAG AAG ACC CT-3′); Reverse, M13R(-24)16S-HR1+2 (5′-AACAGCTATGACCATGCCCRCGGTCGCCCCA AC-3′). These primers were made up to a final volume of 25 μL using distilled water. If BLAST analysis (in the NCBI basic local alignment search tool) indicated that a sequenced 16S fragment belonged to Thunnus spp., we PCR-amplified a fragment of the mitochondrial control region (CR, approximately 236 bp) from the same DNA sample. CR amplification was conducted in a mixture containing 5 ng template DNA, 12.5 μL of 2× Taq PCR MasterMix (GN-PCR201-01, Genomix), and 12.5 μmol of each forward and reverse primer—Forward, Tuna-CR_F; Reverse, Tuna-CR_R1 or Tuna-CR_R2 []—made up to a final volume of 25 μL using distilled water. Thermal cycling began with one cycle at 95 °C for 4 min, followed by 35 cycles of denaturation at 95 °C for 30 s, 47–55 °C for 30 s, and 72 °C for 30 s and, finally, a single extension step at 72 °C for 7 min. PCR products were purified using a PCR DNA Fragment Extraction Kit (Geneaid, Taipei, Taiwan). The amplified mitochondrial fragments were subjected to Sanger sequencing, performed by Mission Biotech. (Taipei, Taiwan) using M13 sequencing primers. Primer sequences linked to the amplified fragments were trimmed before constructing the contigs using CodonCode Aligner. The mitochondrial sequences we generated in this study have not been submitted to GenBank as they do not come from voucher specimens.
2.3. Data Analysis
Edited 16S sequences were first aligned using ClustalW in MEGA11 [], and then the haplotypes were determined in DnaSP 6. Species identity for each 16S haplotype was achieved by comparing them (by BLAST) to reference sequences in the NCBI GenBank database. Following the approach of both Armani et al. [] and Horreo et al. [], only matches displaying full sequence coverage and 100% similarity, and with unambiguous species-level scientific names, were considered positive fish identifications. If more than one fish species was shown as a positive match, all of them were considered potential candidates (Table 2).
All CR sequences used in the study of Mitchell and Hellberg (2016) were downloaded to serve as reference sequences, and then our CR fragments and reference sequences were aligned in ClustalW. A neighbor-joining (NJ) analysis was then conducted based on Kimura two-parameter (K2P) distances and 1 × 103 bootstrapping replicates in MEGA11 []. According to the phylogenetic species concept [], monophyly is a prerequisite for species recognition, so our specimens were authenticated based on the reference species with which they clustered and formed a monophyletic group (with high statistical support, i.e., bootstrapping value ≥ 70) in the NJ phenogram.
2.4. Comparison of Analytical Results and Product Labels
We compared the molecular identification of each sample to the ingredient list of the sampled cans. Since a Taiwanese government-approved standard for common names of fishes does not exist, the English names of labeled Chinese names were ascertained from the fish database of Taiwan (https://fishdb.sinica.edu.tw/ (accessed on 26 October 2021)). Although the Chinese symbols 鰹魚could broadly refer to any species from the genera Auxis (in Chinese: 花鰹屬), Euthynnus (in Chinese: 巴鰹屬), and Katsuwonus (in Chinese: 正鰹屬), skipjack tuna is the only species generally termed 鰹魚 and that can be used legally in Taiwan to make canned tuna (Table 1). Therefore, we assumed that if 鰹魚 appeared on the ingredient list of a canned tuna product, it specifically represented skipjack tuna. Many of the imported products displayed labeling in Chinese and the language of source, but in those cases we exclusively relied on the Chinese label since Chinese is the only official language in Taiwan.
A sample was judged as displaying inconsistent labeling if the fish name in the ingredient list could not be linked unambiguously to a Thunnus species or skipjack tuna. It was then deemed mislabeled if the molecularly authenticated species it contained did not match the ingredient list on the label. Where a vernacular name used in an ingredient list refers to more than one species, a case of mislabeling was assigned when the molecularly authenticated species did not correspond to any fishes bearing that vernacular name. Finally, we determined a product as being true canned tuna if it contained Thunnus species or skipjack tuna.
3. Results
We observed inconsistent labeling in 11 of 65 canned tuna products destined for human consumption, but no such problem with cat food products. Inconsistent labeling reflected canned tuna products also claiming to be made from oriental bonito (Sarda orientalis) (in Chinese: 東方齒鰆) or displaying the ambiguous vernacular name 煙仔虎, which can refer to either skipjack tuna or oriental bonito (Figure 1, Table 2).
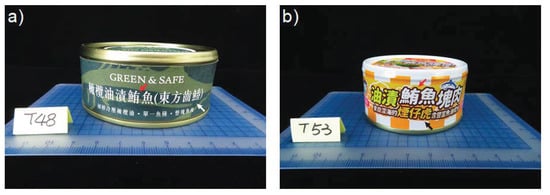
Figure 1.
