Abstract
Background/Objectives: Respiratory infections are a major global public health problem, with potentially serious consequences. Indeed, they remain one of the main causes of morbidity and mortality in children under 5 in developing countries. Etiological information on respiratory infections is crucial for prevention and case management strategies. This review describes the etiology of respiratory infections reported in studies conducted in sub-Saharan African countries. Methods: PubMed, HINARI and Google Scholar search engines were used for bibliographic research, and only data from sub-Saharan Africa were considered. Articles published between 2010 and 2023, in English or French, were included in this review. Results: After a thorough search, 2175 documents were identified. Critical review and removal of duplicates identified 347 full-text studies, which underwent rigorous evaluation. A total of 50 articles were retained, with studies conducted in 24 sub-Saharan African countries, most of them in Cameroon (12%). Thirty-three (66%) were cross-sectional studies, and thirty-seven (74%) were hospital-based surveys. Respiratory syncytial virus was most frequently identified (0.6% to 59%), followed by rhinovirus (7.5% to 73%). The most frequent bacteria were Streptococcus pneumoniae (1–96%) and Haemophilus influenzae (2.5–54%). Conclusions: This study suggests that acute respiratory infections in sub-Saharan Africa, mainly in children, are primarily caused by viruses and a few bacteria.
1. Introduction
Respiratory viral infections are increasingly recognized as major contributors to hospitalization and mortality in all age groups worldwide, with a serious form of illness particularly in infants and immunocompromised individuals [,]. Annually, lower respiratory tract infections (LRTIs) cause approximately four million deaths worldwide and impart annual global inpatient and outpatient costs of approximately EUR 5 billion [,].
Most epidemiological knowledge is based on data from developed countries. In contrast, the burden of acute respiratory infections (ARI) is particularly heavy among children in developing countries, with high rates of hospital admissions and mortality [,]. Indeed, it is estimated that about 126 to 156 million cases of acute lower respiratory tract infections (ALRTI) such as pneumonia and bronchiolitis occur in children worldwide each year, causing around 1.4 million deaths, over 95% of which occur in Africa and Southeast Asia [].
Upper respiratory tract infections are commonly caused by viruses or bacteria. Respiratory viruses are more often responsible for upper tract ARIs than bacteria in children under 5 years of age []. Common symptoms include nasal congestion, cough, sore throat, and fever. However, bacteria are less identified because of low sensitivity of bacterial culture, particularly in patients with community-acquired pneumonia []. Respiratory viruses such as respiratory syncytial virus, Influenza viruses (A and B), parainfluenza viruses, human adenovirus, human coronaviruses OC43 and 229E, rhinovirus and metapneumovirus are currently recognized as common etiologies of ARI in young children in developed countries [].
Recent use of molecular diagnostic techniques has identified other respiratory viruses associated with ARI, including human metapneumovirus, human Bocavirus, human coronavirus NL63, and human coronavirus HKU1 []. In addition, human rhinovirus is implicated in the majority of cold cases and often induces lower respiratory tract infections []. A better understanding of the range of pathogens responsible for ARI is therefore essential for clinical case management and the design of preventive strategies aimed at reducing childhood morbidity and mortality.
Lower respiratory tract infection (LRTI) is common in the elderly, children under five years of age and people who are immunocompromised or suffering from co-morbidity []. People with symptoms suggestive of LRTIs can contract tuberculosis (TB) and/or other bacterial and viral infections []. Over the years, the most severe cases of pneumonia have been associated with Mycobacterium tuberculosis, with little information on other relevant bacterial pathogens []. Some common pathogens causing LRTIs other than Mycobacterium tuberculosis include: Streptococcus pneumoniae, Haemophilus influenzae, Klebsiella pneumoniae and Staphylococcus aureus [].
The viral and bacterial etiologies of ARIs have been well documented in Northern Hemisphere countries [,]. However, few studies are available in Africa []. Thus, the present study aims to summarize the literature related to the etiology of respiratory infections in sub-Saharan African countries and to identify information gaps to improve essential knowledge on the subject.
We focused our research on sub-Saharan Africa, as epidemiological, socioeconomic and vaccine policy factors in North Africa would probably be very different []. Indeed, the distinction between North Africa and sub-Saharan Africa is climatically and ecologically significant because of the natural barrier created by the Sahara Desert, the world’s largest desert with a harsh, hot climate [].
2. Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was followed for our review []. This review was registered on the Open Science Framework; Registration DOI: https://doi.org/10.17605/OSF.IO/EXRCS (https//osf.io/nsd5m/, accessed on 29 July 2024).
2.1. Search Strategy
This review considers data from documents published online (articles, reports, etc.) that reported information on both viral and bacterial etiology of ARIs in sub-Saharan Africa, by searching the online bibliographic databases PubMed, HINARI and Google Scholar using the following key terms: “Acute respiratory infections”, “Upper respiratory infections”, “Lower respiratory infections”, “Viruses”, “Bacteria”, “Respiratory syndrome”, “Influenza syndrome”, “sub-Saharan Africa”, “Prevalence/Proportion”, and “etiology”. The reference list of selected articles was used as a lead for identifying further studies. The Boolean operators “AND” and “OR” were used to combine two or more terms. The search was limited to studies published in English or French, involving patients of any age in sub-Saharan Africa, in which pathogens were identified using immunofluorescence assays (IFA), Polymerase Chain Reactions (PCR), viral cultures, bacterial cultures, or a combination of these methods.
We also adopted the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist []. Two research questions guided this review: (1) What is the etiology of respiratory infections in sub-Saharan Africa, viral and/or bacterial? (2) What are the positive proportions of these pathogens in each study?
2.2. Study Selection
This review compiles studies focused on ARIs caused by viruses and/or bacteria. We only considered data from sub-Saharan Africa reported in papers published between 2010 and 2023, in English or French.
2.3. Inclusion Criteria
Studies included were cohort, analytical, prospective, retrospective, and cross-sectional investigations reporting the proportion of respiratory viruses and bacteria in hospital and/or community settings. In the case of repeated studies, where the same population was recruited and examined over the same period, only the most recent or most complete study was included.
