- Systematic Review
Perampanel-Induced Psychosis and Psychosis-like Symptoms: A Systematic Review
- Petar Z. Taslaković,
- Miloš N. Milosavljević and
- Srđan Stefanović
- + 1 author
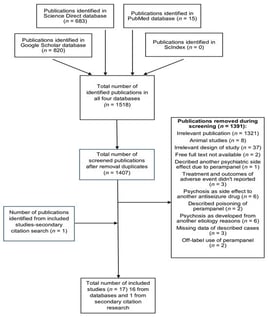
Background/Objectives: This study aimed to investigate whether therapy with perampanel is associated with the development of psychosis or psychosis-like symptoms in patients with epilepsy. Methods: We conducted systematic electronic searches in PubMed, Google Scholar, ScienceDirect, and Scindex databases. We included articles published as case reports/case series, as well as conference abstracts and letters from the editor, if they contained enough data for analysis and quality assessment. The main inclusion criteria relate to patients who experienced psychosis or psychosis-like symptoms described by the authors during perampanel therapy or during its recent use. Results: Publications (n = 17) describing a total of 33 patients who met the inclusion criteria were included. Patient ages ranged from 11 to 70 years, and the majority of them were female (66.67%). A confirmed personal psychiatric history was identified in 60.61% of patients. The time interval between the initiation of perampanel and the onset of adverse events varied significantly across cases. The most frequently reported symptom was aggression (75.75%), followed by irritability (30.30%), while delusions or hallucinations were observed in 8 patients (24.24%). Conclusions: Clinicians should be aware that psychosis or psychosis-like symptoms may represent dose-dependent adverse effects of perampanel with a satisfactory prognosis. Identified risk factors for these developments were positive personal psychiatric history, antiseizure polytherapy at high doses, women’s gender, and focal epilepsies with secondary generalization, mainly manifested as tonic–clonic seizures. Early recognition of symptoms, followed by drug discontinuation, dose reduction, symptomatic treatment, or a combination of the mentioned strategies, is crucial for achieving better outcomes.
3 February 2026



![Inhibitory effects of mirabegron on specific [3H]NMS binding in rat tissues ((A): bladder, (B): submaxillary gland, brain, bladder, and heart) (Reproduced with permission from Ref. [31] 2021, Elsevier).](https://mdpi-res.com/cdn-cgi/image/w=281,h=192/https://mdpi-res.com/futurepharmacol/futurepharmacol-06-00007/article_deploy/html/images/futurepharmacol-06-00007-g001-550.jpg)