Taiwanese canned tuna products displaying inconsistent labeling. “鮪” (red arrows) in the Chinese labels declares both of these canned tuna products as legally being made from Thunnus fishes or skipjack tuna (Katsuwonus pelamis) (in Chinese: 正鰹). (a) Oriental bonito (Sarda orientalis) (in Chinese: 東方齒鰆) (white arrow) in the label indicates the product contains that species. (b) The Chinese vernacular name 煙仔虎 (black arrow) may represent both oriental bonito and skipjack tuna.
We successfully amplified the 16S fragment from all 90 samples, resulting in eight haplotypes (Supplementary Information S2). All haplotypes could be identified to species-level by BLAST analysis, but only haplotypes Hap_A and Hap_E specifically relate to oriental bonito and skipjack tuna, respectively. More than one species was identified by BLAST analysis for the remaining six haplotypes, but based on the number of BLAST hits we assume Hap_B represents kawakawa (Euthynnus affinis) (in Chinese: 巴鰹), Hap_C is skipjack tuna, Hap_D and Hap_F are Thunnus species, Hap_H is either longtail tuna (T. tonggol) (in Chinese: 長腰鮪) or bigeye tuna (T. obesus), and Hap_G is either bullet tuna or frigate tuna (Auxis thazard) (in Chinese: 扁花鰹).
Our BLAST analysis of 16S sequences revealed that 31 of our samples contained Thunnus fishes. However, the success rate of CR amplification from those samples was quite low (5/31; 16%). The aligned CR dataset (Supplementary Information S3) for NJ analysis is 256 bp in length and contains 47 taxa, including 159 variable sites and 131 parsimony-informative sites. The NJ analysis of CR sequences supports that samples T1, T7, and T56 are yellowfin tuna, and that sample T34 is longtail tuna, but we could not authenticate sample D2G based on its CR sequence (Figure 2).
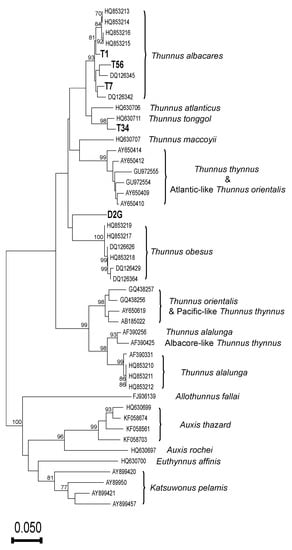
Figure 2.
Neighbor-joining (NJ) tree of the K2P model of 47 taxa inferred from 256 bp of mitochondrial control region (CR) sequences with 1000 bootstrapping replicates. Each terminal is labeled with the GenBank accession number or sample code. Bootstrapping values ≥ 70 are indicated on the respective branches.
Excluding the canned cat food samples that were all accurately labeled, 25 of the remaining 65 canned tuna products were mislabeled and a further three were potential mislabeling cases. Our BLAST analysis confirmed that sample T36 contained Thunnus fish, but did not reveal which species. We found the labeling of sample T48 to be misleading. In Chinese, the symbol “鮪” (for tuna) is never associated with oriental bonito, so it is unreasonable for the symbol for oriental bonito to be placed in parentheses following “鮪魚” (representing “tuna fish”) on the label for this sample. We observed a similar issue for sample T4, since neither skipjack tuna nor oriental bonito can be regarded as a type of “鮪” (Thunnus spp.). Since the ingredient statement on the label fails to clearly indicate which species is contained in the can, it is difficult to judge whether or not these two samples are mislabeled. Notably, many of the products identified as exhibiting inconsistent labeling were also found to be mislabeled. The mislabeling rate of canned products for human consumption was 38% (25/65). Mislabeling was even more pervasive among cat food products, with a rate of 68% (17/25). The main reason for this high mislabeling rate of cat food products is that many claim to contain Thunnus fishes but are in actual fact made from skipjack tuna. The overall mislabeling rate for the 90 tested products of this study is ~47% (42/90).
Of the 65 human food products we tested, 37 (57%) legitimately contained either Thunnus fishes or skipjack tuna, and 20 out of 25 cat food products are true canned tuna. Overall, the proportion of true canned tuna products is about 63.33% (57/90) in this study.