2.4. Exclusion Criteria
There were no age or gender restrictions (Figure 1). Exclusion criteria were mainly: (i) respiratory infections of non-human origin; (ii) comparison of PCR kits for identification of respiratory pathogens; and (iii) studies on respiratory infection management policy. Endnote software version X9 Bld 12062, was used to remove duplicates and manage records during the screening process.
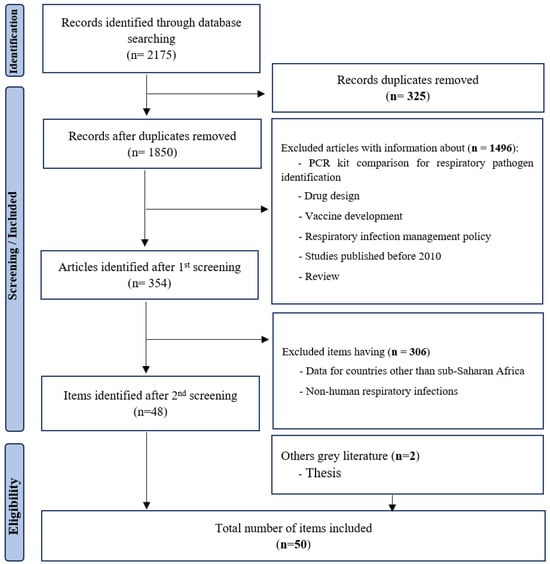
Figure 1.
Summary of search strategy (PRISMA flow diagram).
2.5. Data Extraction
Full versions of selected articles were downloaded and reviewed by two study authors. Data were extracted using a predefined form with the following information: (i) references; (ii) sample collection period; (iii) year of publication; (iv) study country; (v) age range; (vi) study objective; (vii) zone/sample size; (viii) study framework; (ix) type of sampling; (x) diagnostic methods; (xi) proportion of pathogens; and (xii) type of study.
2.6. Data Summary
We synthesized the data by summarizing the main findings of each study. Given the variety of study types included in the review, ranging from simple descriptive to analytical studies, we have considered a synthesis more appropriate rather than a formal meta-analysis. Tables were created to list all the pathogens found in each study, together with relevant study information as mentioned above on data extraction.
3. Results
3.1. Literature Review
The published articles included in this review were those from studies with samples collected from 2006 to 2022. To filter articles for this review, we initially identified a total of 2168 articles from PubMed, HINARI and Google Scholar that fit with our initial search strategy. Of these, 50 studies were included and 2125 were excluded, after screening each article (Figure 1).
Of the 50 studies included, 9 focused on viral and bacterial strains responsible for pneumonia in children and the elderly [,,,,], and 41 focused on the surveillance and epidemiology of viral or bacterial strains responsible for respiratory infections. Among them, 36 studies were carried out in outpatients, whereas 14 studies were from hospitalized patients (Table 1 and Table 2). All viruses and bacteria found in hospitalized patients were also identified in outpatients.

Table 1.
Summary of published studies carried out in outpatients.

Table 2.
Summary of published studies carried out in hospitalized patients.
Many of these studies were carried out among children under 5 years of age. Articles excluded were related to comparisons of amplification kits, respiratory infection management policy, data from countries other than Africa, and those concerning non-human respiratory infections.
3.2. Features of Included Studies
The 50 studies involved a total of 81,621 patients. Sample size ranged from 91 to 14,119 ARI patients per study. The included studies were conducted in 24 sub-Saharan African countries (Figure 2).
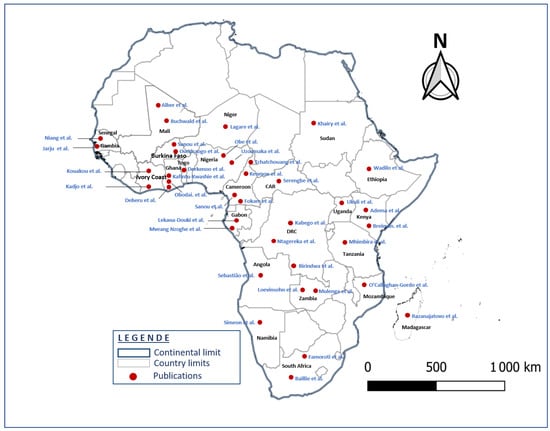
Figure 2.
Geographical identification of the 50 included studies associated with the 24 countries (map generating with QGIS 3.16.0).
A total of 6 published studies were conducted in Cameroon; 4 in Ghana, Gabon and Burkina Faso; 3 each in the Democratic Republic of the Congo (DRC), Nigeria, Zambia and Kenya; 2 studies each in South Africa, Ivory Coast, Niger and Mali, respectively; and one study in each of the following countries: Senegal, Tanzania, Ethiopia, Central African Republic (CAR), Angola, Mozambique, Uganda, Togo, Namibia, Madagascar, Gambia and Sudan.
We identified 33 (66%) cross-sectional (descriptive and case–control) studies, 8 (16%) prospective or longitudinal studies, 6 (12%) retrospective studies, 2 (4%) cohort studies and 1 (2%) analytical study (Figure 3).
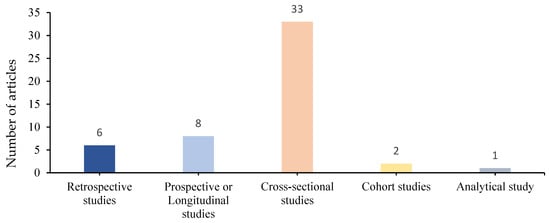
Figure 3.
Distribution of different types of studies.
Considering settings in which these published studies were focused, there were 37 (74%) hospital-based studies, 11 (22%) community-based studies, and 2 (4%) were not indicated. The study setting was urban in 42 (84%) studies, and mixed (rural, semi-rural and pre-urban) in 8 (16%) studies.
