Simple Summary
To date, no specific analyses focusing on penile-sparing surgery for local tumour recurrence after previous glansectomy or partial penectomy have been reported. We addressed this void and we considered a retrospective series of consecutive patients treated at a single institution. We focused on: (1) treatment feasibility, (2) complications, and (3) oncological outcomes.
Abstract
We tested the feasibility and oncological outcomes after penile-sparing surgery (PSS) for local recurrent penile cancer after a previous glansectomy/partial penectomy. We retrospectively analysed 13 patients (1997–2022) with local recurrence of penile cancer after a previous glansectomy or partial penectomy. All patients underwent PSS: circumcision, excision, or laser ablation. First, technical feasibility, treatment setting, and complications (Clavien–Dindo) were recorded. Second, Kaplan–Meier plots depicted overall and local recurrences over time. Overall, 11 (84.5%) vs. 2 (15.5%) patients were previously treated with glansectomy vs. partial penectomy. The median (IQR) time to disease recurrence was 56 (13–88) months. Six (46%) vs. two (15.5%) vs. five (38.5%) patients were treated with, respectively, local excision vs. local excision + circumcision vs. laser ablation. All procedures, except one, were performed in an outpatient setting. Only one Clavien–Dindo 2 complication was recorded. The median follow-up time was 41 months. Overall, three (23%) vs. four (30.5%) patients experienced local vs. overall recurrence, respectively. All local recurrences were safely treated with salvage surgery. In conclusion, we reported the results of a preliminary analysis testing safety, feasibility, and early oncological outcomes of PSS procedures for patients with local recurrence after previous glansectomy or partial penectomy. Stronger oncological outcomes should be tested in other series to optimise patient selection.
1. Introduction
Penile-sparing surgery (PSS) is the recommended strategy for patients with localized penile cancer, whenever feasible, due to its efficacy to remove the entire tumour while preserving as much of the penis as possible [1]. PSS is associated with higher rates of local recurrence (10–55%), but similar overall survival, compared with partial or radical penectomy [2,3,4,5]. Several PSS procedures have been recently developed for penile cancer patients, varying from less invasive techniques, such as topical chemotherapy or laser ablation, to more aggressive treatments like glansectomy or partial penectomy [6]. However, despite the rates of local recurrence varying according to the PSS technique used [5,7], even with glansectomy approximately 4–12.8% of patients experience local recurrence during follow-up [8,9]. Historically, total amputation has been offered to those patients who exhibit local recurrence after previous glansectomy/partial penectomy, compromising the functional results of previous PSS [10]. However, some of those patients with localised recurrence could be amenable to repeat PSS procedures, without compromising oncological control of the disease. This said, to the best of our knowledge, no specific analyses focusing on this management strategy exist to date and only sporadic cases have been reported by previous authors [11,12].
We hypothesised that a group of selected patients with disease recurrence after previous glansectomy/partial penectomy could be safely treated with a new PSS procedure.
To address this void, we focused on a consecutive series (1997–2022) of patients with penile cancer recurrence after glansectomy or partial penectomy and we tested the surgical feasibility of another PSS and subsequent recurrence rates over time.
2. Materials and Methods
2.1. Patients
This study respected the ethical guidelines of the Declaration of Helsinki. A retrospective analysis of all penile cancer patients treated at our centre between 1997 and 2022 (n = 263) was performed and we selected men submitted to glansectomy or partial penectomy (n = 174; 66%). Of those, we focused on patients who exhibited local recurrence during follow-up (n = 35; 20.1%) and who were treated with total penectomy (n = 22; 62.9%; Supplementary Materials, Table S1) or PSS (n = 13; 37.1%; Table 1). The latter were included in the final analyses.

Table 1.
Clinical characteristics of 13 penile cancer patients, previously treated with glansectomy or partial penectomy, and subsequently treated with penile-sparing surgery for penile cancer recurrence between 2001 and 2022. Data are shown as medians for continuous variables or as counts and percentages (%) for categorical variables. IQR: interquartile range; CCI: Charlson Comorbidity Index; BMI: Body Mass Index; HIV: human immunodeficiency virus; HPV: human papillomavirus.
