Abstract
Background: Early diagnosis of Alzheimer’s disease (AD) is constrained by invasive and costly tests. Aggregation of -amyloid and the A42/A40 ratio in cerebrospinal fluid (CSF) and blood are key biomarkers. Fluorescent probes can report aggregate states, and artificial intelligence (AI) can extract subtle patterns from spectral and blood data. This review synthesizes how probes and AI can identify aggregates and assess the A42/A40 ratio in body fluids to facilitate earlier AD diagnosis. Methods: PRISMA-compliant searches were conducted in Scopus, PubMed, Web of Science, and IEEE Xplore. Results: Twenty-eight studies met inclusion criteria. Plasma A42/A40 was lower in PET-positive individuals by ∼7–18%, with higher performance for mass spectrometry (mean AUC ≈ 0.80) than immunoassays (AUC ≈ 0.71). CSF A42/A40 showed larger group differences (∼50% reductions in PET+) and stronger PET concordance, outperforming plasma. Fluorescent probes—including AN-SP and CRANAD-28—were sensitive to early aggregates and showed in vivo imaging potential, but evidence is largely preclinical or from small cohorts. AI/ML approaches frequently achieved within-study accuracies >90% (e.g., 94–100% in spectral tasks), yet external validation and head-to-head tests of ratio alone versus ratio + AI remain scarce. Conclusions: Plasma A42/40 —particularly by mass spectrometry—currently provides the most reproducible fluid approximation to amyloid PET (mean AUC ≈ 0.80). Fluorescent probes sensitively detect oligomeric A species and show in vivo potential, but evidence remains largely preclinical or from small cohorts. AI/ML methods can extract additional signal from spectral and multivariate blood data, yet consistent incremental gains over optimized Aβ42/40 assays have not been demonstrated due to limited external validation and head-to-head comparisons.
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the leading cause of dementia worldwide [1]. Despite extensive research efforts, its exact cause remains unclear, and no disease-modifying treatments are currently available. The “amyloid hypothesis”, the most widely accepted explanation for AD, proposes that accumulation and aggregation of -amyloid (A) peptides initiate a cascade of neurotoxic oligomeric assemblies, neuroinflammation, and synaptic alterations. Pathologically, AD is characterized by extracellular deposition of A peptides, particularly the A40 and A42 isoforms, and the intracellular accumulation of neurofibrillary tangles composed of hyperphosphorylated tau [2,3,4,5,6]. These pathological hallmarks contribute to synaptic dysfunction, neuronal death, and chronic neuroinflammation, ultimately resulting in cognitive decline [6,7]. Over the past two decades, soluble A oligomers (AOs)—formed early in the aggregation process—have emerged as the principal neurotoxic species in AD, exhibiting disease-dependent accumulation in the brains of affected individuals and driving synaptic dysfunction and neurodegeneration [8,9,10,11]. Detecting these AOs at the earliest stages could therefore enable timely diagnosis and therapeutic intervention.
Currently, CSF assays and positron emission tomography (PET) imaging are the main tools used to detect A aggregates and assess amyloid burden in the brain. Although these techniques provide reliable assessments, they are invasive, expensive, and not widely accessible, limiting their use in early-stage screening and routine clinical practice.
In recent years, increasing attention has turned to blood-based biomarkers as a less invasive and more scalable alternative. In particular, plasma A42/40 has shown a stronger correlation with amyloid PET imaging than absolute A levels [12,13]. Several studies have demonstrated that a reduced A42/40 in plasma is associated with a higher risk of developing dementia [1,12,13,14,15,16,17,18,19,20,21,22].
Among the various strategies for detecting A aggregates, fluorescence-based methods offer unique advantages in terms of sensitivity, versatility, and potential for in situ and real-time analysis. Fluorescent probes can be designed to respond to different stages of amyloid aggregation by exhibiting changes in fluorescence intensity, emission wavelength, or lifetime [23]. These probes include environment-sensitive dyes such as Thioflavin T and Congo Red, solvatochromic fluorophores, molecular rotors, and aggregation-induced emission (AIE) fluorogens. Compared to standard immunoassay-based techniques like ELISA and SIMOA, which rely on antibodies, fluorescent probes often offer simpler assay design and direct optical readout. In contrast to PET tracers, fluorescence methods are more cost-effective and compatible with high-throughput or point-of-care platforms. These advantages make fluorescence spectroscopy a promising alternative for detecting early-stage amyloid aggregates, especially when integrated with advanced data analysis techniques. However, the interpretation of fluorescence emission spectra from amyloid-binding probes is often complicated by spectral overlap, background autofluorescence, and dynamic heterogeneity of aggregates. These challenges necessitate advanced computational approaches capable of resolving subtle patterns in complex data.