Pathogens were identified in a variety of respiratory samples, including nasal swabs, oropharyngeal swabs, nasopharyngeal aspirates, induced sputum, tracheal aspirates, bronchoalveolar lavage swabs, urine, blood and pulmonary aspirates.
Respiratory viruses were detected using immunofluorescence tests, multiplex/simplex RT-PCR, conventional PCR, blood culture and viral cultures (Table 1). RT-PCR was the most frequently used diagnostic method. For the detection of individual bacteria, only bacterial cultures and PCR were performed.
3.3. Etiology of Pathogens Detected
All the respiratory pathogens identified in these studies were viral and bacterial (Table 1 and Table 2). Among 50 studies reviewed, human respiratory syncytial virus was the most frequently identified, with a proportion ranging from 0.6 to 59%, followed by human rhinovirus (7.5–73%), Influenza A/B virus (0.9–69.1%), human adenovirus (0.9–30.8%), human parainfluenza virus 1–4 (2–24%), enterovirus (2.9–25.5%), human coronaviruses (1.4–13.9%), human metapneumovirus (1–23.3%), SARS-CoV-2 (0.4–44%) and human bocavirus (1.4–16.2%) (Table 3).

Table 3.
Proportion of pathogens identified in the 43 articles studied, conducted in several countries.
Among the bacteria detected (Table 1 and Table 2), the most prevalent were Streptococcus pneumoniae (1–96%), followed by Haemophilus influenzae type b (2.5–54%), and Klebsiella pneumoniae (1.4–49.9%). Other bacterial species, notably Staphylococcus aureus (1.7–12.2%), Pseudomonas aeruginosa (1.4–37.5%), Mycobacterium tuberculosis (6.5%), Salmonella typhi (1.6%) and other very rarely identified bacteria, such as: M. catarrhalis (46.2%), B. Pertussis (0.1%), and Enterobacter sp. (22.2%) (Table 3).
4. Discussion
Respiratory infections constitute one of the major public health problems with an important socioeconomic impact [,]. Recently, SARS-CoV-2 infection, with clinical manifestations similar to those of common respiratory viruses, showed how often a respiratory infection may become pandemic and revealed the fragility of healthcare systems, particularly in sub-Saharan Africa []. Thus, knowledge of the etiology of respiratory pathogens is essential for better management of infections.
This review updates known information on respiratory infections of viral and/or bacterial etiology in sub-Saharan Africa over the last twelve years. The overall goal of this systematic review was to inform public health actors and researchers on the etiology of respiratory infections (viral and bacterial) in Africa and to provide information that can support actions to optimize decision-making by health authorities for the control of these infections.
A wide variety of detection techniques were found in this review, including molecular viral detection and bacterial culture, which is the universal and reference method for the characterization of respiratory infection pathogens. However, other tools such as neutrophil to lymphocyte ratio (NLR) have been recently successfully tested for an early differential diagnosis of pneumonia’s etiology in children in Egypt and Italy [,] and could be used in sub-Saharan Africa, mainly where financial resources are limited. As demonstrated in adults [,], NLR is a relevant diagnostic tool that reflects the imbalance between innate and adaptive immunity, and its recent pediatric application confirms its potential in the early identification of respiratory infectious causes. The results highlight a predominance of human respiratory syncytial virus and a strong association between human rhinovirus and Influenza A/B virus in children aged below 5 years, presenting with influenza-like illness. The other most frequently detected viruses were adenovirus and all four types of human Parainfluenza virus. This study also showed respiratory infections of bacterial origin, with the most frequently identified species being Streptococcus pneumoniae and Haemophilus influenzae, mainly in bacterial culture as well as in sputum and Brancoalveolar lavage (BAL) samples in adults. These findings may not globally reflect the real picture of different pathogens associated with respiratory infections. Thus, comparison with available WHO African Region reports is needed.
Little or no data were found on the etiology of respiratory infections in many sub-Saharan African countries. Of the 48 countries in sub-Saharan Africa (wikipedia.org/wiki/Afique_sub-saharienne, accessed on 28 September 2023), the 50 studies included in this review were carried out in only 24 countries, the majority of which were in Central and West Africa (Figure 2). No published studies were carried out in the Republic of the Congo, although it borders two (Cameroon and Democratic Republic of the Congo) of the five countries where the number of deaths from childhood pneumonia was the highest []. This lack of data could probably be due to the poor implementation of respiratory infection surveillance activities.
The pattern of predominance of human respiratory syncytial virus in this study is consistent with that reported by several previous systematic reviews [,,,]. Regardless of various factors, including screening test, type of sample tested, age of children, type of education, and severity of infection, most studies indicated that human respiratory syncytial virus is the predominant causative agent of cases of respiratory diseases such as bronchiolitis, asthma, and wheezing with an incidence of 50–80% []. Rhinovirus and Influenza A/B, the second most common viruses observed, have long been considered a cause of benign respiratory tract infections such as the common cold [].
We found five studies that presented cases of viral and bacterial co-infections at rates of around 14% in our review [,,,,]. Although Streptococcus pneumoniae is known to be more prevalent in superinfection in some respiratory syndromes, such as Influenza [,], Haemophilus influenzae and Klebsiella spp. were also identified mostly in co-infection. This observation correlates with the review by Lansbury et al., who also showed that Klebsiella pneumoniae and Haemophilus influenzae were among the most frequent co-infecting bacterial pathogens even in patients with COVID-19 [,]. Staphylococcus aureus was one of the least present, as expected []. Irrespective of testing issues, co-infection with other respiratory pathogens has important implications for diagnosis and prognosis.
Seasonality and study duration could clearly also lead to variability in the proportion of viruses/bacteria responsible for respiratory infections.
5. Conclusions
This review shows that a number of viruses are associated with ARIs in children and adults in sub-Saharan Africa. The WHO’s global strategy for the control of ARI in children under 5 years of age must rigorously consider the importance of both viral and bacterial cases. Moreover, the results highlight the lack of data for several sub-Saharan African countries. Further high-quality studies are needed to determine the role of viruses and bacteria in ARI. In this vein, an approach combining the scientific studies and institutional reports should be considered to provide adequate epidemiological and etiological information.