2.2. Penile-Sparing Surgery
During the study period, several PSS techniques were used, based on the location and the dimension of the lesion, the preference of the surgeons, and the availability of the technologies. PSS consisted of any of the following: circumcision, local excision [12], or laser ablation (either CO2 [13] or thulium–yttrium–aluminium–garnet (Tm:YAG) lasers [14]).
Follow-up after PSS respected the European Association of Urology (EAU) guidelines [1]. Physical examination was performed every 3 months in the first 2 years and every 6 months in the following 3 years. Patients were also advised to perform regular self-examination. Follow-up imaging scans also respected the EAU guidelines [1].
2.3. Variable Definitions and Statistical Analyses
Variables recorded included: age at surgery, year of diagnosis, Charlson Comorbidity Index (CCI), Body Mass Index (BMI), smoking status, HIV and HPV infections, type of previous surgery, tumour size (mm), lesion site, type of surgery, margin status, TNM stage, and tumour grade. Surgical complications were graded according to the Clavien–Dindo classification [15]. Descriptive statistics relied on tests of medians and proportions for, respectively, continuously coded and categorical variables. We conducted a two-step analysis.
First, we focused on the technical feasibility of PSS after glansectomy or partial penectomy. Specifically, we registered the PSS technique used, as well as the treatment setting (outpatient vs. inpatient) and complications. Second, we tested for overall disease recurrences (either distant vs. regional vs. local) as well as local recurrences over time. Here, Kaplan–Meier plots were used. All statistical tests were two-sided with a level of significance set at p < 0.05 and were performed using the R software environment for statistical computing and graphics (version 3.4.1; http://www.r-project.org/).
3. Results
3.1. Descriptive Analyses (Table 1)
The median (interquartile range: IQR) age at surgery was 60 (53–63) years (Table 1). CCI was 1, 2, and ≥3 in, respectively, in four (30.5%), four (30.5%), and five (39%) patients. The median (IQR) tumour size at the time of the previous surgery was 25 (20–30) mm. In consequence, 11 (84.5%) vs. 2 (15.5%) patients underwent glansectomy vs. partial penectomy. Histology at initial surgery was the following: squamous cell (84%) vs. verrucous (7.5%) vs. epidermoid (7.5%) carcinoma. Moreover, T-stage stratification revealed the following distribution: Tx (7.5%) vs. Tis (7.5%) vs. T1 (39%) vs. T2 (46%). Additionally, 7.5% vs. 15% vs. 30.5% vs. 47% of men had Gx vs. G1 vs. G2 vs. G3 tumour grade, respectively. Last, only one patient (7.5%) had previous N1 disease.
3.2. Perioperative Findings (Table 2)

Table 2.
Perioperative findings of 13 penile cancer patients previously treated with glansectomy or partial penectomy, and subsequently treated with penile-sparing surgery for penile cancer recurrence between 2001 and 2022. iLND: inguinal lymph node dissection; sLND: sentinel lymph node dissection; N.A.: not available; PeIN: penile intraepithelial neoplasia.
The median (IQR) time from glansectomy/partial penectomy to disease recurrence was 56 (13–88) months. The median (IQR) tumour size was 7 (5–15) mm. Overall, 10 (77%) vs. 2 (15.5%) vs. 1 (7.5%) recurrences were located at, respectively, neoglans vs. neoglans + foreskin vs. distal urethra. Two exemplificative cases are depicted in Figure 1. In consequence, six (46%) vs. two (15.5%) vs. five (38.5%) patients were treated with, respectively, local excision vs. local excision + circumcision vs. laser ablation. PSS procedures were performed in outpatient vs. inpatient settings in 12 (92.5%) vs. 1 (7.5%) cases. Specifically, patient 13 had an 18 mm recurrence at the level of the neoglans and was treated with wide local excision under general anaesthesia. This patient had a length of stay of 4 days and required antibiotic therapy for a Clavien–Dindo grade 2 complication. All other patients did not experience complications after PSS. Final histology was available for 10 (77%) men. All tumours were squamous cell carcinoma. Four (30.5%) vs. one (7.5%) vs. one (7.5%) vs. four (30.5%) tumours were PeIN vs. Ta vs. Tis vs. T1, respectively. Last, five (38.5%) vs. four (30.5%) vs. one (7.5%) vs. three (23.5%) lesions were Gx vs. G1 vs. G2 vs. G3, respectively.
