Bipolar Electrochemical Analysis of Chirality in Complex Media through Miniaturized Stereoselective Light-Emitting Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Enantioselective HPLC
2.3. Circular Dichroism
2.4. Electrochemistry
3. Results
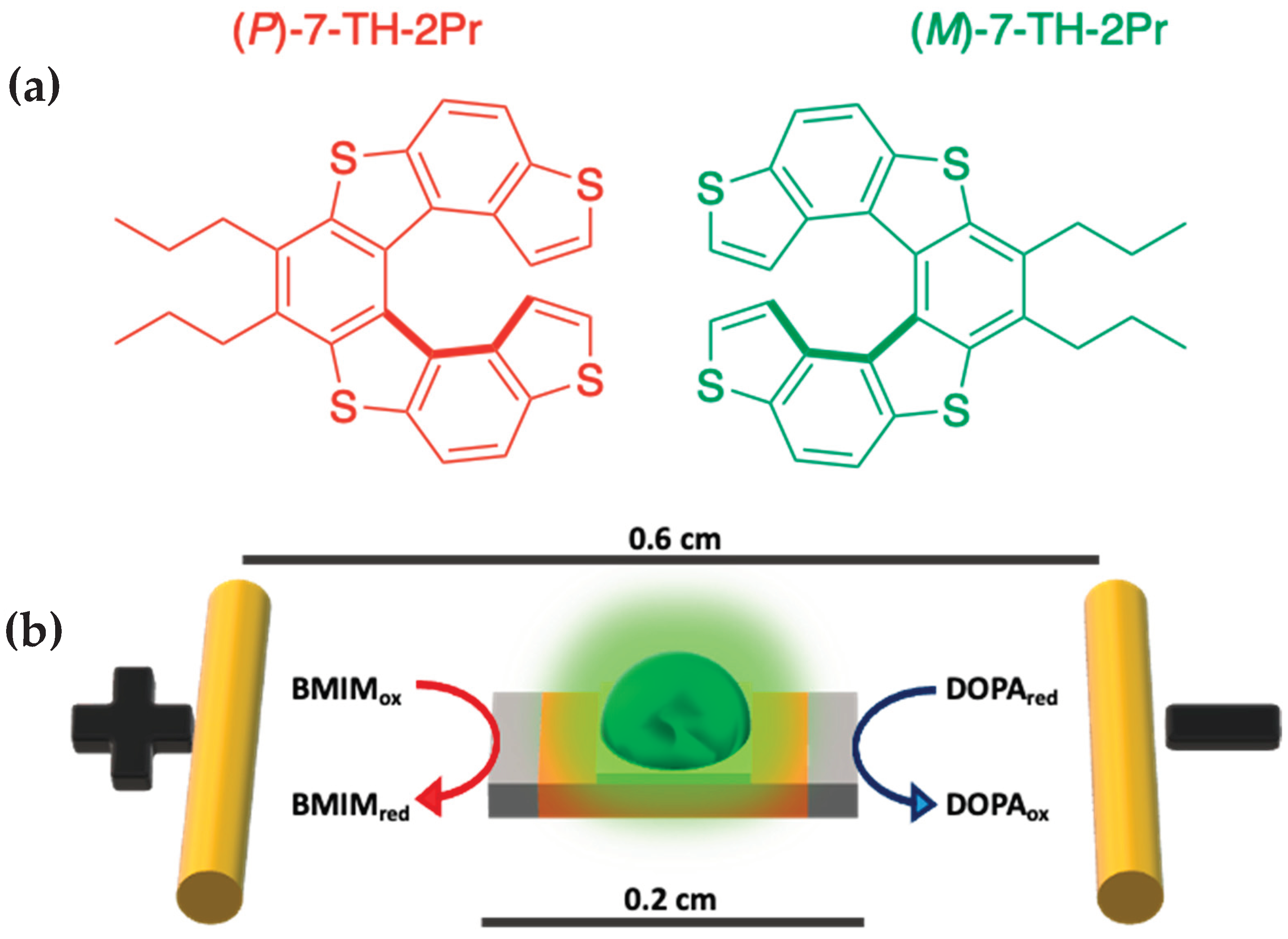
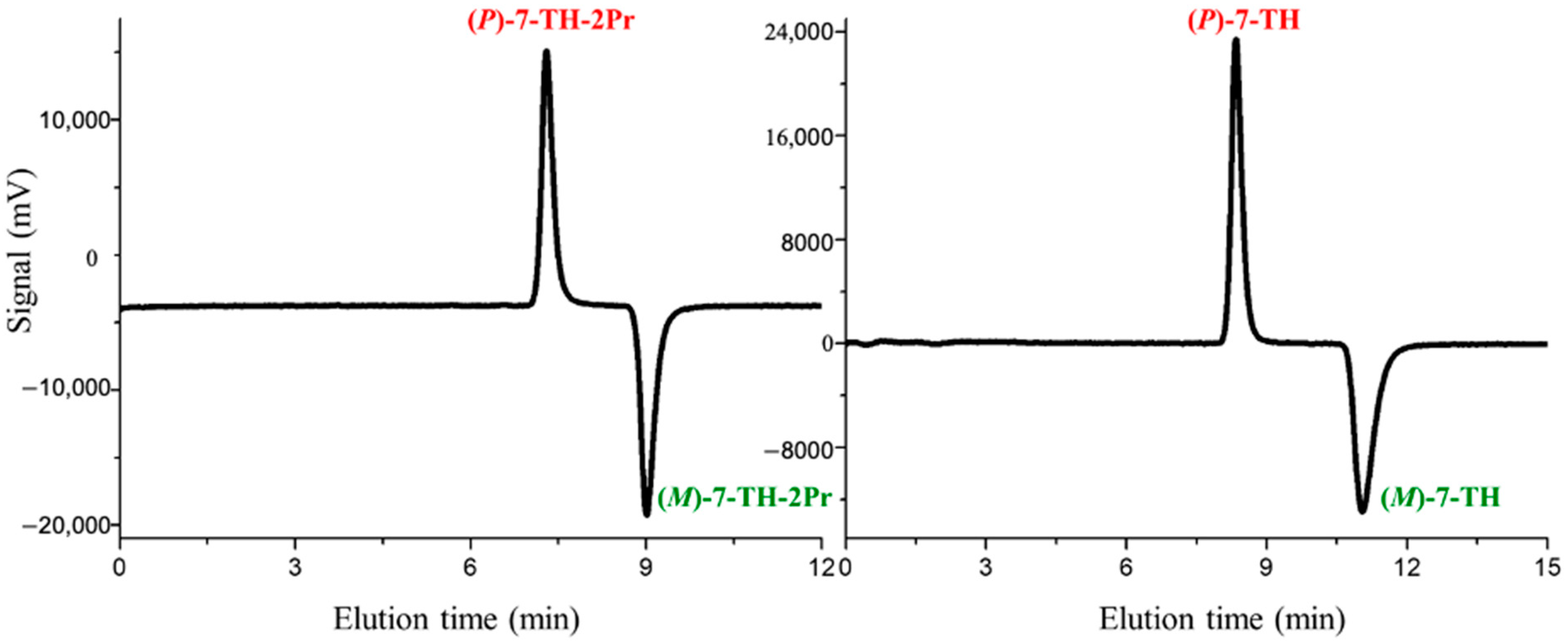
3.1. HLC Separation and Absolute Configuration Assignment of the Enantiomers of 7-TH-2Pr
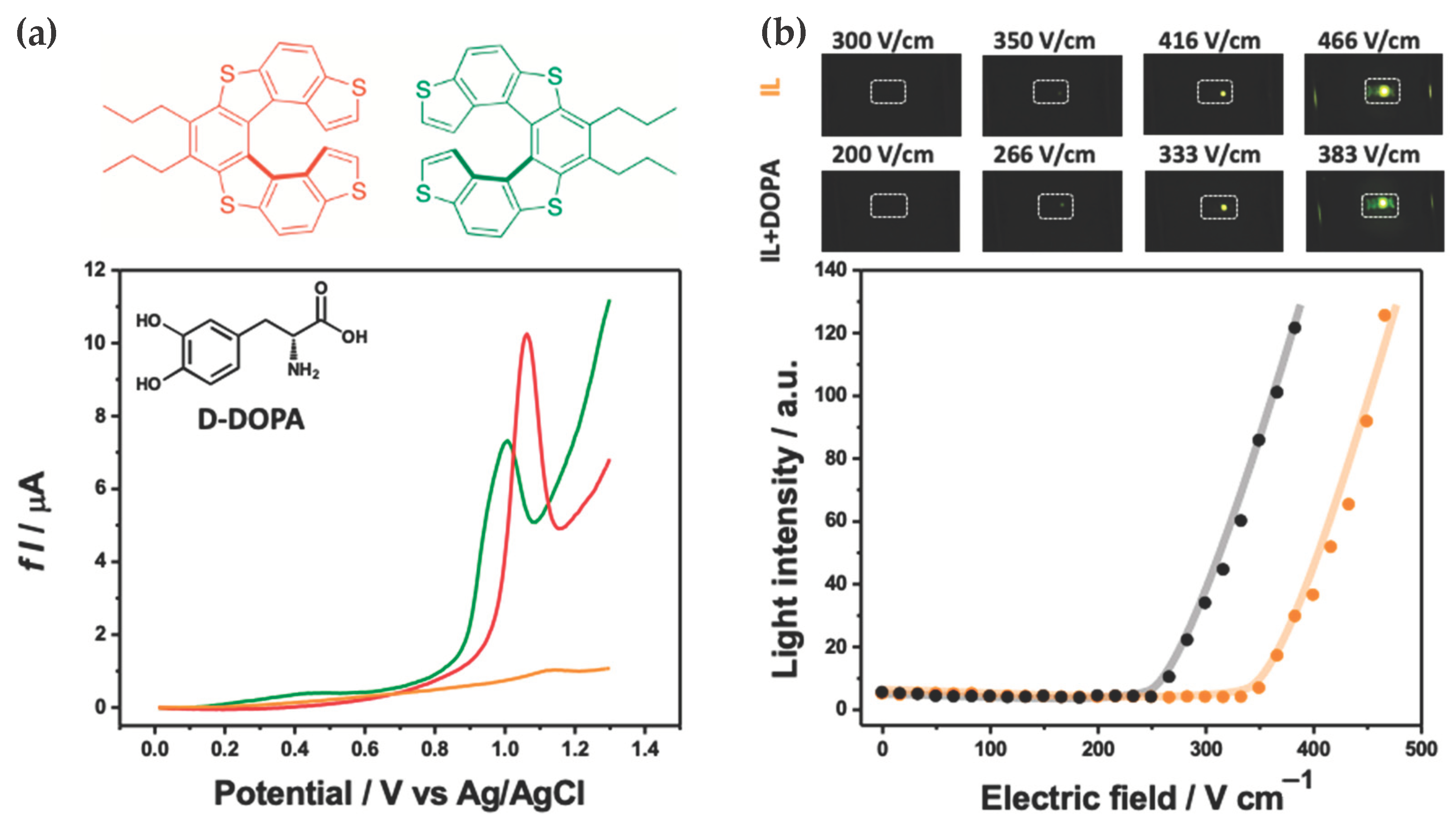
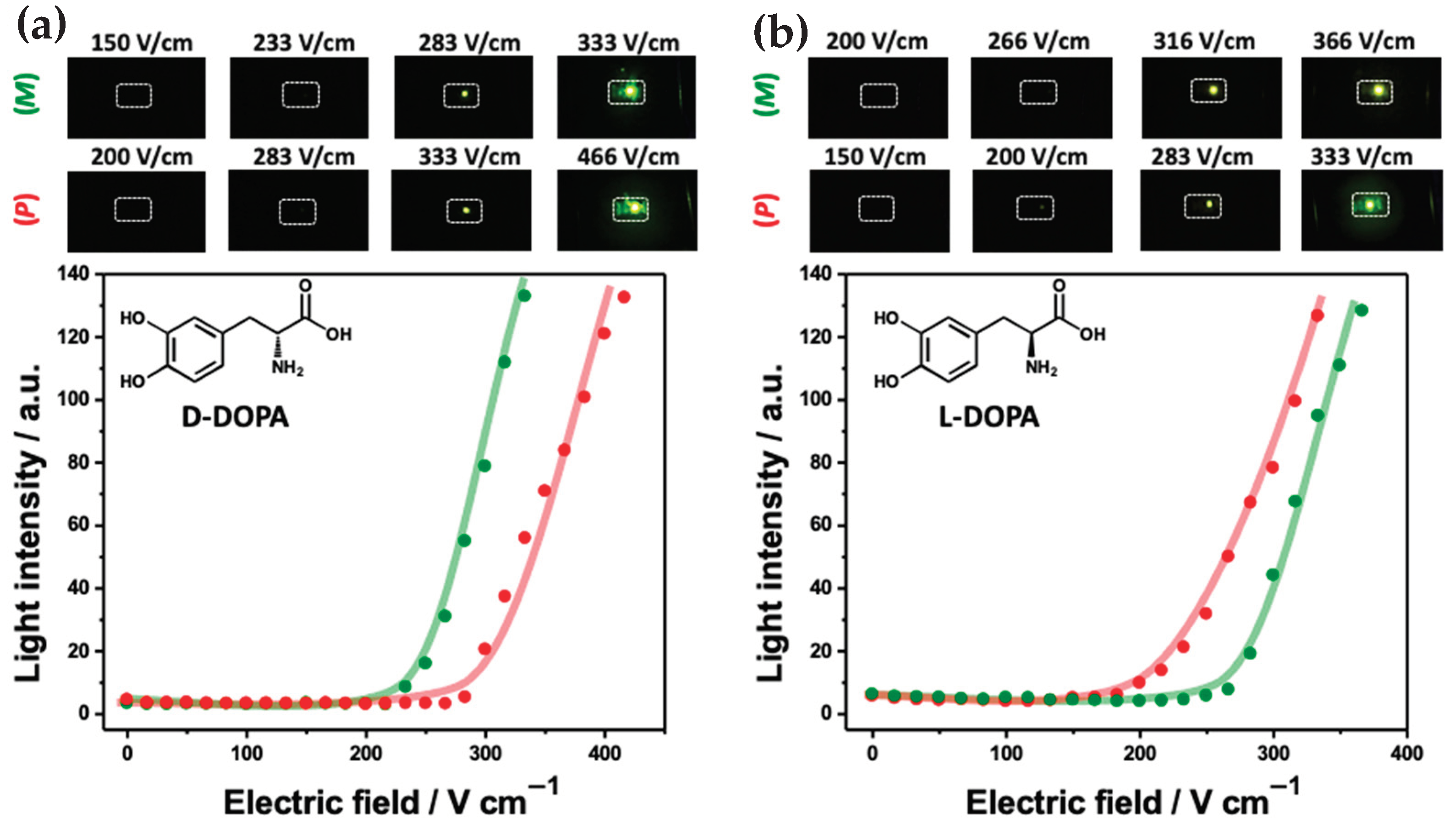
3.2. Bipolar Stereoselective Recognition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, C.S.; Elmayergi, B.H. Chiral Environmental Contaminants. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 1–25. [Google Scholar]
- Xia, N.; Wang, Q.; Liu, L. Nanomaterials-Based Optical Techniques for the Detection of Acetylcholinesterase and Pesticides. Sensors 2014, 15, 499–514. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, J.; Ying, Y.; She, Y.; Wang, J.; Ping, J. Carbon nanomaterial-enabled pesticide biosensors: Design strategy, biosensing mechanism, and practical application. Trends Anal. Chem. 2018, 106, 62–83. [Google Scholar] [CrossRef]
- Cheng, W.; Tang, X.; Zhang, Y.; Wu, D.; Yang, W. Applications of metal-organic framework (MOF)-based sensors for food safety: Enhancing mechanisms and recent advances. Trends Food Sci. Technol. 2021, 112, 268–282. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, C.; Lan, L.; Ping, J.; Ye, Z.; Ying, Y. Nanomaterial-based biosensors for agro-product safety. Trends Anal. Chem. 2021, 143, 116369. [Google Scholar] [CrossRef]
- Raghavan, V.S.; O’Driscoll, B.; Bloor, J.M.; Li, B.; Katare, P.; Sethi, J.; Gorthi, S.S.; Jenkins, D. Emerging graphene-based sensors for the detection of food adulterants and toxicants—A review. Food Chem. 2021, 355, 129547. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Dourandish, Z.; Khalilzadeh, M.A.; Jang, H.W.; Venditti, R.A.; Varma, R.S.; Shokouhimehr, M. Recent Developments in Polymer Nanocomposite-Based Electrochemical Sensors for Detecting Environmental Pollutants. Ind. Eng. Chem. Res. 2021, 60, 1112–1136. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, B.; Costa-García, A.; De La Escosura-Muñiz, A. Electrochemical (Bio) Sensors for Pesticides Detection Using Screen-Printed Electrodes. Biosensors 2020, 10, 32. [Google Scholar] [CrossRef]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of pesticide residue analysis in fruits and vegetables. Pre-treatment, extraction and detection techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef]
- Tajik, S.; Orooji, Y.; Ghazanfari, Z.; Karimi, F.; Beitollahi, H.; Varma, R.S.; Jang, H.W.; Shokouhimehr, M. Nanomaterials modified electrodes for electrochemical detection of Sudan I in food. J. Food Meas. Charact. 2021, 15, 3837–3852. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current advances in treatment technologies for removal of emerging contaminants from water—A critical review. Coord. Chem. Rev. 2021, 442, 213993. [Google Scholar] [CrossRef]
- Siwal, S.S.; Zhang, Q.; Saini, A.K.; Gupta, V.K.; Roberts, D.; Saini, V.; Coulon, F.; Pareek, B.; Thakur, V.K. Recent advances in bio-electrochemical system analysis in biorefineries. J. Environ. Chem. Eng. 2021, 9, 105982. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Thakur, S.; Saini, R.V.; Saini, A.K.; Singh, P.; Kumar Thakur, V.; Nguyen, V.H.; Khan, A.A.P.; Asiri, A.M. Synergistic photocatalytic mitigation of imidacloprid pesticide and antibacterial activity using carbon nanotube decorated phosphorus doped graphitic carbon nitride photocatalyst. J. Taiwan Inst. Chem. Eng. 2020, 113, 142–154. [Google Scholar] [CrossRef]
- Prakash, J.; Parveen, A.; Mishra, Y.K.; Kaushik, A. Nanotechnology-assisted liquid crystals-based biosensors: Towards fundamental to advanced applications. Biosens. Bioelectron. 2020, 168, 112562. [Google Scholar] [CrossRef] [PubMed]