6. Study Limits
This study has several limitations: (1) Only publications in English or French were taken into account, excluding data published in Portuguese, which is the official language of five African countries (Angola, Cape Verde, Guinea-Bissau, Mozambique and São Tomé and Principe), and in Spanish, the official language of Equatorial Guinea. (2) The unpublished literature also constitutes an information bias in this systematic review. Finally, we did not assess the statistical quality of the studies by meta-analysis but included all articles that met the inclusion criteria. (3) Although the WHO reports are an essential source for global surveillance of respiratory infections, producing annual reports on regional or continental global health statistics [], we have chosen to focus on more detailed scientific sources specific to our study context, with more detailed information on etiological specificity.
Author Contributions
J.E.D.L. and P.I.M. conceived and designed the study and initiated the manuscript; F.K.-K., P.B., E.M.L. and F.R.N. supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This review is a prelude to the activities of the COPANFLU project funded by IRD as part of the “Jeunes Equipes Associées à l’IRD” program.
Acknowledgments
Many thanks to Donatien MOUKASSA, Segun Isaac OYEDEJI, Régis DOSSOU-YOVO, Léa G. NGANGOUE, Grâce FILA-FILA, Cynthia NKOUA ep. GOMA, Hosanna L. LENGUIYA, Henri OBA, Aldi Fred MANDIANGOU, Novy Charel BOBOUAKA, Dachel EYENET, Igor J. LOUZOLO, Matthieu Fritz, Valchy B. MIEGAKANDA, Wivine S. MOUELLET, Brel J. NGATALI SAYA, Ghislain DZERET, Lucette N. Macosso, Christelle BIKOUMOU, Max B. KIAMESSO, Vishnou R. AMPIRI, Yann MAVOUNGOU, Tarcisse BALOKI, Georcil AHOUET, Isaac Samuel ONYANKOUANG, Faly A. SOLOKA, Reiche Golmard ELENGA, Durel BABISSAT, Jordy Ahmed DONIAMA, Christ M. Stéphane VEMBE MAHOUNGA, Georges TSOUMOU-GOUENDE, Bertivie C. MATONGO, Fibland OKANDZE, Ines BAKOUMA, Princesse B. BANZOUZI, Laureate MADINGOU, Amour MOUANDA, Devane TSIAMBOULOU, Vardi AKOUALA, Divin NZILA, Placide J. MALOUONA, Eudes F. BOPOPO, Nhorica F. NGOMA, Emmanuel D. MAVOUNGOU, Mavie NGALOLI, Riche ENGAMBE, Narcis PIKEINE, Caleb MOUPASSA, Belle S. MOUKALA, Joyce E. LOUKANOU, Davel W. BATAMBIKA, Orphée O. LOUVILAT, Mondesir KOKO, Blaise MUAKA-MATALA, and Lesmiens T. KIMBATSA for their assistance with documentary research and reading of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, S.; Zhang, W.; Tang, Y.W. Molecular diagnosis of viral respiratory infections. Curr. Infect. Dis. Rep. 2011, 13, 149–158. [Google Scholar] [CrossRef]
- Obando-Pacheco, P.; Justicia-Grande, A.J.; Rivero-Calle, I.; Rodríguez-Tenreiro, C.; Sly, P.; Ramilo, O.; Mejías, A.; Baraldi, E.; Papadopoulos, N.; Nair, H.; et al. Respiratory syncytial virus seasonality: A global overview. J. Infect. Dis. 2018, 217, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Jarju, S.; Greenhalgh, K.; Wathuo, M.; Banda, M.; Camara, B.; Mendy, S.; Sowe, G.; Dahaba, P.; Jammeh, L.; Bajinka, Y.; et al. Viral etiology, clinical features and antibiotic use in children <5 years of age in The Gambia presenting with influenza-like illness. Pediatr. Infect. Dis. J. 2020, 39, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Bardsley, M.; Morbey, R.A.; Hughes, H.E.; Beck, C.R.; Watson, C.H.; Zhao, H.; Ellis, J.; Smith, G.; Elliot, A. Epidemiology of respiratory syncytial virus in children younger than 5 years in England during the COVID-19 pandemic, measured by laboratory, clinical, and syndromic surveillance: A retrospective observational study. Lancet Infect. Dis. 2023, 23, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, S.; Traoré, B.; Nene Bi, Z.A.B.; Yonli, F.T.; Kima, D.; Bonané, P.; Congo, L.; Ouédraogo Traoré, R.; Yé, D.; Marguet, C.; et al. Viral etiology of respiratory tract infections in children at the pediatric hospital in Ouagadougou (Burkina Faso). PLoS ONE 2014, 9, e110435. [Google Scholar] [CrossRef]
- Sanou, A.M.; Cissé, A.; Millogo, T.; Sagna, T.; Tialla, D.; Williams, T.; Nzussouo, T.; Tarnagda, Z. Systematic review of articles on etiologies of acute respiratory infections in children aged less than five years in sub-Saharan Africa, 2000–2015. EC Microbiol. 2016, 6, 556–571. [Google Scholar]
- Sonego, M.; Pellegrin, M.C.; Becker, G.; Lazzerini, M. Risk factors for mortality from acute lower respiratory infections (ALRI) in children under five years of age in low and middle-income countries: A systematic review and meta-analysis of observational studies. PLoS ONE 2015, 10, e0116380. [Google Scholar] [CrossRef]
