Absolute Configuration Sensing of Chiral Aryl- and Aryloxy-Propionic Acids by Biphenyl Chiroptical Probes
Abstract
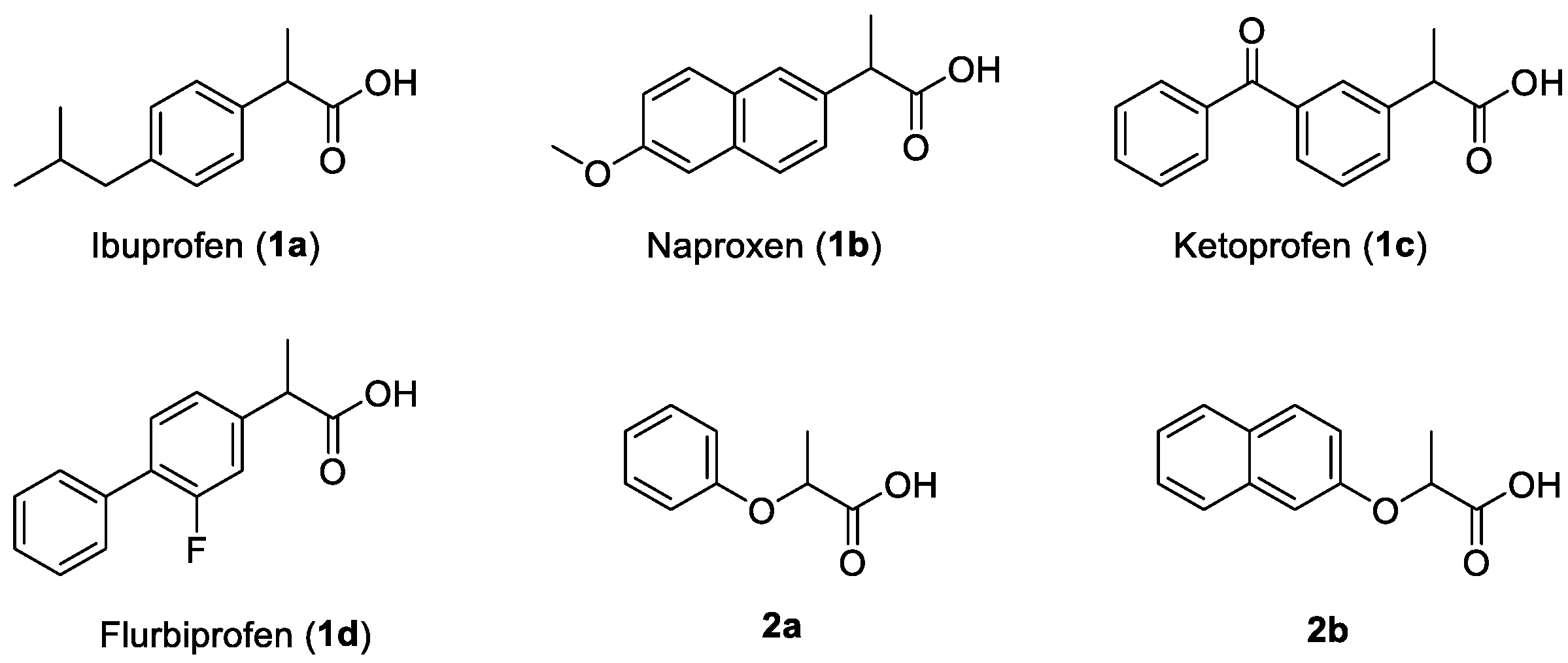
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
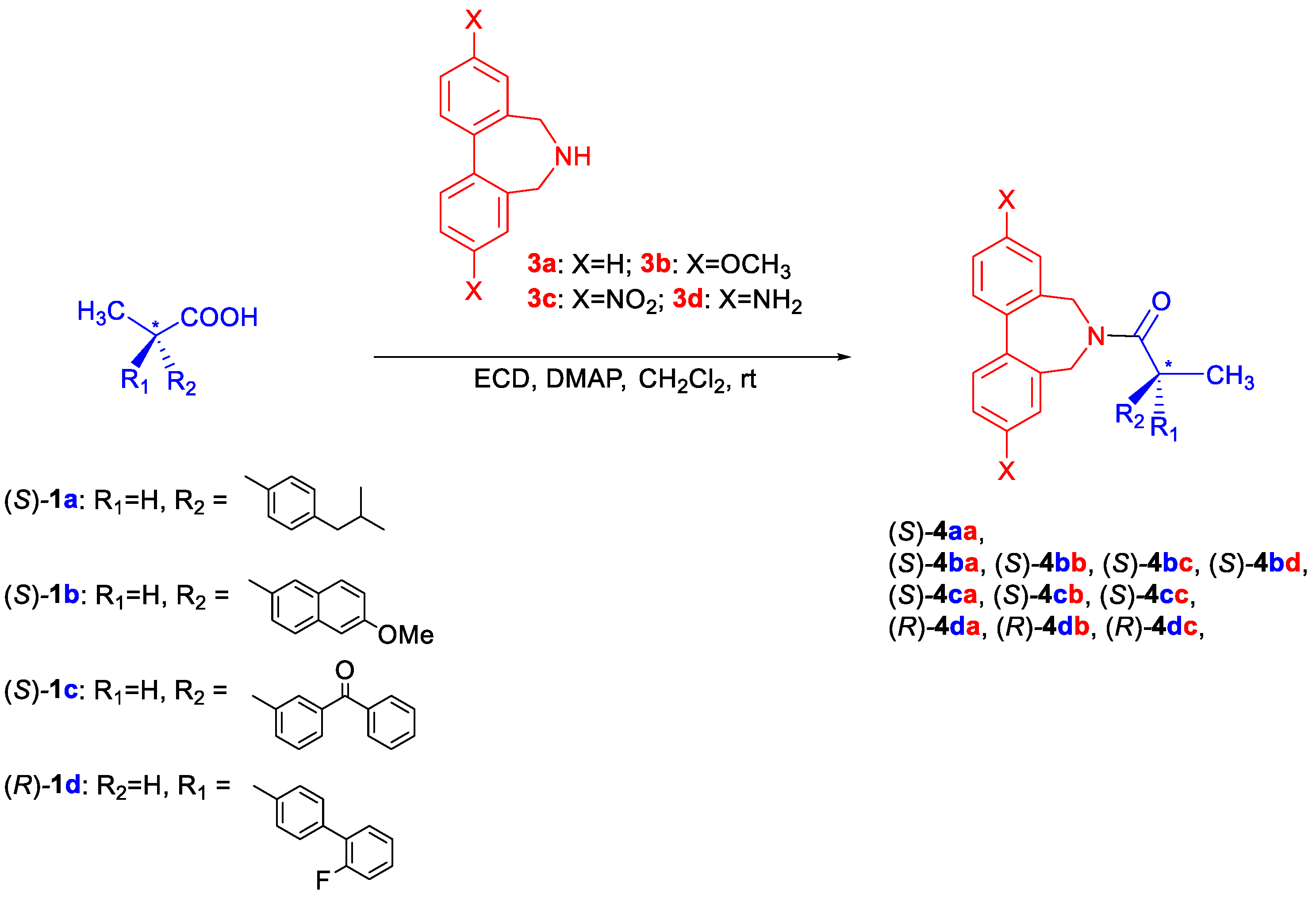
2.2. General Procedure for the Synthesis of Biphenyl Amides 4 and 5
3. Results and Discussion
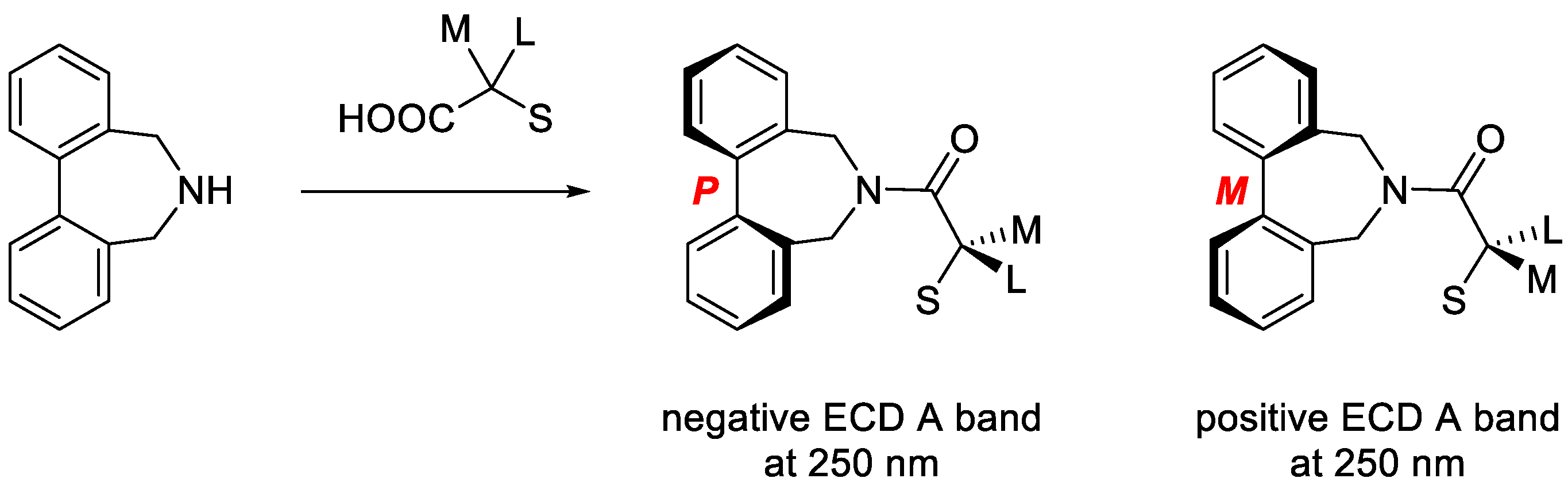
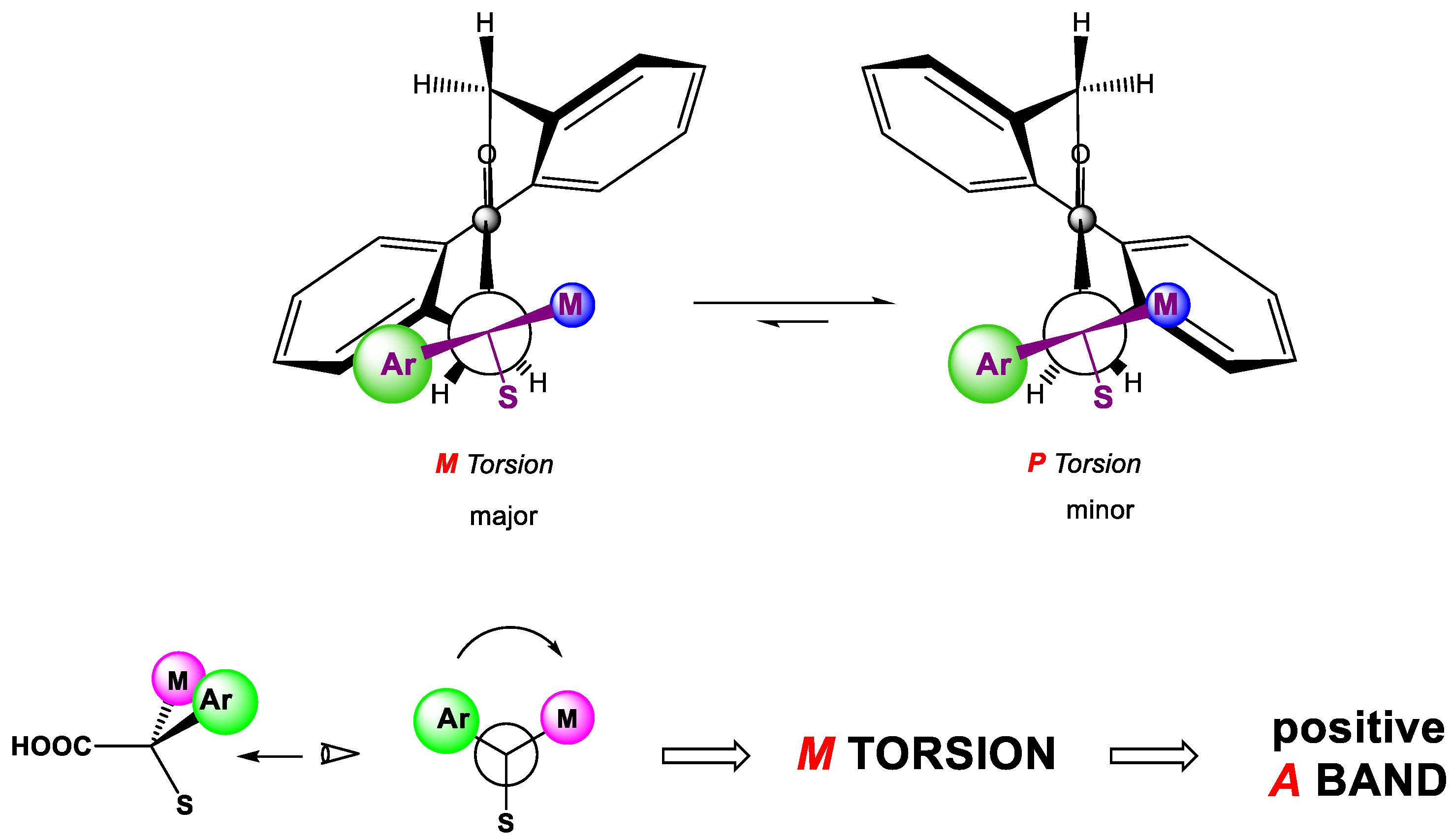
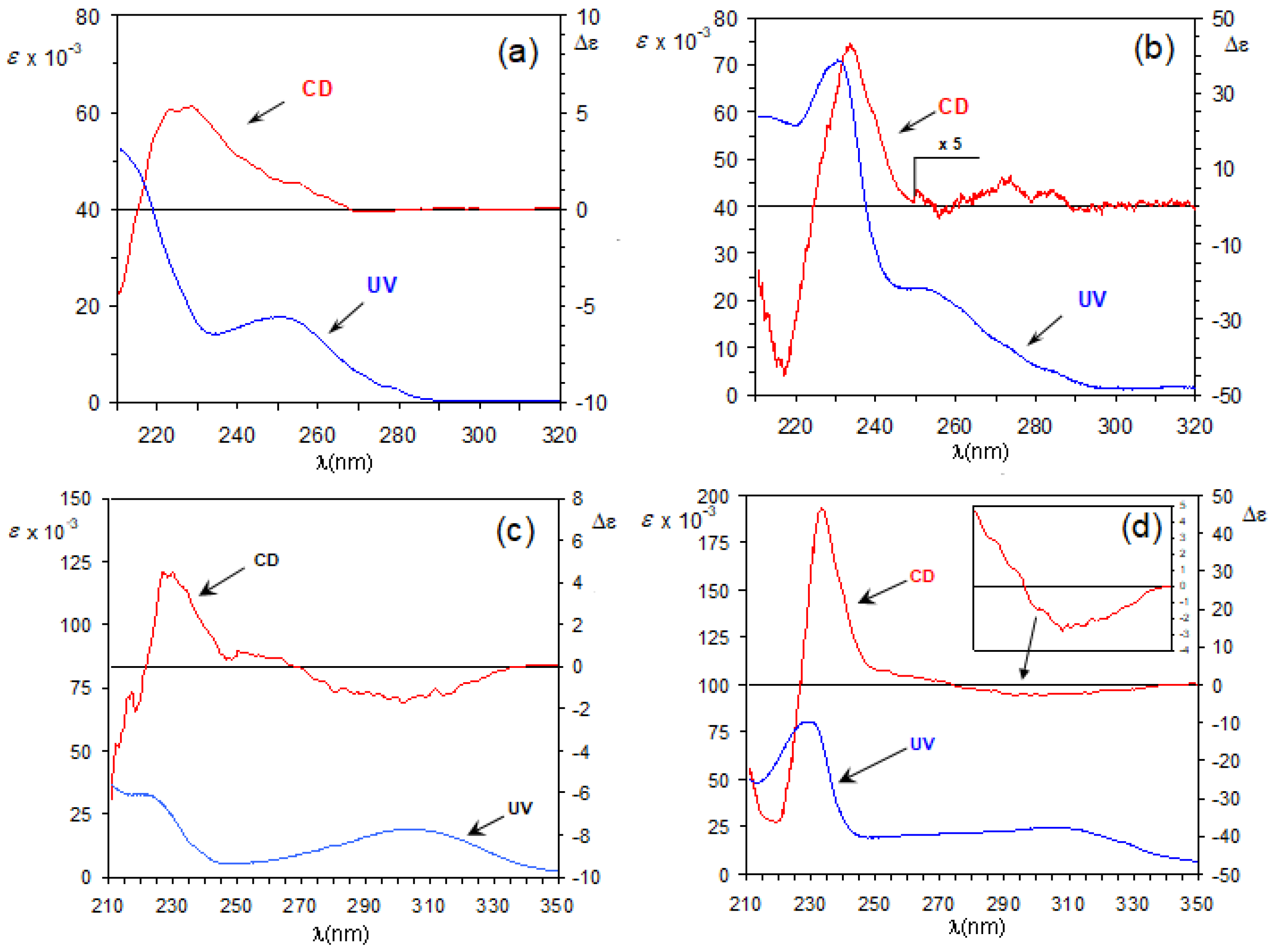
3.1. The Biphenyl Amide Probe
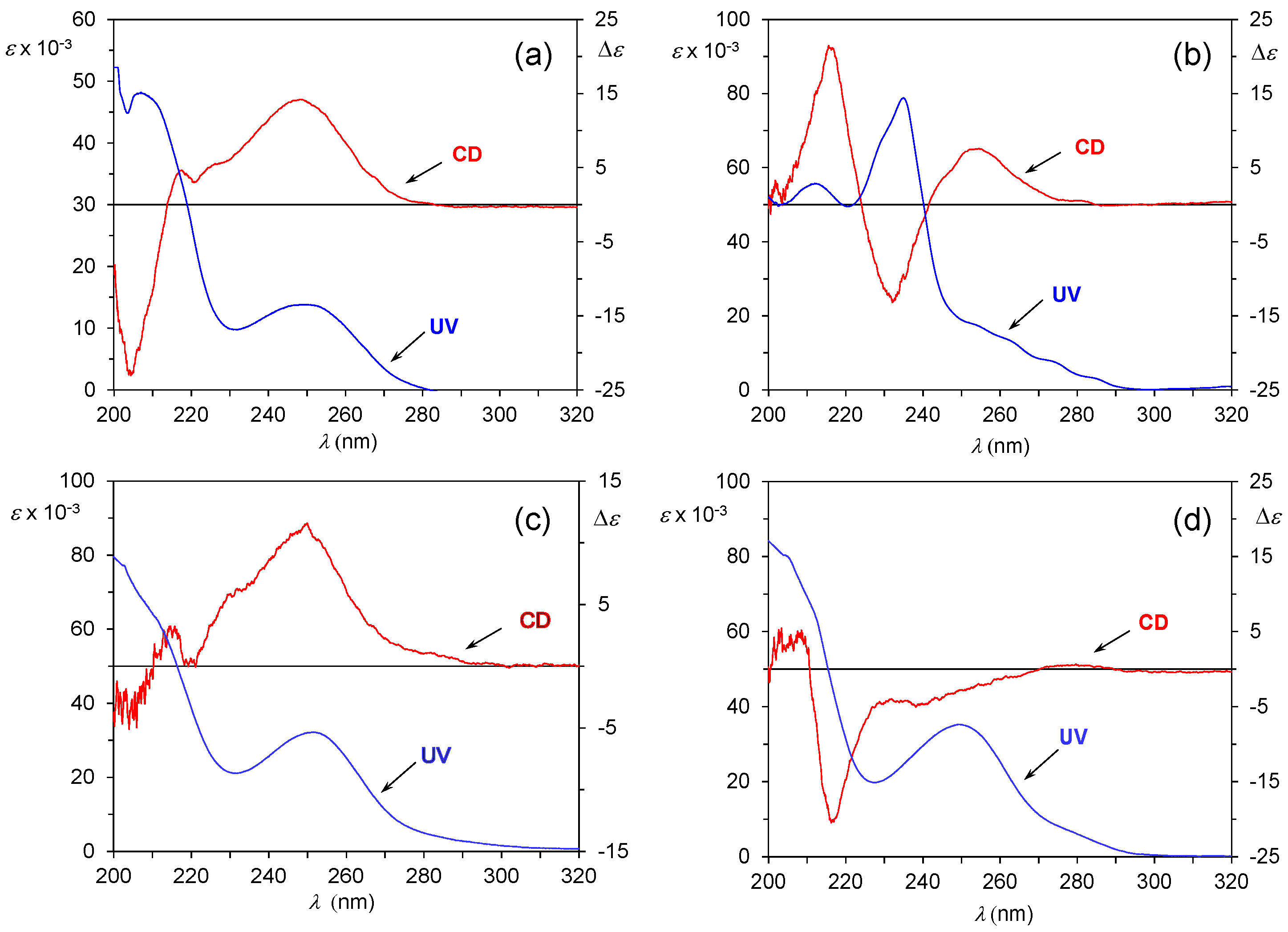
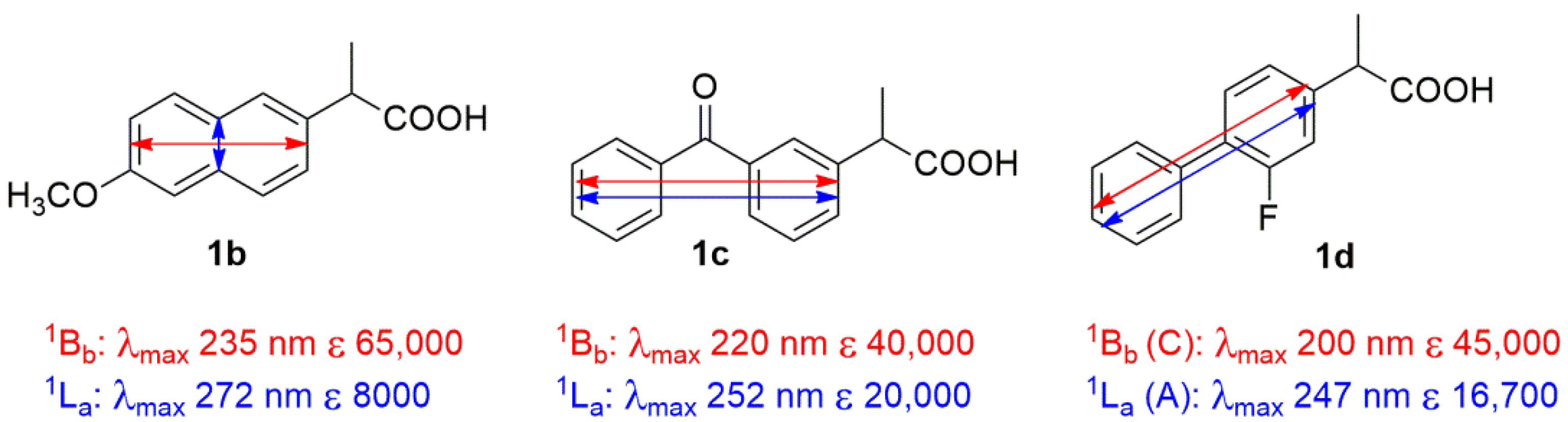
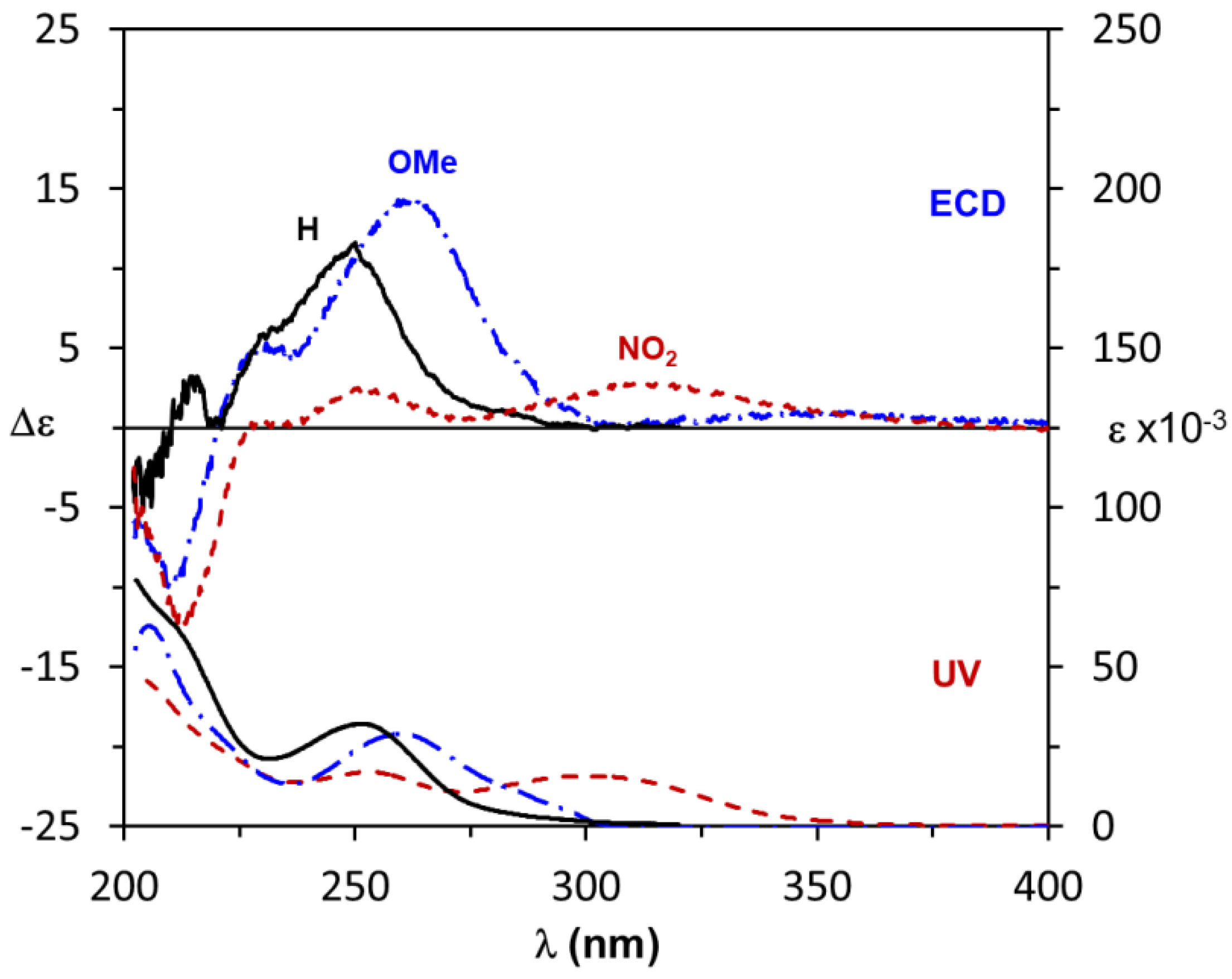
3.2. Assignment of the Absolute Configuration to 2-Arylpropionic Acids
3.3. Assignment of the Absolute Configuration to 2-Aryloxypropionic Acids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References and Note
- Finefield, J.M.; Sherman, D.H.; Kreitman, M.; Williams, R.M. Enantiomeric Natural Products: Occurrence and Biogenesis. Angew. Chem. Int. Ed. 2012, 51, 4802–4836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, G.-Q.; You, Q.-D.; Cheng, J.-F. (Eds.) Chiral Drugs: Chemistry and Biological Action; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 9781118075647. [Google Scholar]
- Francotte, E.; Lindner, W. (Eds.) Chirality in Drug Research, 1st ed.; Wiley: Hoboken, NJ, USA, 2006; Volume 33, ISBN 9783527310760. [Google Scholar]
- Brenna, E.; Fuganti, C.; Serra, S. Enantioselective Perception of Chiral Odorants. Tetrahedron Asymmetry 2003, 14, 1–42. [Google Scholar] [CrossRef]
