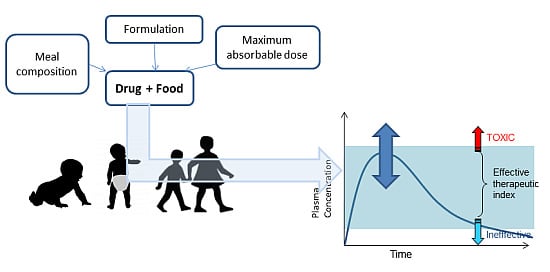
Influence of Food on Paediatric Gastrointestinal Drug Absorption Following Oral Administration: A Review
Abstract
:1. Introduction
2. Clinical Measurement of Food–Drug Interactions
2.1. Regulatory Guidelines for Food–Drug Interaction Studies
3. Paediatric Diet and Composition of Food
3.1. Regulatory Advice on Meal Composition
3.2. Paediatric Feeding Patterns
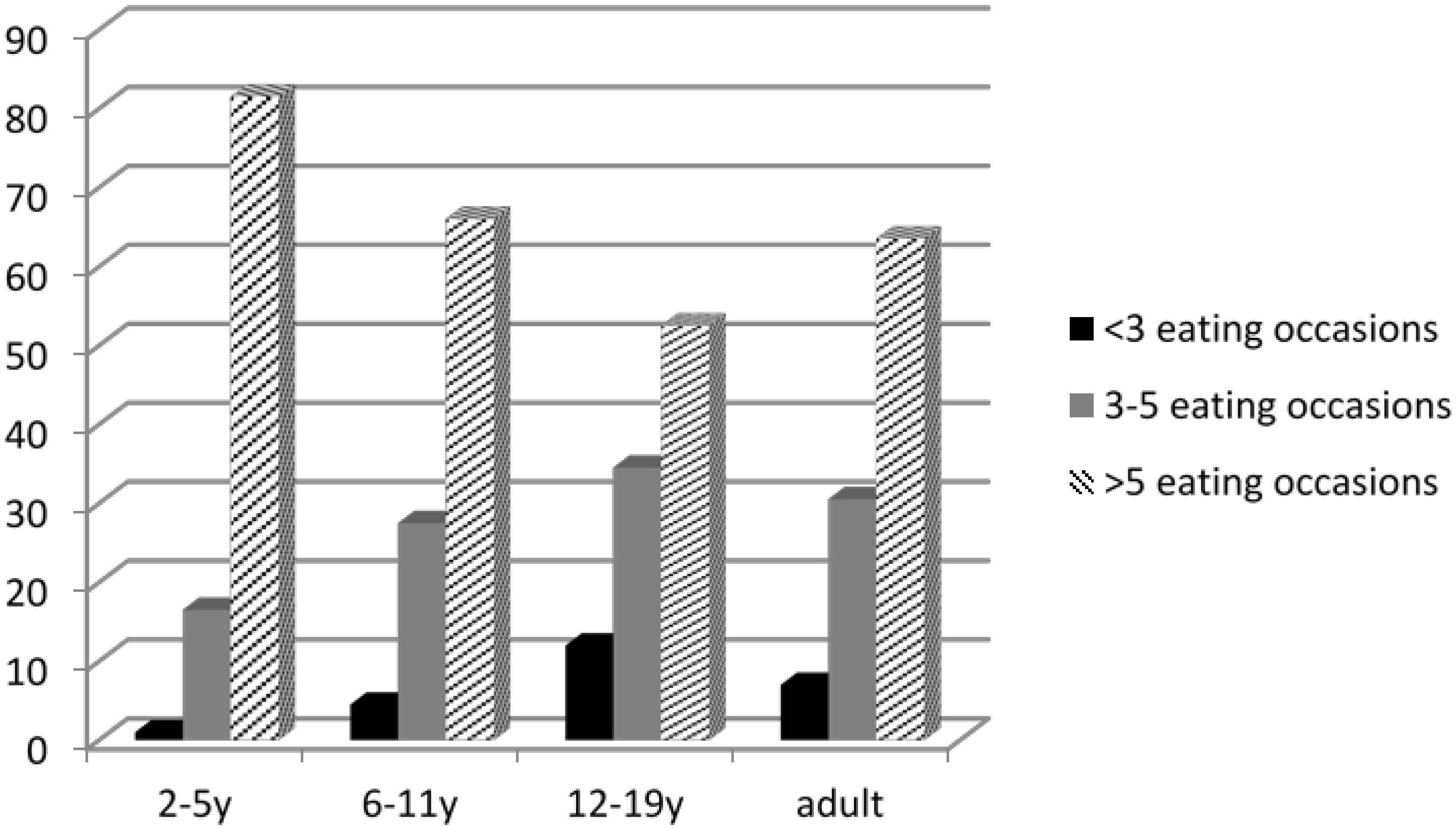
3.3. Volume of Food Consumed
4. Physiological and Anatomical Differences in Paediatric Populations that Affect Drug–Food Interactions
4.1. Gastric Emptying
4.1.1. Calorific Content and GE
4.1.2. Volume and GE
4.1.3. Osmolality and GE
4.1.4. Viscosity and GE
4.1.5. Temperature and GE
4.2. Gastrointestinal Transit Times
4.3. Splanchnic Blood Flow
4.4. Membrane Interactions
4.5. Review of Food Effects in Paediatric Populations
| Drug | Formulation | Dose | Age Range | Meal | Change in Parameter | Comments | Ref | Comparable Adult Data | Adult Ref |
|---|---|---|---|---|---|---|---|---|---|
| Amoxicillin | oral suspension | 15 or 25 mg/kg | 4 m–45 m (mean 27 months) | 4oz milk or formula (Similac or Infamil) | Cmax decreased at 15mg/kg dosing Cmax unchanged at 25 mg/kg dosing AUC unchanged all doses | [42] | Adult study at 500mg dose showed no impact of food (standard breakfast) | [43] | |
| Ampicillin | oral suspension | 15 or 25 mg/kg | 4 m–45 m (mean 27 months) | 4oz milk or formula (Similac or Infamil) | Cmax unchanged AUC unchanged | [42] | Adult study at 500mg dose showed reduction in Cmax and AUC with food (standard breakfast) | [43] | |
| Cefpodoxime proxetil | oral suspension | 10 mg/kg | 5 m–12 y | Age-appropriate meal (volume and composition) | Tmax prolonged Cmax unchanged | Cefpodoxime can be administered without regard for food | [44] | Cmax and AUC eleveated in the fed states for all meals (high protein, low protein, high fat, low fat). | [45] |
| Cephalexin | suspension and capsule | 25 mg/kg | 3–14 y | Standard hospital meal | Cmax reduced (not significant) AUC increased (not significant) | Concomitant administration of food does not substantially affect absorption | [46] | Absorption is delayed but AUC is not appreciably altered | [47] |
| Clarithromycin | oral suspension | 7.5 mg/kg | AUC unchanged | [48] | The extent of absorption is relatively unaffected by the presence of food | [49] | |||
| Desmopressin | oral lyophilisate (MELT) | 120 mg | mean age 12.7 y | Standardised meal | Cmax unchanged AUC unchanged | Bioequivalence established, even with concomitant food-intake | [50] | AUC and Cmax are reduced with food in adults (for the tablet formulation but not the MELT) | [51] |
| Didanosine | 50 or 150 mg/m2 | Cmax reduced AUC unchanged | Take in the fasted state | [52] | AUC and Cmax are substantially reduced with some formulations if taken with food | [53] | |||
| Griseofulvin | oral suspension | 10 or 15 mg/kg/day | 19 m–11 y (mean 4.8 y) | 120 mL whole milk | Cmax increased AUC increased | Drug should be administered with whole milk or other food containing fat for optimum bioavailability | [54] | Cmax increased AUC increased | [55] |
| Lumefantrine | dispersible or crushed tablets (Coartem ®) with 10mL water | 0.25–12.4 y | Categorised as: none; breast feeding; liquid (soup, broth); pancake; porridge or other | Cmax increased (greater increased for crushed tablet) Pancake increased the exposure to a greater extent than milk | Consumption of food at the time of dosing remains advisable | [56] | Cmax and AUC increased when given with food | [57] | |
| 6-mercaptopurine | 75 mg/m2 | 250 mL milk and 50 g biscuits | Tmax prolonged Cmax significantly reduced AUC significantly reduced | 6-MP should be taken in a fasting state to optimize drug absorption in children undergoing chemotherapy for ALL | [58] | Tmax prolonged Cmax reduced | [59] | ||
| 6-mercaptopurine | 4 year old child (n = 1) | Milk or fruit squash | In the presence of milk Cmax reduced AUC reduced | Child required increased dose of mercaptopurine when taken with milk | [60] | Tmax prolonged Cmax reduced | [59] | ||
| 6-mercaptopurine | Breakfast (milk or yogurt plus cereal, or sandwiches) | Cmax unchanged AUC unchanged | Insufficient data for a recommendation | [61] | Tmax prolonged Cmax reduced | [59] | |||
