Abstract
The pinewood nematode (PWN), Bursaphelenchus xylophilus, infects susceptible pine species and causes pine wilt disease (PWD). The first visible symptoms are yellowing and drooping of pine needles due to compromised biochemical reactions of photosynthesis, as a result of damage to the tree’s water column. In vitro cultures are useful tools to study minute biochemical changes because they easily enable reproducibility and genetic homogeneity. In the present work, in vitro maritime pine (Pinus pinaster) shoot cultures were used to simulate PWD, by infecting with PWN in asepsis. Changes in the levels of photopigments, i.e., chlorophyll a and b, carotenoids, and stress related anthocyanins, were followed through spectrophotometry. Infection with the PWN led to a 30% decrease in shoot concentrations of chlorophyll a and a 50% reduction on chlorophyll b. Concentrations of carotenoids increased by 70%, while for anthocyanins no statistically significant changes were observed. PWN phytophagy seems to trigger chlorophyll degradation and production of carotenoids, most probably as a response to oxidative stress. This preliminary study allows gauging the impacts of PWN infection in pine, at the initial stages of PWD, as a contribution to developing, for example, an early detection tool for this phytoparasite.
Keywords:
anthocyanins; carotenoids; chlorophyll; in vitro shoots; photosynthesis; pine wilt disease 1. Introduction
The pinewood nematode (PWN) Bursaphelenchus xylophilus is a microscopic pine phytoparasite vectored by the longhorn beetle, Monochamus spp., to susceptible pine species [1]. Despite being generally mycophagous, the PWN shifts to a phytophagous lifestyle when in contact with pine internal tissues and begins feeding on resin canals and vascular tissues [2]. With the increase in PWN population, due to a quick and massive nematode reproduction, the damage to pine internal tissues causes embolism events that block water transport to the canopy. The reduction in water supply leads to the first visible symptoms of pine wilt disease (PWD), pine needle yellowing and drooping [3]. Needle chlorosis is a result of impaired biochemical photosynthetic reactions. The lower water translocation within plant tissues leads to reduced leaf water potential, changing the capacity of the water splitting complex PSII, a reduction of net photosynthetic rate and stomatal conductance, leading to the accumulation of reactive oxygen species (ROS) which results in excessive oxidative stress, known to affect chlorophyll and carotenoids levels [4]. Chlorophyll, a pivotal photopigment in photosynthesis, allows turning light into energy in plants. It consists of a chlorin ring encasing a magnesium atom, other attached side chains, and a hydrocarbon tail formed by a phytol ester. Chlorophylls are many related green pigments, but the most studied are chlorophyll a (Chl a), used in oxygenic photosynthesis, and chlorophyll b (Chl b), an accessory pigment that helps in photosynthesis by absorbing light energy. Chemically they differ for having an aldehyde (Chl b) instead of a methyl group (Chl a) at the C7 position. Their absorption spectrum is thus different, making differentiation and quantification possible through spectrophotometry. Carotenoids are protectant pigments with essential roles in plants against photooxidative damage by quenching toxic oxygen species [5].
Given the ecological impacts of PWD on forest ecosystems and the high economic costs to the wood industry, uncovering specific biochemical mechanisms of PWN infection and proliferation are of the utmost importance. Conducting experiments in natural conditions is limited to the infected areas and is highly dependent on environmental conditions, as well as deeply influenced by biotic and abiotic variables. Under greenhouse conditions, environmental variation is more easily controlled, however, pine natural genetic diversity still greatly influences experimental outcomes [4].
In vitro shoot cultures present a valuable alternative, because it is an easily accessible laboratory system, free from contaminations, where environmental and nutritional conditions are accurately measured and controlled. The use of in vitro culture systems, benefits from a significant reduction in genetic variation, because pine microshoots are clones of the mother plant, allows for the study of single variables within the host/nematode combination, and plant material can be obtained in large amounts without occupying much space [6,7]. These factors support the use of in vitro systems, as useful biotechnological tools, for phytopathological studies and monitorization of PWD progression.
The present study aims to determine the impact of PWN infection on photopigment concentration, by using in vitro P. pinaster shoots. The levels of chlorophyll and carotenoids were quantified through spectrophotometry, along with the concentration of anthocyanins, a known marker for photo-oxidative stress.
Using the pine/PWN co-culture lab tool allows the establishment of a host/parasite system under laboratory conditions, that enables an easier analysis of fine biochemical changes which take place during infection. This study aims to uncover physiology based PWN infection markers and contribute to the development of early PWD detection systems.
