Pediatric Spinal Solitary Fibrous Tumor: A Systematic Review of a Rare Condition
Abstract
Highlights
- Pediatric SFTs are a rare tumor with only five cases reported in the literature, all of which show good functional outcomes and no recurrence at follow-up.
- Gross total resection remains of primary importance for the treatment of SFTs; adjuvant therapies (radio and/or chemotherapy) are lacking standardized pediatric protocols, with a predilection of radiotherapy if not contraindicated.
- Management of SFTs in the pediatric population must be tailored to each patient and surgery should be the first choice.
- The role of radiotherapy, chemotherapy and targeted agents in improving long-term disease control is still controversial.
Abstract
1. Introduction
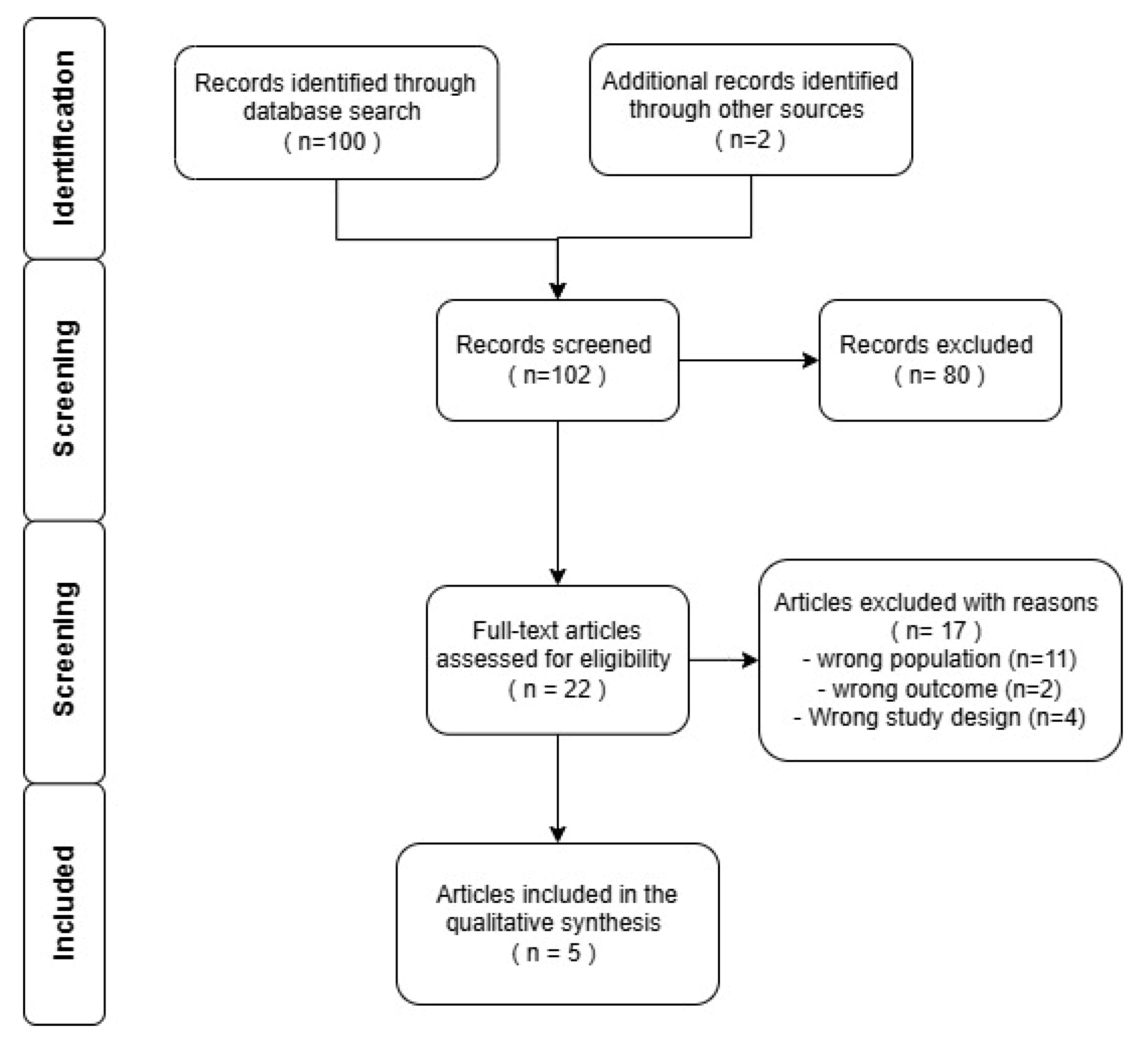
2. Materials and Methods
3. Results
4. Discussion
4.1. Radiological and Histological Characteristics
4.2. Molecular Characterization
4.3. Oncological Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klemperer, P.; Coleman, B.R. Primary neoplasms of the pleura. A report of five cases. Am. J. Ind. Med. 1992, 22, 4–31. [Google Scholar] [CrossRef] [PubMed]
- Kinslow, C.J.; Wang, T.J.C. Incidence of extrameningeal solitary fibrous tumors. Cancer 2020, 126, 4067. [Google Scholar] [CrossRef] [PubMed]
- Kinslow, C.J.; Bruce, S.S.; Rae, A.I.; Sheth, S.A.; McKhann, G.M.; Sisti, M.B.; Bruce, J.N.; Sonabend, A.M.; Wang, T.J.C. Solitary-fibrous tumor/hemangiopericytoma of the central nervous system: A population-based study. J. Neurooncol. 2018, 138, 173–182. [Google Scholar] [CrossRef]
- Ahmad, Z.; Tariq, M.U.; Din, N.U. Meningeal solitary fibrous tumor/hemangiopericytoma: Emphasizing on STAT 6 immunohistochemistry with a review of literature. Neurol. India 2018, 66, 1419–1426. [Google Scholar] [CrossRef]
- Haas, R.L.; Walraven, I.; Lecointe-Artzner, E.; van Houdt, W.J.; Strauss, D.; Schrage, Y.; Hayes, A.J.; Raut, C.P.; Fairweather, M.; Baldini, E.H.; et al. Extrameningeal solitary fibrous tumors-surgery alone or surgery plus perioperative radiotherapy: A retrospective study from the global solitary fibrous tumor initiative in collaboration with the Sarcoma Patients EuroNet. Cancer 2020, 126, 3002–3012. [Google Scholar] [CrossRef]
- Aridi, T.; Tawil, A.; Hashem, M.; Khoury, J.; Raad, R.A.; Youssef, P. Unique Presentation and Management Approach of Pleural Solitary Fibrous Tumor. Case Rep. Surg. 2019, 2019, 9706825. [Google Scholar] [CrossRef]
- Kim, J.M.; Choi, Y.-L.; Kim, Y.J.; Park, H.K. Comparison and evaluation of risk factors for meningeal, pleural, and extrapleural solitary fibrous tumors: A clinicopathological study of 92 cases confirmed by STAT6 immunohistochemical staining. Pathol. Res. Pr. 2017, 213, 619–625. [Google Scholar] [CrossRef]
- Harrison-Phipps, K.M.; Nichols, F.C.; Schleck, C.D.; Deschamps, C.; Cassivi, S.D.; Schipper, P.H.; Allen, M.S.; Wigle, D.A.; Pairolero, P.C. Solitary fibrous tumors of the pleura: Results of surgical treatment and long-term prognosis. J. Thorac. Cardiovasc. Surg. 2009, 138, 19–25. [Google Scholar] [CrossRef]
