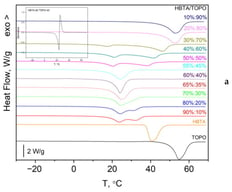
The development of deep eutectic solvents (DESs) is a key issue for the realization of green and efficient metal extraction processes. The present study aims to experimentally construct the phase diagram of the binary system consisting of tri-n-octylphosphine oxide (TOPO) and 4,4,4-trifluoro-1-phenyl-1,3-butanedione (HBTA) and, thus, determine its eutectic composition for the solvent extraction of Li
+. Differential scanning calorimetry was used to characterize the phase transitions (melting temperatures and enthalpies) over the entire composition range of the binary mixture. Its eutectic composition was established at HBTA:TOPO mass ratio of 60:40. For further validation of the eutectic composition from the experimentally measured thermal effects for melting of different HBTA:TOPO mass ratios, a Tammann diagram was also constructed. Only mixtures with HBTA:TOPO mass ratios of 70:30, 60:40 (eutectic composition), and 50:50 were liquids at 30 °C, while at room temperature of 25 °C, the 70:30 mixture formed crystals. All three mixtures, which were liquids at 30 °C, were found to extract Li
+ effectively. However, at a room temperature of 25 °C, only the eutectic mixture (60:40 mass ratio) extracted Li
+ effectively, while the mixture with HBTA:TOPO mass ratio of 50:50 formed crystals when mechanically agitated and, therefore, was deemed as unsuitable for Li
+ extraction.



![Comparison of spectra from various halogen light sources with the standard solar spectrum [32].](https://mdpi-res.com/cdn-cgi/image/w=281,h=192/https://mdpi-res.com/thermo/thermo-06-00011/article_deploy/html/images/thermo-06-00011-g001-550.jpg)