4. Discussion
According to Article 28 of the Act Governing Food Safety and Sanitation in Taiwan, public labeling, promotion and advertisement of foods or food additives, cleansers, utensils, containers or packaging designated by the central competent authority shall not be false, exaggerated or misleading. The 11 cases of inconsistent labeling we identified among our 90 samples, which display “鮪” (for tuna) on their labels but also list scombrids other than Thunnus species or skipjack tuna as an ingredient, obviously mislead customers into believing these products contain true canned tuna. For this study, we solely relied on the information on Chinese labeling, but we also noted conflicting information between Chinese and source-language labeling of imported products. For example, the ingredient statement in Japanese of sample T42 clearly declares that it is made from albacore tuna (T. alalunga), but its Chinese label only states that it contains Thunnus fishes (in Chinese: 鮪魚). Similarly, the Japanese label of sample T59 indicates skipjack tuna as an ingredient (in Japanese: かつお), but its Chinese label specifies yellowfin tuna (in Chinese: 黃鰭鮪) (Table 2). Such conflicting labeling of imported products not only confuses consumers but may also circumvent legal controls.
The “one species-one name” policy is critical to the authentication of fishery products []. Clearly, usage of scientific names could enable investigators to easily judge if a product is mislabeled. Under European Union labeling regulations, including the species’ scientific name on fishery product labels is mandatory []. However, scientific names are not required on Taiwanese fishery products nor are such names familiar to the public. Xiong et al. [] and Chang et al. [] advocated the adoption of the Chinese-Latin Dictionary of Fish Names (https://fishdb.sinica.edu.tw/chi/chinesequer1.php (accessed on 26 October 2021)) as a standard list of fishes in Chinese corresponding to scientific nomenclature. This Dictionary indeed clarifies that the Chinese symbols 東方齒鰆 (in English: oriental bonito) correspond to Sarda orientalis, but it does not include other Chinese vernacular names. Thus, any official “one species-one name” standard should not only contain scientific and Chinese names, but also incorporate vernacular names.
Notably, our success rates for amplifying the two mitochondrial DNA fragments differed considerably—100% for 16S, but only 16% for CR. The canning process is known to damage DNA molecules, with Quinteiro et al. [] documenting that most DNA segments extracted from canned tuna are <100 bp in length. Therefore, it is not surprising that amplification of the 85-bp 16S region was more successful than the 236-bp CR fragment (Binominal Generalized Logical Model, p < 0.01).
Apart from haplotypes Hap_A and Hap_C, a single species was not identified by BLAST for the other 16S haplotypes. There are a number of possible reasons for that outcome. First, DNA degradation through the canning process limits molecular authentication based on longer sequences, such as via conventional DNA barcoding. Accordingly, shorter DNA segments must be targeted, but they contain less information and so are less likely to unambiguously assign a specific species []. Second, molecular identification based on mitochondrial sequences is very sensitive to gene flow and incomplete lineage sorting [,,]. The tribe Thunnus comprises very closely related species, some of which display genetic introgression [,]. Consequently, though Hap_D, Hap_E, and Hap_H are all clearly form Thunnus fishes, their exact species identity remains unclear. Though conventional DNA barcoding can distinguish Thunnus fishes [,,], it would be problematic to amplify the ~650 bp barcode from the degraded DNA of canned samples. Third, a reliable database is crucial to accurate DNA-based identification []. GenBank does not guarantee that deposited sequences display correct species names. For example, the BLAST result for Hap_C matches multiple sequences for skipjack tuna sequences and one for yellowfin tuna (GenBank accession number: KM055376), implying that accession KM055376 is very likely misidentified. Hence, as highlighted in a number of studies [,,], a reliable and complete DNA reference database for authenticating seafood resources is sorely needed.
In this study, we found that many canned tuna products in Taiwan are made from oriental bonito, kawakawa, bullet tuna, or frigate tuna instead of legitimate Thunnus fishes or skipjack tuna. Although oriental bonito was never found in canned cat food products, the other three substituted fishes were identified in both human and cat food samples. These same four species have also been reported as illegitimate tuna substitutes in other studies [,,,,,]. Though istiophorid fishes have been reported as mislabeled Thunnus products in other studies [,], we did not detect them in this study.
Our NJ analysis of CR sequences, including five haplotypes generated in this study, further revealed that both yellowfin tuna and longtail tuna are used in canned tuna products. Yellowfin tuna is one of the commonest canned tunas [,], so it is not surprising that three of our five CR haplotypes clustered with yellowfin tuna sequences (sample T34 was identified as longtail tuna, and sample D2G could not be identified to species level). Our difficulties with amplifying the CR region mean that the specific Thunnus composition of canned tuna products in Taiwan remains unclear. Identifying canned tuna products to species level is important because certain Thunnus fishes have higher mercury levels [], posing a human health risk. Therefore, mitochondrial regions other than CR, such as ATP synthase membrane subunit 8 (ATP8), ATP6, and COIII could be considered [], or smaller CR fragments could be targeted.