- Tasdemir, A.; Soyuer, I.; Yurci, A.; Karahanli, I.; Akyildiz, H. A huge solitary fibrous tumor localized in the pancreas: A young women. J. Pancreas 2012, 13, 304–307. [Google Scholar]
- Spasevska, L.; Janevska, V.; Janevski, V.; Noveska, B.; Zhivadinovik, J. Solitary Fibrous Tumor of the Pancreas: A Case Report and Review of the Literature. Prilozi 2016, 37, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, A.; Varaldo, M.; Ferrarazzo, C.; Tunesi, G.; Mencoboni, M. Solitary fibrous tumor. Rare Tumors 2010, 2, e64. [Google Scholar] [CrossRef] [PubMed]
- Demicco, E.G.; Park, M.S.; Araujo, D.M.; Fox, P.S.; Bassett, R.L.; Pollock, R.E.; Lazar, A.J.; Wang, W.L. Solitary fibrous tumor: A clinicopathological study of 110 cases and proposed risk assessment model. Mod. Pathol. 2012, 25, 1298–1306. [Google Scholar] [CrossRef]
- Cox, D.P.; Daniels, T.; Jordan, R.C.K. Solitary fibrous tumor of the head and neck. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 79–84. [Google Scholar] [CrossRef]
- Chung, H.R.; Tam, K.; Han, A.Y.; Obeidin, F.; Nakasaki, M.; Chhetri, D.K.; St John, M.A.; Kita, A.E. Solitary Fibrous Tumors of the Head and Neck: A Single-Institution Study of 52 Patients. OTO Open 2022, 6, 2473974X221098709. [Google Scholar] [CrossRef]
- Dorfman, D.M.; To, K.; Dickersin, G.R.; Rosenberg, A.E.; Pilch, B.Z. Solitary fibrous tumor of the orbit. Am. J. Surg. Pathol. 1994, 18, 281–287. [Google Scholar] [CrossRef]
- Künzel, J.; Hainz, M.; Ziebart, T.; Pitz, S.; Ihler, F.; Strieth, S.; Matthias, C. Head. and neck solitary fibrous tumors: A rare and challenging entity. Eur. Arch. Otorhinolaryngol. 2016, 273, 1589–1598. [Google Scholar] [CrossRef]
- White, G.Z.; Cox, E.L.; Schwartz, E.J.; Korkigian, S.A. Rare Solitary Fibrous Tumor in the Pediatric Neck: A Case Report and Review of the Literature. Cureus 2017, 9, e1140. [Google Scholar] [CrossRef]
- Haas, R.L.; Walraven, I.; Lecointe-Artzner, E.; van Houdt, W.J.; Scholten, A.N.; Strauss, D.; Schrage, Y.; Hayes, A.J.; Raut, C.P.; Fairweather, M.; et al. Management of meningeal solitary fibrous tumors/hemangiopericytoma; surgery alone or surgery plus postoperative radiotherapy? Acta Oncol. 2021, 60, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S.S.; Scheithauer, B.W.; Nascimento, A.G.; Hirose, T.; Davis, D.H. Solitary fibrous tumor of the meninges: A lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am. J. Clin. Pathol. 1996, 106, 217–224. [Google Scholar] [CrossRef]
- Ge, H.J.; Yao, J.J.; Li, L.; Li, B.W.; Ge, C.; Liu, H.; Li, Y.; Yin, H.F. Clinicopathological features of spinal solitary fibrous tumor. Zhonghua Bing Li Xue Za Zhi 2022, 51, 875–880. [Google Scholar] [PubMed]
- Yao, Z.G.; Wu, H.B.; Hao, Y.H.; Wang, X.F.; Ma, G.Z.; Li, J.; Li, J.F.; Lin, C.H.; Zhong, X.M.; Wang, Z.; et al. Papillary Solitary Fibrous Tumor/Hemangiopericytoma: An Uncommon Morphological Form With NAB2-STAT6 Gene Fusion. J. Neuropathol. Exp. Neurol. 2019, 78, 685–693. [Google Scholar] [CrossRef]