- Gadsby, N.J.; McHugh, M.P.; Russell, C.D.; Mark, H.; Morris, A.C.; Laurenson, I.F.; Hill, A.T.; Templeton, K.E. Development of two real-time multiplex PCR assays for the detection and quantification of eight key bacterial pathogens in lower respiratory tract infections. Clin. Microbiol. Infect. 2015, 21, 788.e1–788.e13. [Google Scholar] [CrossRef]
- Ayar, G.; Sahin, S.; Yazici, M.U.; Parlakay, A.Ö.; Tezer, H. RSV pneumonia in the pediatric intensive care unit. J. Pediatr. Inf. 2014, 8, 12–17. [Google Scholar] [CrossRef]
- Dube, F.S.; Kaba, M.; Robberts, F.J.; Ah Tow, L.; Lubbe, S.; Zar, H.J.; Nicol, M.P. Respiratory microbes present in the nasopharynx of children hospitalized with suspected pulmonary tuberculosis in Cape Town, South Africa. BMC Infect. Dis. 2016, 16, 597. [Google Scholar] [CrossRef]
- Deberu, O.; Nkrumah, B.; Sylverken, A.A.; Sambian, D.; Acheampong, G.; Amuasi, J.; Stebleson, A.; Agboyie, D.; Yenbaree, M.; Mensah, S.; et al. Common bacteria in sputum or gastric lavage of patients presenting with signs and symptoms of lower respiratory tract infections. Pan Afr. Med. J. 2021, 38, 383. [Google Scholar] [CrossRef] [PubMed]
- Yassine, H.M.; Sohail, M.U.; Younes, N.; Nasrallah, G.K. Systematic Review of the Respiratory Syncytial Virus (RSV) Prevalence, Genotype Distribution, and Seasonality in Children from the Middle East and North Africa (MENA) Region. Microorganisms 2020, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Haddad-Boubaker, S.; Mefteh, K.; Mejri, C.; Bouaffsoun, A.; El Moussi, A.; Boutiba, I.; Mnuf, K.; Slim, A.; Kechrid, A.; Smaoui, H. High genotypic diversity of Rhinoviruses obtained from Tunisian children with severe acute respiratory infection. J. Infect. Dev. Ctries. 2021, 15, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Lagare, A.; Maïnassara, H.B.; Issaka, B.; Sidiki, A.; Tempia, S. Viral and bacterial etiology of severe acute respiratory illness among children <5 years of age without influenza in Niger. BMC Infect. Dis. 2015, 15, 515. [Google Scholar] [CrossRef]
- Gessner, B.D.; Shindo, N.; Briand, S. Seasonal influenza epidemiology in sub-Saharan Africa: A systematic review. Lancet Infect. Dis. 2011, 11, 223–235. [Google Scholar] [CrossRef]
- Wikipédia, L’encyclopédie Libre. Afrique. Available online: http://fr.wikipedia.org/w/index.php?title=Afrique&oldid=207325236 (accessed on 28 August 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tchatchouang, S.; Nzouankeu, A.; Kenmoe, S.; Ngando, L.; Penlap, V.; Fonkoua, M.C.; Pefura-Yone, E.; Njouom, R. Bacterial aetiologies of lower respiratory tract infections among adults in Yaoundé, Cameroon. BioMed Res. Int. 2019, 2019, 4834396. [Google Scholar] [CrossRef]
- Lagare, A.; Ousmane, S.; Dano, I.D.; Issaka, B.; Issa, I.; Mainassara, H.B.; Testa, J.; Tempia, S.; Mamadou, S. Molecular detection of respiratory pathogens among children aged younger than 5 years hospitalized with febrile acute respiratory infections: A prospective hospital-based observational study in Niamey, Niger. Health Sci. Rep. 2019, 2, e137. [Google Scholar] [CrossRef]
- Birindwa, A.M.; Kasereka, J.K.; Gonzales-Siles, L.; Geravandi, S.; Mwilo, M.; Tudiakwile, L.K.; Mwinja, N.; Muhigirwa, B.; Kashosi, T.; Manegabe, J.; et al. Bacteria and viruses in the upper respiratory tract of Congolese children with radiologically confirmed pneumonia. BMC Infect. Dis. 2021, 21, 837. [Google Scholar] [CrossRef]
- Mhimbira, F.; Hiza, H.; Mbuba, E.; Hella, J.; Kamwela, L.; Sasamalo, M.; Ticlla, T.; Said, K.; Mhalu, G.; Chiryamkubi, M.; et al. Prevalence and clinical significance of respiratory viruses and bacteria detected in tuberculosis patients compared to household contact controls in Tanzania: A cohort study. Clin. Microbiol. Infect. 2018, 25, 107.e1–107.e7. [Google Scholar] [CrossRef]
- Razanajatovo, N.H.; Guillebaud, J.; Harimanana, A.; Rajatonirina, S.; Ratsima, E.H.; Andrianirina, Z.Z.; Rakotoariniaina, H.; Andriatahina, T.; Orelle, A.; Ratovoson, R.; et al. Epidemiology of severe acute respiratory infections from hospital-based surveillance in Madagascar, November 2010 to July 2013. PLoS ONE 2018, 13, e0205124. [Google Scholar] [CrossRef]
- Lekana-Douki, S.E.; Mouinga-Ondémé, A.; Nkoghe, D.; Drosten, C.; Drexler, J.F.; Kazanji, M.; Leroy, E.M. Early introduction and delayed dissemination of pandemic influenza, Gabon. Emerg. Infect. Dis. 2013, 19, 644–647. [Google Scholar] [CrossRef]
- Lekana-Douki, S.E.; Nkoghe, D.; Drosten, C.; Ngoungou, E.B.; Drexler, J.F.; Leroy, E.M. Viral etiology and seasonality of influenza-like illness in Gabon, March 2010 to June 2011. BMC Infect. Dis. 2014, 14, 373. [Google Scholar] [CrossRef]
- Breiman, R.F.; Cosmas, L.; Njenga, M.K.; Williamson, J.; Mott, J.A.; Katz, M.A.; Erdman, D.; Schneider, E.; Oberste, M.S.; Neatherlin, J.; et al. Severe acute respiratory infection in children in a densely populated urban slum in Kenya, 2007–2011. BMC Infect. Dis. 2015, 15, 95. [Google Scholar] [CrossRef]