| Methotrexate | 15 mg/m2 | 3–15 years | Milky meal = milk, cornflakes sugar, bread and butter Or citrus meal = orange juice, fresh orange, bread, butter and jam | Milky meal: Tmax prolonged Cmax and AUC significantly reduced Citrus meal: Cmax and AUC unchanged | Methotrexate absorption is delayed by food, particularly milk. For maximum absorption methotrexate should not be taken at meal times. | [62] | Tmax prolonged AUC unchanged | [63] | |
| Penicillin V (phenoxymethylpenicillin) | dispersed in water 23 mg/mL (Calciopen) | 20 mg/kg | 6 m–5 years | Breakfast | Cmax significantly reduced AUC significantly reduced | Dosing Penicillin V with food will reduce its exposure | [10] | Slight alteration in pharmacokinetics such that it is recommended to dose penicillin V in the fasted state | [64] |
| Penicillin V (phenoxymethylpenicillin) | Suspension | infants and children | milk | Cmax reduced AUC reduced | Dosing Penicillin V with milk will reduce its exposure | [65] | Slight alteration in pharmacokinetics such that it is recommended to dose penicillin V in the fasted state | [64] | |
| Propylthiouracil | Not stated | 100–280 mg/m2 | Not stated | Tmax prolonged Cmax reduced AUC variable | Propylthiouracil administration in the fasting state is advisable | [66] | Cmax unchanged AUC unchanged | [67] | |
| Theophylline | slow release products | Standard breakfast of cornflakes, rye bread, butter, salami and low fat milk | Effects dependent upon formulation | Food effect is dependent upon formulation. Caution advised if switching brand. | [68] | Food has substantial but variable effects on absorption from modified-release formulations in adults. | [69] | ||
| 6-thioguanine | Not stated | 40 mg/m2 | 1–16 years | Standard breakfast of cereal with milk, toast and a glass of milk | Tmax prolonged Cmax significantly reduced AUC significantly reduced | Although there is a reduction in exposure with food there was no difference in 6-TGN concentrations after 4 weeks. Taking the drug on an empty stomach may not be necessary. | [70] |
5. Physico-Chemical Food–Drug Interactions
5.1. pH Effects
5.2. Viscosity
5.3. Binding/Chelation
5.4. Thermal Degradation
6. Formulation Influence on Food Effects
6.1. Review of Food Effects in Paediatric Formulations
| Drug | Formulation | Dose | Food/Meal | Timing of Dose | Change in Parameter | Remarks from Reference | Reference |
|---|---|---|---|---|---|---|---|
| Clobazam | crushed tablet | 20 mg | Applesauce | Cmax unchanged AUC unchanged | Clobazam tablets can be given crushed with applesauce. Administration of clobazam with a high-fat meal did not affect clobazam exposure | [91] | |
| Lansoprazole | enteric coated granules (capsule contents) | 30 mg | 1 tablespoon of yogurt or 1 tablespoon Ensure® pudding or 1 tablespoon Cottage cheese | overnight fast —3 h post dose | Tmax prolonged (for cottage cheese) Cmax unchanged AUC unchanged | The bioavailability when administered in yogurt, Ensure® pudding and cottage cheese, was similar to that of the intact capsule in these healthy adult volunteers | [92] |
| Lansoprazole | enteric coated granules (capsule contents) | 30 mg | 180mL orange juice without pulp or tomato juice; or soft food (1 tablespoon of strained pears) | overnight fast —3 h post dose | Tmax prolonged (for orange juice only) Cmax unchanged AUC unchanged | The bioavailability when administered in orange juice, tomato juice, or a small amount of strained pears, was similar to that of the intact capsule in these healthy adult volunteers | [93] |
| Levetircetam | crushed tablet | 500 mg | 4oz applesauce 120 mL enteral nutrition formula (Sustacal®) | overnight fast —4 h post dose | Cmax unchanged AUC unchanged | The overall rate and extent of absorption was not significantly impaired after crushing and mixing of the tablet with either a food vehicle or a typical ENF product | [94] |
| Methylphenidate | extended release granules (capsule contents) | 20 mg | 1 level tablespoon applesauce (15 mL) | No data available | Cmax unchanged AUC unchanged | The bioavailability of methylphenidate was not altered by sprinkling their contents onto a small amount of applesauce | [95] |
| Morphine | extended release granules (capsule contents) | 60 mg | 2 tablespoons applesauce | overnight fast —4 h post dose | Cmax unchanged AUC unchanged | The bioavailability when sprinkled onto applesauce was similar to that of the intact capsule in adults | [96] |