We observed the terms 白身鮪魚 or 鮪魚白肉 commonly in the ingredient statements of our cat food samples (Table 2), reflecting the high mislabeling rate (17/25) among cat food products. However, most of the cat food samples (20/25) still represented true canned tuna, albeit not the species that might be expected. To date, there is no official definition for either of these two Chinese terms. They may be translated as “light tuna”, which often refers to yellowfin tuna or skipjack tuna, but could actually be any fishes mentioned in the Code of Federal Regulation Title 21 (CFR 161.190) and with flesh color in the Munsell color system ≥5.3 []. If those terms were to be officially recognized as translations of light tuna, then the mislabeling rate of cat food we report herein would be much lower (down to 8/25) (Binominal Generalized Logical Model, p < 0.01). Accordingly, we implore the responsible authorities to clearly define the terms for use in canned product labeling.
Although we found that 63.33% of our samples are true canned tuna, this outcome may not reflect the actual adulteration level of canned tuna products in Taiwan. First, we selected only one small piece of tissue from each can, but the mixing of tuna species in cans has been found in the European market []. An assessment of how prevalent the mixing of tuna species is in cans in the Taiwanese market would be needed to determine how close our calculated adulteration level is to the real scenario. Second, we solely sampled major brands, so there are some that remain to be assessed, especially of cat food. Moreover, seasonality in scombrid catch may alter the species composition of adulterated canned tuna products. Thus, more comprehensive and long-term monitoring of the species composition of canned tuna products is needed.
5. Conclusions
We report an overall mislabeling rate of 46.67% among the 90 samples of this study, with 63.33% of sampled canned products being true canned tuna legitimately made from Thunnus fish or skipjack tuna. In many cases, the labels of the sampled canned tuna products would confuse customers as to what species they contain. Either they contain species such as oriental bonito that do not conform to Taiwanese legislation, or ill-defined terms such as 白身鮪魚 or Chinese vernacular names are used. We assert that a standard list of scientific names and their corresponding Chinese and vernacular names conforming to the “one species-one name” principle, as well as clear definitions of terms for use in canned tuna labeling, is crucial to tackling fish product adulteration. We found that ~37% of investigated canned tuna products contain illegitimate species. In many cases, the manufacturers have substituted so-called “pseudo-tunas”, such as oriental bonito, kawakawa, bullet tuna, and frigate tuna, for legal species, i.e., Thunnus species and skipjack tuna. Our study demonstrates that a pair of primers targeting a short segment (85 bp) of 16S performs well in amplifying DNA extracted from canned food samples. However, the limited information content provided by this short sequence hampered molecular identification to species level, especially given the close phylogenetic relationships and potential for gene flow among Thunnus species. Moreover, the CR fragment we targeted largely proved uninformative, likely owing to the extreme DNA fragmentation caused by high heat treatment during the canning process. Our previous study of seafood adulteration in conveyor-belt sushi restaurants revealed no case of tuna fraud in such establishments [], so such adulteration appears to be more common in canned products. A large-scale and long-term monitoring study would help fully establish the extent of canned tuna fraud in the Taiwanese market.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods10112655/s1, Supplementary Information S1: The photos of all sampled canned tuna items, Supplementary Information S2: The 16S haplotypes, Supplementary Information S3: The aligned CR sequences for constructing NJ phylogenetic analysis.
Author Contributions
All authors contributed to the study conception and design. Conceptualization, C.-H.C. and Y.-C.W.; methodology, C.-H.C. and Y.-C.W.; software, Y.-T.K. and T.-T.H.; validation, C.-H.C., Y.-T.K. and T.-T.H.; formal analysis, Y.-T.K. and T.-T.H.; investigation, Y.-T.K. and T.-T.H.; resources, Y.-T.K. and T.-T.H.; data curation, Y.-T.K. and T.-T.H.; writing—original draft preparation, C.-H.C.; writing—review and editing, C.-H.C.; visualization, C.-H.C.; supervision, Y.-C.W.; project administration, C.-H.C. and Y.-C.W.; funding acquisition, C.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology, Taiwan, MOST 109-2621-B-029-006 and 110-2621-B-029-005.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the first author.
Acknowledgments
The authors are grateful to John O’Brien for editing assistance. Lisa, Do-Do, Bagel, Spot, and Jiu-Jiu happily ate the cat food samples after tissue collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action; FAO: Rome, Italy, 2020; Available online: http://www.fao.org/state-of-fisheries-aquaculture (accessed on 26 October 2021).