- Bobossi Serengbe, G.; Gody, J.C.; Fioboy, R.; Nakoune, E. Étiologie virale des infections respiratoires aiguës de l’enfant à Bangui. Arch. Pediatr. 2015, 22, 324–325. [Google Scholar] [CrossRef]
- Kenmoe, S.; Tchendjou, P.; Vernet, M.A.; Moyo-Tetang, S.; Mossus, T.; Njankouo-Ripa, M.; Kenne, A.; Beng, V.P.; Vabret, A.; Njouom, R. Viral etiology of severe acute respiratory infections in hospitalized children in Cameroon, 2011–2013. Influenza Other Respir. Viruses 2016, 10, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Uzoamaka, M.; Ngozi, O.; Johnbull, O.S.; Martin, O. Bacterial etiology of lower respiratory tract infections and their antimicrobial susceptibility. Am. J. Med. Sci. 2017, 354, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Niang, M.N.; Diop, N.S.; Fall, A.; Kiori, D.E.; Sarr, F.D.; Sy, S.; Goudiaby, D.; Barry, M.A.; Fall, M.; Dia, N. Respiratory viruses in patients with influenza-like illness in Senegal: Focus on human respiratory adenoviruses. PLoS ONE 2017, 12, e0174287. [Google Scholar] [CrossRef] [PubMed]
- Famoroti, T.; Sibanda, W.; Ndung’u, T. Prevalence and seasonality of common viral respiratory pathogens, including Cytomegalovirus in children, between 0–5 years of age in KwaZulu-Natal, an HIV endemic province in South Africa. BMC Pediatr. 2018, 18, 240. [Google Scholar] [CrossRef]
- Kadjo, H.A.; Adjogoua, E.; Dia, N.; Adagba, M.; Abdoulaye, O.; Daniel, S.; Kouakou, B.; Ngolo, D.C.; Coulibaly, D.; Ndahwouh, T.N.; et al. Detection of non-influenza viruses in acute respiratory infections in children under five-year-old in Cote d’Ivoire (January–December 2013). Afr. J. Infect. Dis. 2018, 12, 78–88. [Google Scholar] [CrossRef]
- Sanou, A.M.; Wandaogo, S.C.M.; Poda, A.; Tamini, L.; Kyere, A.E.; Sagna, T.; Ouedraogo, M.S.; Pauly, M.; Hübschen, J.M.; Muller, C.P.; et al. Epidemiology and molecular characterization of influenza viruses in Burkina Faso, sub-Saharan Africa. Influenza Other Respir. Viruses 2018, 12, 490–496. [Google Scholar] [CrossRef]
- Obodai, E.; Odoom, J.K.; Adiku, T.; Goka, B.; Wolff, T.; Biere, B.; Schweiger, B.; Reiche, J. The significance of human respiratory syncytial virus (HRSV) in children from Ghana with acute lower respiratory tract infection: A molecular epidemiological analysis, 2006 and 2013–2014. PLoS ONE 2018, 13, e0203788. [Google Scholar] [CrossRef]
- Lekana-Douki, S.E.; Behillil, S.; Enouf, V.; Leroy, E.M.; Berthet, N. Detection of human bocavirus-1 in both nasal and stool specimens from children under 5 years old with influenza-like illnesses or diarrhea in Gabon. BMC Res. Notes 2018, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Kabego, L.; Balol’Ebwami, S.; Kasengi, J.B.; Miyanga, S.; Bahati, Y.L.; Kambale, R.; de Beer, C. Human respiratory syncytial virus: Proportion, viral co-infections and risk factors for lower respiratory tract infections in children under 5 years of age at a general hospital in the Democratic Republic of Congo. J. Med. Microbiol. 2018, 67, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Kenmoe, S.; Vernet, M.A.; Le Goff, J.; Penlap, V.B.; Vabret, A.; Njouom, R. Molecular characterization of human adenovirus associated with acute respiratory infections in Cameroon from 2011 to 2014. Virol. J. 2018, 15, 153. [Google Scholar] [CrossRef] [PubMed]
- Adema, I.W.; Kamau, E.; Nyiro, J.U.; Otieno, G.P.; Lewa, C.; Munywoki, P.K.; Nokes, D.J. Surveillance of respiratory viruses among children attending a primary school in rural coastal Kenya. Wellcome Open Res. 2020, 5, 63. [Google Scholar] [CrossRef]
- Buchwald, A.G.; Tamboura, B.; Tennant, S.M.; Haidara, F.C.; Coulibaly, F.; Doumbia, M.; Diallo, F.; Keita, A.M.; Sow, S.O.; Kotloff, K.L.; et al. Epidemiology, risk factors, and outcomes of respiratory syncytial virus infections in newborns in Bamako, Mali. Clin. Infect. Dis. 2020, 70, 59–66. [Google Scholar] [CrossRef]
- Obe, O.A.; Mutiu, B.W.; Amoo, A. Respiratory Syncytial Virus Infection among Children in Lagos, Nigeria. J. Clin. Immunol. Microbiol. 2021, 2, 1–11. [Google Scholar] [CrossRef]
- Kouakou, V.; Kadjo, H.; Oulo, N.A.; N’guessan, F.D.; N’Douba, A. Surveillance of Respiratory Syncytial Virus in Children Aged 0–5 years in Côte d’Ivoire. Am. J. BioScience 2021, 9, 185. [Google Scholar] [CrossRef]
- Kenmoe, S.; Sadeuh-Mba, S.A.; Vernet, M.A.; Beng, V.P.; Vabret, A.; Njouom, R. Molecular epidemiology of Enteroviruses and Rhinoviruses in patients with acute respiratory infections in Yaounde, Cameroon. Influenza Other Respir. Viruses 2021, 15, 641–650. [Google Scholar] [CrossRef]