- O’Neill, A.C.; Tirumani, S.H.; Do, W.S.; Keraliya, A.R.; Hornick, J.L.; Shinagare, A.B.; Ramaiya, N.H. Metastatic Patterns of Solitary Fibrous Tumors: A Single-Institution Experience. Am. J. Roentgenol. 2017, 208, 2–9. [Google Scholar] [CrossRef]
- Baldi, G.G.; Stacchiotti, S.; Mauro, V.; Dei Tos, A.P.; Gronchi, A.; Pastorino, U.; Duranti, L.; Provenzano, S.; Marrari, A.; Libertini, M.; et al. Solitary fibrous tumor of all sites: Outcome of late recurrences in 14 patients. Clin. Sarcoma Res. 2013, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Xie, Y.Y.; Chen, W.; Peng, Z.F.; Yuan, X.R.; Li, X.J.; Feng, C.Y.; Wang-Gou, S.Y. Solitary Fibrous Tumor of Central Nervous System: Clinical and Prognostic Study of 24 Cases. World Neurosurg. 2017, 99, 584–592. [Google Scholar] [CrossRef]
- Khalifa, J.; Ouali, M.; Chaltiel, L.; Le Guellec, S.; Le Cesne, A.; Blay, J.Y.; Cousin, P.; Chaigneau, L.; Bompas, E.; Piperno-Neumann, S.; et al. Efficacy of trabectedin in malignant solitary fibrous tumors: A retrospective analysis from the French Sarcoma Group. BMC Cancer 2015, 15, 700. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Libertini, M.; Negri, T.; Palassini, E.; Gronchi, A.; Fatigoni, S.; Poletti, P.; Vincenzi, B.; Dei Tos, A.P.; Mariani, L.; et al. Response to chemotherapy of solitary fibrous tumour: A retrospective study. Eur. J. Cancer 2013, 49, 2376–2383. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Negri, T.; Libertini, M.; Palassini, E.; Marrari, A.; De Troia, B.; Gronchi, A.; Dei Tos, A.P.; Morosi, C.; Messina, A.; et al. Sunitinib malate in solitary fibrous tumor (SFT). Ann. Oncol. 2012, 23, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Patel, S.R.; Ludwig, J.A.; Trent, J.C.; Conrad, C.A.; Lazar, A.J.; Wang, W.L.; Boonsirikamchai, P.; Choi, H.; Wang, X.; et al. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumor. Cancer 2011, 117, 4939–4947. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Gokgoz, N.; Samman, B.; Andrulis, I.L.; Wunder, J.S.; Demicco, E.G. RNA expression profiling reveals PRAME, a potential immunotherapy target, is frequently expressed in solitary fibrous tumors. Mod. Pathol. 2021, 34, 951–960. [Google Scholar] [CrossRef]
- Brunori, A.; Cerasoli, S.; Donati, R.; Giangaspero, F.; Chiappetta, F. Solitary fibrous tumor of the meninges: Two new cases and review of the literature. Surg. Neurol. 1999, 51, 636–640. [Google Scholar] [CrossRef]
- Singla, R.; Singh, P.K.; Khanna, G.; Suri, V.; Agarwal, D.; Chandra, P.S.; Kale, S.S.; Mahapatra, A.K. An institutional review of 10 cases of spinal hemangiopericytoma/solitary fibrous tumor. Neurol. India 2020, 68, 448–453. [Google Scholar] [CrossRef]
- Ferrari, A.; Brennan, B.; Casanova, M.; Corradini, N.; Berlanga, P.; Schoot, R.A.; Ramirez-Villar, G.L.; Safwat, A.; Guillen Burrieza, G.; Dall’Igna, P.; et al. Pediatric Non-Rhabdomyosarcoma Soft Tissue Sarcomas: Standard of Care and Treatment Recommendations from the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG). Cancer Manag. Res. 2022, 14, 2885–2902. [Google Scholar] [CrossRef] [PubMed]
- Albert, G.W.; Gokden, M. Solitary fibrous tumors of the spine: A pediatric case report with a comprehensive review of the literature. J. Neurosurg. Pediatr. PED 2017, 19, 339–348. [Google Scholar] [CrossRef]