- Engel, K.-H. Chirality: An Important Phenomenon Regarding Biosynthesis, Perception, and Authenticity of Flavor Compounds. J. Agric. Food Chem. 2020, 68, 10265–10274. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P. Current Status of Chirality in Agrochemicals: Chirality in Agrochemicals. Pest. Manag. Sci. 2018, 74, 2389–2404. [Google Scholar] [CrossRef]
- Maia, A.S.; Ribeiro, A.R.; Castro, P.M.L.; Tiritan, M.E. Chiral Analysis of Pesticides and Drugs of Environmental Concern: Biodegradation and Enantiomeric Fraction. Symmetry 2017, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.S. Environmental fate processes and biochemical transformations of chiral emerging organic pollutants. Anal. Bioanal. Chem. 2006, 386, 544–558. [Google Scholar] [CrossRef]
- Edwards, A.; Jenkinson, S. 8.3 Perspective and Concepts: Overview of Techniques for Assigning Stereochemistry. In Comprehensive Chirality; Elsevier: Amsterdam, The Netherlands, 2012; pp. 39–53. ISBN 9780080951683. [Google Scholar]
- Berova, N.; Polavarapu, P.L.; Nakanishi, K.; Woody, R.W. (Eds.) Comprehensive Chiroptical Spectroscopy: Applications in Stereochemical Analysis of Synthetic Compounds, Natural Products, and Biomolecules; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; ISBN 9781118120392. [Google Scholar]
- Polavarapu, P.L. Chiroptical Spectroscopy: Fundamentals and Applications; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2017; ISBN 9781315374888. [Google Scholar]