- Ntagereka, P.B.; Basengere, R.A.; Baharanyi, T.C.; Kashosi, T.M.; Buhendwa, J.-P.C.; Bisimwa, P.B.; Kusinza, A.B.; Mugumaarhahama, Y.; Shukuru, D.W.; Patrick, S.B.; et al. Molecular evidence of coinfection with acute respiratory viruses and high proportion of SARS-CoV-2 among patients presenting flu-like illness in Bukavu city, Democratic Republic of Congo. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 1553266. [Google Scholar] [CrossRef]
- Kafintu-Kwashie, A.A.; Nii-Trebi, N.I.; Obodai, E.; Neizer, M.; Adiku, T.K.; Odoom, J.K. Molecular epidemiological surveillance of viral agents of acute lower respiratory tract infections in children in Accra, Ghana. BMC Pediatr. 2022, 22, 364. [Google Scholar] [CrossRef]
- Kolawole, O.; Oguntoye, M.; Dam, T.; Chunara, R. Etiology of respiratory tract infections in the community and clinic in Ilorin, Nigeria. BMC Res. Notes 2017, 10, 712. [Google Scholar] [CrossRef]
- Ukuli, Q.A.; Erima, B.; Mubiru, A.; Atim, G.; Tugume, T.; Kibuuka, H.; Mworozi, E.; Ducatez, M.F.; Wabwire-Mangen, F.; Byarugaba, D.K. Molecular characterisation of human adenoviruses associated with respiratory infections in Uganda. BMC Infect. Dis. 2023, 23, 435. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Njenga, M.K.; Bigogo, G.; Aura, B.; Aol, G.; Audi, A.; Jagero, G.; Muluare, P.O.; Gikunju, S.; Nderitu, L.; et al. Etiology and Incidence of viral and bacterial acute respiratory illness among older children and adults in rural western Kenya, 2007–2010. PLoS ONE 2012, 7, e43656. [Google Scholar] [CrossRef] [PubMed]
- Fokam, J.; Takou, D.; Nka, A.D.; Ka’e, A.C.; Yagai, B.; Chenwi, C.A.; Semengue, E.N.J.; Beloumou, G.A.; Ndjeyep, S.C.D.; Abba, A.; et al. Epidemiological, virological and clinical features of SARS-CoV-2 among individuals during the first wave in Cameroon: Baseline analysis for the EDCTP PERFECT-Study RIA2020EF-3000. J. Public Health Afr. 2022, 13, 2142. [Google Scholar] [CrossRef] [PubMed]
- Dorkenoo, A.M.; Gbeasor-Komlanvi, F.A.; Gbada, K.; Zida-Compaore, W.I.C.; Teou, D.; Konu, Y.R.; Lack, F.; Sadio, A.J.; Tchankoni, M.K.; Dagnra, A.C.; et al. Prevalence of malaria and COVID-19 in febrile patients in Lomé, Togo in 2020. Acta Parasitol. 2022, 67, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Alber, D.; Haidara, F.C.; Luoma, J.; Adubra, L.; Ashorn, P.; Ashorn, U.; Badji, H.; Cloutman-Green, E.; Diallo, F.; Ihamuotila, R.; et al. SARS-CoV-2 infection and antibody seroprevalence in routine surveillance patients, healthcare workers and general population in Kita region, Mali: An observational study 2020–2021. BMJ Open 2022, 12, e060367. [Google Scholar] [CrossRef]
- Khairy, A.; Elhussein, N.; Elbadri, O.; Mohamed, S.; Malik, E.M. Epidemiology of COVID-19 among Children and Adolescents in Sudan 2020–2021. Epidemiologia 2023, 4, 247–254. [Google Scholar] [CrossRef]
- Mulenga, L.B.; Hines, J.Z.; Fwoloshi, S.; Chirwa, L.; Siwingwa, M.; Yingst, S.; Wolkon, A.; Barradas, D.T.; Favaloro, J.; Zulu, J.E.; et al. Prevalence of SARS-CoV-2 in six districts in Zambia in July, 2020: A cross-sectional cluster sample survey. Lancet Glob. Health 2021, 9, e773–e781. [Google Scholar] [CrossRef]
- Wadilo, F.; Feleke, A.; Gebre, M.; Mihret, W.; Seyoum, T.; Melaku, K.; Howe, R.; Mulu, A.; Mihret, A. Viral etiologies of lower respiratory tract infections in children <5 years of age in Addis Ababa, Ethiopia: A prospective case–control study. Virol. J. 2023, 20, 163. [Google Scholar]
- Baillie, V.L.; Moore, D.P.; Mathunjwa, A.; Baggett, H.C.; Brooks, A.; Feikin, D.R.; Hammitt, L.L.; Howie, S.R.; Knoll, M.D.; Kotloff, K.L.; et al. Epidemiology of the rhinovirus (RV) in African and Southeast Asian children: A case-control pneumonia etiology study. Viruses 2021, 13, 1249. [Google Scholar] [CrossRef]
- Simusika, P.; Bateman, A.C.; Theo, A.; Kwenda, G.; Mfula, C.; Chentulo, E.; Monze, M. Identification of viral and bacterial pathogens from hospitalized children with severe acute respiratory illness in Lusaka, Zambia, 2011–2012: A cross-sectional study. BMC Infect. Dis. 2015, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Loevinsohn, G.; Hardick, J.; Sinywimaanzi, P.; Fenstermacher, K.Z.J.; Shaw-Saliba, K.; Monze, M.; Gaydos, C.A.; Rothman, R.E.; Pekosz, A.; Thuma, P.E.; et al. Respiratory pathogen diversity and co-infections in rural Zambia. Int. J. Infect. Dis. 2021, 102, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Yugbaré, S.O.O.; Ouédraogo, R.; Nenebi, A.; Traoré, B.; Congo, L.; Yonli, F.; Kima, D.; Bonané, P.; Yé, D.; Plantier, J.C.; et al. Infections à virus respiratoire syncytial (VRS) au CHU pédiatrique Charles de Gaulle de Ouagadougou, Burkina Faso. Bull. Soc. Pathol. Exot. 2016, 109, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kenmoe, S. Prévalence et Diversité Génétique des Virus Respiratoires au Cameroun. 2017. Available online: http://www.theses.fr/2017NORMC417/document (accessed on 9 September 2025).
