Abstract
Dietary interventions during chemotherapy hold promise for clinical and supportive care outcomes. We systematically investigated the feasibility, safety, and efficacy of nutritional counseling conducted during chemotherapy. Studies prospectively implemented nutrition counseling during chemotherapy. Articles were identified from three databases—EMBASE, Cochrane Library, and SCOPUS—from inception to 1 October 2024. Feasibility, safety, and efficacy of outcome data were extracted. Among 44 publications, 39 studies recruited 98 ± 80 participants (range 15–360); 38/39 (97%) were randomized controlled trials. One-third (31%) were among patients with breast cancer. Interventions were divided into individualized nutritional counseling (n = 21), nutrition counseling plus exercise (n = 13), and nutrient-specific dietary patterns (n = 10). Many had goals to achieve established nutrition guidelines. Feasibility was high based on attendance at counseling sessions, retention, and/or food log analysis. Overall, there were minimal adverse events related to the interventions. Many studies showed between-group differences favoring the intervention group for body weight (8/24, gain or loss, according to goals), nutritional status (8/9), quality of life (3/10 without and 6/9 with exercise), cancer-related fatigue (7/10), chemotherapy tolerance (6/11), and treatment responses (3/13). In conclusion, nutritional interventions were feasible and safe for patients undergoing chemotherapy and demonstrated preliminary efficacy to improve nutritional status, fatigue, chemotherapy tolerance, and other outcomes.
1. Introduction
There are approximately 20 million new cancer cases every year worldwide []. Treatments for cancer and their side effects can significantly impact a person’s quality of life. Fatigue, pain, and other treatment-induced side effects and toxicities lead to dose-reductions of life-saving treatment as well as persistent symptoms that limit daily activities and reduce overall well-being for years after treatment [].
Dietary interventions may help manage and reduce the side effects of chemotherapy, improve overall quality of life during treatment, and improve tumor response to treatment [,]. Furthermore, by making informed choices about their nutrition, patients can actively participate in their cancer care and experience a greater sense of independence [,]. Guidelines on nutrition for cancer patients include nutrient quantity and diet composition [,,]. In regard to quantity, clinical practice tends to focus on adequate calorie and protein intake to prevent muscle loss, sarcopenia, and malnutrition, including oral nutrition supplements if nutrient intake is not adequate from food alone [,,]. In regard to dietary composition, guidelines do not recommend specific dietary patterns but encourage a broad range of tolerable foods to meet nutrient needs while discouraging dietary supplements because of their potential to interfere with chemotherapy efficacy [,,,]. Guidelines also lack more specific recommendations that target specific clinical and supportive care outcomes during chemotherapy. Further, they lack guidance on nutrient timing (e.g., intermittent fasting), a more recent discussion in the nutrition field [,].
Translational nutrition research is beginning to show that specific dietary patterns have large effects on tumor development. For example, work from Augenlicht et al. has shown that Western-style diets, which are replete in macro- and micronutrients, promote tumor growth in rodent models [,]. In addition, work by Yee et al. showed that omega-3 fatty acid consumption influences breast tissue composition in humans [,]. Thus, dietary patterns that are more specific than current recommendations have potential to influence clinical and supportive care outcomes.
Herein, to guide the development of more specific dietary recommendations for various target outcomes, we sought to gain a better understanding of the nutritional interventions that have been tested during chemotherapy treatment. Establishment of feasibility and efficacy for dietary interventions for specific outcomes—weight management, quality of life, etc.—can enhance our understanding of the role of nutrition in cancer care, refine clinical practices, and support the development of targeted interventions that optimize patient outcomes and well-being. The aim of this systematic review is to evaluate the feasibility, safety, and efficacy of dietary interventions on health outcomes in patients undergoing active chemotherapy treatment for cancer.
2. Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) reporting guidelines [] and the protocol was pre-registered in the PROSPERO database (CRD42023417079). While the pre-registered protocol describes all dietary interventions during chemotherapy and radiation, our preliminary search identified over 88 disparate articles. Thus, we narrowed the focus of this review to only studies among patients undergoing active chemotherapy and excluded dietary interventions that focused on therapeutic diets focused on influencing biomarkers of cancer progression (i.e., intermittent fasting and ketogenic diets). This approach is in accordance with the European Society for Clinical Nutrition and Metabolism guidelines for clinical practice in cancer patients []. An ethics approval was not necessary because we were not collecting new data from human participants.
2.1. Eligibility Criteria
Studies were eligible if they matched the following inclusion criteria as specified by population, intervention, study design, and outcomes: (a) population: human participants of any age with any cancer diagnosis undergoing active chemotherapy treatment. (b) Intervention: any standardized dietary pattern that modulated nutrient composition or quantity (except ketogenic diet, which is a short-term therapeutic diet being tested to enhance antineoplastic activities of treatment, as well as other clinical indications). Studies involving enteral nutrition, dietary supplements, or functional foods were excluded. Interventions that also included physical activity components were included. (c) Study design: prospectively assigned randomized controlled trials, cross-over studies, or single-armed studies. (d) Outcomes: feasibility/adherence to an intervention, safety, and/or clinical outcomes as presented by the researchers. Only full-text articles published in peer-reviewed journals were included; articles were excluded if they were not published in English, conference abstracts or posters, theses or dissertations, or protocols only.
2.2. Search Strategy
Systematic research was conducted using three databases—EMBASE, Cochrane Library, and SCOPUS—from inception to 1 October 2024. A combination of MeSH (Medical Subject Headings) terms and keywords related to nutritional interventions during chemotherapy was consistently applied for each database search entry (Table S1). Search results from all three databases were imported into EndNote (Clarivate, Philadelphia, PA, USA) and scanned for duplicates. Screening of the titles and abstracts was carried out collaboratively by SJ and AO and independently by AK, followed by a full-text review. Discrepancies in eligibility for full-text articles were resolved by all authors responsible for screening (SJ, AO, and AK). The reference lists of identified papers and relevant review articles were manually checked for additional studies fulfilling the inclusion criteria.
2.3. Data Extraction and Risk of Bias Assessment
The data extraction procedure followed the PRISMA statement []. SJ and AK independently extracted the study design, year published, sample size, participants’ demographics (i.e., age, sex), cancer type and chemotherapy treatment regimen, intervention type and frequency of intervention, feasibility in the form of adherence and/or retention, safety in the form of adverse events, and health outcomes as reported in the published studies. Any discrepancies were resolved with discussion. We inductively decided which outcomes to report in the results section; we reported outcomes with the highest frequency among all studies. Two reviewers (SJ and AK) independently assessed risk of bias using Cochrane Risk of Bias 2.0 for randomized controlled trials [], identifying “high risk,” “some concerns,” and “low risk” of bias for each clinical trial, and differences were resolved with discussion (Figure S1).
