Abstract
Previous studies about the COVID-19 pandemic on STEMI patient outcomes have conflicting results. It remains unclear if this may be attributed to regional differences and/or differences during COVID-19 wave periods. Using the American Heart Association Get With The Guidelines–Coronary Artery Disease registry data, we evaluated (1) time metrics related to STEMI system goals and (2) regional variation in STEMI incidence and in-hospital mortality during pandemic wave time periods. The study included all patients 18–100 years old admitted with STEMI (n = 72,516) to 1 of 435 American Heart Association Get With The Guidelines–Coronary Artery Disease hospitals (1 October 2019–31 December 2021). Of these, 70.8% were male and 73.0% non-Hispanic White, with a median age of 63 (IQR 18) years. Compared to pre-pandemic time frames, patients with STEMI had a higher risk profile, delayed time to treatment, were treated with fibrinolytic therapy or primary PCI, and were transferred for primary PCI at similar rates, and had higher adjusted in-hospital mortality (during the second wave in the South and Midwest). Preservation of STEMI systems of care resulted in an overall lower in-hospital mortality rate than predicted, although opportunities exist to improve treatment delays. Regional differences in mortality rates require further study.
1. Introduction
ST-elevation myocardial infarction (STEMI) is a life-threatening emergency requiring rapid diagnosis and reperfusion therapies to prevent immediate and long-term devastating patient outcomes. Timely identification and treatment of STEMI are critical because delayed therapy or misdiagnosed MI doubles the risk of death [1].
The coronavirus (SARS-CoV-2) emerged in 2020 as a new threat to individuals with cardiac disease: emergency medical services (EMS) calls, emergency department (ED) visits, and hospital admissions for acute cardiac conditions decreased substantially [2,3,4]. Prior to the pandemic, there were over 3.5 million ED visits for STEMI per year in the United States (US) for suspected STEMI [5]. In the first 10 weeks of the COVID pandemic (mid-March 2020 through May 2020), ED visits for MI declined by 23% compared with the preceding 10-week period (January 2020-mid-March 2020) [6]. EMS calls and hospital admissions decreased sharply for conditions such as acute coronary syndrome (ACS), decompensated heart failure, and atrial fibrillation [2,4,7] while out-of-hospital sudden cardiac arrest and resultant death increased [7]. Of the >500,000 total deaths reported in the US between March and April 2020 (i.e., early pandemic period), 87,001 were considered excess deaths, of which >50% were attributed to causes other than COVID-19, including heart disease, diabetes, Alzheimer’s disease, and cerebrovascular disease [8]. The direct impact of COVID-19 on STEMI outcomes remains unclear, in part related to the unknown number of patients who did not seek care.
In response to this concern, and within Mission: Lifeline, the American Heart Association’s (AHA) national initiative to advance the systems of care for patients with STEMI [9], the AHA issued guidance to ensure that patients with STEMI continue to receive life-saving treatments while maintaining patient and healthcare worker safety [10]. The American Heart AHA Get With The Guidelines–Coronary Artery Disease registry (GWTG-CAD) [11] enrolls patients admitted with STEMI in over 700 participating hospitals across the US. Using these national data, the purpose of our study was to describe (1) time metrics related to STEMI system of care goals and (2) regional variation related to the incidence of STEMI and in-hospital mortality of patients presenting to hospitals pre-pandemic and during specified pandemic wave time frames. We hypothesized there would be a delay in STEMI diagnosis and treatment, and an increase in in-hospital mortality during pandemic wave periods compared to pre-pandemic. Moreover, we hypothesized that there would be geographical variation in mortality related to specific wave periods. This is a comprehensive report of national data to describe the impact of the COVID-19 pandemic on STEMI systems of care.
2. Materials and Methods
2.1. Data Sources and Collection
The AHA GWTG-CAD Registry is a voluntary quality improvement program in the US that has been described previously [12,13]. Participating hospitals upload clinical data of consecutive patients admitted with STEMI or non-ST-elevation myocardial infarction (NSTEMI). Program information and data elements collected in the case report form are available at: https://www.heart.org/en/professional/quality-improvement. Because data were used primarily at the local site for quality improvement, sites were granted a waiver of informed consent under the Common Rule. Patients, therefore, are neither recruited nor consented, and no patient identifiers are collected. The data collection and coordination for GTWG programs are managed by IQVIA (Parsippany, NJ, USA). The data for the social vulnerability index (SVI), ranging from 0 (least vulnerable) to 1 (most vulnerable), were from the Center for Disease Control (CDC)/Agency for Toxic Substances and Disease Registry (ATSDR) SVI database at: https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html (accessed on 2 July 2025). Patient-level GWTG-CAD data and SVI data are linked by county. Characteristics of the CAD registry hospitals are shown in Supplementary Table S1. The number of hospitals within the registry by CDC region is shown in Supplementary Table S2. Supplementary Table S3 includes the number of STEMI patients in the CAD registry during analogous pre-pandemic time frames.
2.2. Study Period, Population, and Patient Selection
The study population included all patients between 18 and 100 years old admitted to GWTG-CAD hospitals from 1 October 2019 to 31 December 2021 (Figure 1). A discharge diagnosis of STEMI or STEMI equivalent was defined as STEMI of the anterior wall, inferior wall, other sites, or unspecified site. Patients diagnosed with STEMI and discharged from the hospital with valid data for sex (male and female; other excluded n = 58), race/ethnicity, and disposition status, and who were admitted to hospitals with patients from all wave time frames were included. A total of 72,516 patients from 435 sites were included in the study after applying the inclusion–exclusion criteria.
