Novel Chirogenic Systems and Sensing Materials for Stereoselective Sensors Development
A topical collection in Chemosensors (ISSN 2227-9040). This collection belongs to the section "Materials for Chemical Sensing".
Viewed by 49171Editors
Interests: sensors; induced chirality; chirality transfer; supramolecular chirality; chiral chromophores; circular dichroism; chiral materials and surfaces
Special Issues, Collections and Topics in MDPI journals
Interests: chiral compounds; synthesis of macrocycles; host-guest chemistry; chirality induction; cucurbiturils
Interests: chemical sensors; porphyrins; corroles; sensor arrays; supramolecular chemistry; nanostructured materials; thin films
Special Issues, Collections and Topics in MDPI journals
Interests: porphyrinoid synthesis; chiral porphyrin aggregates; chiral materials; stereoselective sensors
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Chirality (from the Greek word “kheir", meaning “hand”) is a fundamental concept that has been recognised in chemistry since the second half of nineteenth century. In fact, hard as they try, nobody could ever overestimate its importance, that extends from the synthesis of organic and inorganic compounds, pharmaceutical and biologically active molecules, theoretical studies and technological application, to understanding the basic principles and origin of the emergence of Life on our planet.
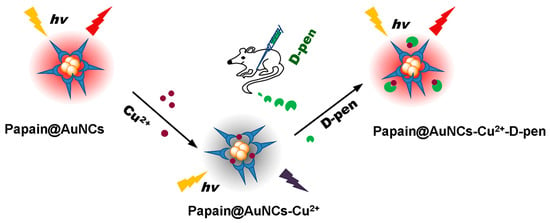
A chiral species is a single molecule or molecular assembly that cannot be superimposed with its mirror image. Chiral molecules are consequently present as two stereoisomers, called enantiomers, and, importantly, they are the main building blocks of living organism. On a daily basis, chiral molecules are conventionally used and produced by pharmaceutical, food, agrochemical, perfume, and cosmetics industries. As a result, chiral waste becomes an extremely important issue at present. Chiral compounds can be ecologically hazardous, due to their high biological activity, creating a global pollution problem. It is of note, that enantiomers have a different impact on living organisms making it extremely important to differentiate these stereoisomers, which is extremely a difficult and challenging task and usually requires highly specific and costly instruments. Yet, the stereoisomerism of contaminants is presently not considered in detail. For example, ~25% of all pesticides produced are chiral compounds and in many cases they are used as racemic mixtures, while about 70–80% of medical drugs are enantiopure molecules. In this context, the development of portable chemical sensors devices which are reliable, sensitive and rapid, capable of fast, simple and real-time in situ and on site analysis for sensing and discrimination of chiral molecules presents an attractive breakthrough target compared to existing standard instrumental methods.
Therefore, the aim of this Topical Collection is to highlight and overview all aspects of chiral pollution on environment and corresponding detection by using modern analytical approaches.
This issue will also include the design and fabrication of organic/inorganic as well as hybrid sensitive materials. Different aspects will be covered ranging, for example, from the synthesis of the proper building blocks and their characterization to their eventual deposition in solid-state.
All types of papers, including comprehensive reviews on general environmental issues, full experimental or theoretical papers, comments, and others are welcome for consideration.
Prof. Dr. Victor Borovkov
Prof. Dr. Riina Aav
Prof. Dr. Roberto Paolesse
Dr. Manuela Stefanelli
Dr. Donato Monti
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Chemosensors is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2000 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- chiral pollutants
- environment
- chirality
- stereoisomerism
- enantiomers
- chirality sensors
- self-assembly
- supramolecular systems