3. Results
3.1. Study Design
The systematic search identified 1361 articles from the EMBASE, Cochrane Library, and SCOPUS databases (Figure 1). After the removal of duplicates, evaluation of each article, and review of reference lists from the identified articles and relevant review articles, 44 publications met the full inclusion criteria and were included in this review [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
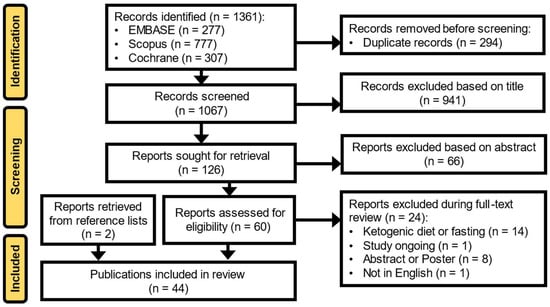
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
3.2. Study Characteristics
The included studies were Phase I and Phase II clinical trials; there were no Phase III trials (Table 1) [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. From the 44 publications, there were 39 trials because some of the studies resulted in more than one manuscript (i.e., Abdollahi et al. [,], Bourdel-Marchasson et al. [,], Sanft et al. [,], Kenkhuis et al. [,], and Kleckner et al. [,]). On average, the nutritional intervention studies recruited 98 participants, with a range of 15 (Maurer et al. [])–360 (Jacot et al. []) individuals. Publications arose from studies conducted all over the world, including the United States (n = 9) [,,,,,,,,], China (n = 7) [,,,,,,], the Netherlands (n = 6) [,,,,,], France (n = 4) [,,,], Iran (n = 3) [,,], the United Kingdom (n = 3) [,,], Germany (n = 2) [,], Brazil (n = 2) [,], Denmark (n = 2) [,], India (n = 2) [,], Italy (n = 2) [], Canada (n = 1) [], Korea (n = 1) [], and Thailand (n = 1) []. The most common patient population was breast cancer (n = 12/39, 31%) [,,,,,,,,,,,], followed by mixed cancer types (n = 9) [,,,,,,,,]; hematologic cancers (n = 4) [,,,]; head, neck, and/or gastric cancer (n = 4) [,,,]; gynecological cancers (n = 3) [,,]; colorectal cancer (n = 3) [,,]; pancreatic cancer (n = 1) []; and lung cancer (n = 1) []. Six studies recruited patients undergoing concurrent [,,,] or sequential [,] radiation. Only five studies were published before 2010 (i.e., Ollenschläger et al., 1992 [], Ovensen et al., 1993 [], Loprinzi et al., 1996 [], Gardner et al., 2008 [], and Demark-Wahnefried et al., 2008 []).

Table 1.
Characteristics of the studies included in this systematic review.
3.3. Nutritional Interventions
The types of nutritional interventions varied greatly among studies (Table 1). More than half of the 39 total trials examined individualized nutritional counseling (n = 30), 19 without [,,,,,,,,,,,,,,,,,,] and 11 with [,,,,,,,,,,] added exercise regimens. All interventions were administered by a registered dietitian, trained coordinator, or unlicensed nutritionist throughout a patient’s cancer treatment; however, the interventions differed in dietary goals and format of counseling (e.g., face-to-face, telephone, video call, or a combination). These studies tended to set recommendations for minimum protein (~0.8–2.0 g/kg body weight) and calorie (~25–50 kcal/kg body weight) intake and/or encourage adherence to published guidelines, such as the European Society for Parenteral and Enteral Nutrition (ESPEN) [] or the World Cancer Research Foundation (WCRF) []. Nine trials examined dietary patterns, including a Mediterranean diet (or a variation, n = 3) [,,], a protein-rich home delivery program (n = 2) [,], high energy density [], a neutropenic diet [], a plant-based high-protein diet [], or an anti-inflammatory diet [].
3.4. Feasibility
There was not a standard manner in which studies reported feasibility. For example, some studies measured the percentage of counseling sessions attended (e.g., Bourdel-Marchasson et al. []), the number of participants who provided data at all time points (e.g., Keum et al. []), number of servings/amount of nutrients consumed from food logs (e.g., Ovesen et al. [], Gardner et al. []), score on a diet composition-related questionnaire (e.g., Kleckner et al. []), or serum-based biomarkers (e.g., carotenoids, Djuric et al. []). Nutritional counseling resulted in high adherence among the 39 interventions in regard to number of sessions attended and/or compliance with goals, depending on what was reported (Table 2). Retention in the nutritional counseling trials was the lowest for one program with energy and protein goals among patients with esophageal cancer undergoing chemoradiotherapy [] (67.9%); all others had ≥80% [,,,,] or 90% retention [,,,,,,,,,,,,]. Interventions incorporating exercise in addition to nutritional counseling reported slightly less adherence than nutritional counseling alone, and adherence to the exercise components was lower than to dietary counseling sessions. Retention for these studies was 70–<80% for four studies [,,,], 80–<90% for two studies [,], and ≥90% for six studies [,,,,,]. Interventions that included counseling sessions [,,,] reported high attendance (≥80% [,,,]). In regard to adhering to dietary recommendations, all [,,,,,,,,,,,,,,,,] but one [] of the 18 studies that reported these data demonstrated that participants changed their diets in the direction of the recommendations even if total adherence was not achieved; Kenkhuis et al. [] studied a nutrition and exercise intervention and they noted no within-group or between-group changes in diet quality. Interventions tended to lead to higher calorie and/or protein intake compared to the control group, consistent with goals [,,,,,,,], or a decreased energy intake, consistent with goals []. Participants also increased fruit and vegetable intake in some [,,,] but not all [] studies over time in the intervention group, consistent with recommendations, compared to the control group. Those that tested a Mediterranean diet during treatment saw a within-group increase in Mediterranean diet adherence score or similar measure in the intervention arm and not the control arm [,,].

Table 2.
Feasibility, safety, and efficacy outcomes of nutrition studies conducted during chemotherapy.
The trials that tested dietary patterns had high feasibility on average. Retention was >90% in six [,,,,,] of eight [,,,,,,,] trials, though in Bille et al. [] there was a high number of dropouts between consent and initiation of the study; retention was 100% for participants who began the intervention. Studies that utilized a home delivery service to facilitate adherence reported that food provision was indeed a facilitator to adherence [,]; in IJmker-Hemink et al. [] participants reported a median burden level of 2 on a scale from 0 to 10 [].
3.5. Safety
Adverse events were reported in most studies, but events were primarily attributed to chemotherapy treatment rather than the intervention itself (Table 2). Nutritional counseling interventions appeared safe, with some studies indicating a lower incidence of chemotherapy-related side effects in the intervention group compared to the control [,,,,,]. Most studies reported no significant differences in side effects between intervention and control groups.
3.6. Primary Health Outcomes Assessed
The primary outcomes of the nutritional intervention clinical trials included feasibility as well as a variety of clinical and supportive care outcomes (Table 1). As many of these studies were Phase I or proof-of-concept studies, many declared the primary outcome measure to be feasibility (n = 7) [,,,,,,]; Puklin et al. [] specifically described changes in physical activity and diet quality attributable to the intervention. Common primary supportive care outcomes (or primary purpose of the manuscript) included quality of life (n = 10) [,,,,,,,,,], body mass and composition (n = 15) [,,,,,,,,,,,,,], cancer-related fatigue (n = 5) [,,,,], and nutritional status (n = 5) [,,,,]. The following primary outcomes were reported by a single study each: clinically relevant decrease in skeletal muscle area [], anaerobic threshold measured by cardiopulmonary exercise testing (CPET) [], chemotherapy-induced nausea and vomiting (CINV) [], gastrointestinal side effects [], infection [], one-year survival [], mortality [], and relative dose intensity [].