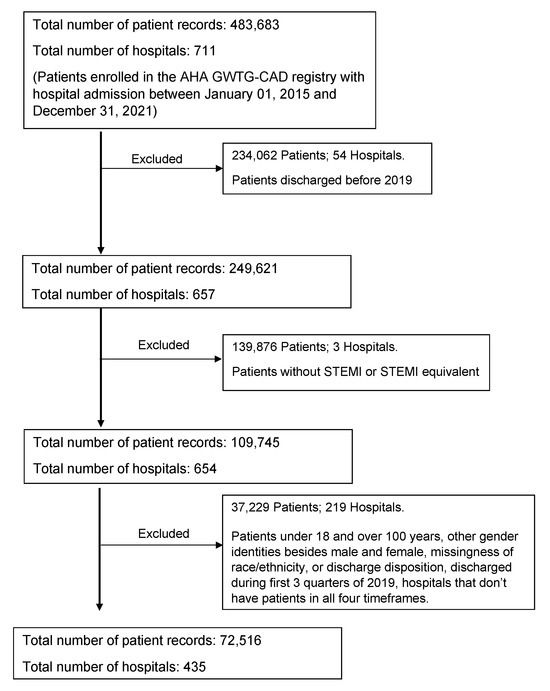
Figure 1.
Inclusion–exclusion criteria flowchart of the study.
2.3. Outcomes and Covariates
The primary outcomes included the following timed process metrics: for receiving centers only, first medical contact (FMC) to cardiac catheterization lab (Cath Lab) activation for EMS patients; door-to-Cath Lab activation for walk-in patients; FMC-to-device time for EMS patients; door-to-device time for walk-in patients; symptom onset-to-device time for EMS and walk-in patients. For both receiving and referral centers, FMC-to-electrocardiogram (ECG) time for EMS patients; door-to-ECG time for walk-in patients; and ED dwell time for both EMS and walk-in patients. In-hospital all-cause mortality was the secondary outcome. Only date times that have a precision of hours and minutes are used to calculate timed process metrics (e.g., Cath Lab activation date time, arrival date time, and EMS FMC date time for FMC to Cath Lab activation). Values for all timed process metrics less than 0 or greater than 1440 min are excluded [10,11]. Categorical variables of five time intervals, including FMC to device ≤ 90 min for EMS patients, door-to-device ≤ 90 min for walk-in patients, door to ECG ≤ 10 min for walk-in patients, ED ≤ 30 min for EMS patients, and ED ≤ 30 min for walk-in patients, were created. COVID-19 pandemic waves included time frames to encompass specific CDC COVID-19 peaks and case trends. Time frames were (1) pre-pandemic: 1 October 2019–29 February 2020; (2) wave 1: 1 March 2020–31 October 2020; (3) wave 2: 1 November 2020–31 March 2021; and (4) wave 3: 1 April 2021–31 December 2021 [14]. Each COVID-19 pandemic wave was the main independent variable, with pre-pandemic as the reference group. The reason for the non-system delay was defined as the need for additional personal protective equipment (PPE) for suspected/confirmed infectious disease.
2.4. Statistical Analysis
Descriptive statistics were presented as percentages for categorical variables and medians (with interquartile range) for continuous variables. Characteristics of patients and hospitals were stratified by pre-pandemic and COVID-19 waves. Missing values of insurance status were assigned to the “other/not documented” category. Missing values of SVI are imputed by the median. Missing values of cardiac arrest prior to arrival, heart failure on FMC, and cardiogenic shock on FMC were managed by multiple imputations by chained equations. Between-group differences were assessed using a chi-square test for categorical variables and Kruskal–Wallis followed by the Dunn test for continuous variables. Kruskal–Wallis, a non-parametric test, ranks all the observations from two or more groups and sums the ranks from one of the groups, which is then compared with the expected rank sum. It should be noted that with a sufficient sample size, the difference in ranks will be large enough to be significant even though the medians are equal. A linear trend of the in-hospital mortality across pre-pandemic and pandemic waves was assessed for each CDC region using the Mann–Kendall test. For in-hospital mortality, generalized estimating equations (GEE) were used to calculate odds ratios (ORs) for different pandemic waves, accounting for within-hospital clustering, using robust sandwich estimators for the variance. Three models were fitted, including unadjusted, multivariable-adjusted (adjusting for age, race/ethnicity, cardiac arrest prior to arrival, cardiogenic shock on FMC, and heart failure on FMC), and extended multivariable-adjusted (adjusting for all covariates from the multivariable-adjusted model and diabetes, hypertension, left ventricular function (LVF) assessment, insurance, and SVI). Additional models were also fitted on different subsets of the study population, including sex (male and female) and different regions (Midwest, Northeast, South, West). Sensitivity analyses excluding cardiac arrest prior to arrival and cardiogenic shock on FMC (attributed to the pandemic) from the adjusted covariate list stratified by males and females were also performed. Statistical significance was assessed at α = 0.05. Data analysis was performed using the open-source software R (4.2.0) and Python (3.7.16) by the American Heart Association’s Data Science Analytics team.