| Rabeprazole | enteric coated granules (capsule contents) | 10 mg | 1 tablespoon of yogurt 1 tablespoon applesauce 5mL infant formula | overnight fast —4 h post dose | Cmax unchanged AUC unchanged | The bioavailability of rabeprazole granules was similar for all food stuffs evaluated | [97] |
| Azithromycin | paediatric suspension (cherry-banana) (40mg/mL) | 500 mg | High fat breakfast and 227 mL of whole milk, ingested within a 20 min period | overnight fast —4 h post dose | Cmax increased AUC unchanged | The suspension formulation may be administered without regard to meals, increasing the convenience of once-daily dosing regimens | [98] |
| Everolimus | dispersible tablet | 1.5 mg | Standardized, high-fat breakfast | overnight fast —4 h post dose | Tmax prolonged Cmax reduced AUC unchanged | Administer the dispersible tablet to each patient on a consistent basis either with or without food. | [99] |
| Glycopyrrolate | oral solution (1 mg/5mL) | 2 mg | FDA High fat breakfast | overnight fast —4 h post dose | Cmax reduced AUC reduced | Administer at least one hour before or after meals | [100] |
| Ibuprofen | chewable tablets | 200 mg | Standardised breakfast plus 240 mL whole milk total calorie content = 650 calories | overnight fast —4 h post dose | Tmax prolonged Cmax reduced AUC slightly decreased | [101] | |
| Nelfinavir | powder to mix with food | 100–800 mg | Standardised breakfast | overnight fast —4 h post dose | Tmax prolonged Cmax increased AUC increased | Recommended that patients take nelfinavir with a meal or snack | [102] |
| Nitazoxanide | Suspension (25 mL of 100 mg/5 mL) | 500 mg | High fat high calorie standardised breakfast and 240 mL whole milk | overnight fast —4 h post dose | Tmax prolonged Cmax unchanged AUC increased | [103] | |
| Paracetamol | Suspension (42 mL of 24 mg/mL ) | 1008 mg | Light calorie low fat breakfast | meal 2.5 h prior to dosing (semi-fed state) | Tmax prolonged Cmax unchangedAUC unchanged | Food had a significant effect on the early exposure and onset of therapeutic level of paracetamol from the paediatric suspension | [88] |
| Ritonavir | oral solution | 600 mg | 514 KCal; 9% fat, 12% protein and 79% carbohydrate) | Tmax prolonged Cmax decreased AUC decreased | [104] | ||
| Rufinamide | oral suspension (40 mg/mL) | 400 mg | High fat meal | overnight fast—4 h post dose | Tmax prolonged Cmax increased AUC increased | The rufinamide suspension is bioequivalent to the approved tablets | [105] |
| Sertraline HCl | oral solution | 100 mg | Standardised breakfast | overnight fast—4 h post dose | Cmax unchanged AUC unchanged | The pharmacokinetics of the sertraline oral solution are similar under fed and fasted conditions | [106] |
| Topiramate | oral solution (20 mL 5mg/mL) | 100 mg | High fat low calorie meal | overnight fast—4 h post dose | Tmax prolonged Cmax unchanged AUC unchanged | A high-fat, high-calorie meal delays absorption of liquid topiramate without changing overall topiramate exposure when compared to fasted conditions. | [107] |
| Tripanavir | oral solution 100 mg/mL (co administered with 200 mg ritonavir) | 500 mg of | Cmax reduced slightly AUC unchanged | Oral solution can be administered to patients either with or without food. The current label recommends the tipranavir capsules be taken with food. | [108] |
7. Predicting a Food Effect
7.1. Theoretical Models
7.2. Physical Models
7.2.1. Solubility/Dissolution Testing
7.3. In Silico Models
8. Conclusions
Conflicts of Interest
References
- Won, C.S.; Oberlies, N.H.; Paine, M.F. Mechanisms underlying food-drug interactions: Inhibition of intestinal metabolism and transport. Pharmacol. Ther. 2012, 136, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Akram, G.; Mullen, A.B. Paediatric nurses’ knowledge and practice of mixing medication into foodstuff. Int. J. Pharm. Pract. 2012, 20, 191–198. [Google Scholar] [CrossRef] [PubMed]
- BNF for Children 2011–2012. Pharmaceutical Press, 2012. Available online: http://www.pharmpress.com/product/9780853699590/bnfc (accessed on 3 July 2012).
- FDA. Guidance for Industry: Food-Effect Bioavailability and Fed Bioequivalence Studies; U.S. Department of Health and Human Services, Center for Drug Evaluation and Research (CDER), 2002; Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070241.pdf (accessed on 2 July 2012).