3.7. Efficacy
The most common clinical outcomes were body weight/body mass index (BMI), nutritional status, quality of life, cancer-related fatigue, chemotherapy toxicity, and chemotherapy responses/survival. These outcomes are summarized here.
3.7.1. Body Weight or Body Composition
Body weight and/or BMI was reported by 25 studies [,,,,,,,,,,,,,,,,,,,,,,,,]. Nine studies saw no between-group differences in changes in body weight/BMI [,,,,,,,,]. Body weight was higher in the intervention vs. control group for nine studies (weight gain or attenuation in weight loss), consistent with goals [,,,,,,,,] (for the LAM-6 remission induction chemotherapy regimen only in Ollenschläger et al. [] and for the intervention vs. control periods for Bille et al. []). There were four trials that aimed to reduce body weight, specifically fat mass, during chemotherapy, and all showed a significant between-group reduction in weight [,,,]. Demark-Wahnefried et al. [] noted no significant lean body mass losses in any group over time and no between-group differences, except the diet plus exercise arm, which had a decrease in waist circumference over time. Ford et al. [] did not report body weight but observed that a greater protein intake was associated with increased muscle mass (as measured using appendicular lean soft tissue index).
3.7.2. Nutritional Status
Nutritional status was measured in nine studies [,,,,,,,,]. Studies monitored nutrition status using albumin levels [,,,,,], the Patient-Generated Subjective Global Assessment (PG-SGA) [,,], and/or the Nutritional Risk Score []. Higher nutritional indexes were seen in the intervention vs. control group in eight [,,,,,,,] of nine [,,,,,,,,] studies; Keum et al. [] reported no between-group differences but observed that participants who used the Noom smartphone application experienced greater improvements in nutritional status.
3.7.3. Quality of Life
Quality of life was measured using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C30 [,,,,,,,,,,], a Functional Assessment of Chronic Illness Therapy (FACIT) questionnaire [,,,], the 36-item Short Form Survey [], a single-item question [], the Concise Quality of Life Index [], the Thai-Modified Function Living Index Cancer Questionnaire [], the 5-level EuroQual (EQ-5D-5L) questionnaire [], or the Functional Living Index-Emesis [] (Table 2). Ten [,,,,,,,,,] of 11 [,,,,,,,,,] nutritional counseling studies without added exercise saw no differences between groups for quality of life. Najafi et al. [] reported higher quality of life in the intervention group, though both groups had stable scores over time, and Sukaraphat et al. [] reported greater quality of life in the intervention vs. the control group post-intervention. The exercise-plus-nutrition interventions were associated with higher quality of life vs. the control group for six [,,,,,] of nine [,,,,,,,,] studies. Both the high protein [] and the Mediterranean diet [] interventions led to greater quality of life vs. the control, but the difference did not reach statistical significance.
3.7.4. Cancer-Related Fatigue
Cancer-related fatigue was measured in eight studies [,,,,,,,]. These trials used the EORTC QLQ-C30 fatigue scale [], Multidimensional Fatigue Inventory [,,,], the Chinese version of the Brief Fatigue Inventory [], the FACIT-F fatigue subscale [], or the Fatigue Symptom Inventory []. Improvements in fatigue were observed in the intervention vs. control group for six studies [,,,,,] and were similar between groups in two studies of nutrition plus exercise [,].
3.7.5. Chemotherapy Tolerance
Eleven studies [,,,,,,,,,,] reported tolerability to chemotherapy by means of relative dose intensity or chemotherapy completion rate [,,,,,], chemotherapy interruptions or postponements [,,,], or dose-limiting toxicities []. Despite the broad range of nutritional interventions, there were between-group differences favoring the intervention group or intervention period for six of the studies [,,,,,]; there were no between-group differences for five studies [,,,,].
3.7.6. Response to Chemotherapy
Tumor response and/or survival were reported by 13 publications [,,,,,,,,,,,,]. These outcomes included tumor size or response to treatment [,,,,,], remission status [,], and survival or mortality statistics [,,,,,,]. Between-group differences favoring the intervention group were seen in three studies—one encouraging adequate protein, calories, and micronutrients according to Dutch recommendations []; one encouraging macronutrients and American food group recommendations []; and one testing naturopathy, yoga, and a specific provided Indian diet []—with no between-group differences in 10 studies [,,,,,,,,,]. Keum et al. [] observed a greater tumor response rate for “above average” Noom smartphone application users vs. non-Noom users.
3.7.7. Other Outcomes
Several other outcomes were not commonly reported but were noteworthy. Abdollahi et al. [], de Lima Bezzera et al. [], and White et al. [] targeted gastrointestinal side effects such as nausea, vomiting, and diarrhea, and notable improvements were observed for the nutritional counseling intervention vs. control group. Cao et al. [] performed a nurse-led multidomain intervention among patients with head and neck cancers and saw significant reduction in the incidence of nausea and rate of vomiting attributable to the intervention. The neutropenic diet intervention, which assessed infection and mortality rates, revealed that the diet did not prevent major infections or death [].
4. Discussion
The aim of this systematic review was to evaluate the feasibility, safety, and preliminary efficacy of dietary interventions in patients undergoing active chemotherapy treatment for cancer. The results suggest individualized nutritional counseling to meet current nutrition guidelines and/or mitigate chemotherapy-induced side effects can improve patient outcomes while being safe and feasible alone or when combined with exercise interventions. In addition, more specific dietary patterns, including an Indian naturopathy dietary pattern [], the Mediterranean diet [,], and a plant-based high-protein diet [], were feasible. Nutritional counseling and dietary pattern interventions tended to be effective for managing body weight during treatment, whether it was to (a) increase body weight or attenuate weight loss or (b) minimize increases in fat mass (Table 2). Nutritional counseling interventions consistently improved nutritional status and nutritional indexes. In regard to quality of life, interventions were more effective if exercise was combined with nutrition counseling. Three-quarters (6/8) of nutritional counseling or specific dietary patterns improved cancer-related fatigue. In regard to clinical outcomes, there were between-group differences favoring the intervention group or intervention period for approximately half (6/11) of the studies assessing tolerance to chemotherapy over a broad range of nutritional interventions, and 3/13 interventions favored the intervention group in regard to tumor response and/or survival metrics, with the others showing no between-group differences. None of these trials were Phase III definitive trials, and most of these efficacy measures were not primary outcomes, though these data provide support for future trials testing nutritional interventions during chemotherapy treatment.
The Academy of Nutrition and Dietetics [], the European Society of Enteral and Parenteral Nutrition (ESPEN) [,], and the American Cancer Society [] publish nutrition guidelines for patients with cancer undergoing chemotherapy, with less formal guidelines for the geriatric oncology population [,]. These guidelines aim to detect, treat, and prevent malnutrition, specifically with 25–30 kcal/kg body weight/day and 1.0–1.5 g protein/kg body weight/day with micronutrient needs, in accordance with the Recommended Dietary Allowance (RDA) for age [,,]. The Mediterranean diet, a high-protein plant-based diet, and an anti-inflammatory diet support these guidelines and can be tailored to a patient’s preferences (e.g., flavors, protein choices), circumstances (e.g., socioeconomic status, social patterns), and clinical needs. The wide range of energy and protein recommendations among our included studies (e.g., 0.8–1.2 g protein/kg body weight in Sukaraphat et al. [] and 2.0 g/kg in Ford et al. []) may have resulted in the differences in efficacy for some of the reported outcomes, such as changes in body weight.