3. Results
3.1. Patient Population
Between 1 October 2019, through 31 December 2021, a total of 72,516 patients hospitalized with STEMI and entered into the GWTG-CAD registry were evaluated (Table 1). The cohort had a median age of 63 years (IQR: 18), with 29.2% female, 73.0% non-Hispanic White, 11.3% Black, and 7.7% Hispanic. Most had health insurance coverage (including 46.8% with private insurance, 25.5% with Medicare, 11.4% with Medicaid) and median SVI was 0.5 (IQR: 0.4). Compared to the pre-pandemic cohort (8.3%), patients in waves 2 and 3 had higher proportions of heart failure on FMC (9.6%, 9.7%). Patients in wave 3 also had significantly higher rates of cardiogenic shock (8.3%) compared to pre-pandemic rates (7.5%). More individuals were transported by EMS during all waves (waves 1–3 [49.4, 48.9%, 48.9%]) compared to pre-pandemic (46.9%). The overall SARS-CoV-2 infection rate at admission was 1.3%.

Table 1.
Baseline characteristics of patients hospitalized with STEMI by established pre-pandemic vs. pandemic wave time periods.
3.2. Reperfusion Therapy
Patients with STEMI during waves 1–3 received reperfusion therapy (i.e., fibrinolytics 5%, primary PCI 86.9%) at similar rates as pre-pandemic. There were no differences in the proportion of patients transferred from a STEMI referring hospital to a STEMI receiving center during the pandemic waves compared with the pre-pandemic period (22.7%). There were higher rates of non-system reasons for delay, specifically of need for additional PPE for suspected/confirmed infectious disease across waves 1–3 compared to the pre-pandemic period.
3.3. Time Metrics
We compared timed process metrics among all STEMI patients hospitalized pre- and during pandemic waves 1–3. Median and interquartile range (IQR) values for timed process metrics and the proportion of patients achieving recommended time metrics are presented in Table 2. FMC-to-Cath lab activation times among patients transported by EMS to receiving hospitals were longer for each pandemic wave compared to the pre-pandemic period (p < 0.002). For walk-in patients, door-to-Cath Lab activation median times were significantly longer in wave 3 compared to the pre-pandemic period. FMC-to-device times in receiving centers for EMS patients were longer among waves 1–3 compared to the pre-pandemic period (p < 0.001). A significantly higher proportion of EMS patients met the target FMC-to-device time of ≤90 min pre-pandemic compared to each of the pandemic waves (p < 0.001). Among walk-in patients, a significantly higher proportion of pre-pandemic patients met the target of door-to-device time of ≤90 min compared to pandemic waves 2 and 3 (p < 0.001). In receiving centers, symptom onset-to-device times for EMS transported patients were longer for waves 1–3 compared to the pre-pandemic period (p < 0.001). Among walk-in patients, symptom onset-to-device times were lower pre-pandemic compared to all waves 1–3 (p < 0.016). In both receiving and referral centers, there was no significant difference between pandemic waves for FMC to ECG time among EMS patients, but waves 2–3 had a significantly longer door-to-ECG time among walk-in patients. In both receiving and referral centers, for walk-in patients, there was a significantly higher proportion of patients meeting the target of ≤10 min for door-to-ECG times in the pre-pandemic period compared to waves 2–3. In both receiving and referral centers, for both EMS and walk-in patients, the ED time of the three pandemic waves was significantly longer than the pre-pandemic period. Among EMS patients, a significantly higher proportion of pre-pandemic patients met the target of ≤30 min for ED time compared to those in waves 1–3 (p < 0.001). Among walk-in patients, a significantly higher proportion of pre-pandemic patients met the target of ≤30 min for ED time compared to waves 1–2.

Table 2.
Timing metrics of all patients hospitalized with STEMI by established pre-pandemic vs. pandemic wave time periods.
3.4. In-Hospital Mortality
Patients had higher rates of mortality during pandemic wave 2 (8.0%) compared to the pre-pandemic period (6.8%) (p < 0.001) (Table 1), with a higher odds ratio (OR 1.12 [CI 1.01, 1.25], p = 0.030) compared with the pre-pandemic period, after adjusting for age, race/ethnicity, diabetes, hypertension, cardiac arrest prior to arrival, heart failure at FMC, cardiogenic shock at FMC, LVF assessment, insurance status, and SVI (Table 3). This increase was limited to males (OR 1.19 [CI 1.04, 1.36]) and not seen in females (Supplementary Table S4a,b). Sensitivity analysis also demonstrated mortality increase for males only (OR 1.22 [CI 1.08, 1.39]) (Supplementary Table S5a,b). Figure 2 shows observed in-hospital mortality and adjusted odds ratios with 95% confidence intervals by pandemic waves and CDC regions after adjusting for age, race/ethnicity, diabetes, hypertension, cardiac arrest prior to arrival, heart failure at FMC, cardiogenic shock at FMC, LVF assessment, insurance status, and SVI. There were significantly higher proportions of in-hospital mortality among the South (7.0%) and West (8.5%) regions compared to the Northeast (reference 6.2%) region (Table 4). The Midwest and South regions exhibited a higher odds ratio of in-hospital mortality in pandemic wave 2 compared to the pre-pandemic period (Supplementary Table S6a–d).

Table 3.
Multivariable analysis of in-hospital mortality among COVID waves.
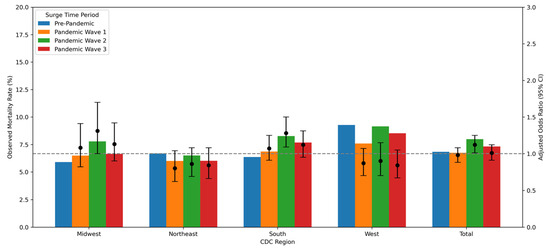
Figure 2.