- Sanou, A.M. Epidemiology and Molecular Characterization of Viruses and Bacteria Detected in Acute Respiratory Infections in Children under Five in Burkina Faso. Master’s Thesis, Université Nazi BONI, Bobo-Dioulasso, Burkina Faso, 2018. Available online: https://bibliovirtuelle.u-naziboni.bf/biblio/opac_css/docnume/UFR-SJPEG/IDR-2018-SAN-EPI.pdf (accessed on 13 December 2017).
- O’Callaghan-Gordo, C.; Bassat, Q.; Morais, L.; Díez-Padrisa, N.; Machevo, S.; Nhampossa, T.; Nhalungo, D.; Sanz, S.; Quintó, L.; Alonso, P.L.; et al. Etiology and epidemiology of viral pneumonia among hospitalized children in rural Mozambique: A malaria endemic area with high prevalence of human immunodeficiency virus. Pediatr. Infect. Dis. J. 2011, 30, 39–44. [Google Scholar] [CrossRef]
- Jones, A.H.; Ampofo, W.; Akuffo, R.; Doman, B.; Duplessis, C.; Amankwa, J.A.; Sarpong, C.; Sagoe, K.; Agbenohevi, P.; Puplampu, N.; et al. Sentinel surveillance for influenza among severe acute respiratory infection and acute febrile illness inpatients at three hospitals in Ghana. Influenza Other Respir. Viruses 2016, 10, 367–374. [Google Scholar] [CrossRef]
- Simeon, P.; Godman, B.; Kalemeera, F. Antibiotics’ susceptibility patterns of bacterial isolates causing lower respiratory tract infections in ICU patients at referral hospitals in Namibia. Hosp. Pract. 2021, 49, 356–363. [Google Scholar] [CrossRef]
- Nzoghe, A.M.; Padzys, G.S.; Siawaya, A.C.M.; Yattara, M.K.; Leboueny, M.; Houechenou, R.M.A.; Bongho, E.C.; Mba-Mezemze, C.; Ndjindji, O.M.; Biteghe-Bi-Essone, J.C.; et al. Dynamic and features of SARS-CoV-2 infection in Gabon. Sci. Rep. 2021, 11, 9672. [Google Scholar]
- Sebastião, C.S.; Parimbelli, P.; Mendes, M.; Sacomboio, E.; Morais, J.; de Vasconcelos, J.N.; Brito, M. Prevalence and Risk Factors of SARS-CoV-2 Infection among Parturients and Newborns from Luanda, Angola. Pathogens 2021, 10, 1494. [Google Scholar] [CrossRef]
- Anjorin, A.A.; Abioye, A.I.; Asowata, O.E.; Soipe, A.; Kazeem, M.I.; Adesanya, I.O.; Raji, M.A.; Adesanya, M.; Oke, F.A.; Lawal, F.J.; et al. Comorbidities and the COVID-19 pandemic dynamics in Africa. Trop. Med. Int. Health 2021, 26, 2–13. [Google Scholar] [CrossRef]
- Omran, A.; Awad, H.; Ibrahim, M.; El-Sharkawy, S.; Elfiky, S.; Rezk, A.R. Lung Ultrasound and Neutrophil Lymphocyte Ratio in Early Diagnosis and Differentiation between Viral and Bacterial Pneumonia in Young Children. Children 2022, 9, 1457. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci 2022, 23, 3636. [Google Scholar] [CrossRef]
- de Jager, C.P.; Wever, P.C.; Gemen, E.F.; Kusters, R.; van Gageldonk-Lafeber, A.B.; van der Poll, T.; Laheij, R.J. The Neutrophil-Lymphocyte Count Ratio in Patients with Community-Acquired Pneumonia. PLoS ONE 2012, 7, e46561. [Google Scholar] [CrossRef]
- Cataudella, E.; Giraffa, C.M.; Di Marca, S.; Pulvirenti, A.; Alaimo, S.; Pisano, M.; Terranova, V.; Corriere, T.; Ronsisvalle, M.L.; Di Quattro, R.; et al. Neutrophil-To-Lymphocyte Ratio: An Emerging Marker Predicting Prognosis in Elderly Adults with Community-Acquired Pneumonia. J. Am. Geriatr. Soc. 2017, 65, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Simões, E.A.; Rudan, I.; Gessner, B.D.; Azziz-Baumgartner, E.; Zhang, J.S.F.; Feikin, D.R.; Mackenzie, G.A.; Moiïsi, J.C.; Roca, A.; et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: A systematic analysis. Lancet 2013, 381, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Gul, A.; Javed, U.; Urooj, A.; Amin, S.; Fatima, Z. Etiology of acute viral respiratory infections common in Pakistan: A review. Rev. Med. Virol. 2019, 29, e2024. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory syncytial virus—A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, L.; Deng, X.; Liang, R.; Su, M.; He, C.; Hu, L.; Su, Y.; Ren, J.; Yu, F.; et al. Recent advances in the detection of respiratory virus infection in humans. J. Med. Virol. 2020, 92, 408–417. [Google Scholar] [CrossRef]
- Van der Zalm, M.M.; Uiterwaal, C.S.; Wilbrink, B.; Koopman, M.; Verheij, T.J.; van der Ent, C.K. The influence of neonatal lung function on rhinovirus-associated wheeze. Am. J. Respir. Crit. Care Med. 2011, 183, 262–267. [Google Scholar] [CrossRef]
- Zhu, X.; Ge, Y.; Wu, T.; Zhao, K.; Chen, Y.; Wu, B.; Zhu, F.; Zhu, B.; Cui, L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020, 285, 198005. [Google Scholar] [CrossRef]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Proportion and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Davis, B.; Rothrock, A.N.; Swetland, S.; Andris, H.; Davis, P.; Rothrock, S.G. Viral and atypical respiratory co-infections in COVID-19: A systematic review and meta-analysis. J. Am. Coll. Emerg. Physicians Open 2020, 1, 533–548. [Google Scholar] [CrossRef]
- World Health Organization (WHO). World Health Statistics. Available online: https://www.who.int/data/gho/publications/world-health-statistics (accessed on 3 October 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).