- Superchi, S.; Rosini, C.; Mazzeo, G.; Giorgio, E. Determination of Molecular Absolute Configuration: Guidelines for Selecting a Suitable Chiroptical Approach. In Comprehensive Chiroptical Spectroscopy; Berova, N., Polavarapu, P.L., Nakanishi, K., Woody, R.W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; Volume 2, pp. 421–447. ISBN 9781118120392. [Google Scholar]
- Superchi, S.; Scafato, P.; Gorecki, M.; Pescitelli, G. Absolute Configuration Determination by Quantum Mechanical Calculation of Chiroptical Spectra: Basics and Applications to Fungal Metabolites. CMC 2018, 25, 287–320. [Google Scholar] [CrossRef]
- Mándi, A.; Kurtán, T. Applications of OR/ECD/VCD to the Structure Elucidation of Natural Products. Nat. Prod. Rep. 2019, 36, 889–918. [Google Scholar] [CrossRef]
- Vergura, S.; Santoro, E.; Masi, M.; Evidente, A.; Scafato, P.; Superchi, S.; Mazzeo, G.; Longhi, G.; Abbate, S. Absolute Configuration Assignment to Anticancer Amaryllidaceae Alkaloid Jonquailine. Fitoterapia 2018, 129, 78–84. [Google Scholar] [CrossRef]
- Belviso, S.; Santoro, E.; Lelj, F.; Casarini, D.; Villani, C.; Franzini, R.; Superchi, S. Stereochemical Stability and Absolute Configuration of Atropisomeric Alkylthioporphyrazines by Dynamic NMR and HPLC Studies and Computational Analysis of HPLC-ECD Recorded Spectra. Eur. J. Org. Chem. 2018, 2018, 4029–4037. [Google Scholar] [CrossRef]
- Superchi, S.; Casarini, D.; Laurita, A.; Bavoso, A.; Rosini, C. Induction of a Preferred Twist in a Biphenyl Core by Stereogenic Centers: A Novel Approach to the Absolute Configuration of 1,2- and 1,3-Diols. Angew. Chem. Int. Ed. Engl. 2001, 40, 451–454. [Google Scholar] [CrossRef]