Observed in-hospital mortality rates and adjusted odds ratios with 95% confidence intervals by COVID-19 wave and CDC region (adjusted for age, race/ethnicity, diabetes mellitus, hypertension, cardiac arrest prior to arrival, heart failure on FMC, LVF, cardiogenic shock on FMC, insurance status, and Social Vulnerability Index). The primary (left) y-axis represents the observed mortality rate (%), and the secondary (right) y-axis represents the adjusted odds ratio. Each bar corresponds to the observed mortality rate within a wave, while the scatter points indicate the adjusted odds ratios with error bars showing the 95% confidence intervals. The dashed horizontal line at odds ratio = 1 serves as a reference.

Table 4.
Proportion of in-hospital mortality rate per CDC region. * indicates statistically significant.
4. Discussion
In this large-scale study of patients with STEMI during the COVID-19 pandemic in the US, unique in its size and evaluation of STEMI system goals and outcomes across the country during waves of COVID-19 infection between 1 March 2020 and 31 December 2021, patients had a higher risk profile, delayed FMC to device despite presenting via EMS more often, similar treatment with reperfusion therapy, and higher in-hospital mortality only during one wave period compared to the pre-pandemic period, with higher adjusted in-hospital mortality in the Midwest and South, although the overall incidence of documented SARS-CoV-2 virus was only 1.3%.
Many studies have previously reported on patients experiencing STEMI during the SARS-CoV-2 outbreak. Several of these studies, however, were limited to patients with concomitant SARS-CoV-2 infection during the early period of the pandemic. Within the North American COVID-19 Myocardial Infarction (NACMI) Registry, 230 patients with COVID-19 infection and 495 with suspected infection, all presenting with STEMI, exhibited a higher risk profile, were less likely to undergo coronary angiography and PCI, experienced increased time to treatment (door-to-balloon time) and higher in-hospital mortality (of 33.0%) compared to matched control patients with STEMI pre-pandemic [15]. In another study of NACMI patients with STEMI and COVID-19, 74% had one culprit lesion, 14% had multiple culprits, and 12% had no culprit identified [16]. Investigators discovered this cohort had thrombus grade burden, multivessel thrombotic disease, and post-PCI thrombus-related complications. Similarly, within the AHA COVID-19 Cardiovascular Disease Registry, of 15,621 COVID-19 hospitalizations in 2020, 54 patients (0.4%) had STEMI, of whom only 50.0% received reperfusion therapy [17]. In-hospital mortality was 41% in patients with STEMI compared to 16% in those without STEMI.
During the waves of the COVID pandemic, patients who presented with STEMI had a higher risk factor profile compared to the pre-pandemic patients. This may be partly attributable to barriers in access to care throughout the waves of the pandemic and to less effective, more delayed treatment. These data demonstrate delays in presentation (e.g., symptom onset-to-device) and delays in care on arrival, as described in other studies [18]. Delays in time to treatment for STEMI have also been noted, both in patients with and without COVID-19 infection [19,20]. In a study within the Vancouver Coastal Health Authority STEMI database, while the incidence of STEMI was similar, FMC-to-balloon time was delayed compared to pre-pandemic, and the delay increased across the three waves of the pandemic [21]. In a more recent study of patients transported by EMS to the ED for suspected ACS, however, there were no significant differences in ECG markers of ischemia (ST-elevation, ST-depression, T-wave inversion, and/or Q-waves), adverse events (death, heart failure, reinfarction), or prehospital time intervals (9-1-1 call to EMS arrival or to ED arrival) between pre-pandemic and pandemic periods [22].
Most prior studies have also noted an increase in in-hospital mortality in patients with STEMI with [19] and without [23,24] COVID-19 infection. In a retrospective, cross-sectional study of all 15,244 hospitalizations involving 14,724 patients with acute MI in a large multistate healthcare system, the risk-adjusted mortality rate among patients with STEMI increased substantially throughout 2020 [23]. However, the COVID-19 infection rate and time to treatment were not reported. In the International Study on Acute Coronary Syndromes–ST-elevation Myocardial Infarction (ISACS-STEMI) COVID-19 worldwide registry, designed to estimate the impact of the COVID-19 pandemic on patients with STEMI treated by PCI [24], among 16,683 patients undergoing PCI in 109 centers located on four continents during the pandemic in 2020, there was a significant age-related reduction in procedures and longer ischemic time, with a larger effect in elderly than in younger patients. There was also a significantly higher 30-day mortality during the pandemic period, especially among elderly patients compared to young patients [24].