- Tamburrini, G.; Gessi, M.; Colosimo, C., Jr.; Lauriola, L.; Giangaspero, F.; Di Rocco, C. Infantile myofibromatosis of the central nervous system. Childs Nerv. Syst. 2003, 19, 650–654. [Google Scholar] [CrossRef]
- Jallo, G.I.; Roonprapunt, C.; Kothbauer, K.; Freed, D.; Allen, J.; Epstein, F. Spinal solitary fibrous tumors: A series of four patients: Case report. Neurosurgery 2005, 57, E195. [Google Scholar] [CrossRef]
- Lee, J.-C.; Fletcher, C.D.M. Malignant fat-forming solitary fibrous tumor (so-called ‘lipomatous hemangiopericytoma’): Clinicopathologic analysis of 14 cases. Am. J. Surg. Pathol. 2011, 35, 1177–1185. [Google Scholar] [CrossRef]
- Robinson, D.R.; Wu, Y.M.; Kalyana-Sundaram, S.; Cao, X.; Lonigro, R.J.; Sung, Y.S.; Chen, C.L.; Zhang, L.; Wang, R.; Su, F.; et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat. Genet. 2013, 45, 180–185. [Google Scholar] [CrossRef]
- Dauleac, C.; Vasiljevic, A.; Berhouma, M. How to differentiate spinal cord hemangiopericytoma from common spinal cord tumor? Neurochirurgie 2020, 66, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.U.; Din, N.U.; Abdul-Ghafar, J.; Park, Y.-K. The many faces of solitary fibrous tumor; diversity of histological features, differential diagnosis and role of molecular studies and surrogate markers in avoiding misdiagnosis and predicting the behavior. Diagn. Pathol. 2021, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhou, Z.; Zhang, D.; Yang, J.; Liu, C.; Wang, T.; Wu, Z.; Yang, C.; Wei, H.; Zhao, J.; et al. Surgical management of spinal solitary fibrous tumor/hemangiopericytoma: A case series of 20 patients. Eur. Spine J. 2018, 27, 891–901. [Google Scholar] [CrossRef]
- Martin-Broto, J.; Mondaza-Hernandez, J.L.; Moura, D.S.; Hindi, N. A Comprehensive Review on Solitary Fibrous Tumor: New Insights for New Horizons. Cancers 2021, 13, 2913. [Google Scholar] [CrossRef]
- Thway, K.; Hayes, A.; Ieremia, E.; Fisher, C. Heterologous osteosarcomatous and rhabdomyosarcomatous elements in dedifferentiated solitary fibrous tumor: Further support for the concept of dedifferentiation in solitary fibrous tumor. Ann. Diagn. Pathol. 2013, 17, 457–463. [Google Scholar] [CrossRef]
- Chmielecki, J.; Crago, A.M.; Rosenberg, M.; O’Connor, R.; Walker, S.R.; Ambrogio, L.; Auclair, D.; McKenna, A.; Heinrich, M.C.; Frank, D.A.; et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat. Genet. 2013, 45, 131–132. [Google Scholar] [CrossRef]
- Mohajeri, A.; Tayebwa, J.; Collin, A.; Nilsson, J.; Magnusson, L.; von Steyern, F.V.; Brosjö, O.; Domanski, H.A.; Larsson, O.; Sciot, R.; et al. Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosomes Cancer 2013, 52, 873–886. [Google Scholar] [CrossRef]