- Santoro, E.; Vergura, S.; Scafato, P.; Belviso, S.; Masi, M.; Evidente, A.; Superchi, S. Absolute Configuration Assignment to Chiral Natural Products by Biphenyl Chiroptical Probes: The Case of the Phytotoxins Colletochlorin A and Agropyrenol. J. Nat. Prod. 2020, 83, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Superchi, S.; Bisaccia, R.; Casarini, D.; Laurita, A.; Rosini, C. Flexible Biphenyl Chromophore as a Circular Dichroism Probe for Assignment of the Absolute Configuration of Carboxylic Acids. J. Am. Chem. Soc. 2006, 128, 6893–6902. [Google Scholar] [CrossRef]
- Vergura, S.; Scafato, P.; Belviso, S.; Superchi, S. Absolute Configuration Assignment from Optical Rotation Data by Means of Biphenyl Chiroptical Probes. Chem. Eur. J. 2019, 25, 5682–5690. [Google Scholar] [CrossRef] [PubMed]
- Vergura, S.; Pisani, L.; Scafato, P.; Casarini, D.; Superchi, S. Central-to-Axial Chirality Induction in Biphenyl Chiroptical Probes for the Stereochemical Characterization of Chiral Primary Amines. Org. Biomol. Chem. 2018, 16, 555–565. [Google Scholar] [CrossRef]
- Sagiv, J.; Yogev, A.; Mazur, Y. Application of Linear Dichroism to the Analysis of Electronic Absorption Spectra of Biphenyl, Fluorene, 9,9’-Spirobifluorene, and [6.6]Vespirene. Interpretation of the Circular Dichroism Spectrum of [6.6]Vespirene. J. Am. Chem. Soc. 1977, 99, 6861–6869. [Google Scholar] [CrossRef]