In the present study, limited by a questionably low COVID-19 infection rate in patients with STEMI, it is of interest to note that adjusted in-hospital mortality was increased in wave 2 compared to the pre-pandemic period. Whether this can be attributed in part to an increased COVID-19 infection rate and/or a low vaccination rate in wave 2 compared to wave 3 is unknown; therefore, these ideas are hypothesis-generating. In the NACMI registry, none of the 22 vaccinated patients expired in the hospital, whereas an in-hospital death was recorded in 37 (22%) of unvaccinated patients (p = 0.009) [19]. It is also unclear why the mortality increase only occurred in men, but etiologies of STEMI other than plaque rupture and coronary occlusion in women may play a role. Possible further explanations include sex-specific differences in STEMI pathophysiology (plaque morphology and thrombus burden) [25,26] and delay in seeking care for fear of exposure to healthcare-seeking behaviors [27]. Within the COVID-19 NACMI Registry, women more often had STEMI without an identified culprit lesion than men (33% vs. 18%, p < 0.001). The use of PCI was significantly higher in men, whereas medical therapy was higher in women, although in-hospital mortality was similar [28]. However, with respect to increased time to treatment across all waves (albeit absolute differences in several metrics were small and less likely to be clinically significant), it was anticipated that mortality would be increased in all waves compared with the pre-pandemic period. Furthermore, as shown in the observational cohort study using data from the National Center for Health Statistics, there was an increase in ischemic heart disease deaths in the early phases of the pandemic [18]. Although the incidence of OHCA was similar throughout the waves of the pandemic to pre-pandemic, perhaps there was a decrease in the survival rate prior to hospital arrival. This may also explain the observation that mortality was decreased during wave 1 compared to pre-pandemic in the Northeast and West. Delayed care-seeking or overwhelmed EMS may have contributed to an increase in OHCA; therefore, patients with less severe presentations may have reached the hospital during the initial wave, resulting in survival bias. In the Lombardia Cardiac Arrest Registry in Italy, patients with COVID-19 accounted for 77.4% of the increase in cases of out-of-hospital cardiac arrest observed in 2020, and an increase of 14.9% in out-of-hospital deaths in 2020 compared with 2019 among patients in whom resuscitation attempts by EMS were unsuccessful [7]. Moreover, a prior study within the AHA GWTG-CAD registry from 2018 to 2021 [29] in all patients with STEMI, including those with non-system delays, noted that although both time to treatment and mortality had increased during the pandemic (attributed to inherent delays related to EMS, PPE, decreased staffing), mortality during quarter 4 of 2019, pre-pandemic, was 7.2%, which was higher than previously reported in the earlier registry studies [12,30]. The earlier studies, however, excluded patients with non-system reasons for delay; thus, in-hospital mortality may have been significantly increased. Nonetheless, importantly, when time-to-treatment goals were met, mortality was significantly lower.
It is also difficult to determine why changes in mortality differed across geographical regions, but the prevalence of SARS-CoV-2 infection, the proportion of rurality, differences in transfer and transport time, and variations in the development of STEMI systems of care may play a role. Notably, there was no change in the proportion of STEMI patients in the South and an increased proportion in the West throughout the pandemic in comparison to pre-pandemic and the absolute number of patients with STEMI decreased in comparison to analogous pre-pandemic time periods for all waves in all regions (Supplementary Table S3). In a study across 51 New York State hospitals during the early pandemic, PCI for STEMI was decreased, and symptom onset-to-arrival increased in high-density COVID areas but not in low-density areas [31]. It has also been suggested that the geographic areas most impacted by COVID-19 may not remain stable due to changing public health mandates both over time and in different geographic regions [32]. A study using an emerging hot spot analysis to examine the spatiotemporal trends in COVID-19 death rates in the US from March 2020 to May 2021 revealed that, over the three phases of the pandemic (first wave, second wave, and post-vaccine deployment), hot spots shifted from high-density population areas with more socially vulnerable individuals to the states with less strict social distancing and then to the states with low vaccination rates [32]. These data suggested that the factors associated with COVID-19 death rates, including local infection and testing rates, social distancing interventions, and other social, environmental, geographical (urban versus rural), and health risk factors, varied over time. It was noted that these findings can inform the planning of public health initiatives. However, since the COVID-19 infection rate was low on admission in this study population, it is unclear how these regional changes would affect mortality in patients with STEMI.
It is important to highlight several findings from this AHA voluntary, quality improvement registry that support the importance of STEMI systems of care. Despite the inherent delays in care during the pandemic noted above, there was no change in the proportion of patients receiving reperfusion therapy with either fibrinolytic agents or, importantly, with primary PCI, although the overall reperfusion incidence of 83% is lower than in the earlier AHA registry studies. In addition, there was no change in the proportion of patients transferred to a STEMI receiving center for PCI throughout the pandemic waves compared to pre-pandemic, which likely relates to the relatively stable in-hospital mortality. Indeed, within the AHA functioning regional STEMI systems of care, with regular meetings of all stakeholders, including STEMI Referral Hospitals, Receiving Centers, and EMS, planning of how best and where to care for STEMI patients during the pandemic could occur. Certainly, what we have learned from the review of the process metrics and outcomes throughout the waves of the pandemic across the country will allow us to best prepare and respond to the next (ongoing) pandemic or disaster [33], and continue to maintain and optimize the quality of care and outcomes for patients with STEMI. It should be noted, however, that long transfer delays both pre-pandemic and during the pandemic provide key opportunities for systems improvement.
Limitations
This study has several limitations. The registry is a voluntary quality improvement project with the objective of assisting participating hospitals to improve their processes of care. It uses hospital-reported data, although audits are performed, and the sample of the study is not from random sampling. There is potential for selection bias given the voluntary nature of the registry. All multivariable analyses are subject to potential confounding. They, moreover, do not include any hospital-level covariates. Small and rural hospitals that rely on fibrinolysis and frequently need to transfer patients to PCI-capable facilities are underrepresented in the registry. This suggests that the findings may not be generalizable. It is possible that hospitals most impacted by the pandemic were less likely to voluntarily report data, in which case, we may underestimate the overall impact of the pandemic on STEMI patients. Hospitals under greater pandemic-related stress may have been less able to participate consistently or submit complete data, potentially leading to underrepresentation of more severely impacted institutions. We may, furthermore, not fully understand the true rate of COVID-19 infection or vaccinations in our data, given the various sources of reporting used, including a lack of reporting from individuals using home testing to determine infection.