- Brill, R.W.; Hobday, A.J. Tunas and their fisheries: Safeguarding sustainability in the twenty-first century. Rev. Fish Biol. Fish. 2017, 27, 691–695. [Google Scholar] [CrossRef]
- Guillotreau, P.; Squires, D.; Sun, J.; Compeán, G.A. Local, regional and global markets: What drives the tuna fisheries? Rev. Fish Biol. Fish. 2017, 27, 909–929. [Google Scholar] [CrossRef]
- Bojolly, D.; Doyen, P.; Fur, B.L.; Christaki, U.; Verrez-Bagnis, V.; Grard, T. Development of a qPCR method for the identification and quantification of two closely related tuna species, bigeye tuna (Thunnus obesus) and yellowfin tuna (Thunnus albacares), in canned tuna. J. Agric. Food Chem. 2017, 65, 913–920. [Google Scholar] [CrossRef]
- Mata, W.; Chanmalee, T.; Punyasuk, N.; Thitamadee, S. Simple PCR-RFLP detection method for genus- and species-authentication of four types of tuna used in canned tuna industry. Food Control 2020, 108, 106842. [Google Scholar] [CrossRef]
- Ram, J.L.; Ram, M.L.; Baidoun, F.F. Authentication of canned tuna and bonito by sequence and restriction site analysis of polymerase chain reaction products of mitochondrial DNA. J. Agric. Food Chem. 1996, 44, 2460–2467. [Google Scholar] [CrossRef]
- 107年度«建構完整食品標示管理體系計畫»—«宣稱鮪魚罐頭之標示說明會» (in Chinese). Taiwan. Available online: https://www.ieatpe.org.tw/upload/img-Y29132534-0001 (accessed on 26 October 2021).
- Standard for Canned Tuna and Bonito CXS 70-1981, Codex Alimentarius FAO-WHO. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B70-1981%252FCXS_070e.pdf (accessed on 26 October 2021).
- Code of Federal Regulations CFR 21. Sec. 161.190. United State Food and Drug Administration; USA. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=161.190 (accessed on 26 October 2021).
- 水産物缶詰及び水産物瓶詰の日本農林規格 (in Japanese) Japan. Available online: https://www.maff.go.jp/j/jas/jas_kikaku/attach/pdf/kikaku_itiran2-158.pdf (accessed on 26 October 2021).
- European Regulation (Council Regulation (EEC) No 1536/92). FAO, European Union. 1992. Available online: https://www.ecolex.org/details/legislation/council-regulation-eec-no-153692-laying-down-common-marketing-standards-for-preserved-tuna-and-bonito-lex-faoc018868/ (accessed on 26 October 2021).
- Blanco-Fernandez, C.; Garcia-Vazquez, E.; Machado-Schiaffino, G. Seventeen years analysing mislabelling from DNA barcodes: Towards hake sustainability. Food Control 2020, 123, 107723. [Google Scholar] [CrossRef]
- da Silva, C.F.; Daneluz, C.M.; Camacho-Oliveira, R.B.; do Prado, F.D.; Foresti, F.; Rodrigues, C.E., Jr.; Porto-Foresti, F. DNA Barcode reveals mislabelling in the identification of marine fish swimming bladders for commercialization. Forensic Sci. Int. 2019, 299, 41–43. [Google Scholar] [CrossRef]
- Delpiani, G.; Delpiani, S.M.; Antoni, M.Y.D.; Ale, M.C.; Fischer, L.; Lucifora, L.O.; de Astarloa, J.M.D. Are we sure we eat what we buy? Fish mislabelling in Buenos Aires province, the largest sea food market in Argentina. Fish Res. 2020, 221, 105373. [Google Scholar] [CrossRef]
- Helgoe, J.; Oswald, K.J.; Quattro, J.M. A comprehensive analysis of the mislabeling of Atlantic cod (Gadus morhua) products in Spain. Fish. Res. 2020, 222, 105400. [Google Scholar] [CrossRef]
- Kusche, H.; Hanel, R. Consumers of mislabeled tropical fish exhibit increased risks of ciguatera intoxication: A report on substitution patterns in fish imported at Frankfurt Airport, Germany. Food Control 2021, 121, 107647. [Google Scholar] [CrossRef]
- Minoudi, S.; Karaiskou, N.; Avgeris, M.; Gkagkavouzis, K.; Tarantilid, P.; Triantafyllidou, D.; Palilis, L.; Avramopoulou, V.; Tsikliras, A.; Barmperis, K.; et al. Seafood mislabeling in Greek market using DNA barcoding. Food Control 2020, 113, 107213. [Google Scholar] [CrossRef]
- Pardo, M.Á.; Jiménez, E. DNA barcoding revealing seafood mislabeling in food services from Spain. J. Food Compos. Anal. 2020, 91, 103521. [Google Scholar] [CrossRef]