- Bunnenberg, E.; Djerassi, C.; Mislow, K.; Moscowitz, A. Inherently Dissymmetric Chromophores and Circular Dichroism. J. Am. Chem. Soc. 1962, 84, 2823–2826. [Google Scholar] [CrossRef]
- Mislow, K.; Bunnenberg, E.; Records, R.; Wellman, K.; Djerassi, C. Inherently Dissymmetric Chromophores and Circular Dichroism. II. J. Am. Chem. Soc. 1963, 85, 1342–1349. [Google Scholar] [CrossRef]
- Rashidi-Ranjbar, P.; Sandström, J. The UV and CD Spectra of a 60∘ Twisted Bridged Biphenyl: An Experimental and CNDO/S Study. J. Mol. Struct. 1991, 246, 25–32. [Google Scholar] [CrossRef]
- Collins, A.N.; Sheldrake, G.N.; Crosby, J. (Eds.) Chirality in Industry II; Wiley: Chichester, UK, 1997; ISBN 9780471935957. [Google Scholar]
- Haga, T.; Crasby, K.E.; Schussler, J.R.; Palmer, C.J.; Yoshii, H.; Kimura, F.; Kurihara, N. Aryloxypropanoate Herbicides in Chirality in Agrochemicals; Kurihara, N., Miyamoto, J., Eds.; Wiley Series in Agrochemicals and Plant Protection; Wiley: New York, NY, USA, 1998; ISBN 9780471981213. [Google Scholar]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.-C. Mechanism of Action of Fibrates on Lipid and Lipoprotein Metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, N.; Miyamoto, J.; Paulson, G.D.; Zeeh, B.; Skidmore, M.W.; Hollingworth, R.M.; Kuiper, H.A. Pesticides Report 37: Chirality in Synthetic Agrochemicals: Bioactivity and Safety Consideration (Technical Report). Pure Appl. Chem. 1997, 69, 2007–2026. [Google Scholar] [CrossRef]