In particular, AI-based methods (machine learning and related approaches) have emerged as promising approaches to resolve subtle patterns in high-dimensional, noisy fluorescence data, improving identification and quantification of A oligomers in biological fluids. Several studies demonstrate how AI techniques—such as Random Forest (RF), XGBoost, Principal Component Analysis (PCA), and Linear Discriminant Analysis (LDA)—have been effectively applied to multichannel fluorescence data and spectral decomposition for accurate classification of AD-related patterns, achieving classification accuracies exceeding 90% [24,25,26]. Moreover, AI approaches have been used to integrate multiple biomarker modalities, including A42/40, improving diagnostic accuracy and enabling patient stratification [27,28,29].
Building on these considerations, this systematic review addresses the following primary research question:
How can fluorescent probes and AI-based tools facilitate the early diagnosis of Alzheimer’s disease by identifying -amyloid aggregates and assessing the A42/A40 ratio in body fluids?
In addition, the review explores the following sub-questions:
- How accurately can measurement of A42/40 in plasma detect AD compared to PET imaging?
- Biomarkers: What specific biomarkers have shown efficacy in the early detection of -amyloid aggregates?
- Which ML techniques have contributed to the detection of A aggregates and biomarkers in early-stage AD?
2. Methods
To adequately address the research question and sub-questions, this systematic review was conducted in accordance with the fundamentals of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology. The following steps outline the approach taken:
2.1. Search Strategy
A literature search was conducted in Scopus, PubMed, Web of Science, and IEEE Xplore. The search included studies published from January 2014 to December 2024. The queries were carefully designed to capture all relevant literature and included Boolean operators, synonyms, and Medical Subject Headings (MeSH) terms. To structure the search effectively, we categorized keywords into five thematic groups: (1) Technology: Keywords related to techniques and measurement methods, such as biomarker and fluorescence spectroscopy, were included; (2) Analysis: This category focused on analytical methodologies, including terms like machine learning, data science, and spectral analysis; (3) Field of research: Keywords pertaining to the biological context, such as protein measurements, amyloid aggregates, and A42/40, were prioritized; (4) Zone: This group targeted the measurement medium, using terms such as plasma, blood, CSF; and (5) Exclusion terms: Irrelevant keywords, including terms such as nanoparticles, cancer, and tumor, were excluded to refine the scope of the search and ensure relevance to the research question.
Examples of search queries for PubMed database include:
| (biomarker* OR spectroscop* OR fluorescen* OR “biosensor”) |
| AND |
| (“machine learning” OR “deep learning” OR “data science” OR “artificial intelligence” OR spectr* analys*) |
| AND |
| (amyloid* aggregate OR amyloid* plaques OR amyloid* fibril* OR “abeta42/abeta40” OR amyloid* autofluorescence OR “early diagnosis” OR “Alzheimer” OR “A42/40”) |
| AND |
| (“blood” OR “plasma” OR “CSF” OR “biological sample” OR “serum” OR “cerebrospinal fluid”) |
| NOT |
| (nanoparticl* OR “nanocluster” OR tumor* OR “cancer” OR “cholesterol”) |
The query was adapted for each database. Figure 1 shows how many papers were returned in each database. Only works published since 2014 were used, in some database this requirement was possible to add in the query, like Scopus, in others not, like IEEE Xplore. After obtaining all the results, the articles were uploaded to Zotero to proceed with the elimination of duplicates.
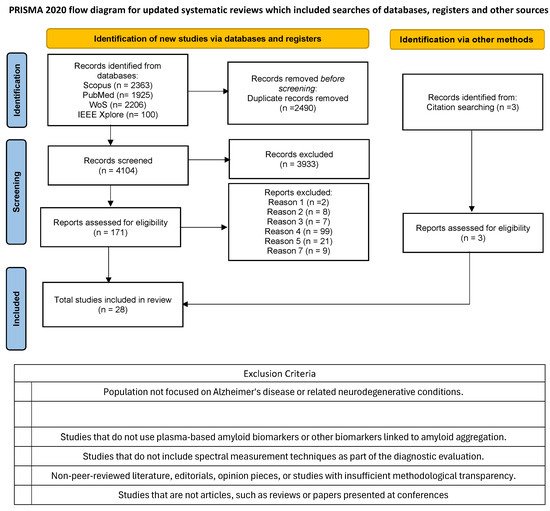
Figure 1.
PRISMA 2020 Flow Diagram for updated systematic review.