2. Material and Methods
2.1. Chemicals
Pure ethanol (96%), hydrogen peroxide (30%), HPLC-grade methanol (99.9%), hydrochloric acid (HCl, fuming, 37%), sucrose (99.5%), 6-benzylaminopurine (99%), indole-3-butyric acid (98%) and activated charcoal powder suitable for cell culture were acquired from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Establishment of In Vitro Pine Shoot Cultures
Certified maritime pine seeds were made available by Instituto da Conservação da Natureza e das Florestas (ICNF) through Centro Nacional de Sementes Florestais (CENASEF), the national forest seeds center located in Amarante, Portugal. Seeds were cleaned by placing 20–30 seeds in 5 mL microtubes and washing vigorously with tap water for 5 min. Afterwards, 3 mL of a commercial detergent solution (10 drops per 100 mL distilled water) was added to the microtubes, mixed vigorously for 10 min, and dipped in an ultrasonic bath for 5 min. Pine seeds were then rinsed with running tap water and surface sterilized by adding 3 mL pure ethanol (96%) to the microtubes, mixing vigorously and dipping in an ultrasonic bath for 10 min. Under a flow hood, the seeds were washed 3× with 3 mL of sterile distilled water. Using a mechanical lathe, the outer seed coat was opened, and the sterilized pine nuts were hydrated overnight in sterile ultrapure water at room temperature [6]. A stratification step was performed by setting the hydrated pine nuts in wet filter paper inside closed containers and keeping at 4 °C for 7 days to synchronize germination. Afterwards, pine nuts were maintained in darkness, at 24 ± 1 °C until germination. Germinants were then transferred to a growth chamber at 24 ± 1 °C and 16 h light photoperiod.
Shoot cultures were initiated by sectioning the upper portion (hypocotyl and cotyledon) of each germinant and separately inoculating in semi-solid multiplication medium consisting of Schenk and Hildebrandt [8] (SH) medium supplemented with 30 g/L of sucrose, 0.5 mg/L of 6-benzylaminopurine (BAP) and 0.1 mg/L indole-3-butyric acid (IBA) [6]. The pH was set to 5.8, before the addition of 0.8% (w/v) agar and autoclaved at 121 °C for 15 min. For microshoot elongation, shoot clusters were transferred to elongation medium consisting of semi-solid SH medium without plant growth regulators but supplemented with 3 mg/L of activated charcoal. Routine subculture of P. pinaster microshoots was performed monthly to Combiness ® (Belgium) microboxes [9.7 cm base diameter per 8 cm height and green filter (XXL+) on the lid for air exchange] containing ca. 100 mL of culture medium [6]. Maritime pine shoot cultures in multiplication or elongation media were kept under controlled environmental conditions of 16 h of light at 24 ± 1 °C and 8 h of darkness at 18 °C.
2.3. Infection of In Vitro Pine Shoots with the Pinewood Nematode
In vitro pine shoots obtained from a single germinant (clones) were used for infection with the PWN. A suspension of B. xylophilus isolate Bx0.13.003, regularly maintained at the Plant Nematology Lab of the National Institute for Agrarian and Veterinary Research (INIAV, I.P.) at Oeiras, Portugal, was used for infection under asepsis [9]. Surface sterilization was performed in 500 µL aliquots containing 3000 to 3500 mixed life stage PWNs. Under a flow hood, nematodes were re-suspended in 20 mL hydrogen peroxide solution (20%, v/v), for 15 min using a 20 µm mesh sieve. Following, PWNs were washed 5 times with 20 mL of sterile water and then resuspended in 1 mL sterile water. Success of the sterilization step was assessed by inoculating a 100 µL aliquot of sterilized PWN suspension on potato dextrose agar (PDA) plates and maintaining for 4 days at 25 °C.
Maritime pine microshoots routinely maintained in elongation SH culture medium were used for infection with sterile PWN suspensions. Under a flow hood, microshoots were sub-cultured using SH culture medium (without phytohormones nor activated charcoal) and a 100 µL suspension with 250 ± 50 sterilized PWNs was added to the small hole in the culture medium where the cut end of the shoot was placed. The established co-cultures were maintained in the conditions described above for 30 days. Afterwards, microshoots with PWD symptoms were collected, immediately frozen in liquid nitrogen, and kept at −80 °C until analysis.