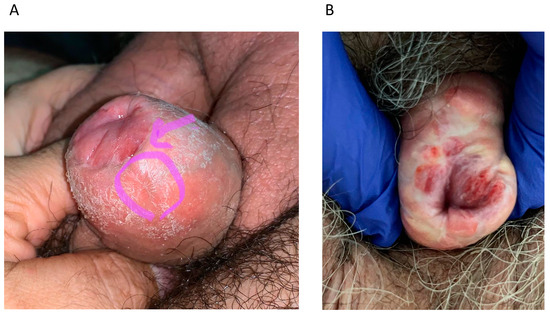
Figure 1.
(A) Patient 1: Local recurrence at the level of the neoglans + foreskin at 56 months after glansectomy that underwent circumcision + excision. (B) Patient 3: Local recurrence at the level of the neoglans + foreskin at 9 months after glansectomy that underwent circumcision + excision.
3.3. Findings at Follow-Up (Table 3)

Table 3.
Findings at follow-up of 13 penile cancer patients previously treated with glansectomy or partial penectomy, and subsequently treated with penile-sparing surgery for penile cancer recurrence between 2001 and 2022. iLND: inguinal lymph node dissection; sLND: sentinel lymph node dissection; NED: no evidence of disease; PeIN: penile intraepithelial neoplasia.
The median (IQR) follow-up time was 41 (10–72) months. During follow-up, three (23%) vs. four (30.5%) patients experienced local (Figure 2a) vs. overall (Figure 2b) recurrence, respectively. Specifically, patient 3 was only treated with bilateral inguinal lymph node dissection for isolated nodal recurrent disease 3 months after PSS. Of all patients that exhibited local recurrence (n = 3), two (66.5%) vs. one (33.5%) underwent penectomy vs. wide local excision. Specifically, patient 9 experienced a pT1G2 squamous tumour 31 months after PSS and required penectomy and concomitant sentinel lymph node dissection. Moreover, patient 10 was treated with penectomy for a pT2G3 verrucous carcinoma that recurred 10 months after laser ablation. Last, patient 2 experienced a pT1aG1 squamous tumour that recurred 7 months after PSS. Wide local excision and sentinel lymph node dissection were performed. No patients died during the study period. Local and overall recurrence survival rates for patients previously treated with glansectomy/partial penectomy who underwent another PSS vs. radical penectomy for disease recurrence are depicted in the Supplementary Materials, Figure S1.
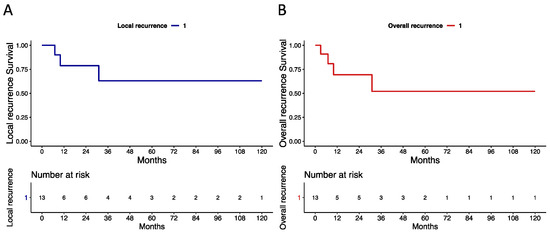
Figure 2.
Kaplan–Meier plots depicting recurrence-free survival rates in 13 patients with recurrent penile cancer treated with penile-sparing surgery after previous glansectomy or partial penectomy (1997–2022). (A) Local recurrence. (B) Overall recurrence (any local, regional, or systemic recurrence).
4. Discussion
Glansectomy and partial penectomy are effective treatments for localised penile cancer, permitting oncological control over time, while simultaneously preserving patient sexual and urinary functions [16]. Unfortunately, approximately 4–12.8% of patients treated with these treatment modalities experience local recurrence during follow-up [8,9]. Historically, radical penectomy has been considered the treatment of choice for recurrent disease in these cases. However, a subgroup of patients with limited local recurrence could be considered for a new PSS procedure, without compromising the functional outcomes of previous conservative surgery [10]. To date, no systematic analyses have been conducted and only sporadic cases were previously reported by some authors [3,4]. We analysed 13 consecutive patients treated with PSS for local recurrence after glansectomy or partial penectomy between 1997 and 2022 with a specific focus on (1) technical feasibility and (2) oncological outcomes. Our results show several important findings.