A total of 28 trials tested individualized nutrition counseling programs to promote adherence to established guidelines while mitigating treatment-related side effects and toxicities (Table 1). These studies imply that, despite the existence of general guidelines, directed, personalized education, interpretation, and/or implementation is needed from a dietitian to achieve adherence. This is likely because patients have diverse nutrition needs based on cancer type, pre-treatment body composition, menopausal status, barriers to consuming food, etc. For example, cancers such as head and neck and colorectal cancers more directly interfere with the ability to consume and absorb nutrients; these patients may require modified texture or food types. Some types of cancers are associated with weight gain (e.g., breast cancer) [] and some are associated with weight loss (e.g., pancreatic cancer), and therefore dietitians may have different priorities for energy needs [,]. Furthermore, different symptom profiles such as nausea, mucositis, and diarrhea require individualized nutrition strategies to meet nutrient goals. As nutrition recommendations become more specific, interventions may become contraindicated or require modifications.
This systematic review highlights gaps in the literature. Nutrition in oncology as a field is beginning to move beyond macronutrient recommendations to more specific nutrient recommendations and dietary patterns. More research into the Mediterranean diet [,,], plant-based high-protein diets [], anti-inflammatory diets [], and other diets can further guide individualized, structured programs for patients. Overweight status is highly prevalent at large as well as in the oncology population []. Studies specifically examining relationships between BMI at presentation and controlled weight change through treatment are needed. In addition, approximately half of patients with cancer are older adults, some of whom have different metabolic profiles (e.g., decreased anabolic response to protein) and goals of treatment (e.g., minimize dose reductions, maintain independence []); nutrition interventions should be tested specifically in older adults with relevant physical and functional outcomes []. More research should explore the integration of digital platforms to support dietary monitoring and adherence (such as the smartphone app in Keum et al. [] and a web-based program in Chan et al. [] recruiting men undergoing chemotherapy or radiation). Ketogenic diets and intermittent fasting regimens were beyond the scope of this review but are an active area of research, mostly as a short-term intervention to improve clinical outcomes [,,,]. While whole-food plant-based diet tends to meet society guidelines for optimizing nutrition and reducing future chronic disease risk, only a few studies have begun to rigorously test the effects (e.g., Campbell et al. []).
There is a plethora of mechanisms by which nutritional interventions can improve outcomes, many of which are active areas of research. First, nutritional counseling and many dietary patterns confer biological and metabolic benefits such as correcting nutritional deficiencies [,,], reducing chronic inflammation [,], improving mitochondrial energy metabolism [], and combating oxidative stress []. Indeed, switching from a Western diet to a healthy diet after colorectal cancer initiation improved colon health and symptoms in a mouse model []. Future research should look into the ability of dietary interventions during chemotherapy to improve the health of the gut microbiome [] and regulate circadian rhythms []. Second, nutritional interventions can confer psychological benefits, including increases in self-efficacy and mood [], as well as expectancy effects []. Third, dietary interventions can result in social benefits by bringing people together, whether it is with friends, family, the clinical care team, or peers in support groups [,].
Sex differences in cancer involve variations in the incidence, survival rates, and treatment response between males and females across various cancer types [,]. Research suggests that genetic, hormonal, and biological factors contribute to these differences []. Sex introduces variations in dietary outcomes attributed to metabolic differences, as seen in animal studies [], as well as in organ-specific and hormone-related cancers. We noted a scarcity of research that systematically investigates how these interventions affect males and females differently. Understanding how dietary changes influence cancer outcomes in a sex-specific manner could have profound implications for lifestyle behaviors, treatment strategies, and cancer survivorship.
This work has important clinical implications. There is now clear evidence that individual dietary counseling to meet calorie and protein recommendations can mitigate side effects, maintain weight/fat-free mass, maximize tolerated dose, prevent treatment delays, mitigate reductions in performance status, and improve quality of life during treatment (Table 2) [,]. While ensuring that minimum protein and calorie needs are being met should remain the first priority to prevent malnutrition [,,,], many patients may be in a position to adopt more detailed dietary patterns in regard to macro- and micronutrients, food groups, and nutrient timing. Unfortunately, many cancer treatment centers have inadequate availability of licensed nutrition practitioners [,,]. A clinical workflow that includes dietitians in outpatient cancer care for all patients undergoing chemotherapy, with tools to self-promote adherence, is needed to improve implementation of guidelines and leverage the benefits of optimizing nutrition throughout cancer care.
This review has both strengths and limitations. First, we compiled all interventions conducted during chemotherapy treatment, gathering an all-encompassing understanding of the current state of nutritional interventions in this population. The risk of bias for these studies tended to be low (Figure S1), with potential risks being not preregistering the study (especially for older studies) and incomplete description of the randomization process. One limitation of this systematic review is that the interventions, control groups, and outcomes (types and methods of measuring them) exhibited considerable heterogeneity, precluding the ability to conduct a useful meta-analysis. Meta-analyses are instrumental in generating a composite effect size for an intervention in comparison to a control group []. However, given the diverse nature of the studies included in this review, combining the data in a meta-analysis would not yield meaningful insights to guide further intervention development or inform clinical implementation. Second, adherence to interventions was not standardized, and therefore it was not possible to quantitatively assess the relative feasibility of interventions. However, we were able to qualitatively assess feasibility. Third, each cancer type, stage, and process has wildly different implications for baseline patient characteristics and needs, and there are different side effects of the disease process itself. Dietitians individualize recommendations based on these factors in addition to chemotherapy-related side effects and needs. Due to the limited number of studies that tested the same intervention within the same populations, we are not yet at the point where we can make more specific recommendations based on disease type, age, sex, etc. Fourth, our review did not include studies testing oral nutritional supplements, which are common and efficacious for many patients []. Finally, some publications that tested nutritional interventions during cancer treatment were not eligible for our review because they recruited patients undergoing either chemotherapy or radiation treatment (e.g., Movahed et al. [], Chan et al. []). However, by excluding these we are able to report results for only patients undergoing active chemotherapy.
5. Conclusions
In conclusion, this systematic review shows that dietary interventions that include or go beyond current nutritional guidelines during chemotherapy treatment tend to be feasible, safe, and effective for a multitude of clinical and supportive care outcomes. Further, more definitive research is necessary to establish an evidence base that is strong enough for inclusion in guidelines and incorporation into the clinical infrastructure to improve the outcomes for patients undergoing chemotherapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol32010003/s1, Table S1: Search terms; Figure S1: Risk of bias assessment.