2.4. Quantification of Chlorophyll, Carotenoids and Anthocyanins
For the quantification of photosynthetic pigments, infected in vitro pine shoots were ground in liquid nitrogen and then 100 mg of the macerate were added to 1 mL of HPLC-grade methanol in 2 mL microtubes. Afterwards, the microtubes were vigorously shaken, vortexed, and stored at −20 °C for 48 h. Microtubes were then centrifuged at 12,000× g for 20 min at 4 °C and the supernatant collected and analyzed. Quantification of chlorophyll and carotenoids was performed through spectrophotometry by measuring the supernatant’s absorbance at 665.2, 652.4 and 470 nm [10]. For chlorophyll, the following formulas were used ((1) to (3)).
chlorophyll a (Chl a) = 16.72 × A665.2 − 9.16 × A652.4,
chlorophyll b (Chl b) = 34.09 × A652.4 − 15.28 × A665.2,
Total chlorophyll (Chl a + b) = 1.44 × A665.2 + 24.93 × A652.4,
While for carotenoids, formula (4) was used.
carotenoids (Car) = [(1000 × A470) – (1.63 × Chl a) – (104.96 × Chl b)]/221,
Results were expressed as µg of pigment/g of shoot fresh weight (FW).
For anthocyanins, 10 µL of HCl (37%) were added to 990 µL of the extract in a 2 mL microtube. After vigorously shaking, the acidified extract was used to quantify anthocyanins by measuring the absorbance at 536 and 600 nm [11]. The absorbance at 600 nm was subtracted from absorbance at 536 nm to account for phaeophytin interference, and anthocyanins were quantified by using the extinction coefficient 33,000 cm/M. Results were expressed as µmol/g FW and represent the mean ± SE of three samples [11].
2.5. Statistical Analysis
Statistical processing was performed with version 2019 of Origin Graphing & Analysis software (OriginLab, Northampton, MA, USA). Statistical significance of data was determined with one-way ANOVA and individual means were compared using the Tukey’s Post-Hoc test with p < 0.05. Results were presented as average and standard error value of 3 experimental replicates each with 3 technical replicates.
3. Results and Discussion
3.1. Establishment of Pine In Vitro Shoots and Infection with the Pinewood Nematode
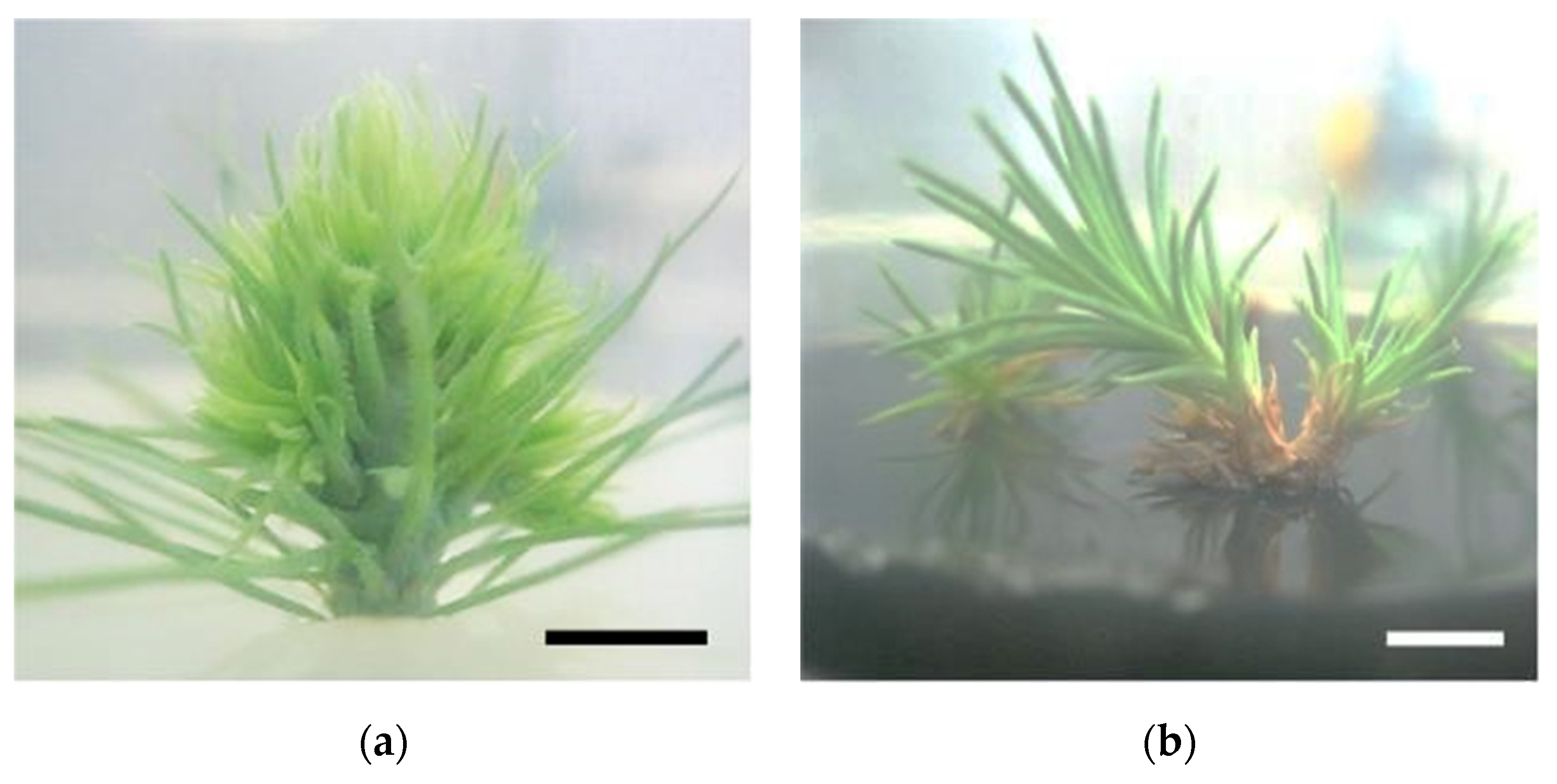
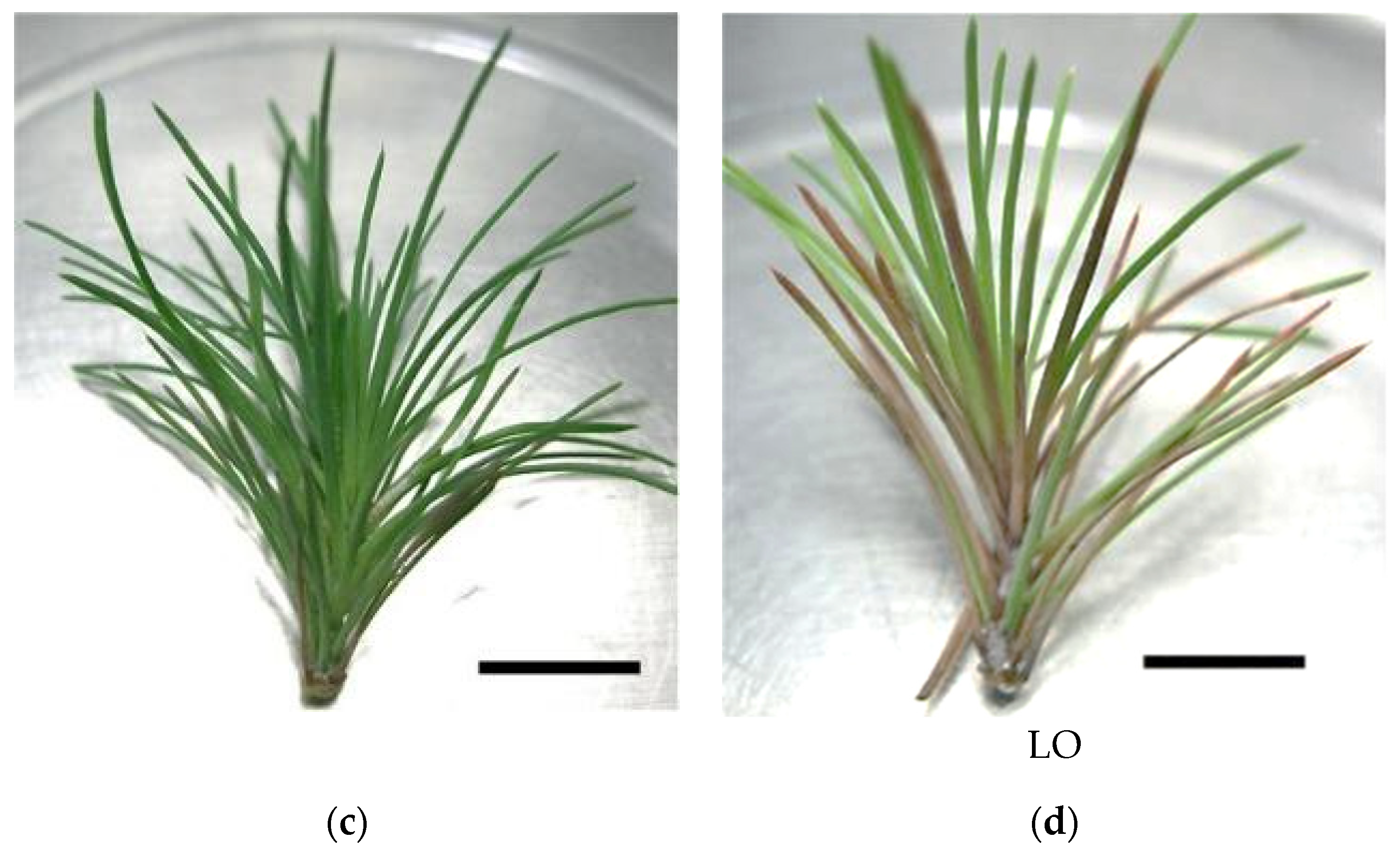
In vitro P. pinaster shoots were established by culturing the upper portion of the aseptic pine seedling (with ca. 1 month after germination) on SH medium with phytohormones (0.1 mg/L IBA and 0.5 mg/L BAP). Within 4 to 6 weeks, several meristems began emerging from