First, of all patients who experienced local recurrence after glansectomy or partial penectomy, 37% were treated with PSS. This percentage appears to be encouraging since approximately a third of patients could avoid immediate penile amputation. Moreover, this percentage could also be underestimated since the gold standard treatment for local recurrence after glansectomy/partial penectomy is represented by total penectomy. Due to the lack of specific recommendations for PSS after glansectomy/partial penectomy, the accurate selection of candidates appears to be a key factor. Unfortunately, due to the lack of information about postoperative surgical margins and the small number of patients analysed, only hypothetical considerations could be derived from this analysis. Specifically, patient age, education, comorbidities, sexual life, and compliance with strict follow-up schemes appear to be crucial. Moreover, other tumour characteristics, such as a long time to recurrence from previous surgery, small lesion size, low tumour T stage and grade, as well as recurrence location should be considered. Indeed, in our series, compared with patients immediately treated with radical penectomy, patients treated with PSS had smaller and more superficial tumours. Moreover, the time to disease recurrence was significantly lower for patients submitted to total amputation. Last, surgeon experience and hospital volume appear to be important when recommending PSS for recurrent disease. However, other reports testing the oncological safety and technical feasibility of PSS after glansectomy/partial penectomy are urgently required to optimise patient selection and promote wider use of PSS for this patient category.
Second, we demonstrated that PSS for local recurrence after glansectomy/partial penectomy is technically feasible. According to tumour characteristics, clinician preference, and availability of technologies, several PSS procedures could be safely performed in this patient category. Specifically, all our patients were treated with either excision [12] or laser ablation [13,14]. Moreover, the vast majority of the surgeries were performed in outpatient settings and only one patient with an 18 mm lesion necessitated general anaesthesia and hospital recovery. Additionally, only one Clavien–Dindo 2 complication that was easily treated with antibiotic therapy was observed. Our findings encourage the use of PSS for selected patients with disease recurrence after previous glansectomy/partial penectomy. However, future analyses should focus on other important outcomes such as operative time, patient satisfaction, and sexual and urinary function before recommending implementation in daily practice. Moreover, until clear evidence of the superiority of one PSS technique over the others is demonstrated, all PSS procedures should be encouraged for treating local recurrences after glansectomy or partial penectomy.
Third, we tested early oncological outcomes after PSS for local recurrence after glansectomy/partial penectomy. Here, we observed that 23% and 30.5% of patients experienced local and overall recurrence over time, respectively. Our findings could indicate the safety of PSS in local control of recurrent disease for this patient category. Specifically, all patients who experienced local recurrence after PSS (n = 3) were safely re-treated with penectomy or wide excision. Conversely, only one patient presented isolated nodal recurrence (N1) and necessitated bilateral inguinal lymph node dissection. Our results also indicate that immediate penile amputation, at the time of the first local recurrence after glansectomy/partial penectomy, could probably be avoided in this patient category and only offered to those who exhibit another local recurrence during time (salvage setting). However, we advocate for accurate patient selection and strict follow-up of patients who are candidates for these treatment modalities. Moreover, our findings should be considered exploratory at best, since only a limited number of patients (n = 13) over a long time span (1997–2022) were treated at one referral centre. Last, the limited follow-up available for this patient cohort (median: 41 months) is not enough for testing major oncological endpoints such as tumour progression and cancer-specific mortality. In consequence, we advocate testing the oncological safety of another PSS procedure for tumour recurrence after a previous glansectomy or partial penectomy in a series of patients with longer follow-up data.
Taken together, we reported the technical feasibility and oncological outcomes of PSS for local recurrence of patients previously treated with glansectomy or partial penectomy for penile cancer. We observed that approximately a third of patients with local recurrence could be treated with PSS without compromising oncological control of the disease. Moreover, for those patients who experience another recurrence over time, salvage penectomy could be safely offered.
Despite its novelty, our study has limitations. First, the current data are retrospective and influenced by inherent selection bias. Second, as previously stated, we were unable to fit multivariable Cox models predicting recurrence rates over time due to a low number of patients and events. Third, we created heterogeneity among patients by including several PSS techniques (real-life scenarios). Fourth, information about surgical margin status was unavailable after PSS [17,18,19]. Fifth, some important pathological features, such as lymphovascular invasion and T1 sub-classification (T1a vs. T1b) [20,21,22] were unavailable. Sixth, as previously stated, information about patient satisfaction and sexual and urinary function after PSS were not recorded. Last, we did not perform a systematic comparison between patients who were immediately treated with total penectomy at first local recurrence after glansectomy or partial penectomy vs. those re-treated with PSS. Specifically, we only reported Kaplan–Meier plots depicting local and overall recurrence survival rates in these two groups (Supplementary Materials, Figure S1), without extensively discussing our misleading findings (i.e., lower overall recurrence rates in PSS-treated patients), which are, in our opinion, a product of selection bias.