- FDA. Guidance for Industry: Exposure-Response Relationships—Study Design, Data Analysis and Regulatory Applications; U.S. Department of Health and Human Services; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER), 2003. [Google Scholar]
- EMA. ICH Topic E11. Clinical Investigation of Medicineal Products in the Paediatric Population. CPMP/ICH/2711/99, 2011. [Google Scholar]
- EMA. Guideline on the role of pharmacokinetics in the development of medicinal products in the paediatric population. EMEA/CHMP/EWP/147013/2004, 2006. [Google Scholar]
- Rolan, P.E.; Mercer, A.J.; Weatherley, B.C.; Holdich, T.; Meire, H.; Peck, R.W.; Ridout, G.; Posner, J. Examination of some factors responsible for a food-induced increase in absorption of atovaquone. Br. J. Clin. Pharmacol. 1994, 37, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Crounse, R.G. Human pharmacology of griseofulvin: The effect of fat intake on gastrointestinal absorption. J. Investig. Dermatol. 1961, 37, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Finkel, Y.; Bolme, P.; Eriksson, M. The effect of food on the oral absorption of penicillin V preparations in children. Acta Pharmacol. Toxicol. (Copenh) 1981, 49, 301–304. [Google Scholar] [CrossRef] [PubMed]
- EMA. Concept Paper on Extrapolation of Efficacy and Safety in Medicine Development; EMA/129698/2012; Human Medicines Development and Evaluation: UK, 2012; Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/04/WC500142358.pdf (accessed on 28 November 2012).
- Klein, S.; Butler, J.; Hempenstall, J.M.; Reppas, C.; Dressman, J.B. Media to simulate the postprandial stomach I. Matching the physicochemical characteristics of standard breakfasts. J. Pharm. Pharmacol. 2004, 56, 605–610. [Google Scholar] [CrossRef] [PubMed]
- US Department of Agriculture. Meals and Snacks: Distribution of Meal Patterns and Snack Occasions, by Gender and Age, What We Eat in America NHANES 2009–2010. Available online: http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0910/Table_33_DMP_GEN_09.pdf (accessed on 30 October 2012).
- WHO Working Group on the Growth Reference Protocol; WHO Task Force on Methods for the Natural Regulation of Fertility. Growth of healthy infants and the timing, type, and frequency of complementary foods. Am. J. Clin. Nutr. 2002, 76, 620–627. [Google Scholar]
- Klein, S.; Butler, J.; Hempenstall, J.M.; Reppas, C.; Dressman, J.B. Media to simulate the postprandial stomach I. Matching the physicochemical characteristics of standard breakfasts. J. Pharm. Pharmacol. 2004, 56, 605–610. [Google Scholar] [CrossRef] [PubMed]
- WHO. Complementary Feeding of Young Children in Developing Countries. Availble online: http://www.who.int/nutrition/publications/infantfeeding/WHO_NUT_98.1/en/index.html (accessed on 31 October 2012).
- Francis, D.E.M. Nutrition for Children; Blackwell Scientific Publications: Oxford, UK, 1986; ISBN 13: 9780632014781. [Google Scholar]
- Kossena, G.A.; Charman, W.N.; Wilson, C.G.; O’Mahony, B.; Lindsay, B.; Hempenstall, J.M.; Davison, C.L.; Crowley, P.J.; Porter, C.J. Low dose lipid formulations: effects on gastric emptying and biliary secretion. Pharm. Res. 2007, 24, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Kaye, J.L. Review of paediatric gastrointestinal physiology data relevant to oral drug delivery. Int. J. Clin. Pharm. 2011, 33, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Khanna, N.N.; Soda, D.M.; Tsuzuki, O.; Stern, L. Pharmacokinetics of acetaminophen in the human neonate: Formation of acetaminophen glucuronide and sulfate in relation to plasma bilirubin concentration and D-glucaric acid excretion. Pediatrics 1975, 55, 818–825. [Google Scholar] [PubMed]
- Hassan, M.; Ljungman, P.; Bolme, P.; Ringden, O.; Syruckova, Z.; Bekassy, A.; Stary, J.; Wallin, I.; Kallberg, N. Busulfan bioavailability. Blood 1994, 84, 2144–2150. [Google Scholar] [PubMed]
- Silverio, J.; Poole, J.W. Serum concentrations of ampicillin in newborn infants after oral administration. Pediatrics 1973, 51, 578–580. [Google Scholar] [PubMed]
- Jusko, W.J.; Khanna, N.; Levy, G.; Stern, L.; Yaffe, S.J. Riboflavin absorption and excretion in the neonate. Pediatrics 1970, 45, 945–949. [Google Scholar] [PubMed]
- Toublanc, N.; Sargentini-Maier, M.L.; Lacroix, B.; Jacqmin, P.; Stockis, A. Retrospective population pharmacokinetic analysis of levetiracetam in children and adolescents with epilepsy: dosing recommendations. Clin. Pharmacokinet. 2008, 47, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Garzi, A.; Messina, M.; Frati, F.; Carfagna, L.; Zagordo, L.; Belcastro, M.; Parmiani, S.; Sensi, L.; Marcucci, F. An extensively hydrolysed cow’s milk formula improves clinical symptoms of gastroesophageal reflux and reduces the gastric emptying time in infants. Allergol. Immunopathol. 2002, 30, 36–41. [Google Scholar]
- McClure, R.J.; Newell, S.J. Effect of fortifying breast milk on gastric emptying. Arch. Dis. Child. Fetal Neonatal Ed. 1996, 74, F60–F62. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.N.; Stubbs, D.F. The volume and energy content of meals as determinants of gastric emptying. J. Physiol. 1975, 245, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Sunesen, V.H.; Vedelsdal, R.; Kristensen, H.G.; Christrup, L.; Mullertz, A. Effect of liquid volume and food intake on the absolute bioavailability of danazol, a poorly soluble drug. Eur. J. Pharm. Sci. 2005, 24, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.N.; Smith, J.L.; Jiang, C.L. Effect of Meal Volume and Energy Density on the Gastric-Emptying of Carbohydrates. Gastroenterology 1985, 89, 1326–1330. [Google Scholar] [PubMed]
- Collins, P.J.; Horowitz, M.; Maddox, A.; Myers, J.C.; Chatterton, B.E. Effects of increasing solid component size of a mixed solid/liquid meal on solid and liquid gastric emptying. Am. J. Physiol. 1996, 271, G549–G554. [Google Scholar] [PubMed]
- Meeroff, J.C.; Go, V.L.W.; Phillips, S.F. Control of Gastric-Emptying by Osmolality of Duodenal Contents in Man. Gastroenterology 1975, 68, 1144–1151. [Google Scholar] [PubMed]
- Little, T.J.; Gopinath, A.; Patel, E.; McGlone, A.; Lassman, D.J.; D’amato, M.; McLaughlin, J.T.; Thompson, D.G. Gastric emptying of hexose sugars: role of osmolality, molecular structure and the CCK1 receptor. Neurogastroenterol. Motil. 2010, 22, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, Y.; Kusano, M.; Kawamura, O.; Zai, H.; Kuribayashi, S.; Higuchi, T.; Nagoshi, A.; Maeda, M.; Mori, M. High-viscosity liquid meal accelerates gastric emptying. Neurogastroenterol. Motil. 2007, 19, 879–886. [Google Scholar] [CrossRef] [PubMed]
- WHO. Feeding and Nutrition of Infants and Young Children. Available online: http://www.who.int/nutrition/publications/infantfeeding/9289013540/en/index.html (accessed on 31 October 2012).