the main shoot (Figure 1a). Shoot masses of P. pinaster could be detached from the main shoot cluster and sub-cultured in fresh phytohormone supplemented SH medium, for culture maintenance. For shoot elongation, shoot masses were transferred to SH medium without phytohormones but with activated charcoal, which acts as an adsorbent of toxicants. After 3 to 4 subculture steps (12 to 16 weeks) microshoots could be individualized and grown to ca. 5 cm (Figure 1b). These shoots were then used to establish the in vitro co-cultures, i.e., infection with the PWN, in SH medium. Within the first 20 to 26 days, the typical symptoms of PWD, namely needle chlorosis and drooping, were detected when compared to the control in vitro cultures (Figure 1c,d).
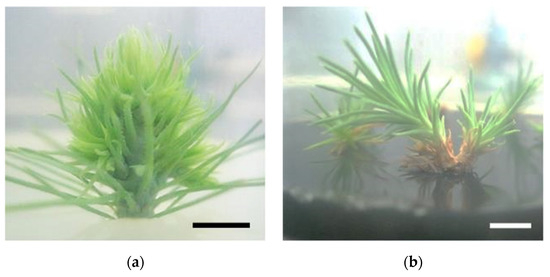
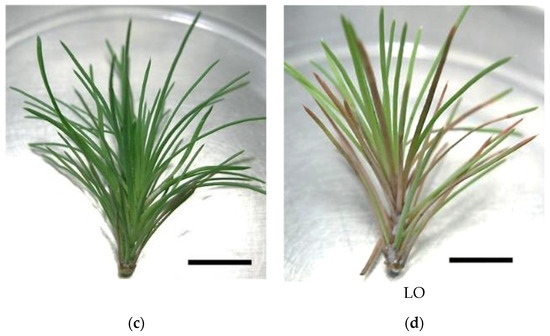
Figure 1.
Pinus pinaster seedling shoot cultured in multiplication medium [culture medium supplemented with 0.5 µg/L of 6-benzylaminopurine (BAP) and 0.1 µg/L of indole-3-butyric acid (IBA)] (a), in vitro microshoots in elongation medium (culture medium supplemented with 3 g/L of activated charcoal) (b), and in vitro explants before (c) and after (d) pinewood nematode infection. Bar = 1 cm.