5. Conclusions
We reported the results of a preliminary analysis testing safety, feasibility, and early oncological outcomes of PSS procedures for patients with local recurrence after previous glansectomy or partial penectomy. Stronger oncological outcomes should be tested in other studies to optimise patient selection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15194807/s1, Table S1: Clinical characteristics of 22 penile cancer patients, previously treated with glansectomy or partial penectomy, and subsequently treated with radical penectomy for penile cancer recurrence between 2001 and 2022. Data are shown as medians for continuous variables or as counts and percentages (%) for categorical variables. IQR: interquartile range. Figure S1: Kaplan–Meier plots depicting recurrence-free survival rates in patients with recurrent penile cancer after previous glansectomy or partial penectomy that underwent penile-sparing surgery (n = 13) vs. radical penectomy (n = 22) between 1997 and 2022. (A) Local recurrence. (B) Overall recurrence (any local, regional, or systemic recurrence).
Author Contributions
Conceptualization, F.M. and S.L.; methodology, F.A.M. and S.L.; software, O.B.; formal analysis, F.A.M. and M.L.P.; investigation, G.M.; resources, S.G.; data curation, M.T. and E.L.; writing—original draft preparation, M.F. (Matteo Fontana), A.C., D.C. and E.V.; writing—review and editing, M.C.R.; visualization, M.F. (Matteo Ferro) and F.N.; supervision, O.d.C.; project administration, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the European Institute of Oncology (protocol code UID 5361 and date of approval 7 September 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hakenberg, O.W.; Compérat, E.M.; Minhas, S.; Necchi, A.; Protzel, C.; Watkin, N. EAU guidelines on penile cancer: 2014 update. Eur. Urol. 2015, 67, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Lindner, A.K.; Schachtner, G.; Steiner, E.; Kroiss, A.; Uprimny, C.; Steinkohl, F.; Horninger, W.; Heidegger, I.; Madersbacher, S.; Pichler, R. Organ-sparing surgery of penile cancer: Higher rate of local recurrence yet no impact on overall survival. World J. Urol. 2020, 38, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Djajadiningrat, R.S.; van Werkhoven, E.; Meinhardt, W.; van Rhijn, B.W.; Bex, A.; van der Poel, H.G.; Horenblas, S. Penile sparing surgery for penile cancer—Does it affect survival? J. Urol. 2014, 192, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, A.; Chipollini, J.; Yan, S.; Ottenhof, S.R.; Tang, D.H.; Draeger, D.; Protzel, C.; Zhu, Y.; Ye, D.-W.; Hakenberg, O.W.; et al. Penile Sparing Surgery for Penile Cancer: A Multicenter International Retrospective Cohort. J. Urol. 2018, 199, 1233–1237. [Google Scholar] [CrossRef]
- Luzzago, S.; Serino, A.; Aurilio, G.; Mistretta, F.A.; Piccinelli, M.L.; Lorusso, V.; Morelli, M.; Bianchi, R.; Catellani, M.; Cozzi, G.; et al. Penile-sparing surgery for patients with superficial or initially invasive squamous cell carcinoma of the penis: Long-term oncological outcomes. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 736.e1–736.e7. [Google Scholar] [CrossRef]
- Anastasiadis, E.; Ayres, B.; Watkin, N. Update on penile sparing surgery for penile cancer. Curr. Opin. Urol. 2021, 32, 1–7. [Google Scholar] [CrossRef]
- Kokorovic, A.; Duplisea, J.; Qiao, W.; McCormick, B.; Adibi, M.; Papadopoulos, J.; Ramirez, G.; Rao, P.; Tamboli, P.; Pettaway, C. Oncologic outcomes and subsequent treatment following organ sparing surgery for penile carcinoma: The University of Texas M.D. Anderson Cancer Center Experience. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 302.e19–302.e27. [Google Scholar] [CrossRef]
- Albersen, M.; Parnham, A.; Joniau, S.; Sahdev, V.; Christodoulidou, M.; Castiglione, F.; Nigam, R.; Malone, P.; Freeman, A.; Jameson, C.; et al. Predictive factors for local recurrence after glansectomy and neoglans reconstruction for penile squamous cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 141–146. [Google Scholar] [CrossRef]