- Ferorelli, S.; Loiodice, F.; Tortorella, V.; Conte-Camerino, D.; De Luca, A.M. Carboxylic Acids and Skeletal Muscle Chloride Channel Conductance: Effects on the Biological Activity Induced by the Introduction of Methyl Groups on the Aromatic Ring of Chiral α-(4-Chloro-Phenoxy)Alkanoic Acids. Il Farmaco 2001, 56, 239–246. [Google Scholar] [CrossRef]
- Buser, H.-R.; Poiger, T.; Müller, M.D. Occurrence and Environmental Behavior of the Chiral Pharmaceutical Drug Ibuprofen in Surface Waters and in Wastewater. Environ. Sci. Technol. 1999, 33, 2529–2535. [Google Scholar] [CrossRef]
- Schneiderheinze, J.M.; Armstrong, D.W.; Berthold, A. Plant and soil enantioselective biodegradation of racemic phenoxyalkanoic herbicides. Chirality 1999, 11, 330–337. [Google Scholar] [CrossRef]
- Cai, X.; Liu, W.; Sheng, G. Enantioselective Degradation and Ecotoxicity of the Chiral Herbicide Diclofop in Three Freshwater Alga Cultures. J. Agric. Food Chem. 2008, 56, 2139–2146. [Google Scholar] [CrossRef]
- Buser, H.R.; Muller, M.D. Enantioselective analyses of persistent and modern pesticides. A step toward sustainable agriculture. Chimia 1997, 51, 694–700. [Google Scholar]
- Sanganyado, E.; Lu, Z.; Fu, Q.; Schlenk, D.; Gan, J. Chiral Pharmaceuticals: A Review on Their Environmental Occurrence and Fate Processes. Water Res. 2017, 124, 527–542. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B. Pharmacologically Active Compounds in the Environment and Their Chirality. Chem. Soc. Rev. 2010, 39, 4466. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.R.; Castro, P.M.L.; Tiritan, M.E. Chiral Pharmaceuticals in the Environment. Environ. Chem. Lett. 2012, 10, 239–253. [Google Scholar] [CrossRef]