3.2. Quantification of Photopigments
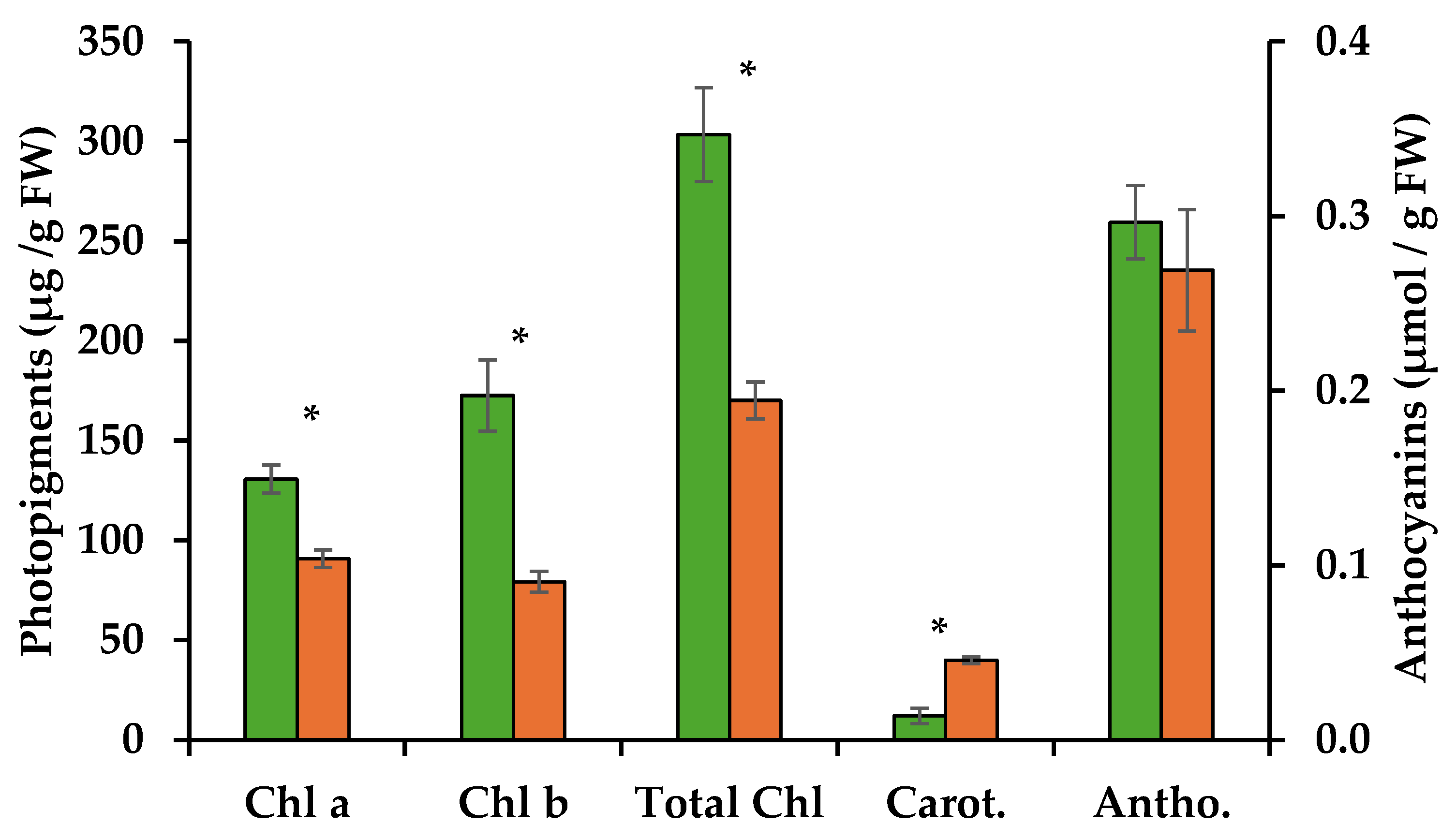
Methanolic extracts of the control and the PWN-infected shoots were used to quantify photosynthetic pigments and anthocyanins. Infection by the PWN resulted in a 31% decrease in chlorophyll a, with 130.7 µg/g FW for control shoots as opposed to 90.8 µg/g FW for PWN-infected shoots. A stronger decrease was observed for chlorophyll b, with extracts of the control shoots containing 172.5 µg/g FW compared to PWN infected shoots, 79.3 µg/g FW, representing a 54% decrease (Table 1). Total chlorophyll content was thus 44% lower on infected P. pinaster shoots (Figure 2 and Table 1).

Table 1.
Percentage variation of chlorophyll, carotenoids and anthocyanins in extracts of in vitro pine shoots after infection with the pinewood nematode (PWN).
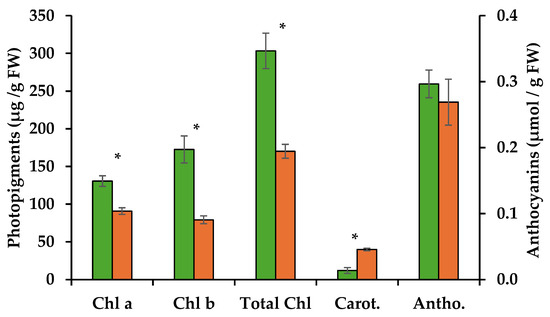
Figure 2.