- Mirchia, K.; Choudhury, A.; Joseph, T.; Birrueta, J.O.; Phillips, J.J.; Bhaduri, A.; Crouch, E.E.; Perry, A.; Raleigh, D.R. Meningeal solitary fibrous tumor cell states phenocopy cerebral vascular development and homeostasis. Neuro Oncol. 2024, 27, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Koelsche, C.; Schweizer, L.; Renner, M.; Warth, A.; Jones, D.T.; Sahm, F.; Reuss, D.E.; Capper, D.; Knösel, T.; Schulz, B.; et al. Nuclear relocation of STAT6 reliably predicts NAB2-STAT6 fusion for the diagnosis of solitary fibrous tumour. Histopathology 2014, 65, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Vivero, M.; Fletcher, C.D.; Mertens, F.; Hornick, J.L. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod. Pathol. 2014, 27, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Dagrada, G.P.; Spagnuolo, R.D.; Mauro, V.; Tamborini, E.; Cesana, L.; Gronchi, A.; Stacchiotti, S.; Pierotti, M.A.; Negri, T.; Pilotti, S. Solitary fibrous tumors: Loss of chimeric protein expression and genomic instability mark dedifferentiation. Mod. Pathol. 2015, 28, 1074–1083. [Google Scholar] [CrossRef]
- Ingram, J.L.; Antao-Menezes, A.; Mangum, J.B.; Lyght, O.; Lee, P.J.; Elias, J.A.; Bonner, J.C. Opposing actions of Stat1 and Stat6 on IL-13-induced up-regulation of early growth response-1 and platelet-derived growth factor ligands in pulmonary fibroblasts. J. Immunol. 2006, 177, 4141–4148. [Google Scholar] [CrossRef]
- Barron, L.; Wynn, T.A. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G723–G728. [Google Scholar] [CrossRef]
- Ouladan, S.; Trautmann, M.; Orouji, E.; Hartmann, W.; Huss, S.; Büttner, R.; Wardelmann, E. Differential diagnosis of solitary fibrous tumors: A study of 454 soft tissue tumors indicating the diagnostic value of nuclear STAT6 relocation and ALDH1 expression combined with in situ proximity ligation assay. Int. J. Oncol. 2015, 46, 2595–2605. [Google Scholar] [CrossRef]
- Yokoi, T.; Tsuzuki, T.; Yatabe, Y.; Suzuki, M.; Kurumaya, H.; Koshikawa, T.; Kuhara, H.; Kuroda, M.; Nakamura, N.; Nakatani, Y.; et al. Solitary fibrous tumour: Significance of p53 and CD34 immunoreactivity in its malignant transformation. Histopathology 1998, 32, 423–432. [Google Scholar] [CrossRef]
- Manara, M.C.; Pasello, M.; Scotlandi, K. CD99: A Cell Surface Protein with an Oncojanus Role in Tumors. Genes 2018, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Maitra, A.; Hansel, D.E.; Argani, P.; Ashfaq, R.; Rahman, A.; Naji, A.; Deng, S.; Geradts, J.; Hawthorne, L.; House, M.G.; et al. Global expression analysis of well-differentiated pancreatic endocrine neoplasms using oligonucleotide microarrays. Clin. Cancer Res. 2003, 9, 5988–5995. [Google Scholar]
- Manara, M.C.; Bernard, G.; Lollini, P.L.; Nanni, P.; Zuntini, M.; Landuzzi, L.; Benini, S.; Lattanzi, G.; Sciandra, M.; Serra, M.; et al. CD99 acts as an oncosuppressor in osteosarcoma. Mol. Biol. Cell 2006, 17, 1910–1921. [Google Scholar] [CrossRef]
- Hasegawa, T.; Matsuno, Y.; Shimoda, T.; Hirohashi, S.; Hirose, T.; Sano, T. Frequent expression of bcl-2 protein in solitary fibrous tumors. Jpn. J. Clin. Oncol. 1998, 28, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, I.; Saito, T.; Kitamura, Y.; Arai, K.; Kawaguchi, M.; Takahashi, K.; Hara, N. Primary solitary fibrous tumor (SFT) in the retroperitoneum. Urol. Oncol. 2008, 26, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Baer, R. Bcl-2 breathes life into embryogenesis. Am. J. Pathol. 1994, 145, 7–10. [Google Scholar]