- Prida, V.; Sepúlveda, M.; Quezada-Romegialli, C.; Harrod, C.; Gomez-Uchida, D.; Cid, B.; Canales-Aguirre, C.B. Chilean salmon sushi: Genetics reveals product mislabeling and a lack of reliable information at the point of sale. Foods 2020, 9, 1699. [Google Scholar] [CrossRef]
- Staffen, C.F.; Staffen, M.D.; Becker, M.L.; Löfgren, S.E.; Muniz, Y.C.N.; de Freitas, R.H.A.; Marrero, A.R. DNA barcoding reveals the mislabeling of fish in a popular tourist destination in Brazil. PeerJ 2017, 5, e4006. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Yuan, F.; Huang, M.; Lu, L.; Xiong, X.; Wen, J. DNA barcoding revealed mislabeling and potential health concerns with roasted fish products sold across China. J. Food Prot. 2019, 82, 1200–1209. [Google Scholar] [CrossRef]
- Chang, C.-H.; Tsai, M.-L.; Huang, T.-T.; Wang, Y.-C. Authentication of fish species served in conveyor-belt sushi restaurants in Taiwan using DNA barcoding. Food Control 2021, 130, 108264. [Google Scholar] [CrossRef]
- Barendse, J.; Roel, A.; Longo, C.; Andriessen, L.; Webster, L.M.I.; Ogden, R.; Neat, F. DNA barcoding validates species labelling of certified seafood. Curr. Biol. 2019, 29, R198–R199. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, H.-Y.; Ren, Q.; Lin, Y.-S.; Shao, K.-T. DNA barcode identification of fish products in Taiwan: Governmentcommissioned authentication cases. Food Control 2016, 66, 38–43. [Google Scholar] [CrossRef]
- Fernandes, T.J.R.; Amaral, J.S.; Mafra, I. DNA barcode markers applied to seafood authentication: An updated review. Crit. Rev. Food Sci. Nutr. 2020, 25, 1–32. [Google Scholar] [CrossRef]
- Hellberg, R.S.; Isaacs, R.B.; Hernandez, E.L. Identification of shark species in commercial products using DNA barcoding. Fish. Res. 2019, 210, 81–88. [Google Scholar] [CrossRef]
- Liu, C.J.N.; Neo, S.; Rengifo, N.M.; French, I.; Chiang, S.; Ooi, M.; Heng, J.M.; Soon, N.; Yeo, J.Y.; Bungum, H.Z.; et al. Sharks in hot soup: DNA barcoding of shark species traded in Singapore. Fish. Res. 2021, 241, 105994. [Google Scholar] [CrossRef]
- Pappalardo, A.M.; Ferrito, V. DNA barcoding species identification unveils mislabeling of processed flatfish products in southern Italy markets. Fish. Res. 2015, 164, 153–158. [Google Scholar] [CrossRef]
- Wong, E.H.-K.; Hanner, R.H. DNA barcoding detects market substitution in North American seafood. Food Res. Int. 2008, 41, 828–837. [Google Scholar] [CrossRef]
- Ceruso, M.; Mascolo, C.; De Luca, P.; Venuti, I.; Biffali, E.; Ambrosio, R.L.; Smaldone, G.; Sordino, P.; Pepe, T. Dentex dentex Frauds: Establishment of a New DNA Barcoding Marker. Foods 2021, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Paul Greenberg. Four Fish: The Future of the Last Wild Food; Penguin Book: London, UK, 2020. [Google Scholar]
- McCluney, J.K.; Anderson, C.M.; Anderson, J.L. The fishery performance indicators for global tuna fisheries. Nat. Commun. 2019, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Huang, S.Y.; Hanner, R.; Levin, J.; Lu, X. Study of fish products in Metro Vancouver using DNA barcoding methods reveals fraudulent labeling. Food Control 2018, 94, 38–47. [Google Scholar] [CrossRef]
- Vandamme, S.G.; Griffiths, A.M.; Taylor, S.-A.; Muri, C.D.; Hankard, E.A.; Towne, J.A.; Watson, M.; Mariani, S. Sushi barcoding in the UK: Another kettle of fish. PeerJ 2018, 4, e1891. [Google Scholar] [CrossRef]
- Willette, D.A.; Simmonds, S.E.; Cheng, S.H.; Esteves, S.; Kane, T.L.; Nuetzel, H.; Pilaud, N.; Rachmawati, R.; Barber, P.H. Using DNA barcoding to track seafood mislabeling in Los Angeles restaurants. Conserv. Biol. 2017, 31, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.-R.; Hu, R.-R.; Han, J.-X.; Deng, T.-T.; Chen, Y. DNA barcoding and mini-barcoding in authenticating processed animal-derived food: A case study involving the Chinese market. Food Chem. 2020, 309, 125653. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-Q.; Qian, Z.-X.; Ye, Z.-H.; Zhou, A.-N.; Zhao, X.-X.; Zhang, P.-J.; Liu, G.-F.; Yu, X.-P. Widespread mislabeling of nonnative apple snails (Ampullariidae: Pomacea) as native field snails (Viviparidae: Cipangopaludina) on the Chinese food markets. Aquaculture 2021, 530, 735756. [Google Scholar] [CrossRef]