- Roussel, E.; Peeters, E.; Vanthoor, J.; Bozzini, G.; Muneer, A.; Ayres, B.; Sri, D.; Watkin, N.; Bhattar, R.; Parnham, A.; et al. Predictors of local recurrence and its impact on survival after glansectomy for penile cancer: Time to challenge the dogma? BJU Int. 2020, 127, 606–613. [Google Scholar] [CrossRef]
- Saidian, A.; Ceballos, B.; Necchi, A.; Baumgarten, A.S.; Spiess, P.E. Salvage therapy for localized recurrences of penile cancer. Curr. Opin. Urol. 2021, 31, 214–219. [Google Scholar] [CrossRef]
- Bissada, N.K.; Yakout, H.H.; Fahmy, W.E.; Gayed, M.S.; Touijer, A.K.; Greene, G.F.; Hanash, K.A. Multi-institutional long-term experience with conservative surgery for invasive penile carcinoma. J. Urol. 2003, 169, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.S.; McDougal, W.S. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J. Urol. 2011, 186, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Bandieramonte, G.; Colecchia, M.; Mariani, L.; Vullo, S.L.; Pizzocaro, G.; Piva, L.; Nicolai, N.; Salvioni, R.; Lezzi, V.; Stefanon, B.; et al. Peniscopically Controlled CO2 Laser Excision for Conservative Treatment of In Situ and T1 Penile Carcinoma: Report on 224 Patients. Eur. Urol. 2008, 54, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Musi, G.; Russo, A.; Conti, A.; Mistretta, F.A.; Di Trapani, E.; Luzzago, S.; Bianchi, R.; Renne, G.; Ramoni, S.; Ferro, M.; et al. Thulium–yttrium–aluminium–garnet (Tm:YAG) laser treatment of penile cancer: Oncological results, functional outcomes, and quality of life. World J. Urol. 2017, 36, 265–270. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Croghan, S.M.; Compton, N.; E Daniels, A.; Fitzgibbon, L.; Daly, P.J.; Cullen, I.M. Phallus Preservation in Penile Cancer Surgery: Patient-reported Aesthetic & Functional Outcomes. Urology 2021, 152, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.A.; Renshaw, A.A.; Loughlin, K.R. Squamous cell carcinoma of the penis and microscopic pathologic margins: How much margin is needed for local cure? Cancer 1999, 85, 1565–1568. [Google Scholar] [CrossRef]
- Minhas, S.; Kayes, O.; Hegarty, P.; Kumar, P.; Freeman, A.; Ralph, D. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005, 96, 1040–1043. [Google Scholar] [CrossRef]
- Gunia, S.; Koch, S.; Jain, A.; May, M. Does the width of the surgical margin of safety or premalignant dermatoses at the negative surgical margin affect outcome in surgically treated penile cancer? J. Clin. Pathol. 2014, 67, 268–271. [Google Scholar] [CrossRef]
- Slaton, J.W.; Morgenstern, N.; Levy, D.A.; Santos, M.W.; Tamboli, P.; Ro, J.Y.; Ayala, A.G.; Pettaway, C.A. Tumor stage, vascular invasion and the percentage of poorly differentiated cancer: Independent prognosticators for inguinal lymph node metastasis in penile squamous cancer. J. Urol. 2001, 165, 1138–1142. [Google Scholar] [CrossRef]
- Graafland, N.M.; Lam, W.; Leijte, J.A.; Yap, T.; Gallee, M.P.; Corbishley, C.; van Werkhoven, E.; Watkin, N.; Horenblas, S. Prognostic factors for occult inguinal lymph node involvement in penile carcinoma and assessment of the high-risk EAU subgroup: A two-institution analysis of 342 clinically node-negative patients. Eur. Urol. 2010, 58, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Zattoni, F.; Cunico, S.C.; Galetti, T.P.; Luciani, L.; Fandella, A.; Guazzieri, S.; Maruzzi, D.; Sava, T.; Siracusano, S.; et al. Lymphatic and vascular embolizations are independent predictive variables of inguinal lymph node involvement in patients with squamous cell carcinoma of the penis: Gruppo Uro-Oncologico del Nord Est (Northeast Uro-Oncological Group) Penile Cancer Data Bas. Cancer 2005, 103, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).