- Fargen, K.M.; Opalach, K.J.; Wakefield, D.; Jacob, R.P.; Yachnis, A.T.; Lister, J.R. The central nervous system solitary fibrous tumor: A review of clinical, imaging and pathologic findings among all reported cases from 1996 to 2010. Clin. Neurol. Neurosurg. 2011, 113, 703–710. [Google Scholar] [CrossRef]
- Bisceglia, M.; Dimitri, L.; Giannatempo, G.; Carotenuto, V.; Bianco, M.; Monte, V.; D’Angelo, V.; Magro, G. Solitary fibrous tumor of the central nervous system: Report of an additional 5 cases with comprehensive literature review. Int. J. Surg. Pathol. 2011, 19, 476–486. [Google Scholar] [CrossRef]
- Fritchie, K.; Jensch, K.; Moskalev, E.A.; Caron, A.; Jenkins, S.; Link, M.; Brown, P.D.; Rodriguez, F.J.; Guajardo, A.; Brat, D.; et al. The impact of histopathology and NAB2-STAT6 fusion subtype in classification and grading of meningeal solitary fibrous tumor/hemangiopericytoma. Acta Neuropathol. 2019, 137, 307–319. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Saponara, M.; Frapolli, R.; Tortoreto, M.; Cominetti, D.; Provenzano, S.; Negri, T.; Dagrada, G.P.; Gronchi, A.; Colombo, C.; et al. Patient-derived solitary fibrous tumour xenografts predict high sensitivity to doxorubicin/dacarbazine combination confirmed in the clinic and highlight the potential effectiveness of trabectedin or eribulin against this tumour. Eur. J. Cancer 2017, 76, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kawai, A.; Araki, N.; Naito, Y.; Ozaki, T.; Sugiura, H.; Yazawa, Y.; Morioka, H.; Matsumine, A.; Saito, K.; Asami, S.; et al. Phase 2 study of eribulin in patients with previously treated advanced or metastatic soft tissue sarcoma. Jpn. J. Clin. Oncol. 2017, 47, 137–144. [Google Scholar] [CrossRef]
- Schöffski, P.; Ray-Coquard, I.L.; Cioffi, A.; Bui, N.B.; Bauer, S.; Hartmann, J.T.; Krarup-Hansen, A.; Grünwald, V.; Sciot, R.; Dumez, H.; et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: A phase 2 study in four independent histological subtypes. Lancet Oncol. 2011, 12, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Bieg, M.; Moskalev, E.A.; Will, R.; Hebele, S.; Schwarzbach, M.; Schmeck, S.; Hohenberger, P.; Jakob, J.; Kasper, B.; Gaiser, T.; et al. Gene Expression in Solitary Fibrous Tumors (SFTs) Correlates with Anatomic Localization and NAB2-STAT6 Gene Fusion Variants. Am. J. Pathol. 2021, 191, 602–617. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Eckhardt, S.G. Sunitinib: From rational design to clinical efficacy. J. Clin. Oncol. 2007, 25, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Martin-Broto, J.; Hindi, N.; Grignani, G.; Martinez-Trufero, J.; Redondo, A.; Valverde, C.; Stacchiotti, S.; Lopez-Pousa, A.; D’Ambrosio, L.; Gutierrez, A.; et al. Nivolumab and sunitinib combination in advanced soft tissue sarcomas: A multicenter, single-arm, phase Ib/II trial. J. Immunother. Cancer 2020, 8, e001561. [Google Scholar] [CrossRef]