Author Contributions
Conceptualization, A.S.K.; methodology, A.S.K. and B.J.B.; formal analysis, S.J., A.O. and A.S.K., data interpretation: K.M.S., M.M.M., G.G.R. and I.R.K.; writing—original draft preparation, S.J. and A.S.K.; writing—review and editing, S.J., A.O., K.M.S., M.M.M., G.G.R., I.R.K., B.J.B. and A.S.K.; supervision, A.S.K.; funding acquisition, A.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This article was supported by funds through the Maryland Department of Health’s Cigarette Restitution Fund Program (CH-649-CRF to support A.S.K. and I.R.K.). B.J.B. is the recipient of a Victorian Government Early Career Fellowship through the Victorian Cancer Agency (ECRF22019). S.J. was supported by the Nathan Schnaper Intern Program (NSIP) in Translational Cancer Research from the National Cancer Institute (R25CA186872 to Bret Hassel and P30CA134274 to Kevin Cullen). A.O. was supported by the University of Maryland Strategic Partnership “MPowering the State”, a formal collaboration between the University of Maryland College Park and the University of Maryland Baltimore.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data in this project were obtained from publicly available publications.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- World Health Organization; International Agency for Research on Cancer. Cancer Fact Sheets, All Cancers; Globocan: Lyon, France, 2022. [Google Scholar]
- Nekhlyudov, L.; Ganz, P.A.; Arora, N.K.; Rowland, J.H. Going beyong being lost in transition: A decade of progress in cancer survivorship. J. Clin. Oncol. 2017, 35, 1978–1981. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- de van der Schueren, M.A.E.; Laviano, A.; Blanchard, H.; Jourdan, M.; Arends, J.; Baracos, V.E. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: Current evidence and guidance for design of future trials. Ann. Oncol. 2018, 29, 1141–1153. [Google Scholar] [CrossRef]
- Brown, J.K.; Byers, T.; Doyle, C.; Coumeya, K.S.; Demark-Wahnefried, W.; Kushi, L.H.; McTieman, A.; Rock, C.L.; Aziz, N.; Bloch, A.S.; et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J. Clin. 2003, 53, 268–291. [Google Scholar] [CrossRef]
- Harvey, B.I.; Youngblood, S.M.; Kleckner, A.S. Barriers and Facilitators to Adherence to a Mediterranean Diet Intervention during Chemotherapy Treatment: A Qualitative Descriptive Analysis. Nutr. Cancer 2023, 75, 1349–1360. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Bohlke, K.; May, A.M.; Clinton, S.K.; Demark-Wahnefried, W.; Gilchrist, S.C.; Irwin, M.L.; Late, M.; Mansfield, S.; Marshall, T.F.; et al. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2491–2507. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef]
- Kirkham, A.A.; Parr, E.B.; Kleckner, A.S. Cardiometabolic health impacts of time-restricted eating: Implications for type 2 diabetes, cancer, and cardiovascular diseases. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Kalam, F.; James, D.; Li, Y.R.; Coleman, M.F.; Kiesel, V.A.; Cespedes Feliciano, E.M.; Hursting, S.D.; Sears, D.D.; Kleckner, A.S. Intermittent fasting interventions to leverage chrononutrition and metabolic mechanisms for cancer treatment and supportive care outcomes. JNCI Monogr. 2023, 61, 84–103. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Zhang, X.; Li, W.; Houston, M.; Peregrina, K.; Dubin, R.; Ye, K.; Augenlicht, L. Dynamic Intestinal Stem Cell Plasticity and Lineage Remodeling by a Nutritional Environment Relevant to Human Risk for Tumorigenesis. Mol. Cancer Res. 2023, 21, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zimmerman, S.E.; Peregrina, K.; Houston, M.; Mayoral, J.; Zhang, J.; Maqbool, S.; Zhang, Z.; Cai, Y.; Ye, K.; et al. The nutritional environment determines which and how intestinal stem cells contribute to homeostasis and tumorigenesis. Carcinogenesis 2019, 40, 937–946. [Google Scholar] [CrossRef]
- Yee, L.D.; Lester, J.L.; Cole, R.M.; Richardson, J.R.; Hsu, J.C.; Li, Y.; Lehman, A.; Belury, M.A.; Clinton, S.K. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am. J. Clin. Nutr. 2010, 91, 1185–1194. [Google Scholar] [CrossRef]
- Straka, S.; Lester, J.L.; Cole, R.M.; Andridge, R.R.; Puchala, S.; Rose, A.M.; Clinton, S.K.; Belury, M.A.; Yee, L.D. Incorporation of eicosapentaenioic and docosahexaenoic acids into breast adipose tissue of women at high risk of breast cancer: A randomized clinical trial of dietary fish and n-3 fatty acid capsules. Mol. Nutr. Food Res. 2015, 59, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, R.; Najafi, S.; Razmpoosh, E.; Shoormasti, R.S.; Haghighat, S.; Raji Lahiji, M.; Chamari, M.; Asgari, M.; Cheshmazar, E.; Zarrati, M. The Effect of Dietary Intervention Along with Nutritional Education on Reducing the Gastrointestinal Side Effects Caused by Chemotherapy Among Women with Breast Cancer. Nutr. Cancer 2019, 71, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.; Spiro, A.; McGough, C.; Norman, A.R.; Gillbanks, A.; Thomas, K.; Cunningham, D.; O’Brien, M.; Andreyev, H.J. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: A randomised controlled trial. J. Hum. Nutr. Diet. 2011, 24, 431–440. [Google Scholar] [CrossRef]
- Bourdel-Marchasson, I.; Blanc-Bisson, C.; Doussau, A.; Germain, C.; Blanc, J.F.; Dauba, J.; Lahmar, C.; Terrebonne, E.; Lecaille, C.; Ceccaldi, J.; et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: A two-year randomized controlled trial. PLoS ONE 2014, 9, e108687. [Google Scholar] [CrossRef]
- Dai, W.; Wang, S.A.; Wang, K.; Chen, C.; Wang, J.; Chen, X.; Yan, J. Impact of Nutrition Counseling in Head and Neck Cancer Sufferers Undergoing Antineoplastic Therapy: A Randomized Controlled Pilot Study. Curr. Oncol. 2022, 29, 6947–6955. [Google Scholar] [CrossRef]
- de Lima Bezerra, A.D.; Matias de Sousa, I.; Silva de Souza, A.P.; Miranda de Carvalho, A.L.; Trussardi Fayh, A.P. Early nutritional intervention does not prevent long-term adverse events in women with breast cancer: A pilot study. Clin. Nutr. ESPEN 2023, 53, 268–273. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.P.S.; Silva, L.C.D.; Fayh, A.P.T. Nutritional Intervention Contributes to the Improvement of Symptoms Related to Quality of Life in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy: A Randomized Clinical Trial. Nutrients 2021, 13, 589. [Google Scholar] [CrossRef]
