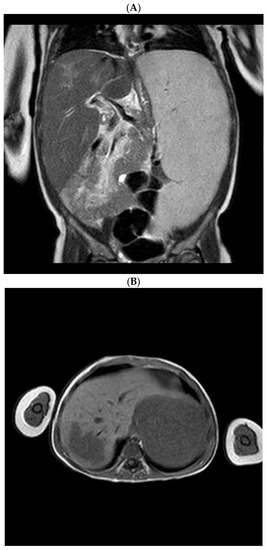
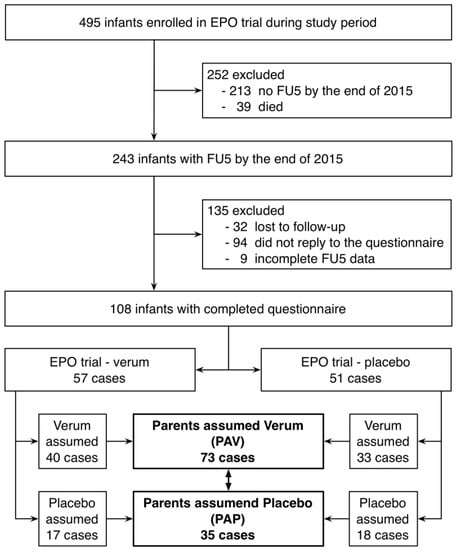
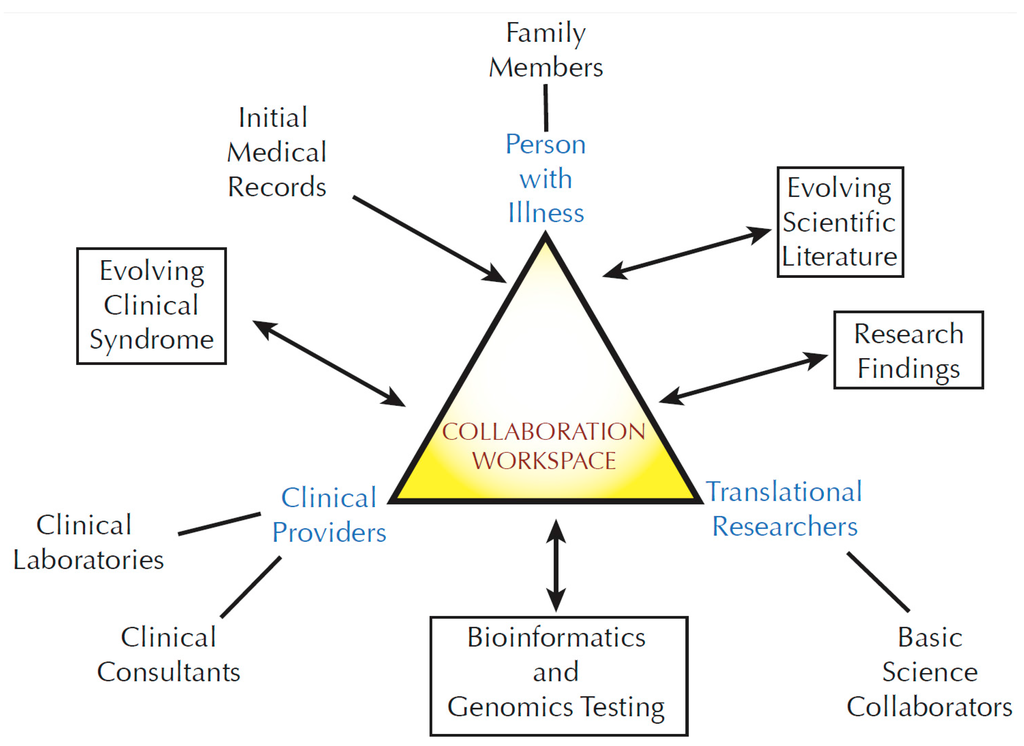
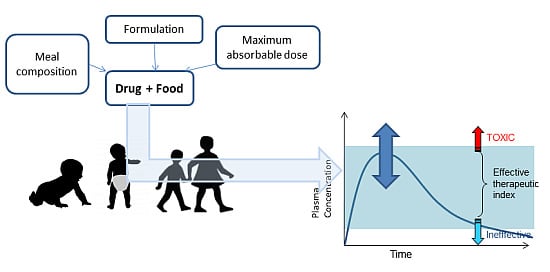
Development of Medicines for Paediatric and Rare Diseases
A topical collection in Children (ISSN 2227-9067).
Viewed by 87486Editors
Interests: melanoma; skin cancer; atypical nevi; reflectance confocal microscopy
Interests: paediatric drug development; regulatory, societal and scientific aspects of drug development; interface of regulatory, scientific, historical and political aspects in drug development and paediatric drug development; medicines for children; drug development for rare, paediatric, and rare paediatric diseases
Special Issues, Collections and Topics in MDPI journals
2. Division of Clinical Pharmacology, Children's National Hospital, Washington, DC 20310, USA
3. Intensive Care and Department of Pediatric Surgery, Erasmus MC Sophia Children's Hospital, 3015 CN Rotterdam, The Netherlands
Interests: developmental pharmacology; neonatal pharmacology; paediatric pharmacology; neonatal medicine; neonatal infectious diseases; pharmacokinetics; pharmacodynamics; neonatal clinical trials
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
The value of licensed and appropriately labeled medicines for children and adults is increasingly a focus of public awareness. This is, for a large part, the result of US and EU legislation on paediatric and orphan diseases. However, paediatric clinical practice has changed less than expected, and the initial enthusiasm has been replaced by a more realistic assessment. Breakthrough therapeutic innovations have been introduced in the last decades, e.g. for cystic fibrosis, metabolic diseases or hormonal defects. However, none of these were triggered by paediatric legislation. The aim of this conference and its related collection is to provide an update on both state-of-the-art methodology and operational challenges in research & development of paediatric and rare diseases. It aims at re-evaluating what is needed for more progress in the development of treatments for both paediatric and rare diseases. We welcome original research, review, opinion papers, editorials, or short communications on the following topics: paediatric legislation, commercial drug development, development of orphan and paediatric drugs, paediatric clinical research, developmental pharmacology, neonatal pharmacology, paediatric pharmacology, neonatal medicine, neonatal infectious diseases, pharmacokinetics, pharmacodynamics, and neonatal clinical trials.
Dr. Klaus Rose
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Children is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2400 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Better Medicines for Children
- Paediatric Drug Development
- Paediatric Formulations
- Juvenile Animal Studies in Drug Development
- Age-Specific Formulations
- Paediatric Clinical Pharmacology
- Neonatal Clinical Pharmacology
- Developmental Pharmacology
- Pharmacokinetics
- Pharmacodynamics
- Neonatal Clinical Trials
- Inclusion of paediatric aspects in drug development
- Children and history of pharmacy
- Children and history of medicine
- Children and society