- Domont, J.; Massard, C.; Lassau, N.; Armand, J.P.; Le Cesne, A.; Soria, J.C. Hemangiopericytoma and antiangiogenic therapy: Clinical benefit of antiangiogenic therapy (sorafenib and sunitinib) in relapsed malignant haemangioperyctoma/solitary fibrous tumour. Investig. New Drugs 2010, 28, 199–202. [Google Scholar] [CrossRef]
- Xie, C.; Wan, X.; Quan, H.; Zheng, M.; Fu, L.; Li, Y.; Lou, L. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018, 109, 1207–1219. [Google Scholar] [CrossRef]
- Hu-Lowe, D.D.; Zou, H.Y.; Grazzini, M.L.; Hallin, M.E.; Wickman, G.R.; Amundson, K.; Chen, J.H.; Rewolinski, D.A.; Yamazaki, S.; Wu, E.Y.; et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin. Cancer Res. 2008, 14, 7272–7283. [Google Scholar] [CrossRef]
- Valentin, T.; Fournier, C.; Penel, N.; Bompas, E.; Chaigneau, L.; Isambert, N.; Chevreau, C. Sorafenib in patients with progressive malignant solitary fibrous tumors: A subgroup analysis from a phase II study of the French Sarcoma Group. (GSF/GETO). Investig. New Drugs 2013, 31, 1626–1627. [Google Scholar] [CrossRef]
- Maruzzo, M.; Martin-Liberal, J.; Messiou, C.; Miah, A.; Thway, K.; Alvarado, R.; Judson, I.; Benson, C. Pazopanib as first line treatment for solitary fibrous tumours: The Royal Marsden Hospital experience. Clin. Sarcoma Res. 2015, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Grohar, P.J.; Segars, L.E.; Yeung, C.; Pommier, Y.; D’Incalci, M.; Mendoza, A.; Helman, L.J. Dual targeting of EWS-FLI1 activity and the associated DNA damage response with trabectedin and SN38 synergistically inhibits Ewing sarcoma cell growth. Clin. Cancer Res. 2014, 20, 1190–1203. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Tortoreto, M.; Bozzi, F.; Tamborini, E.; Morosi, C.; Messina, A.; Libertini, M.; Palassini, E.; Cominetti, D.; Negri, T.; et al. Dacarbazine in solitary fibrous tumor: A case series analysis and preclinical evidence vis-a-vis temozolomide and antiangiogenics. Clin. Cancer Res. 2013, 19, 5192–5201. [Google Scholar] [CrossRef] [PubMed]
- Chaigneau, L.; Kalbacher, E.; Thiery-Vuillemin, A.; Fagnoni-Legat, C.; Isambert, N.; Aherfi, L.; Pauchot, J.; Delroeux, D.; Servagi-Vernat, S.; Mansi, L.; et al. Efficacy of trabectedin in metastatic solitary fibrous tumor. Rare Tumors 2011, 3, e92. [Google Scholar] [CrossRef]
- Haas, R.L.; Walraven, I.; Lecointe-Artzner, E.; Scholten, A.N.; van Houdt, W.J.; Griffin, A.M.; Ferguson, P.C.; Miah, A.B.; Zaidi, S.; DeLaney, T.F.; et al. Radiation Therapy as Sole Management for Solitary Fibrous Tumors (SFT): A Retrospective Study From the Global SFT Initiative in Collaboration With the Sarcoma Patients EuroNet. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1226–1233. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeon, S.H.; Park, C.K.; Park, S.H.; Yoon, H.I.; Chang, J.H.; Suh, C.O.; Kang, S.J.; Lim, D.H.; Kim, I.A.; et al. The Role of Postoperative Radiotherapy in Intracranial Solitary Fibrous Tumor/Hemangiopericytoma: A Multi-institutional Retrospective Study (KROG 18-11). Cancer Res. Treat. 2022, 54, 65–74. [Google Scholar] [CrossRef]