- García-Miranda Ferrari, A.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open. 2021, 3, 100032. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Garkani Nejad, F.; Sheikhshoaie, I.; Nugraha, A.S.; Jang, H.W.; Yamauchi, Y.; Shokouhimehr, M. Performance of metal–organic frameworks in the electrochemical sensing of environmental pollutants. J. Mater. Chem. A 2021, 9, 8195–8220. [Google Scholar] [CrossRef]
- Vikrant, K.; Tsang, D.C.W.; Raza, N.; Giri, B.S.; Kukkar, D.; Kim, K.H. Potential Utility of Metal-Organic Framework-Based Platform for Sensing Pesticides. ACS Appl. Mater. Interfaces 2018, 10, 8797–8817. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, K.S.; Vilian, A.T.E.; Ghoreishian, S.M.; Umapathi, R.; Huh, Y.S.; Han, Y.K. An ultrasensitive electrochemical sensing platform for rapid detection of rutin with a hybridized 2D–1D MXene-FeWO4 nanocomposite. Sens. Actuators B Chem. 2021, 344, 130202. [Google Scholar] [CrossRef]
- Ezhil Vilian, A.T.; Umapathi, R.; Hwang, S.K.; Lee, M.J.; Huh, Y.S.; Han, Y.K. Simple synthesis of a clew-like tungsten carbide nanocomposite decorated with gold nanoparticles for the ultrasensitive detection of tert-butylhydroquinone. Food Chem. 2021, 348, 128936. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Umapathi, R.; Hwang, S.K.; Huh, Y.S.; Han, Y.K. Pd–Cu nanospheres supported on Mo2C for the electrochemical sensing of nitrites. J. Hazard. Mater. 2021, 408, 124914. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Ranjith, K.S.; Lee, S.J.; Hwang, S.K.; Umapathi, R.; Oh, C.W.; Huh, Y.S.; Han, Y.K. Controllable synthesis of bottlebrush-like ZnO nanowires decorated on carbon nanofibers as an efficient electrocatalyst for the highly sensitive detection of silymarin in biological samples. Sens. Actuators B Chem. 2020, 321, 128544. [Google Scholar] [CrossRef]
- Salinas, G.; Arnaboldi, S.; Bouffier, L.; Kuhn, A. Recent advances in bipolar electrochemistry with conducting polymers. ChemElectroChem 2022, 9, e202101234. [Google Scholar] [CrossRef]