- Bateman, D.N. Effects of meal temperature and volume on the emptying of liquid from the human stomach. J. Physiol. 1982, 331, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Amano, Y.; Takahashi, Y.; Mishima, Y.; Moriyama, N.; Miyake, T.; Ishimura, N.; Ishihara, S.; Kinoshita, Y. Gastric emptying of liquid and solid meals at various temperatures. J. Gastroenterol. 2009, 44, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Fadda, H.M.; McConnell, E.L.; Short, M.D.; Basit, A.W. Meal-induced acceleration of tablet transit through the human small intestine. Pharm. Res. 2009, 26, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Edginton, A.N.; Fotaki, N. Oral Drug Absorption in Pediatric Populations. In Oral Drug Absorption: Prediction and Assessment; Dressman, J.B., Reppas, C., Eds.; Informa Healthcare: New York, NY, USA, 2010; pp. 108–126. [Google Scholar]
- Blake, M.J.; Abdel-Rahman, S.M.; Pearce, R.E.; Leeder, J.S.; Kearns, G.L. Effect of Diet on the Development of Drug Metabolism by Cytochrome P-450 Enzymes in Healthy Infants. Pediatr. Res. 2006, 60, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.N.; Thomson, M. Intestinal metabolism and transport of drugs in children: The effects of age and disease. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.-I. Food-drug interactions via human cytochrome P450 3A (CYP3A). Drug Metab. Drug Interact. 2004, 20, 195–217. [Google Scholar] [CrossRef]
- Ginsburg, C.M.; McCracken, G.H., Jr.; Thomas, M.L.; Clahsen, J. Comparative pharmacokinetics of amoxicillin and ampicillin in infants and children. Pediatrics 1979, 64, 627–631. [Google Scholar] [PubMed]
- Eshelman, F.N.; Spyker, D.A. Pharmacokinetics of amoxicillin and ampicillin: Crossover study of the effect of food. Antimicrob. Agents Chemother. 1978, 14, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Kearns, G.L.; Abdel-Rahman, S.M.; Jacobs, R.F.; Wells, T.G.; Borin, M.T. Cefpodoxime pharmacokinetics in children: effect of food. Pediatr. Infect. Dis. J. 1998, 17, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.S.; Heald, D.L.; Barker, K.B.; Patel, R.K.; Spillers, C.R.; Watts, K.C.; Batts, D.H.; Euler, A.R. The effects of gastric pH and food on the pharmacokinetics of a new oral cephalosporin, cefpodoxime proxetil. Clin. Pharmacol. Ther. 1989, 46, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Tetzlaff, T.R.; McCracken, G.H., Jr.; Thomas, M.L. Bioavailability of cephalexin in children: Relationship to drug formulations and meals. J. Pediatr. 1978, 92, 292–294. [Google Scholar] [CrossRef]
- Wise, R. The pharmacokinetics of the oral cephalosporins—A review. J. Antimicrob. Chemother. 1990, 26 (Suppl. E), 13–20. [Google Scholar] [CrossRef] [PubMed]
- Guay, D.R.; Craft, J.C. Overview of the pharmacology of clarithromycin suspension in children and a comparison with that in adults. Pediatr. Infect. Dis. J. 1993, 12 (Suppl. 3), S106–S111. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, K.A. Clinical pharmacokinetics of clarithromycin. Clin. Pharmacokinet. 1999, 37, 385–398. [Google Scholar] [CrossRef] [PubMed]
- De Guchtenaere, A.; Hoebeke, P.; Dehoorne, J.; Raes, A.; van Laecke, E.; Vande Walle, J. 734 Pharmacokinetic Data on Oral Desmopressin Reducing Dosage by Changing to a New Oral Lyophilisate (Melt) Formulation. J. Urol. 2012, 187 (Suppl. 4), e301. [Google Scholar] [CrossRef] [PubMed]
- Rittig, S.; Jensen, A.R.; Jensen, K.T.; Pedersen, E.B. Effect of food intake on the pharmacokinetics and antidiuretic activity of oral desmopressin (DDAVP) in hydrated normal subjects. Clin. Endocrinol. 1998, 48, 235–241. [Google Scholar] [CrossRef]
- Stevens, R.C.; Rodman, J.H.; Yong, F.H.; Carey, V.; Knupp, C.A.; Frenkel, L.M. Effect of food and pharmacokinetic variability on didanosine systemic exposure in HIV-infected children. Pediatric AIDS Clinical Trials Group Protocol 144 Study Team. AIDS Res. Hum. Retrovir. 2000, 16, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Shyu, W.C.; Knupp, C.A.; Pittman, K.A.; Dunkle, L.; Barbhaiya, R.H. Food-induced reduction in bioavailability of didanosine. Clin. Pharmacol. Ther. 1991, 50, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, C.M.; McCracken, G.H., Jr.; Petruska, M.; Olsen, K. Effect of feeding on bioavailability of griseofulvin in children. J. Pediatr. 1983, 102, 309–311. [Google Scholar] [CrossRef]
- Ahmed, I.S.; Aboul-Einien, M.H.; Mohamed, O.H.; Farid, S.F. Relative bioavailability of griseofulvin lyophilized dry emulsion tablet vs. immediate release tablet: A single-dose, randomized, open-label, six-period, crossover study in healthy adult volunteers in the fasted and fed states. Eur. J. Pharm. Sci. 2008, 35, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Borrmann, S.; Sallas, W.M.; Machevo, S.; González, R.; Björkman, A.; Mårtensson, A.; Hamel, M.; Juma, E.; Peshu, J.; Ogutu, B.; et al. The effect of food consumption on lumefantrine bioavailability in African children receiving artemether–lumefantrine crushed or dispersible tablets (Coartem®) for acute uncomplicated Plasmodium falciparum malaria. Trop. Med. Int. Health 2010, 15, 434–441. [Google Scholar] [PubMed]