- Tottie, L.; Baeckström, P.; Moberg, C.; Tegenfeldt, J.; Heumann, A. Molecular Sieve Controlled Diastereoselectivity: Effect in the Palladium-Catalyzed Cyclization of Cis-1,2-Divinylcyclohexane with α-Oxygen-Substituted Acids as Chiral Nucleophiles. J. Org. Chem. 1992, 57, 6579–6587. [Google Scholar] [CrossRef]
- Eliel, E.L.; Wilen, S.H.; Mander, L.N. (Eds.) The groups size was inferred from the Winstein A steric parameters. In Stereochemistry of Organic Compounds; Wiley: New York, NY, USA, 1994; pp. 696–697. ISBN 9780471016700. [Google Scholar]
- As inferred from X-ray structure and computations reported in reference [19], the π-π aryl-aryl interactions give rise to an edge-to-face or T-shape arrangement of the biphenyl and aryl moieties. Anslyn, E.V.; Dougherty, D.A. (Eds.) Modern Physical Organic Chemistry; University Science: Sausalito, CA, USA, 2006; p. 184. ISBN 9781891389313.
- Harada, N.; Nakanishi, K. Exciton Chirality Method and Its Application to Configurational and Conformational Studies of Natural Products. Acc. Chem. Res. 1972, 5, 257–263. [Google Scholar] [CrossRef]
- White, D.P.; Anthony, J.C.; Oyefeso, A.O. Computational Measurement of Steric Effects: The Size of Organic Substituents Computed by Ligand Repulsive Energies. J. Org. Chem. 1999, 64, 7707–7716. [Google Scholar] [CrossRef]