- Shida, N.; Zhou, Y.; Inagi, S. Bipolar electrochemistry: Powerful tool for electrifying functional material synthesis. Acc. Chem. Res. 2019, 52, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Rahn, K.L.; Anand, R.K. Recent advancements in bipolar electrochemistry methods of analysis. Anal. Chem. 2021, 93, 103–123. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Pi, J.; Zhang, L.; Kuhn, A. Bulk electrocatalytic NADH cofactor regeneration with bipolar electrochemistry. Angew. Chem. Int. Ed. 2022, 61, e202111804. [Google Scholar] [CrossRef] [PubMed]
- Ketkaew, M.; Assavapanumat, S.; Klinyod, S.; Kuhn, A.; Wattanakit, C. Bifunctional Pt/Au Janus electrocatalysis for simultaneous oxidation/reduction of furfural with bipolar electrochemistry. Chem. Commun. 2022, 58, 4312–4315. [Google Scholar] [CrossRef] [PubMed]
- Loget, G.; Li, G.; Fabre, B. Logic gates operated by bipolar photoelectrochemical water splitting. Chem. Commun. 2015, 51, 1115–1118. [Google Scholar] [CrossRef]
- Anand, R.K.; Johnson, E.S.; Chui, D.T. Negative dielectrophoretic capture and repulsion of single cells at a bipolar electrode: The impact of faradaic ion enrichment and depletion. J. Am. Chem. Soc. 2015, 137, 776–783. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, Z.; Xu, L.; Su, B.; Fang, Q. Integrating bipolar electrochemistry and electrochemiluminescence imaging with microdroplets for chemical analysis. Biosens. Bioelectron. 2014, 53, 148–153. [Google Scholar] [CrossRef]
- Salinas, G.; Niamlaem, M.; Kuhn, A.; Arnaboldi, S. Recent advances in Electrochemical transduction of chiral information. Curr. Opin. Colloid Interface Sci. 2022, 6, 101626. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Gupta, B.; Benincori, T.; Bonetti, G.; Cirilli, R.; Kuhn, A. Absolute chiral recognition with hybrid wireless electrochemical actuators. Anal. Chem. 2020, 92, 10042–10047. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Salinas, G.; Bonetti, G.; Cirilli, R.; Benincori, T.; Kuhn, A. Bipolar electrochemical measurements of enantiomeric excess with inherently chiral polymer actuators. ACS Meas. Au 2021, 1, 110–116. [Google Scholar] [CrossRef]