- White, N.J.; van Vugt, M.; Ezzet, F. Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine. Clin. Pharmacokinet. 1999, 37, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, R.; Balis, F.M.; Ferrara, P.; Lasorella, A.; Poplack, D.G.; Mastrangelo, R. Influence of food intake on bioavailability of oral 6-mercaptopurine in children with acute lymphoblastic leukemia. Pediatr. Hematol. Oncol. 1986, 3, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Burton, N.K.; Barnett, M.J.; Aherne, G.W.; Evans, J.; Douglas, I.; Lister, T.A. The effect of food on the oral administration of 6-mercaptopurine. Cancer Chemother. Pharmacol. 1986, 18, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Sofianou-Katsoulis, A.; Khakoo, G.; Kaczmarski, R. Reduction in bioavailability of 6-mercaptopurine on simultaneous administration with cow’s milk. Pediatr. Hematol. Oncol. 2006, 23, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Lönnerholm, G.; Kreuger, A.; Lindström, B.; Myrdal, U. Oral Mercaptopurine in Childhood Leukemia: Influence of Food Intake on Bioavailability. Pediatr. Hematol. Oncol. 1989, 6, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, C.R.; Glasgow, J.F.T.; Welshman, S.G.; Bridges, J.M. Can food influence the absorption of methotrexate in children with acute lymphoblastic leukaemia? Lancet 1980, 316, 944–946. [Google Scholar] [CrossRef]
- Kozloski, G.D.; de Vito, J.M.; Kisicki, J.C.; Johnson, J.B. The effect of food on the absorption of methotrexate sodium tablets in healthy volunteers. Arthritis Rheum. 1992, 35, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Stockley’s Drug Interactions; Baxter, K.; Preston, C.L. (Eds.) Royal Pharmaceutical Society of Great Britain, 2012; Available online: http://www.medicinescomplete.com/mc/stockley/current/x00-2781.htm (accessed on 31 October 2012).
- McCracken, G.H., Jr.; Ginsburg, C.M.; Clahsen, J.C.; Thomas, M.L. Pharmacologic evaluation of orally administered antibiotics in infants and children: Effect of feeding on bioavailability. Pediatrics 1978, 62, 738–743. [Google Scholar] [PubMed]
- Okuno, A.; Taguchi, T.; Inyaku, F.; Yano, K.; Suzuki, Y. Pharmacokinetics of propylthiouracil in children and adolescents with Graves disease after a single oral dose. Pediatr. Pharmacol. (N. Y.) 1983, 3, 43–47. [Google Scholar]
- Melander, A.; Wåhlin, E.; Danielson, K.; Hanson, A. Bioavailability of propylthiouracil: Interindividual variation and influence of food intake. Acta Med. Scand. 1977, 201, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S. Effects of food on the absorption of theophylline in children. J. Allergy Clin. Immunol. 1986, 78 4 Pt 2, 704–709. [Google Scholar] [CrossRef]
- Jonkman, J.H. Food interactions with sustained-release theophylline preparations. A review. Clin. Pharmacokinet. 1989, 16, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, D.L.; Patel, N.; Lennard, L.; Lilleyman, J.S. 6-Thioguanine in children with acute lymphoblastic leukaemia: influence of food on parent drug pharmacokinetics and 6-thioguanine nucleotide concentrations. Br. J. Clin. Pharmacol. 2001, 51, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Zent, C.; Smith, P. Study of the effect of concomitant food on the bioavailability of rifampicin, isoniazid and pyrazinamide. Tuber. Lung Dis. 1995, 76, 109–113. [Google Scholar] [CrossRef]
- Lin, J.H. Role of pharmacokinetics in the discovery and development of indinavir. Adv. Drug Deliv. Rev. 1999, 39, 33–49. [Google Scholar] [CrossRef]
- Neuvonen, P.J. The effect of magnesium hydroxide on the oral absorption of ibuprofen, ketoprofen and diclofenac. Br. J. Clin. Pharmacol. 1991, 31, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Mattick, L.R.; Moyer, J.C. Composition of apple juice. J. Assoc. Off. Anal. Chem. 1983, 66, 1251–1255. [Google Scholar] [PubMed]
- Allen, L.V.; Stiles, M.L.; Prince, S.J.; McLaury, H.J.; Sylvestri, M.F. Stability of ramipril in water, apple juice, and applesauce. Am. J. Health-Syst. Pharm. 1995, 52, 2433–2436. [Google Scholar] [PubMed]
- Pao, L.H.; Zhou, S.Y.; Cook, C.; Kararli, T.; Kirchhoff, C.; Truelove, J.; Karim, A.; Fleisher, D. Reduced systemic availability of an antiarrhythmic drug, bidisomide, with meal co-administration: relationship with region-dependent intestinal absorption. Pharm. Res. 1998, 15, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Reppas, C.; Eleftheriou, G.; Macheras, P.; Symillides, M.; Dressman, J.B. Effect of elevated viscosity in the upper gastrointestinal tract on drug absorption in dogs. Eur. J. Pharm. Sci. 1998, 6, 131–139. [Google Scholar] [CrossRef]
- Mimoz, O.; Binter, V.; Jacolot, A.; Edouard, A.; Tod, M.; Petitjean, O.; Samii, K. Pharmacokinetics and absolute bioavailability of ciprofloxacin administered through a nasogastric tube with continuous enteral feeding to critically ill patients. Intensive Care Med. 1998, 24, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.W.; Amsden, G.W. Is it really OK to take this with food? Old interactions with a new twist. J. Clin. Pharmacol. 2002, 42, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Eshra, A.G.; Etman, M.A.; Naggar, V.F. Effect of milk and food on the bioavailability of ketoprofen in man. Int. J. Pharm. 1988, 44, 9–14. [Google Scholar] [CrossRef]