- Krengli, M.; Cena, T.; Zilli, T.; Jereczek-Fossa, B.A.; De Bari, B.; Villa Freixa, S.; Kaanders, J.H.A.M.; Torrente, S.; Pasquier, D.; Sole, C.V.; et al. Radiotherapy in the treatment of extracranial hemangiopericytoma/solitary fibrous tumor: Study from the Rare Cancer Network. Radiother. Oncol. 2020, 144, 114–120. [Google Scholar] [CrossRef] [PubMed]
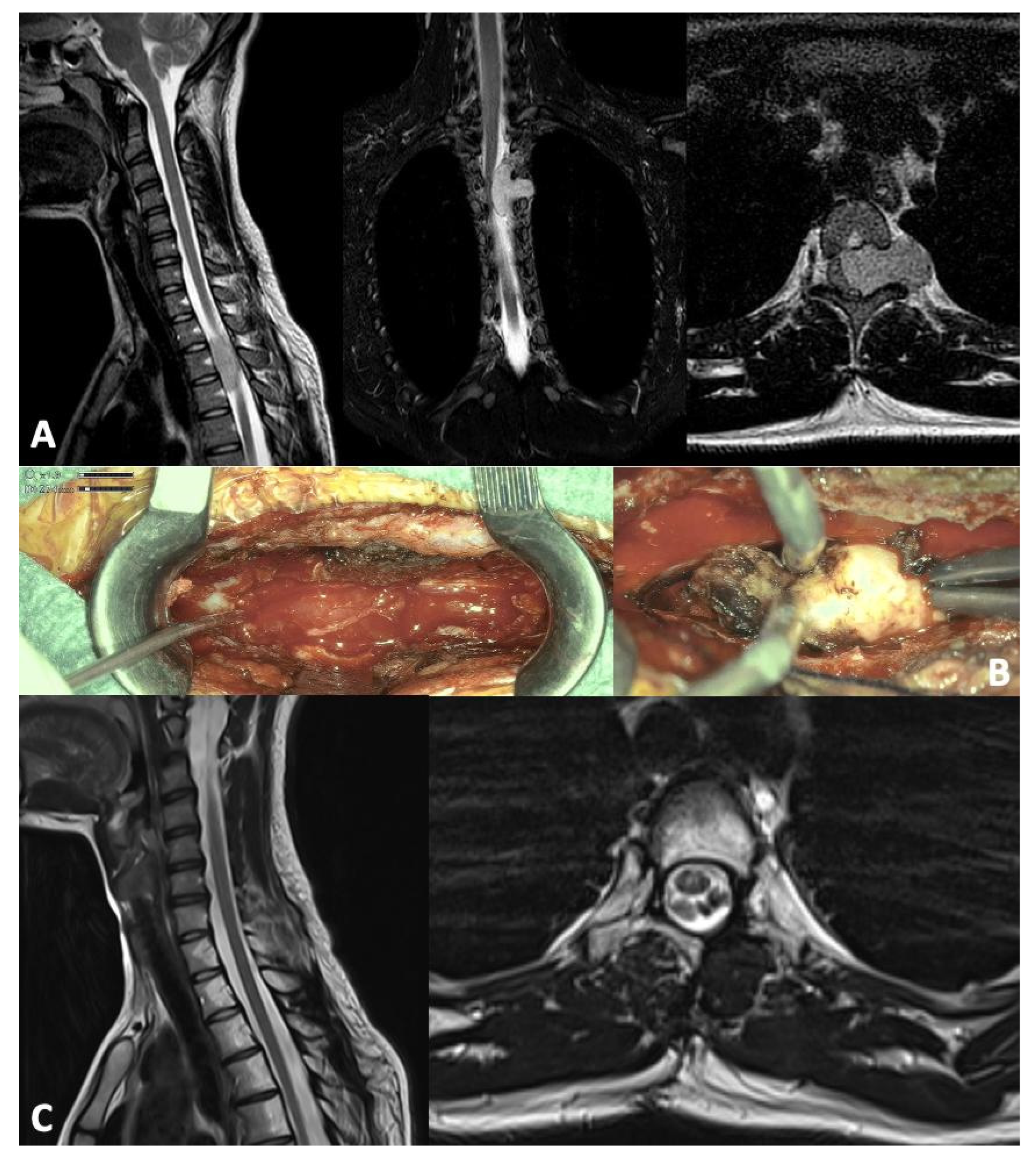

| Authors | Type of Study | Patients | Symptoms | Tumor | Treatment | Outcome | Limitations |
|---|---|---|---|---|---|---|---|
| Albert et al., 2017 [33] | Case report | 1 (10 y.o./M) | Right upper extremity weakness | Intradural extramedullary C1–C3 | GTR | Disease free | Only one pediatric patient |
| Singla et al. 2020 [31] | Case series | 10 (1; 12 y.o./F) | Acute conus cauda syndrome for 3 days | Intradural extramedullary D11–L1 | GTR + RT+ CT (epirubicin, ifosfamide) | Disease free | Heterogeneous population (9 adults, 1 child) |
| Tamburrini et al. 2003 [34] | Case report | 1 (10 months old/M) | Fasciculation in right leg, styosis, hypertonia in legs | Intramedullary from C7 to the conus | Partial resection | Spontaneous regression of residual. Mild paraparesis | Early study, case report |
| Jallo et al. 2005 [35] | Case series | 4 (1; 17 y.o./M) | Scoliosis and spastic paraparesis | Intramedullary D5–D6 (two lesions) | GTR | Disease free, mild scoliosis | Case report, limited FU (1.6 years) |
| Brunori et al. 1999 [30] | Case report | 2 (1; 18 y.o./M) | Paresthesias, limb weakness, urinary retention | Intradural extramedullary from clivus to C3 | GTR | Disease free at 12 months | Early study, limited FU |
| Trezza et al. 2025 | Case report | 1 (17 y.o./F) | Left interscapular unresponsive pain | Extramedullary intradural lesion D3–D4; mild myelopathy and cord compression | GTR + RT (protons; 50.4 Gy) | Disease free at 24 months | Case report |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trezza, A.; Rui, C.B.; Chiaravalli, S.; Biassoni, V.; Schiavello, E.; Vennarini, S.; Orlandi, E.; Carrabba, G.G.; Massimino, M.; Giussani, C.G. Pediatric Spinal Solitary Fibrous Tumor: A Systematic Review of a Rare Condition. Children 2025, 12, 1214. https://doi.org/10.3390/children12091214
Trezza A, Rui CB, Chiaravalli S, Biassoni V, Schiavello E, Vennarini S, Orlandi E, Carrabba GG, Massimino M, Giussani CG. Pediatric Spinal Solitary Fibrous Tumor: A Systematic Review of a Rare Condition. Children. 2025; 12(9):1214. https://doi.org/10.3390/children12091214
Chicago/Turabian StyleTrezza, Andrea, Chiara B. Rui, Stefano Chiaravalli, Veronica Biassoni, Elisabetta Schiavello, Sabina Vennarini, Ester Orlandi, Giorgio G. Carrabba, Maura Massimino, and Carlo G. Giussani. 2025. "Pediatric Spinal Solitary Fibrous Tumor: A Systematic Review of a Rare Condition" Children 12, no. 9: 1214. https://doi.org/10.3390/children12091214
APA StyleTrezza, A., Rui, C. B., Chiaravalli, S., Biassoni, V., Schiavello, E., Vennarini, S., Orlandi, E., Carrabba, G. G., Massimino, M., & Giussani, C. G. (2025). Pediatric Spinal Solitary Fibrous Tumor: A Systematic Review of a Rare Condition. Children, 12(9), 1214. https://doi.org/10.3390/children12091214