Quantification of chlorophyll a (Chl a) and b (Chl b), carotenoids (Carot.) (µg/g FW) and anthocyanins (Antho.) (µmol/g FW) through spectrophotometry, from extracts of in vitro pine shoots without (green bars) and with the pinewood nematode (orange bars). Asterisks over the error bars indicate significant differences between these conditions (p < 0.05).
Chlorophyll a is known to play a central role in the light reactions of photosynthesis, while chlorophyll b is part of the photopigments that absorb light and transfer energy to chlorophyll a. Chlorophyll degradation is often the result of biotic or abiotic stress factors that lead to oxidative stress. Accumulation of reactive oxygen species (ROS) can damage chloroplasts and thylakoid membranes, leading to the breakdown of chlorophyll molecules. However, given its lower relevance for photosynthesis, chlorophyll b tends to be more easily degraded under stress, altering the chlorophyll a:b ratio. In the present work, this was observed, with the chlorophyll a:b ratio changing from 0.76 to 1.14 with PWN infection. Thus, PWN infection seems to lead to reduced chlorophyll contents, probably through ROS accumulation in PWN-infected shoots. A higher chlorophyll a:b ratio can impact photosynthetic efficiency which is known to influence stomatal conductance and leaf water potential [4,12,13]. Indeed, in P. pinaster plantlets infected with the PWN, damage to the oxygen evolving complex (OEC) was seen due to an imbalance between the electron flow from the OEC to the reaction center at the acceptor site of photosystem II [14]. This led to a progressive decrease in the rates of photochemical processes and suggests an overall degradation of chlorophyll photopigments.
In the present work, infection by the PWN also resulted in an increase in carotenoid levels by ca. 70%, from 12 µg/g FW in uninfected shoots to 40 µg/g FW in PWN infected shoots (Figure 2 and Table 1). Carotenoids are important photopigments and serve key roles in photosynthesis, protecting chlorophyll from photooxidation, and as precursors to signaling molecules like abscisic acid. They are synthesized by the plant in response to oxidative stress and act by quenching singlet oxygen and other ROS [15]. The increase in ROS levels is believed to be due to lowering the efficiency in the water splitting process of photosynthesis, induced by PWN infection [4]. Also, increase in carotenoids may support a higher concentration of abscisic acid, previously reported in response to PWN infection in susceptible P. pinaster [16].
Lastly, anthocyanin concentrations showed a slight decrease after infection, but not statistically significant (Figure 2 and Table 1). Anthocyanins are generally produced in response to abiotic stress and in some species to physical damage, for example through herbivory. Their main known function is to protect plants against excessive light, by absorbing the green and yellow wavebands of light, and preventing damage to chlorophyll from the resulting oxidative stress [17]. In the present work, light conditions were controlled and maintained throughout, so changes to anthocyanins due to excess light were not to be expected. However, PWN feeding of internal tissues seems not to stimulate anthocyanin production.
In conclusion, infection of pine tissue with the PWN triggers chlorophyll degradation and stimulates carotenoids production, most probably as a response to excess ROS and oxidative stress in the chloroplast, while not influencing anthocyanin levels. Photopigment levels appear to be good target biomarkers for early detection of PWN infection and should be further analyzed.
Author Contributions
Conceptualization, J.M.S.F.; methodology, J.M.S.F.; software, J.M.S.F.; formal analysis, G.P. and J.M.S.F.; investigation, G.P. and J.M.S.F.; resources, J.M.S.F.; data curation, J.M.S.F.; writing—original draft preparation, G.P. and J.M.S.F.; writing—review and editing, G.P. and J.M.S.F.; funding acquisition, J.M.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
Partly funded by Fundação para a Ciência e a Tecnologia (FCT/MCTES) through project NemACT (2022.00359.CEECIND [18]) and project NemaWAARS (PTDC/ASP-PLA/1108/2021 [19]).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data are available from the corresponding author (Jorge M. S. Faria) upon reasonable request.