- De Lemos, M.L.; Hamata, L.; Jennings, S.; Leduc, T. Interaction between mercaptopurine and milk. J. Oncol. Pharm. Pract. 2007, 13, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, R. Enteral feeding: Drug/nutrient interaction. Clin. Nutr. 2001, 20, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Agency, E.M. ICH Topic Q4B Annex 5 Disintegration Test General Chapter. EMEA/CHMP/ICH/308895/2008, 2008. [Google Scholar]
- Dalenback, J.; Abrahamson, H.; Bjornson, E.; Fandriks, L.; Mattsson, A.; Olbe, L.; Svennerholm, A.; Sjovall, H. Human duodenogastric reflux, retroperistalsis, and MMC. Am. J. Physiol. 1998, 275 3 Pt 2, R762–R769. [Google Scholar] [PubMed]
- Sanaka, M.; Kuyama, Y.; Shimomura, Y.; Qi, J.F.; Okamura, S.; Hao, Y.; Jainguo, C.; Mineshita, S. Gastric emptying of liquids is delayed by co-ingesting solids: A study using salivary paracetamol concentrations. J. Gastroenterol. 2002, 37, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, B.; Albery, T.; Eriksson, A.; Gustafsson, I.; Sjöberg, M. Food effects on tablet disintegration. Eur. J. Pharm. Sci. 2004, 22, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.; Anneveld, B.; Goudappel, G.-J.; Duchateau, G.; Annaert, P.; Augustijns, P.; Zeijdner, E. Food-dependent disintegration of immediate release fosamprenavir tablets: In vitro evaluation using magnetic resonance imaging and a dynamic gastrointestinal system. Eur. J. Pharm. Biopharm. 2011, 77, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Collaku, A.; Heaslip, L.; Yue, Y.; Starkey, Y.-Y.; Clarke, G.; Kronfeld, N. A new rapidly absorbed paediatric paracetamol suspension. A six-way crossover pharmacokinetic study comparingthe rate and extent of paracetamol absorption from a new paracetamol suspension with two marketed paediatric formulations. Drug Dev. Ind. Pharm. 2012, 38, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Charkoftaki, G.; Kytariolos, J.; Macheras, P. Novel milk-based oral formulations: Proof of concept. Int. J. Pharm. 2010, 390, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Kytariolos, J.; Charkoftaki, G.; Smith, J.R.; Voyiatzis, G.; Chrissanthopoulos, A.; Yannopoulos, S.N.; Fatouros, D.G.; Macheras, P. Stability and physicochemical characterization of novel milk-based oral formulations. Int. J. Pharm. 2013, 444, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Research, F. a. D.A. C. f. D.E. a. NDA 202067 Clobazam. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202067Orig1s000ClinPharmR.pdf (accessed on 8 January 2013).
- Chun, A.H.C.; Erdman, K.; Zhang, Y.; Achari, R.; Cavanaugh, J.H. Effect on bioavailability of admixing the contents of lansoprazole capsules with selected soft foods. Clin. Ther. 2000, 22, 231–236. [Google Scholar] [CrossRef]
- Chun, A.H.C.; Erdman, K.; Chiu, Y.-L.; Pilmer, B.L.; Achari, R.; Cavanaugh, J.H. Bioavailability of lansoprazole granules administered in juice or soft food compared with the intact capsule formulation. Clin. Ther. 2002, 24, 1322–1331. [Google Scholar] [CrossRef]
- Fay, M.A.; Sheth, R.D.; Gidal, B.E. Oral absorption kinetics of levetiracetam: The effect of mixing with food or enteral nutrition formulas. Clin. Ther. 2005, 27, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Pentikis, H.S.; Simmons, R.D.; Benedict, M.F.; Hatch, S.J. Methylphenidate Bioavailability in Adults When an Extended-Release Multiparticulate Formulation Is Administered Sprinkled on Food or as an Intact Capsule. J. Am. Acad.Child Adolesc. Psychiatry 2002, 41, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Eliot, L.; Butler, J.; Devane, J.; Loewen, G. Pharmacokinetic evaluation of a sprinkle-dose regimen of a once-daily, extended-release morphine formulation. Clin. Ther. 2002, 24, 260–268. [Google Scholar] [CrossRef]
- Thyssen, A.; Solanki, B.; Treem, W. Randomized, Open-Label, Single-Dose, Crossover, Relative Bioavailability Study in Healthy Adults, Comparing the Pharmacokinetics of Rabeprazole Granules Administered Using Soft Food or Infant Formula as Dosing Vehicle Versus Suspension. Clin. Ther. 2012, 34, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Foulds, G.; Luke, D.R.; Teng, R.; Willavize, S.A.; Friedman, H.; Curatolo, W.J. The absence of an effect of food on the bioavailability of azithromycin administered as tablets, sachet or suspension. J. Antimicrob. Chemother. 1996, 37 (suppl. C), 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, J.M.; Noe, A.; Berthier, S.; McMahon, L.; Langholff, W.K.; Marion, A.S.; Hoyer, P.F.; Ettenger, R.; Rordorf, C. Clinical Development of an Everolimus Pediatric Formulation: Relative Bioavailability, Food Effect, and Steady-State Pharmacokinetics. J. Clin. Pharmacol. 2003, 43, 141–147. [Google Scholar] [CrossRef] [PubMed]
- FDA Centre for Drug Evaluation and Research. NDA 22–571 Glycopyrrolate. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022571Orig1s000ClinPharmR.pdf (accessed on 8 January 2013).