- Keum, J.; Chung, M.J.; Kim, Y.; Ko, H.; Sung, M.J.; Jo, J.H.; Park, J.Y.; Bang, S.; Park, S.W.; Song, S.Y.; et al. Usefulness of Smartphone Apps for Improving Nutritional Status of Pancreatic Cancer Patients: Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, e21088. [Google Scholar] [CrossRef]
- Lin, J.X.; Chen, X.W.; Chen, Z.H.; Huang, X.Y.; Yang, J.J.; Xing, Y.F.; Yin, L.H.; Li, X.; Wu, X.Y. A multidisciplinary team approach for nutritional interventions conducted by specialist nurses in patients with advanced colorectal cancer undergoing chemotherapy: A clinical trial. Medicine 2017, 96, e7373. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Athmann, L.M.; Kardinal, C.G.; O’Fallon, J.R.; See, J.A.; Bruce, B.K.; Dose, A.M.; Miser, A.W.; Kern, P.S.; Tschetter, L.K.; et al. Randomized trial of dietician counseling to try to prevent weight gain associated with breast cancer adjuvant chemotherapy. Oncology 1996, 53, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Haghighat, S.; Raji Lahiji, M.; RazmPoosh, E.; Chamari, M.; Abdollahi, R.; Asgari, M.; Zarrati, M. Randomized Study of the Effect of Dietary Counseling During Adjuvant Chemotherapy on Chemotherapy Induced Nausea and Vomiting, and Quality of Life in Patients With Breast Cancer. Nutr. Cancer 2019, 71, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Ollenschlager, G.; Thomas, W.; Konkol, K.; Diehl, V.; Roth, E. Nutritional behaviour and quality of life during oncological polychemotherapy: Results of a prospective study on the efficacy of oral nutrition therapy in patients with acute leukaemia. Eur. J. Clin. Investig. 1992, 22, 546–553. [Google Scholar] [CrossRef]
- Ovesen, L.; Allingstrup, L.; Hannibal, J.; Mortensen, E.L.; Hansen, O.P. Effects of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: A prospective, randomized study. J. Clin. Oncol. 1993, 11, 2043–2049. [Google Scholar] [CrossRef]
- Regueme, S.C.; Echeverria, I.; Moneger, N.; Durrieu, J.; Becerro-Hallard, M.; Duc, S.; Lafargue, A.; Mertens, C.; Laksir, H.; Ceccaldi, J.; et al. Protein intake, weight loss, dietary intervention, and worsening of quality of life in older patients during chemotherapy for cancer. Support. Care Cancer 2021, 29, 687–696. [Google Scholar] [CrossRef]
- Sukaraphat, N.; Chewaskulyong, B.; Buranapin, S. Dietary counseling outcomes in locally advanced unresectable or metastatic cancer patients undergoing chemotherapy. J. Med. Assoc. Thail. 2016, 99, 1283–1290. [Google Scholar]
- van der Werf, A.; Langius, J.A.E.; Beeker, A.; Ten Tije, A.J.; Vulink, A.J.; Haringhuizen, A.; Berkhof, J.; van der Vliet, H.J.; Verheul, H.M.W.; de van der Schueren, M.A.E. The effect of nutritional counseling on muscle mass and treatment outcome in patients with metastatic colorectal cancer undergoing chemotherapy: A randomized controlled trial. Clin. Nutr. 2020, 39, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.A.; Li, F.; Zhu, J.; Chen, X.; Ren, W.; Gao, B. Multidisciplinary nutritional management improves nutritional and hospitalized outcomes of patients with esophageal cancer undergoing chemoradiotherapy: A randomized control trial. Medicine 2023, 102, e33335. [Google Scholar] [CrossRef]
- White, K.L.; Henson, C.C.; Hann, M.; Eden, M.; Burden, S.T.; Lal, S.; Davidson, S.E.; McLaughlin, J.T. Randomised clinical trial of a gastrointestinal care bundle to reduce symptoms in patients with pelvic cancer undergoing chemoradiotherapy. BMJ Open Gastroenterol. 2020, 7, e000432. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.L.; Wang, Y.Q.; Peng, L.F.; Lin, F.Y.; He, Y.L.; Jiang, Z.Q. Beneficial Effect of Educational and Nutritional Intervention on the Nutritional Status and Compliance of Gastric Cancer Patients Undergoing Chemotherapy: A Randomized Trial. Nutr. Cancer 2017, 69, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.K.; Brown, V.; White, D.; King, D.; Hunt, J.; Wainwright, J.; Emery, A.; Hodge, E.; Kehinde, A.; Prabhu, P.; et al. Multimodal Prehabilitation During Neoadjuvant Therapy Prior to Esophagogastric Cancer Resection: Effect on Cardiopulmonary Exercise Test Performance, Muscle Mass and Quality of Life—A Pilot Randomized Clinical Trial. Ann. Surg. Oncol. 2022, 29, 1839–1850. [Google Scholar] [CrossRef]
- Basen-Engquist, K.M.; Raber, M.; Carmack, C.L.; Arun, B.; Brewster, A.M.; Fingeret, M.; Schembre, S.M.; Harrison, C.; Perkins, H.Y.; Li, Y.; et al. Feasibility and efficacy of a weight gain prevention intervention for breast cancer patients receiving neoadjuvant chemotherapy: A randomized controlled pilot study. Support. Care Cancer 2020, 28, 5821–5832. [Google Scholar] [CrossRef]
- Carayol, M.; Ninot, G.; Senesse, P.; Bleuse, J.P.; Gourgou, S.; Sancho-Garnier, H.; Sari, C.; Romieu, I.; Romieu, G.; Jacot, W. Short- and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: The “APAD1” randomized controlled trial. BMC Cancer 2019, 19, 737. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Case, L.D.; Blackwell, K.; Marcom, P.K.; Kraus, W.; Aziz, N.; Snyder, D.C.; Giguere, J.K.; Shaw, E. Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clin. Breast Cancer 2008, 8, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Djuric, Z.; Ellsworth, J.S.; Weldon, A.L.; Ren, J.; Richardson, C.R.; Resnicow, K.; Newman, L.A.; Hayes, D.F.; Sen, A. A Diet and Exercise Intervention during Chemotherapy for Breast Cancer. Open Obes. J. 2011, 3, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Jacot, W.; Arnaud, A.; Jarlier, M.; Lefeuvre-Plesse, C.; Dalivoust, P.; Senesse, P.; Azzedine, A.; Tredan, O.; Sadot-Lebouvier, S.; Mas, S.; et al. Brief Hospital Supervision of Exercise and Diet During Adjuvant Breast Cancer Therapy Is Not Enough to Relieve Fatigue: A Multicenter Randomized Controlled Trial. Nutrients 2020, 12, 3081. [Google Scholar] [CrossRef]
- Maurer, T.; Belau, M.H.; von Grundherr, J.; Schlemmer, Z.; Patra, S.; Becher, H.; Schulz, K.H.; Zyriax, B.C.; Schmalfeldt, B.; Chang-Claude, J. Randomised controlled trial testing the feasibility of an exercise and nutrition intervention for patients with ovarian cancer during and after first-line chemotherapy (BENITA-study). BMJ Open 2022, 12, e054091. [Google Scholar] [CrossRef]
- Raghunath, K.; Sumathi, C.; Rajappa, S.J.; Mohan, M.; Kumar, U.; Shaik, U.; Botlagunta, M. Impact of naturopathy, yoga, and dietary interventions as adjuvant chemotherapy in the management of stage II and III adenocarcinoma of the colon. Int. J. Color. Dis. 2020, 35, 2309–2322. [Google Scholar] [CrossRef]