- Salinas, G.; Arnaboldi, S.; Bonetti, G.; Cirilli, R.; Benincori, T.; Kuhn, A. Hybrid light-emitting devices for the straightforward readout of chiral information. Chirality 2021, 33, 875–882. [Google Scholar] [CrossRef]
- Salinas, G.; Bonetti, G.; Cirilli, R.; Benincori, T.; Kuhn, A.; Arnaboldi, S. Wireless light-emitting device for the determination of chirality in real samples. Electrochim. Acta 2022, 421, 140494. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Salinas, G.; Karajic, A.; Garrigue, P.; Benincori, T.; Bonetti, G.; Cirilli, R.; Bichon, S.; Gounel, S.; Mano, N.; et al. Direct dynamic read-out of molecular chirality with autonomous enzyme-driven swimmers. Nat. Chem. 2021, 13, 1241–1247. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Salinas, G.; Bonetti, G.; Cirilli, R.; Benincori, T.; Kuhn, A. Bipolar electrochemical rotors for the direct transduction of molecular chiral information. Biosens. Bioelectron. 2022, 218, 114740. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-F.; Shen, Y. Helicene Chemistry, from Synthesis to Applications; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, C.-F. Helicenes: Synthesis and Applications. Chem. Rev. 2012, 112, 1463–1535. [Google Scholar] [CrossRef] [PubMed]
- Gingras, M. One hundred years of helicene chemistry. Part 1: Non-stereoselective syntheses of carbohelicenes. Chem. Soc. Rev. 2013, 42, 968–1006. [Google Scholar] [CrossRef]
- Gingras, M.; Félix, G.; Peresutti, R. One hundred years of helicene chemistry. Part 2: Stereoselective syntheses and chiral separations of carbohelicenes. Chem. Soc. Rev. 2013, 42, 1007–1050. [Google Scholar] [CrossRef] [PubMed]
- Cahn, R.S.; Ingold, C.; Prelog, V. Specification of Molecular Chirality. Angew. Chem. Int. Ed. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Gingras, M. One hundred years of helicene chemistry. Part 3: Applications and properties of carbohelicenes. Chem. Soc. Rev. 2013, 42, 1051–1095. [Google Scholar] [CrossRef]