- FDA Centre for Drug Evaluation and Research. NDA 20–944 Advil (ibuprofen). Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/98/20944_Advil_biopharmr.pdf (accessed on 8 January 2013).
- FDA Centre for Drug Evaluation and Research. Application 020778 Viracept oral powder. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/97/020778ap.pdf (accessed on 8 January 2013).
- FDA Centre for Drug Evaluation and Research. NDA 21–818,21–498/S-003 Nitazoxanide. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021818s000_ClinPharmR.pdf (accessed on 8 January 2013).
- Ibarra, M.; Fagiolino, P.; Vázquez, M.; Ruiz, S.; Vega, M.; Bellocq, B.; Pérez, M.; González, B.; Goyret, A. Impact of food administration on lopinavir-ritonavir bioequivalence studies. Eur. J. Pharm. Sci. 2012, 46, 516–521. [Google Scholar] [CrossRef] [PubMed]
- FDA Centre for Drug Evaluation and Research. NDA 201367 Rufinamide. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/201367Orig1s000ClinPharmR.pdf (accessed on 8 January 2013).
- FDA Centre for Drug Evaluation and Research. NDA 20–990 Sertraline HCl. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/99/20990_Zoloft_biopharmr.pdf (accessed on 8 January 2013).
- FDA Centre for Drug Evaluation and Research. NDA 20844 (S031) Topiramate. Available online: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm129621.pdf (accessed on 8 January 2013).
- FDA Centre for Drug Evaluation and Research. NDA 21–822 Tipranavir. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/021822s000ClinPharmr.pdf (accessed on 8 January 2013).
- Shah, T.; Tse, A.P.Y.; Gill, H.; Wong, I.C.K.; Sutcliffe, A.; Gringras, P.; Appleton, R.; Tuleu, C. Administration of melatonin mixed with soft food and liquids for children with neurodevelopmental difficulties. Dev. Med. Child Neurol. 2008, 50, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N. A quantitative approach to probe the dependence and correlation of food-effect with aqueous solubility, dose/solubility ratio, and partition coefficient (Log P) for orally active drugs administered as immediate-release formulations. Drug Dev. Res. 2005, 65, 55–75. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Galia, E.; Nicolaides, E.; Hörter, D.; Löbenberg, R.; Reppas, C.; Dressman, J.B. Evaluation of various dissolution media for predicting In vivo performance of class I and II drugs. Pharm. Res. 1998, 15, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Ashby, L.J.; Beezer, A.E.; Buckton, G. In vitro dissolution testing of oral controlled release preparations in the presence of artificial foodstuffs. I. Exploration of alternative methodology: microcalorimetry. Int. J. Pharm. 1989, 51, 245–251. [Google Scholar] [CrossRef]
- Sunesen, V.H.; Pedersen, B.L.; Kristensen, H.G.; Müllertz, A. In vivo in vitro correlations for a poorly soluble drug, danazol, using the flow-through dissolution method with biorelevant dissolution media. Eur. J. Pharm. Sci. 2005, 24, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Masaoka, Y.; Sakuma, S.; Yamashita, S. Effect of food intake on the oral absorption of poorly water-soluble drugs: In vitro assessment of drug dissolution and permeation assay system. J. Pharm. Sci. 2006, 95, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Blanquet, S.; Zeijdner, E.; Beyssac, E.; Meunier, J.P.; Denis, S.; Havenaar, R.; Alric, M. A dynamic artificial gastrointestinal system for studying the behavior of orally administered drug dosage forms under various physiological conditions. Pharm. Res. 2004, 21, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Vardakou, M.; Mercuri, A.; Barker, S.A.; Craig, D.Q.; Faulks, R.M.; Wickham, M.S. Achieving antral grinding forces in biorelevant in vitro models: Comparing the USP dissolution apparatus II and the dynamic gastric model with human in vivo data. AAPS PharmSciTech 2011, 12, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Havenaar, R.; Anneveld, B.; Hanff, L.M.; de Wildt, S.N.; de Koning, B.A.E.; Mooij, M.G.; Lelieveld, J.P.A.; Minekus, M. In vitro gastrointestinal model (TIM) with predictive power, even for infants and children? Int. J. Pharm. 2013, 457, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Jantratid, E.; Kesisoglou, F.; Vertzoni, M.; Reppas, C.; Dressman, J.B. Predicting the oral absorption of a poorly soluble, poorly permeable weak base using biorelevant dissolution and transfer model tests coupled with a physiologically based pharmacokinetic model. Eur. J. Pharm. Biopharm. 2012, 82, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.S.; Della Casa Alberighi, O.; Laer, S.; Meibohm, B. Physiologically Based Pharmacokinetic (PBPK) Modeling in Children. Clin. Pharmacol. Ther. 2012, 92, 40–49. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batchelor, H.K. Influence of Food on Paediatric Gastrointestinal Drug Absorption Following Oral Administration: A Review. Children 2015, 2, 244-271. https://doi.org/10.3390/children2020244
Batchelor HK. Influence of Food on Paediatric Gastrointestinal Drug Absorption Following Oral Administration: A Review. Children. 2015; 2(2):244-271. https://doi.org/10.3390/children2020244
Chicago/Turabian StyleBatchelor, Hannah K. 2015. "Influence of Food on Paediatric Gastrointestinal Drug Absorption Following Oral Administration: A Review" Children 2, no. 2: 244-271. https://doi.org/10.3390/children2020244
APA StyleBatchelor, H. K. (2015). Influence of Food on Paediatric Gastrointestinal Drug Absorption Following Oral Administration: A Review. Children, 2(2), 244-271. https://doi.org/10.3390/children2020244