| Arylpropionic Acid a | |||||||
|---|---|---|---|---|---|---|---|
| (S)-1b | (S)-1c | (R)-1d | |||||
| Azepine | X b | λmax c (Δε) | [α]D | λmax c (Δε) | [α]D | λmax c (Δε) | [α]D |
| 3a | H | 253 (+7.5) | +95 | 250 (+11.2) | +121 | 243 (−3.8) | −88 |
| 3b | OMe | 260 (+19.0) | +113 | 262 (+14.2) | +85 | 263 (−11.8) | −139 |
| 3c | NO2 | 306 (+2.8) | +67 | 312 (+2.7) | +153 | 310 (−2.5) | −97 |
| 3d | NH2 | 283 (+5.7) | +69 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergura, S.; Orlando, S.; Scafato, P.; Belviso, S.; Superchi, S. Absolute Configuration Sensing of Chiral Aryl- and Aryloxy-Propionic Acids by Biphenyl Chiroptical Probes. Chemosensors 2021, 9, 154. https://doi.org/10.3390/chemosensors9070154
Vergura S, Orlando S, Scafato P, Belviso S, Superchi S. Absolute Configuration Sensing of Chiral Aryl- and Aryloxy-Propionic Acids by Biphenyl Chiroptical Probes. Chemosensors. 2021; 9(7):154. https://doi.org/10.3390/chemosensors9070154
Chicago/Turabian StyleVergura, Stefania, Stefano Orlando, Patrizia Scafato, Sandra Belviso, and Stefano Superchi. 2021. "Absolute Configuration Sensing of Chiral Aryl- and Aryloxy-Propionic Acids by Biphenyl Chiroptical Probes" Chemosensors 9, no. 7: 154. https://doi.org/10.3390/chemosensors9070154
APA StyleVergura, S., Orlando, S., Scafato, P., Belviso, S., & Superchi, S. (2021). Absolute Configuration Sensing of Chiral Aryl- and Aryloxy-Propionic Acids by Biphenyl Chiroptical Probes. Chemosensors, 9(7), 154. https://doi.org/10.3390/chemosensors9070154