- Gordoa, A.; Carreras, G.; Sanz, N.; Viñas, J. Tuna species substitution in the spanish commercial chain: A knock-on effect. PLoS ONE 2017, 12, e0170809. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, C.G.; Velasco, A.; Perez-Martin, R.I.; Kappel, K.; Schröder, U.; Verrez-Bagnis, V.; Jérôme, M.; Mendes, R.; Silva, H.; Mariani, S.; et al. Tuna labels matter in Europe: Mislabelling rates in different tuna products. PLoS ONE 2018, 13, e0196641. [Google Scholar] [CrossRef]
- Ward, R.D.; Hanner, R.; Hebert, P.D.N. The campaign to DNA barcode all fishes, FISH-BOL. J. Fish Biol. 2009, 74, 329–356. [Google Scholar] [CrossRef]
- Ceruso, M.; Mascolo, C.; De Luca, P.; Venuti, I.; Smaldone, G.; Biffali, E.; Anastasio, A.; Pepe, T.; Sordino, P. A Rapid Method for the Identification of Fresh and Processed Pagellus erythrinus Species against Frauds. Foods 2020, 9, 1397. [Google Scholar] [CrossRef] [PubMed]
- Pollack, S.J.; Kawalek, M.D.; Williams-Hill, D.M.; Hellberg, R.S. Evaluation of DNA barcoding methodologies for the identification of fish species in cooked products. Food Control 2018, 84, 297–304. [Google Scholar] [CrossRef]
- Quinteiro, J.; Sotelo, C.G.; Rehbein, H.; Pryde, S.E.; Medina, I.; Pérez-Martín, R.I.; Rey-Méndez, M.; Mackie, I.M. Use of mtDNA direct polymerase chain reaction (PCR) sequencing and PCR−restriction fragment length polymorphism methodologies in species identification of canned tuna. J. Agric. Food Chem. 1998, 46, 1662–1669. [Google Scholar] [CrossRef]
- Hwang, C.-C.; Lee, Y.-C.; Huang, Y.-R.; Lin, C.-M.; Shiau, C.-Y.; Hwang, D.-F.; Tsai, Y.-H. Biogenic amines content, histamine-forming bacteria and adulteration of bonito in tuna candy products. Food Control 2010, 21, 845–850. [Google Scholar] [CrossRef]
- Servusova, E.; Piskata, Z. Identification of selected tuna species in commercial products. Molecules 2021, 26, 1137. [Google Scholar] [CrossRef]
- Xiong, X.; Xu, W.; Guo, L.; An, J.; Huang, L.; Qian, H.; Cui, X.; Li, Y.; Cao, M.; Xiong, X.; et al. Development of loop-mediated isothermal amplification (LAMP) assay for rapid screening of skipjack tuna (Katsuwonus pelamis) in processed fish products. J. Food Compos. Anal. 2021, 102, 104038. [Google Scholar] [CrossRef]
- Horreo, J.L.; Ardura, A.; Pola, I.G.; Martinez, J.L.; Garcia-Vazquez, E. Universal primers for species authentication of animal foodstuff in a single polymerase chain reaction. J. Sci. Food Agric. 2013, 93, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.K.; Hellberg, R.S. Use of the mitochondrial control region as a potential DNA mini-barcoding target for the identification of canned tuna species. Food Anal. Methods 2016, 9, 2711–2720. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.; Tinacci, L.; Xiong, X.; Castigliego, L.; Gianfaldoni, D.; Guidi, A. Fish species identification in canned pet food by BLAST and Forensically Informative Nucleotide Sequencing (FINS) analysis of short fragments of the mitochondrial 16s ribosomal RNA gene (16S rRNA). Food Control 2015, 50, 821–830. [Google Scholar] [CrossRef]
- Horreo, J.L.; Fitze, P.S.; Jiménez-Valverde, A.; Noriega, J.A.; Pelaez, M.L. Amplification of 16S rDNA reveals important fish mislabeling in Madrid restaurants. Food Control 2019, 96, 146–150. [Google Scholar] [CrossRef]
- Nixon, K.C.; Wheeler, Q.D. An amplification of the phylgenetic species concept. Cladistics 1990, 6, 211–223. [Google Scholar] [CrossRef]
- D’Amico, P.; Armani, A.; Gianfaldoni, D.; Guidi, A. New provisions for the labelling of fishery and aquaculture products: Difficulties in the implementation of Regulation (EU) n. 1379/2013. Mar. Policy 2016, 71, 147–156. [Google Scholar] [CrossRef]
- Xiong, X.; D’Amico, P.; Guardone, L.; Castigliego, L.; Guidi, A.; Gianfaldoni, D.; Armani, A. The uncertainty of seafood labeling in China: A case study on cod, salmon and tuna. Mar. Policy 2016, 68, 123–135. [Google Scholar] [CrossRef]