- Dhbaibi, K.; Favereau, L.; Crassous, J. Enantioenriched Helicenes and Helicenoids Containing Main-Group Elements (B, Si, N, P). Chem. Rev. 2019, 119, 8846–8953. [Google Scholar] [CrossRef]
- Pop, F.; Zigon, N.; Avarvari, N. Main-Group-Based Electro- and Photoactive Chiral Materials. Chem. Rev. 2019, 119, 8435–8478. [Google Scholar] [CrossRef]
- Licandro, E.; Cauteruccio, S.; Dova, D. Thiahelicenes: From basic knowledge to applications. In Advances in Heterocyclic Chemistry; Ramsden, C.A., Scriven, E.F.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 118, pp. 1–46. [Google Scholar] [CrossRef]
- Hoffmann, N. Photochemical reactions applied to the synthesis of helicenes and helicene-like compounds. J. Photochem. Photobiol. C 2014, 19, 1–19. [Google Scholar] [CrossRef]
- Collins, S.K.; Vachon, M.P. Unlocking the potential of thiaheterohelicenes: Chemical synthesis as the key. Org. Biomol. Chem. 2006, 4, 2518–2524. [Google Scholar] [CrossRef]
- Bossi, A.; Falciola, L.; Graiff, C.; Maiorana, S.; Rigamonti, C.; Tiripicchio, A.; Licandro, E.; Mussini, P.R. Electrochemical activity of thiahelicenes: Structure effects and electrooligomerization ability. Electrochim. Acta 2009, 54, 5083–5097. [Google Scholar] [CrossRef]
- Cauteruccio, S.; Dreuw, A.; Licandro, E.; Mussini, P.R. Tetrathiahelicenes: An infinite source of inspiration. In Helicenes: Synthesis, Properties and Applications; Crassous, J., Stará, I.G., Starý, I., Eds.; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Yamada, K.; Nakagawa, H.; Kawazura, H. Thermal racemization of thiaheterohelicenes. Bull. Chem. Soc. Jpn. 1986, 59, 2429–2432. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Cauteruccio, S.; Grecchi, S.; Benincori, T.; Marcaccio, M.; Orbelli Biroli, A.; Longhi, G.; Licandro, E.; Mussini, P.R. Thiahelicene-based inherently chiral films for enantioselective electroanalysis. Chem. Sci. 2019, 10, 1539–1548. [Google Scholar] [CrossRef]
- Licandro, E.; Rigamonti, C.; Ticozzelli, M.T.; Monteforte, M.; Baldoli, C.; Giannini, C.; Maiorana, S. Synthesis and functionalization of novel tetrathia[7]helicenes as new push-pull systems. Synthesis 2006, 21, 3670–3678. [Google Scholar] [CrossRef]