- Sanft, T.; Harrigan, M.; McGowan, C.; Cartmel, B.; Zupa, M.; Li, F.Y.; Ferrucci, L.M.; Puklin, L.; Cao, A.; Nguyen, T.H.; et al. Randomized Trial of Exercise and Nutrition on Chemotherapy Completion and Pathologic Complete Response in Women with Breast Cancer: The Lifestyle, Exercise, and Nutrition Early After Diagnosis Study. J. Clin. Oncol. 2023, 41, 5285–5295. [Google Scholar] [CrossRef] [PubMed]
- Stelten, S.; van Lonkhuijzen, L.; Hartman, Y.A.W.; van Driel, W.J.; Winkels, R.M.; Kenter, G.G.; Buffart, L.M.; Hoedjes, M. Experiences, adherence and satisfaction with a combined exercise and dietary intervention for patients with ovarian cancer undergoing chemotherapy: A mixed-methods study. Gynecol. Oncol. 2022, 165, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Bille, S.J.; Fjalstad, B.W.; Clausen, M.B.; Andreasen, B.J.; Andersen, J.R. The Effect of Special Diets on Weight and Nutritional Intake in Hematological Cancer Patients: A Randomized Study. Nutr. Cancer 2018, 70, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Mattiuzzi, G.; Faderl, S.; Borthakur, G.; Garcia-Manero, G.; Pierce, S.; Brandt, M.; Estey, E. Randomized comparison of cooked and noncooked diets in patients undergoing remission induction therapy for acute myeloid leukemia. J. Clin. Oncol. 2008, 26, 5684–5688. [Google Scholar] [CrossRef]
- IJmker-Hemink, V.; Lize, N.; Beijer, S.; Raijmakers, N.; Wanten, G.; van den Berg, M. Lessons learned from a randomized controlled trial on a home delivered meal service in advanced cancer patients undergoing chemotherapy: A pilot study. BMC Nutr. 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- IJmker-Hemink, V.E.; Kooijman, N.; Kamm, Y.J.L.; Koornstra, R.H.T.; Timmer-Bonte, J.N.H.; Wanten, G.J.A.; van den Berg, M.G.A. The Effect of a Home-delivered Protein-rich Meal Service on Quality of Life in Oncological Patients Undergoing Chemotherapy. Cancer Care Res. Online 2023, 3, e038. [Google Scholar] [CrossRef]
- Jalali, S.M.; Abdollahi, M.; Hosseini, A.; Bozorg, D.K.; Ajami; Azadeh, M.; Moiniafshar, K. The positive effects of Mediterranean-neutropenic diet on nutritional status of acute myeloid leukemia patients under chemotherapy. Front. Biol. 2018, 13, 475–480. [Google Scholar] [CrossRef]
- Kleckner, A.S.; Reschke, J.E.; Kleckner, I.R.; Magnuson, A.; Amitrano, A.M.; Culakova, E.; Shayne, M.; Netherby-Winslow, C.S.; Czap, S.; Janelsins, M.C.; et al. The Effects of a Mediterranean Diet Intervention on Cancer-Related Fatigue for Patients Undergoing Chemotherapy: A Pilot Randomized Controlled Trial. Cancers 2022, 14, 4202. [Google Scholar] [CrossRef] [PubMed]
- Sathiaraj, E.; Afshan, K.; R, S.; Jadoni, A.; Murugan, K.; Patil, S.; Naik, R. Effects of a Plant-Based High-Protein Diet on Fatigue in Breast Cancer Patients Undergoing Adjuvant Chemotherapy—A Randomized Controlled Trial. Nutr. Cancer 2023, 75, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Villarini, A.; Pasanisi, P.; Raimondi, M.; Gargano, G.; Bruno, E.; Morelli, D.; Evangelista, A.; Curtosi, P.; Berrino, F. Preventing weight gain during adjuvant chemotherapy for breast cancer: A dietary intervention study. Breast Cancer Res. Treat. 2012, 135, 581–589. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, G.; Xiu, Y.; Zhao, M. The Effect of Nutritional Support Based on the Dietary Anti-Inflammatory Index on Cancer-Related Fatigue in Lung Cancer Patients Undergoing Chemotherapy. Cancer Nurs. 2023, 46, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, C.; Wang, Y.; Liu, M.; Han, X.; Li, H. A nurse-led multidomain intervention to improve the management of chemotherapy-induced nausea and vomiting in patients with head and neck cancers: A randomized controlled trial. Eur. J. Oncol. Nurs. 2024, 70, 102615. [Google Scholar] [CrossRef]
- Ford, K.L.; Sawyer, M.B.; Ghosh, S.; Trottier, C.F.; Disi, I.R.; Easaw, J.; Mulder, K.; Koski, S.; Porter Starr, K.N.; Bales, C.W.; et al. Feasibility of two levels of protein intake in patients with colorectal cancer: Findings from the Protein Recommendation to Increase Muscle (PRIMe) randomized controlled pilot trial. ESMO Open 2024, 9, 103604. [Google Scholar] [CrossRef]
- Tang, F.; Wang, P.; Ye, Y. Effect of Nutritional Intervention on the Management of Radiotherapy and Chemotherapy for Nasopharyngeal Carcinoma. Nutr. Cancer 2024, 76, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.G.; Hartman, Y.A.W.; Stelten, S.; Kenkhuis, M.F.; van Lonkhuijzen, L.; Kenter, G.G.; Kos, M.; van de Ven, P.M.; Driel, W.J.V.; Winkels, R.M.; et al. Effects of a combined exercise and dietary intervention on clinical outcomes in patients with ovarian cancer: The Physical Activity and Dietary intervention in OVArian cancer (PADOVA) randomized controlled trial. Int. J. Gynecol. Cancer 2024, 1–7. [Google Scholar] [CrossRef]
- Kenkhuis, M.F.; Stelten, S.; Hartman, Y.A.; Brouwer, C.G.; Ten Tusscher, M.R.; van Lonkhuijzen, L.R.; Kenter, G.G.; van Driel, W.J.; Winkels, R.M.; Bekkers, R.L.; et al. Effects of a combined exercise and dietary intervention on body composition, physical functioning and fatigue in patients with ovarian cancer: Results of the PADOVA trial. Br. J. Cancer 2024, 131, 101–109. [Google Scholar] [CrossRef]
- Puklin, L.S.; Ferrucci, L.M.; Harrigan, M.; McGowan, C.; Zupa, M.; Cartmel, B.; Li, F.Y.; Ligibel, J.A.; Spiegelman, D.; Sharifi, M.; et al. Improving lifestyle behaviors during chemotherapy for breast cancer: The Lifestyle, Exercise, and Nutrition Early After Diagnosis (LEANer) Trial. Cancer 2024, 130, 2440–2452. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Silvers, M.A.; Savva, J.; Huggins, C.E.; Truby, H.; Haines, T. Potential benefits of early nutritional intervention in adults with upper gastrointestinal cancer: A pilot randomised trial. Support. Care Cancer 2014, 22, 3035–3044. [Google Scholar] [CrossRef]
- Djuric, Z. The Mediterranean diet: Effects on proteins that mediate fatty acid metabolism in the colon. Nutr. Rev. 2011, 69, 730–744. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thompson, K.L.; Elliott, L.; Fuchs-Tarlovsky, V.; Levin, R.M.; Voss, A.C.; Piemonte, T. Oncology Evidence-Based Nutrition Practice Guideline for Adults. J. Acad. Nutr. Diet. 2017, 117, 297–310.e47. [Google Scholar] [CrossRef] [PubMed]