Acknowledgments
The authors wish to thank Larisa Varela for the technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Evans, H.F.; McNamara, D.G.; Braasch, H.; Chadoeuf, J.; Magnusso, C. Pest Risk Analysis (PRA) for the Territories of the European Union (as PRA Area). EPPO Bull. 1996, 26, 199–249. [Google Scholar] [CrossRef]
- Mamiya, Y. Pathology of the Pine Wilt Disease Caused by Bursaphelenchus Xylophilus. Annu. Rev. Phytopathol. 1983, 21, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Back, M.A.; Bonifácio, L.; Inácio, M.L.; Mota, M.; Boa, E. Pine Wilt Disease: A Global Threat to Forestry. Plant Pathol. 2024, 73, 1026–1041. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Mendes, M.D.; Lima, A.S.; Barbosa, P.M.; Ascensão, L.; Barroso, J.G.; Pedro, L.G.; Mota, M.M.; Figueiredo, A.C. Pinus Halepensis, Pinus Pinaster, Pinus Pinea and Pinus Sylvestris Essential Oils Chemotypes and Monoterpene Hydrocarbon Enantiomers, before and after Inoculation with the Pinewood Nematode Bursaphelenchus Xylophilus. Chem. Biodivers. 2017, 14, e1600153. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M. Plant Pigments: Chlorophylls. In Phenotyping Crop Plants for Physiological and Biochemical Traits; Sudhakar, P., Latha, P., Reddy, P., Eds.; Elsevier: Tirupati, India, 2016; pp. 121–127. [Google Scholar]
- Faria, J.M.S.; Sena, I.; Vieira da Silva, I.; Ribeiro, B.; Barbosa, P.; Ascensão, L.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. In Vitro Co-Cultures of Pinus Pinaster with Bursaphelenchus Xylophilus: A Biotechnological Approach to Study Pine Wilt Disease. Planta 2015, 241, 1325–1336. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Figueiredo, A.C.; Teixeira, D.M.; Inácio, M.L. Infection of In Vivo and In Vitro Pines with the Pinewood Nematode Bursaphelenchus Xylophilus and Isolation of Induced Volatiles. J. Vis. Exp. 2024. [Google Scholar] [CrossRef] [PubMed]
- Schenk, R.U.; Hildebrandt, A.C. Medium and Techniques for Induction and Growth of Monocotyledonous and Dicotyledonous Plant Cell Cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Cavaco, T.; Gonçalves, D.; Barbosa, P.; Teixeira, D.M.; Moiteiro, C.; Inácio, M.L. First Report on the Synergistic Interaction between Essential Oils against the Pinewood Nematode Bursaphelenchus xylophilus. Plants 2023, 12, 2438. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Estorninho, M.; Chozas, S.; Mendes, A.; Colwell, F.; Abrantes, I.; Fonseca, L.; Fernandes, P.; Costa, C.; Máguas, C.; Correia, O.; et al. Differential Impact of the Pinewood Nematode on Pinus Species Under Drought Conditions. Front. Plant Sci. 2022, 13, 841707. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Takanashi, T.; Kanzaki, N.; Komatsu, M.; Levia, D.F.; Kabeya, D.; Tobita, H.; Kitao, M.; Ishida, A. Pine Wilt Disease Causes Cavitation around the Resin Canals and Irrecoverable Xylem Conduit Dysfunction. J. Exp. Bot. 2018, 69, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Carrasquinho, I.; António, C. Primary Metabolite Adjustments Associated With Pinewood Nematode Resistance in Pinus Pinaster. Front. Plant Sci. 2021, 12, 777681. [Google Scholar] [CrossRef]
- Matysiak, R. Content of Carotenoids in Needles of Pinus sylvestris L. Growing in Polluted Area. Dendrobiology 2001, 46, 39–42. [Google Scholar]
- Rodrigues, A.M.; Langer, S.; Carrasquinho, I.; Bergström, E.; Larson, T.; Thomas-Oates, J.; António, C. Pinus Pinaster Early Hormonal Defence Responses to Pinewood Nematode (Bursaphelenchus xylophilus) Infection. Metabolites 2021, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.S. Nature′ s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. BioMed Res. Int. 2004, 2004, 314–320. [Google Scholar] [CrossRef] [PubMed]
- FCT. A Novel Approach for Bioprospecting Sustainable Nematicides against Plant Parasitic Nematodes of AgroeCosysTems (NemAct). PTCris 2023. [Google Scholar] [CrossRef]
- FCT. A Motif to Unveil Mechanisms of Parasitism Gene Regulation in the Pinewood Nematode as a Target for Disease Control and Plant Resistance. PTCris 2022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).