- Kawasaki, T.; Suzuki, K.; Licandro, E.; Bossi, A.; Maiorana, S.; Soai, K. Enantioselective synthesis induced by tetrathia[7]helicenes in conjunction with asymmetric autocatalysis. Tetrahedron Asymmetry 2006, 17, 2050–2053. [Google Scholar] [CrossRef]
- Nakagawa, H.; Obata, A.; Yamada, K.; Kawazura, H. Crystal and molecular structures of tetrathia[7]heterohelicene: Racemate and enantiomer. J. Chem. Soc. Perkin Trans. 1985, 2, 1899–1903. [Google Scholar] [CrossRef]
- Salinas, G.; Pavel, I.A.; Sojic, N.; Kuhn, A. Electrochemistry-based light-emitting mobile systems. ChemElectroChem 2020, 7, 4853–4862. [Google Scholar] [CrossRef]
- Salinas, G.; Dauphin, A.L.; Colin, C.; Villani, E.; Arbault, S.; Bouffier, L.; Kuhn, A. Chemo- and magnetotaxis of self-propelled light-emitting chemoelectronic swimmers. Angew. Chem. Int. Ed. 2020, 59, 7508–7513. [Google Scholar] [CrossRef]
- Salinas, G.; Beladi-Mousavi, S.M.; Gerasimova, L.; Bouffier, L.; Kuhn, A. Wireless imaging of transient redox activity based on bipolar light-emitting electrode arrays. Anal. Chem. 2022, 94, 14317–14321. [Google Scholar] [CrossRef]
- Zhao, Y.; Bouffier, L.; Xu, G.; Loget, G.; Sojic, N. Electrochemiluminescence with semiconductor (nano) materials. Chem. Sci. 2022, 13, 2528–2550. [Google Scholar] [CrossRef]
- Meng, C.; Knežević, S.; Du, F.; Guan, Y.; Kanoufi, F.; Sojic, N.; Xu, G. Recent advances in electrochemiluminescence imaging analysis. eScience 2022, 2, 591–605. [Google Scholar] [CrossRef]
- Fu, Y.; Cui, X.; Zhang, Y.; Feng, T.; He, J.; Zhang, X.; Bai, X.; Cheng, Q. Measurement and correlation of the electrical conductivity of the ionic liquid [BIMIM][TFSI] in binary organic solvents. J. Chem. Eng. Data 2018, 63, 1180–1189. [Google Scholar] [CrossRef]
- Perkin, S.; Crowhurst, L.; Niedermeyer, H.; Welton, T.; Smith, A.M.; Goswami, N.N. Self-assembly in the electrical double layer of ionic liquids. Chem. Commun. 2011, 47, 6572–6574. [Google Scholar] [CrossRef]