5. Conclusions
In conclusion, among a US national sample of STEMI patients presenting to the hospital during waves of the COVID-19 pandemic compared to those pre-pandemic, patients had a higher risk profile, delayed time to treatment despite presenting via EMS more often, similar proportions treated with reperfusion therapy and transfer rates for primary PCI, and higher in-hospital mortality only during one wave period, with adjusted mortality increased in the Midwest and South. The latter finding emphasizes the importance of addressing regional disparities in patient outcomes. Treatment delays during the pandemic underscore the need for targeted strategies to enhance timely STEMI management and inform future planning. Regionalization of STEMI care has remained an important systems approach to address gaps in care and suboptimal outcomes during the COVID-19 pandemic and allows us to prepare for the next pandemic or national disaster.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/covid5080114/s1, Table S1: Characteristics of CAD registry hospitals; Table S2: CAD registry hospitals by CDC region; Table S3: STEMI patients of CAD registry by pre-pandemic periods; Table S4: (a) Multivariable analysis of in-hospital mortality among COVID waves for females, (b) Multivariable analysis of in-hospital mortality among COVID waves for males; Table S5: (a) Sensitivity Analysis: Multivariable analysis of In-hospital mortality among COVID waves for females, (b) Sensitivity Analysis: Multivariable analysis of in-hospital mortality among COVID waves for males; Table S6: (a) Multivariable analysis of in-hospital mortality among COVID waves for Midwest region, (b) Multivariable analysis of in-hospital mortality among COVID waves for Northeast region, (c) Multivariable analysis of in-hospital mortality among COVID waves for South region, (d) Multivariable analysis of in-hospital mortality among COVID waves for West region.
Author Contributions
Conceptualization, J.K.Z.-H. and A.K.J.; methodology, J.K.Z.-H., A.K.J., R.P. and K.T.; software, R.P.; validation, R.P., K.T. and H.H.; formal analysis, R.P. and H.H.; investigation, J.K.Z.-H. and A.K.J.; resources, K.T.; data curation, K.T.; writing—original draft preparation, J.K.Z.-H. and A.K.J.; writing—J.K.Z.-H., A.K.J., A.G., R.P., K.T., M.J.A., P.B., M.B., G.C.F., W.F., C.B.G., T.D.H., H.H., J.J., M.R., T.S., H.S., F.W. and L.W.; visualization, R.P. and H.H.; supervision, J.K.Z.-H. and A.K.J.; project administration, K.T.; funding acquisition, K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the American Heart Association (American Heart Association: 21CA002).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
The AHA GWTG-CAD Registry is a voluntary quality improvement program in the US, which has been described previously [12,13]. Participating hospitals upload clinical data of consecutive patients admitted with STEMI or non-ST-elevation myocardial infarction (NSTEMI). Program information and data elements collected in the case report form are available at: https://www.heart.org/en/professional/quality-improvement (accessed on 2 July 2025).
Conflicts of Interest
Jessica K. Zègre-Hemsey: NIH funding. Abhinav Goyal, Remy Poudel, Kathie Thomas, Murtuza J. Ali, Patricia J. M. Best, William J. French, Timothy D. Henry, Haoyun Hong, James Jollis, Michael Redlener, Travis Spier, Harper Stone, Feras Wahab, Lanjing Wang and Alice K. Jacobs: No disclosures. Mark Bieniarz: Consultant, research funding Edwards Lifesciences; Abiomed, Johnson & Johnson. Gregg C. Fonarow: Consultant for Abbott, Amgen, AstraZeneca, Bayer, Boehringer, Ingelheim, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer. Christopher B. Granger: Abiomed, Alnylam Pharmaceuticals, Amgen, Anthos, Bayer, Boston Scientific Corporation, Cardionomic, Celecor, Janssen Global Services, Medscape, Medtronic, Merck, NephroSynergy, Novartis Pharma, Novo Nordisk, Pfizer Philips, Roche, Tenacio, Veralox Therapeutics. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| COVID-19 | Coronavirus disease of 2019 |
| STEMI | ST-elevation myocardial infarction |
| PCI | Percutaneous coronary intervention |
| EMS | Emergency medical services |
| ACS | Acute coronary syndrome |
References
- Pope, J.H.; Aufderheide, T.P.; Ruthazer, R.; Woolard, R.H.; Feldman, J.A.; Beshansky, J.R.; Griffith, J.L.; Selker, H.P. Missed Diagnoses of Acute Cardiac Ischemia in the Emergency Department. N. Engl. J. Med. 2000, 342, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, H.M. Where Have All the Heart Attacks Gone. New York Times, 6 April 2020.