- Kleckner, A.S.; Magnuson, A. The nutritional needs of older cancer survivors. J. Geriatr. Oncol. 2022, 13, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Mislang, A.R.; Di Donato, S.; Hubbard, J.; Krishna, L.; Mottino, G.; Bozzetti, F.; Biganzoli, L. Nutritional management of older adults with gastrointestinal cancers: An International Society of Geriatric Oncology (SIOG) review paper. J. Geriatr. Oncol. 2018, 9, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Dieli-Conwright, C.M.; Wong, L.; Waliany, S.; Bernstein, L.; Salehian, B.; Mortimer, J.E. An observational study to examine changes in metabolic syndrome components in patients with breast cancer receiving neoadjuvant or adjuvant chemotherapy. Cancer 2016, 122, 2646–2653. [Google Scholar] [CrossRef] [PubMed]
- Slawinski, C.G.V.; Barriuso, J.; Guo, H.; Renehan, A.G. Obesity and Cancer Treatment Outcomes: Interpreting the Complex Evidence. Clin. Oncol. (R. Coll. Radiol.) 2020, 32, 591–608. [Google Scholar] [CrossRef]
- Hurria, A.; Lichtman, S.M.; Gardes, J.; Li, D.; Limaye, S.; Patil, S.; Zuckerman, E.; Tew, W.; Hamlin, P.; Abou-Alfa, G.K.; et al. Identifying vulnerable older adults with cancer: Integrating geriatric assessment into oncology practice. J. Am. Geriatr. Soc. 2007, 55, 1604–1608. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Van Blarigan, E.L.; Langlais, C.S.; Zhao, S.; Ramsdill, J.W.; Daniel, K.; Macaire, G.; Wang, E.; Paich, K.; Kessler, E.R.; et al. Feasibility and Acceptability of a Remotely Delivered, Web-Based Behavioral Intervention for Men With Prostate Cancer: Four-Arm Randomized Controlled Pilot Trial. J. Med. Internet Res. 2020, 22, e19238. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. The Ketogenic Diet: Evidence for Optimism but High-Quality Research Needed. J. Nutr. 2020, 150, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Du, Y.; Meireles, C.; Sharma, K.; Qi, L.; Castillo, A.; Wang, J. Adherence to ketogenic diet in lifestyle interventions in adults with overweight or obesity and type 2 diabetes: A scoping review. Nutr. Diabetes 2023, 13, 16. [Google Scholar] [CrossRef]
- Campbell, E.K.; Fidahusain, M.; Campbell Ii, T.M. Evaluation of an Eight-Week Whole-Food Plant-Based Lifestyle Modification Program. Nutrients 2019, 11, 2068. [Google Scholar] [CrossRef]
- Santos, L.D.; Custodio, D.D.; Silva, A.T.F.; Ferreira, C.C.; Marinho, C.; Caixeta, C.; Souza, V.; Teixeira, R.; Araujo, T.G.; Shivappa, N.; et al. Overweight Women with Breast Cancer on Chemotherapy Have More Unfavorable Inflammatory and Oxidative Stress Profiles. Nutrients 2020, 12, 3303. [Google Scholar] [CrossRef]
- Groschel, C.; Prinz-Wohlgenannt, M.; Mesteri, I.; Karuthedom George, S.; Trawnicek, L.; Heiden, D.; Aggarwal, A.; Tennakoon, S.; Baumgartner, M.; Gasche, C.; et al. Switching to a Healthy Diet Prevents the Detrimental Effects of Western Diet in a Colitis-Associated Colorectal Cancer Model. Nutrients 2019, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Inglis, J.E.; Lin, P.J.; Kerns, S.L.; Kleckner, I.R.; Kleckner, A.S.; Castillo, D.A.; Mustian, K.M.; Peppone, L.J. Nutritional Interventions for Treating Cancer-Related Fatigue: A Qualitative Review. Nutr. Cancer 2019, 71, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Mosher, C.E.; Champion, V.L.; Hanna, N.; Jalal, S.I.; Fakiris, A.J.; Birdas, T.J.; Okereke, I.C.; Kesler, K.A.; Einhorn, L.H.; Given, B.A.; et al. Support service use and interest in support services among distressed family caregivers of lung cancer patients. Psychooncology 2013, 22, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Knippen, K.L.; Mahas, R.; Van Wasshenova, E. Outcome Expectancies, Health Information Seeking, and Cancer Beliefs Associated with Multivitamin/Mineral Use in a National Sample, HINTS-FDA 2015. J. Acad. Nutr. Diet. 2020, 120, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Barak-Nahum, A.; Haim, L.B.; Ginzburg, K. When life gives you lemons: The effectiveness of culinary group intervention among cancer patients. Soc. Sci. Med. 2016, 166, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.F.; Li, Z.; Habedank, M. A Randomized Controlled Trial Testing the Effectiveness of Coping with Cancer in the Kitchen, a Nutrition Education Program for Cancer Survivors. Nutrients 2020, 12, 3144. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y. Pharmacogenomics of sex difference in chemotherapeutic toxicity. Curr. Drug Discov. Technol. 2007, 4, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Lim, H.; Moon, A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol. Ther. 2018, 26, 335–342. [Google Scholar] [CrossRef]
- Robison, L.S.; Albert, N.M.; Camargo, L.A.; Anderson, B.M.; Salinero, A.E.; Riccio, D.A.; Abi-Ghanem, C.; Gannon, O.J.; Zuloaga, K.L. High-Fat Diet-Induced Obesity Causes Sex-Specific Deficits in Adult Hippocampal Neurogenesis in Mice. eNeuro 2020, 7, ENEURO.0391-19.2019. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, E.B.; Claghorn, K.; Dixon, S.W.; Hill, E.B.; Braun, A.; Lipinski, E.; Platek, M.E.; Vergo, M.T.; Spees, C. Inadequate Nutrition Coverage in Outpatient Cancer Centers: Results of a National Survey. J. Oncol. 2019, 2019, 7462940. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, E.B.; Dixon, S.W.; Claghorn, K.; Levin, R.M.; Mills, J.B.; Spees, C.K. Closing the Gap in Nutrition Care at Outpatient Cancer Centers: Ongoing Initiatives of the Oncology Nutrition Dietetic Practice Group. J. Acad. Nutr. Diet. 2018, 118, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.S.; Rice, N.; Kingston, E.; Kelly, A.; Reynolds, J.V.; Feighan, J.; Power, D.G.; Ryan, A.M. A national survey of oncology survivors examining nutrition attitudes, problems and behaviours, and access to dietetic care throughout the cancer journey. Clin. Nutr. ESPEN 2021, 41, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Sa-Nguansai, S.; Pintasiri, P.; Tienchaiananda, P. Efficacy of oral nutritional supplement in cancer patients receiving chemotherapy: A systematic review and meta-analysis of randomized controlled trials. Ann. Palliat. Med. 2024, 13, 260–272. [Google Scholar] [CrossRef]
- Movahed, S.; Seilanian Toussi, M.; Pahlavani, N.; Motlagh, A.G.; Eslami, S.; Nematy, M.; Ghayour-Mobarhan, M.; Khadem-Rezaiyan, M.; Emadzadeh, M.; Varshoee Tabrizi, F.; et al. Effects of medical nutrition therapy compared with general nutritional advice on nutritional status and nutrition-related complications in esophageal cancer patients receiving concurrent chemoradiation: A randomized controlled trial. Mediterr. J. Nutr. Metab. 2020, 13, 265–276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).