- Ivanistsev, V.; O’Connor, S.; Fedorov, M.V. Poly(a)morphic portrait of the electrical double layer in ionic liquids. Electrochem. Commun. 2014, 48, 61–64. [Google Scholar] [CrossRef]
- Matsushita, S.; Yan, B.; Yamamoto, S.; Jeong, Y.S.; Akagi, K. Synthesis of Helical Polyacetylene in Chiral Nematic Liquid Crystals Using Crown Ether Type Binaphthyl Derivatives as Chiral Dopants. J. Am. Chem. Soc. 2005, 42, 14647–14654. [Google Scholar] [CrossRef]
- Rizzo, S.; Arnaboldi, S.; Mihali, V.; Cirilli, R.; Forni, A.; Gennaro, A.; Isse, A.A.; Pierini, M.; Mussini, P.R.; Sannicolò, F. “Inherently Chiral” Ionic-Liquid Media: Effective Chiral Electroanalysis on Achiral Electrodes. Angew. Chem. 2017, 56, 2079–2082. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cauteruccio, S.; Pelliccioli, V.; Grecchi, S.; Cirilli, R.; Licandro, E.; Arnaboldi, S. Bipolar Electrochemical Analysis of Chirality in Complex Media through Miniaturized Stereoselective Light-Emitting Systems. Chemosensors 2023, 11, 131. https://doi.org/10.3390/chemosensors11020131
Cauteruccio S, Pelliccioli V, Grecchi S, Cirilli R, Licandro E, Arnaboldi S. Bipolar Electrochemical Analysis of Chirality in Complex Media through Miniaturized Stereoselective Light-Emitting Systems. Chemosensors. 2023; 11(2):131. https://doi.org/10.3390/chemosensors11020131
Chicago/Turabian StyleCauteruccio, Silvia, Valentina Pelliccioli, Sara Grecchi, Roberto Cirilli, Emanuela Licandro, and Serena Arnaboldi. 2023. "Bipolar Electrochemical Analysis of Chirality in Complex Media through Miniaturized Stereoselective Light-Emitting Systems" Chemosensors 11, no. 2: 131. https://doi.org/10.3390/chemosensors11020131
APA StyleCauteruccio, S., Pelliccioli, V., Grecchi, S., Cirilli, R., Licandro, E., & Arnaboldi, S. (2023). Bipolar Electrochemical Analysis of Chirality in Complex Media through Miniaturized Stereoselective Light-Emitting Systems. Chemosensors, 11(2), 131. https://doi.org/10.3390/chemosensors11020131