- De Filippo, O.; D’aScenzo, F.; Angelini, F.; Bocchino, P.P.; Conrotto, F.; Saglietto, A.; Secco, G.G.; Campo, G.; Gallone, G.; Verardi, R.; et al. Reduced Rate of Hospital Admissions for ACS during Covid-19 Outbreak in Northern Italy. N. Engl. J. Med. 2020, 383, 88–89. [Google Scholar] [CrossRef]
- Hartnett, K.P.; Kite-Powell, A.; DeVies, J.; Coletta, M.A.; Boehmer, T.K.; Adjemian, J.; Gundlapalli, A.V.; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 Pandemic on Emergency Department Visits—United States, January 1, 2019–May 30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Pendyal, A.; Rothenberg, C.; Scofi, J.E.; Krumholz, H.M.; Safdar, B.; Dreyer, R.P.; Venkatesh, A.K. National Trends in Emergency Department Care Processes for Acute Myocardial Infarction in the United States, 2005 to 2015. J. Am. Heart Assoc. 2020, 9, e017208. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.J.; Ritchey, M.D.; Goodman, A.B.; Dias, T.; Twentyman, E.; Fuld, J.; Schieve, L.A.; Imperatore, G.; Benoit, S.R.; Kite-Powell, A.; et al. Potential Indirect Effects of the COVID-19 Pandemic on Use of Emergency Departments for Acute Life-Threatening Conditions—United States, January–May 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Baldi, E.; Sechi, G.M.; Mare, C.; Canevari, F.; Brancaglione, A.; Primi, R.; Klersy, C.; Palo, A.; Contri, E.; Ronchi, V.; et al. Out-of-Hospital Cardiac Arrest during the Covid-19 Outbreak in Italy. N. Engl. J. Med. 2020, 383, 496–498. [Google Scholar] [CrossRef]
- Woolf, S.H.; Chapman, D.A.; Sabo, R.T.; Weinberger, D.M.; Hill, L. Excess Deaths From COVID-19 and Other Causes, March–April 2020. JAMA 2020, 324, 510–513. [Google Scholar] [CrossRef]
- Jacobs, A.K.; Antman, E.M.; Faxon, D.P.; Gregory, T.; Solis, P. Development of Systems of Care for ST-Elevation Myocardial Infarction Patients. Circulation 2007, 116, 217–230. [Google Scholar] [CrossRef]
- Jacobs, A.K.; Ali, M.; Best, P.J.; Bieniarz, M.; Cohen, M.G.; French, W.J.; Fonarow, G.C.; Granger, C.B.; Goyal, A.; Henry, T.D.; et al. Temporary Emergency Guidance to STEMI Systems of Care During the COVID-19 Pandemic. Circulation 2020, 142, 199–202. [Google Scholar] [CrossRef]
- Get with the Guidelines. Coronary Artery Disease. Available online: https://www.heart.org/en/professional/quality-improvement/get-with-the-guidelines/get-with-the-guidelines-coronary-artery-disease (accessed on 2 July 2025).
- Granger, C.B.; Bates, E.R.; Jollis, J.G.; Antman, E.M.; Nichol, G.; O’Connor, R.E.; Gregory, T.; Roettig, M.L.; Peng, S.A.; Ellrodt, G.; et al. Improving Care of STEMI in the United States 2008 to 2012. J. Am. Heart Assoc. 2019, 8, e008096. [Google Scholar] [CrossRef]
- Osho, A.; Fernandes, M.F.; Poudel, R.; de Lemos, J.; Hong, H.; Zhao, J.; Li, S.; Thomas, K.; Kikuchi, D.S.; Zegre-Hemsey, J.; et al. Race-Based Differences in ST-Segment–Elevation Myocardial Infarction Process Metrics and Mortality From 2015 Through 2021: An Analysis of 178 062 Patients from the American Heart Association Get with the Guidelines–Coronary Artery Disease Registry. Circulation 2023, 148, 229–240. [Google Scholar] [CrossRef] [PubMed]
- CDC Museum. COVID-19 Timeline. Available online: https://www.cdc.gov/museum/timeline/covid19.html (accessed on 18 October 2024).
- Garcia, S.; Dehghani, P.; Grines, C.; Davidson, L.; Nayak, K.R.; Saw, J.; Waksman, R.; Blair, J.; Akshay, B.; Garberich, R.; et al. Initial Findings from the North American COVID-19 Myocardial Infarction Registry. J. Am. Coll. Cardiol. 2021, 77, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, P.; Singh, J.; Mancini, G.J.; Stanberry, L.; Bergstedt, S.; Madan, M.; Benziger, C.P.; Ghasemzadeh, N.; Bortnick, A.; Kankaria, R.; et al. Angiographic characteristics of patients with STEMI and COVID-19: Insights from NACMI registry. Am. Heart J. 2024, 271, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.S.; Varshney, A.S.; Goodrich, E.L.; Gong, J.; Ginder, C.; Senman, B.C.; Johnson, M.; Butler, K.; Woolley, A.E.; de Lemos, J.A.; et al. Epidemiology and Management of ST-Segment–Elevation Myocardial Infarction in Patients with COVID-19: A Report from the American Heart Association COVID-19 Cardiovascular Disease Registry. J. Am. Heart Assoc. 2022, 11, e024451. [Google Scholar] [CrossRef]
- Wadhera, R.K.; Shen, C.; Gondi, S.; Chen, S.; Kazi, D.S.; Yeh, R.W. Cardiovascular Deaths During the COVID-19 Pandemic in the United States. J. Am. Coll. Cardiol. 2021, 77, 159–169. [Google Scholar] [CrossRef]
- Garcia, S.; Dehghani, P.; Stanberry, L.; Grines, C.; Patel, R.A.; Nayak, K.R.; Singh, A.; Htun, W.W.; Kabour, A.; Ghasemzadeh, N.; et al. Trends in Clinical Presentation, Management, and Outcomes of STEMI in Patients with COVID-19. J. Am. Coll. Cardiol. 2022, 79, 2236–2244. [Google Scholar] [CrossRef]