- Meusnier, I.; Singer, G.A.C.; Landry, J.-F.; Hickey, D.A.; Hebert, P.D.N.; Hajibabaei, M. A universal DNA mini-barcode for biodiversity analysis. BMC Genom. 2008, 9, 214. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.J.; Tavares, E.S.; Elbourne, R.F. Countering criticisms of single mitochondrial DNA gene barcoding in birds. Mol. Ecol. Resour. 2009, 9, 257–268. [Google Scholar] [CrossRef]
- Weber, A.A.-T.; Stöhr, S.; Chenuil, A. Species delimitation in the presence of strong incomplete lineage sorting and hybridization: Lessons from Ophioderma (Ophiuroidea: Echinodermata). Mol. Phylogenet. Evol. 2019, 131, 138–148. [Google Scholar] [CrossRef]
- Bayona-Vásquez, N.J.; Glenn, T.C.; Uribe-Alcocer, M.; Pecoraro, C.; Díaz-Jaimes, P. Complete mitochondrial genome of the yellowfin tuna (Thunnus albacares) and the blackfin tuna (Thunnus atlanticus): Notes on mtDNA introgression and paraphyly on tunas. Conserv. Genet. Resour. 2018, 10, 697–699. [Google Scholar] [CrossRef]
- Díaz-Arce, N.; Arrizabalaga, H.; Murua, H.; Irigoien, X.; Rodríguez-Ezpeleta, N. RAD-seq derived genome-wide nuclear markers resolve the phylogeny of tunas. Mol. Phylogenet. Evol. 2016, 102, 202–207. [Google Scholar] [CrossRef]
- Abdullah, A.; Rehbein, H. Authentication of raw and processed tuna from Indonesian markets using DNA barcoding, nuclear gene and character-based approach. Eur. Food Res. Technol. 2014, 239, 695–706. [Google Scholar] [CrossRef]
- Lowenstein, J.H.; Amato, G.; Kolokotronis, S.-O. The real maccoyii: Identifying tuna sushi with DNA barcodes—contrasting characteristic attributes and genetic distances. PLoS ONE 2009, 4, e7866. [Google Scholar] [CrossRef]
- Puncher, G.N.; Arrizabalaga, H.; Alemany, F.; Cariani, A.; Oray, I.K.; Karakulak, F.S.; Basilone, G.; Cuttitta, A.; Mazzola, S.; Tinti, F. Molecular identification of Atlantic bluefin tuna (Thunnus thynnus, Scombridae) larvae and development of a DNA character-based identification key for Mediterranean scombrids. PLoS ONE 2015, 10, e0130407. [Google Scholar] [CrossRef]
- Kappel, K.; Schröder, U. Difficulties in DNA barcoding-based authentication of snapper products due to ambiguous nucleotide sequences in public databases. Food Control 2020, 118, 107348. [Google Scholar] [CrossRef]
- Mitchell, A.; Rothbart, A.; Frankham, G.; Johnson, R.N.; Neaves, L.E. Could do better! A high school market survey of fish labelling in Sydney, Australia, using DNA barcodes. PeerJ 2019, 7, e7138. [Google Scholar] [CrossRef]
- Shehata, H.R.; Naaum, A.M.; Garduño, R.A.; Hanner, R. DNA barcoding as a regulatory tool for seafood authentication in Canada. Food Control 2018, 92, 147–153. [Google Scholar] [CrossRef]
- Espiñeira, M.; Gonzalez-Lavín, N.; Vieites, J.M.; Santaclara, F.J. Development of a method for the identification of scombroid and common substitute species in seafood products by FINS. Food Chem. 2009, 117, 698–704. [Google Scholar] [CrossRef]
- Lockley, A.K.; Bardsley, R.G. Novel method for the discrimination of tuna (Thunnus thynnus) and bonito (Sarda sarda) DNA. J. Agric. Food Chem. 2020, 48, 4463–4468. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Lu, J.; Qu, M.; Jiang, Y.; Li, F.; Guo, Y.; Wang, L.; Zhai, Y. Methodology and application of PCR-RFLP for species identification in tuna sashimi. Food Sci. Nutr. 2020, 8, 3138–3146. [Google Scholar] [CrossRef]
- Boughattas, F.; Karoui, R. Mid infrared spectroscopy combined with chemometric tools for the identification of canned tuna species in brine. J. Food Compos. Anal. 2021, 96, 103717. [Google Scholar] [CrossRef]
- Kumar, G. Mercury concentrations in fresh and canned tuna: A review. Rev. Fish Sci. Aquac. 2018, 26, 111–120. [Google Scholar] [CrossRef]
- Amaral, C.R.L.; Maciel, V.; Goldenberg-Barbosa, R.; Silva, D.A.; Amorim, A.; Carvalho, E.F. Tuna fish identification using mtDNA markers. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e471–e473. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).