- Chew, N.W.; Ow, Z.G.W.; Teo, V.X.Y.; Heng, R.R.Y.; Ng, C.H.; Lee, C.-H.; Low, A.F.; Chan, M.Y.-Y.; Yeo, T.-C.; Tan, H.-C.; et al. The Global Effect of the COVID-19 Pandemic on STEMI Care: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2021, 37, 1450–1459. [Google Scholar] [CrossRef]
- Malhi, N.; Moghaddam, N.; Hosseini, F.; Singer, J.; Lee, T.; Turgeon, R.D.; Wong, G.C.; Fordyce, C.B. Care and Outcomes of ST-Segment Elevation Myocardial Infarction Across Multiple COVID-19 Waves. Can. J. Cardiol. 2022, 38, 783–791. [Google Scholar] [CrossRef]
- Steege, N.; Crandell, J.; DeVon, H.A.; Rosamond, W.D.; Wong, E.; Chrownoski, K.; Grover, J.; Zègre-Hemsey, J.K. Differences in ischemic burden features and prehospital time intervals in patients transported by ambulance to the emergency department with suspected acute coronary syndrome pre and during the SARS-CoV-2 Pandemic. Int. Paramed. Pract. 2024, 14, 2–7. [Google Scholar]
- Gluckman, T.J.; Wilson, M.A.; Chiu, S.-T.; Penny, B.W.; Chepuri, V.B.; Waggoner, J.W.; Spinelli, K.J. Case Rates, Treatment Approaches, and Outcomes in Acute Myocardial Infarction During the Coronavirus Disease 2019 Pandemic. JAMA Cardiol. 2020, 5, 1419–1424. [Google Scholar] [CrossRef]
- De Luca, G.; Algowhary, M.; Uguz, B.; Oliveira, D.C.; Ganyukov, V.; Busljetik, O.; Cercek, M.; Jensen, L.O.; Loh, P.H.; Calmac, L.; et al. Age-Related Effects of COVID-19 Pandemic on Mechanical Reperfusion and 30-Day Mortality for STEMI: Results of the ISACS-STEMI COVID-19 Registry. J. Clin. Med. 2023, 12, 2116. [Google Scholar] [CrossRef]
- Lansky, A.J.; Ng, V.G.; Maehara, A.; Weisz, G.; Lerman, A.; Mintz, G.S.; De Bruyne, B.; Farhat, N.; Niess, G.; Jankovic, I.; et al. Gender and the Extent of Coronary Atherosclerosis, Plaque Composition, and Clinical Outcomes in Acute Coronary Syndromes. JACC Cardiovasc. Imaging 2012, 5, S62–S72. [Google Scholar] [CrossRef]
- Kataoka, Y.; Puri, R.; Hammadah, M.; Duggal, B.; Uno, K.; Kapadia, S.R.; Tuzcu, E.M.; Nissen, S.E.; King, P.; Nicholls, S.J. Sex Differences in Nonculprit Coronary Plaque Microstructures on Frequency-Domain Optical Coherence Tomography in Acute Coronary Syndromes and Stable Coronary Artery Disease. Circ. Cardiovasc. Imaging 2016, 9, e004506. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Manzo-Silberman, S.; Algowhary, M.; Uguz, B.; Oliveira, D.C.; Ganyukov, V.; Busljetik, O.; Cercek, M.; Okkels, L.; Loh, P.H.; et al. Gender Difference in the Effects of COVID-19 Pandemic on Mechanical Reperfusion and 30-Day Mortality for STEMI: Results of the ISACS-STEMI COVID-19 Registry. J. Clin. Med. 2023, 12, 896. [Google Scholar] [CrossRef]
- Quesada, O.; Van Hon, L.; Yildiz, M.; Madan, M.; Sanina, C.; Davidson, L.; Htun, W.W.; Saw, J.; Garcia, S.; Dehghani, P.; et al. Sex Differences in Clinical Characteristics, Management Strategies, and Outcomes of STEMI With COVID-19: NACMI Registry. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100360. [Google Scholar] [CrossRef] [PubMed]
- Jollis, J.G.; Granger, C.B.; Zègre-Hemsey, J.K.; Henry, T.D.; Goyal, A.; Tamis-Holland, J.E.; Roettig, M.L.; Ali, M.J.; French, W.J.; Poudel, R.; et al. Treatment Time and In-Hospital Mortality Among Patients With ST-Segment Elevation Myocardial Infarction, 2018–2021. JAMA 2022, 328, 2033–2040. [Google Scholar] [CrossRef]
- Jollis, J.G.; Al-Khalidi, H.R.; Roettig, M.L.; Berger, P.B.; Corbett, C.C.; Doerfler, S.M.; Fordyce, C.B.; Henry, T.D.; Hollowell, L.; Magdon-Ismail, Z.; et al. Impact of Regionalization of ST-Segment–Elevation Myocardial Infarction Care on Treatment Times and Outcomes for Emergency Medical Services–Transported Patients Presenting to Hospitals With Percutaneous Coronary Intervention: Mission: Lifeline Accelerator-2. Circulation 2018, 137, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Hannan, E.L.; Wu, Y.; Cozzens, K.; Friedrich, M.; Tamis-Holland, J.; Jacobs, A.K.; Ling, F.S.; King, S.B.; Venditti, F.J.; Walford, G.; et al. Percutaneous Coronary Intervention for ST-Elevation Myocardial Infarction Before and During COVID in New York. Am. J. Cardiol. 2021, 142, 25–34. [Google Scholar] [CrossRef]
- Park, Y.M.; Kearney, G.D.; Wall, B.; Jones, K.; Howard, R.J.; Hylock, R.H. COVID-19 Deaths in the United States: Shifts in Hot Spots over the Three Phases of the Pandemic and the Spatiotemporally Varying Impact of Pandemic Vulnerability. Int. J. Environ. Res. Public Health 2021, 18, 8987. [Google Scholar] [CrossRef]
- Mallapaty, S. The pathogens that could spark the next pandemic. Nature 2024, 632, 488. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).