In an independent search, it was checked that the query used included all relevant publications. In this search, some adjustments were included: adding the term “oligomers”, separating “amyloid” from “aggregate”, “plaques”, or “fibers”, incorporating synonyms for “autofluorescence” such as “intrinsic fluorescence” or “endogenous fluorescence,” and testing terms like “aggregat*” to capture all variations related to aggregation. To systematically assess the impact of these modifications, we developed a Python 3.12.0 program to compare the results obtained from the revised queries against our initial search. This program identified papers that appeared in the modified searches but were absent from our original dataset. After evaluating these additional papers, we found that none of them provided new relevant information for our bibliographic review, confirming the robustness of our initial search strategy.
This review did not include documents from patent repositories (e.g., WIPO, Espacenet) or clinical trial registries (e.g., ClinicalTrials.gov), as these sources often lack standardized methodological descriptions or validated results. In the context of this review, most entries in these databases do not specify the methodology or analytical techniques employed. Without this information, inclusion and comparison are not feasible, and these registries are outside the scope of our predefined inclusion criteria.
2.2. Eligibility Criteria
The following inclusion and exclusion criteria were followed to select the articles.
2.2.1. Inclusion Criteria
- Research that investigates the relationship between amyloid aggregation and diagnostic outcomes for AD using A42/40.
- Studies focused on early diagnosis of AD using biomarkers for the A aggregates, such as autofluorescence of the amyloid, or other plasma or CSF biomarkers.
- Studies that provide quantitative data on biomarkers or other diagnostic tests associated with AD.
- Studies that combine AI and ML with spectral measurements for analytic determination, regardless of whether the biomarker is directly related to amyloid.
2.2.2. Exclusion Criteria
- Population not focused on AD or related neurodegenerative conditions.
- Studies not addressing early diagnosis or studies that are focused on treatment clinical management, or late-stage disease.
- Studies that do not use plasma-based amyloid biomarkers or other biomarkers linked to amyloid aggregation.
- Studies that do not include spectroscopic techniques as part of the diagnostic evaluation.
- Non-peer-reviewed literature, editorials, opinion pieces, or studies with insufficient methodological transparency.
- Studies that do not apply AI or ML techniques for spectral data analysis or diagnostic purposes.
- Studies that are not articles, such as reviews or papers presented at conferences.
We used Zotero functionalities to merge all references from all databases. After removal of all duplicate articles, a first screening was performed that reviewed the title, abstract, and keywords of each study to determine whether the study is relevant to the review based on the inclusion and exclusion criteria cited in this section. An initial screening was performed, checking the exclusion criteria. If there was some uncertainty, the study was flagged for further discussion. Studies that were not excluded at this first screening were moved on to the full text screening.
In the second stage of screening, the full text of all studies that passed the initial phase was reviewed. This stage also adhered to the established inclusion and exclusion criteria. If any study could not be clearly classified during the screening process, it was subjected to further evaluation by a senior reviewer. In the first screening phase, 171 papers were selected (Figure 1).
2.3. Diagnostic Evaluation Metrics
To support interpretation and comparison across methodologies, this review systematically analyzes key diagnostic performance metrics—such as sensitivity, specificity, accuracy, Spearman’s correlation, and AUC—reported in the included studies. These indicators help evaluate the ability of fluorescence-based probes and AI-assisted analyses to detect early amyloid aggregation, distinguish AD from controls, and correlate with established biomarkers like A42/40.
Accuracy: the proportion of correctly classified cases (both positive and negative) relative to the total number of cases. While widely used, accuracy can be misleading in data sets with imbalanced class distributions.
Sensitivity (true positive rate): Proportion of individuals with AD who are correctly identified as positive. High sensitivity ensures that most true cases are detected, reducing the likelihood of false negatives.
Specificity (true negative rate): Proportion of healthy individuals correctly identified as negative. High specificity minimizes false positives, which is important for avoiding undue anxiety or unnecessary interventions.
Spearman’s rank correlation coefficient (): A nonparametric measure used to assess the strength and direction of a monotonic relationship between two variables, such as between biomarker aggregation levels and A42/40. Unlike Pearson’s correlation, Spearman’s does not assume a linear relationship and is more robust to non-normally distributed data.
Receiver Operating Characteristic (ROC) Curve and Area Under the Curve (AUC): The ROC curve plots the true positive rate (sensitivity) against the false positive rate () at various classification thresholds. The area under the ROC curve (AUC) provides a single-value measure of the discriminatory power of the model; an AUC of 1.0 indicates perfect classification, while an AUC of 0.5 suggests performance equivalent to random chance.
Centiloid: A standardized scale for PET imaging to quantify “global” cortical amyloid burden. The centiloid scale facilitates comparison across studies and tracers by transforming PET measures into a common metric [30].
3. Results
This systematic review aims to (i) summarize biomarkers for detecting amyloid aggregates, with emphasis on oligomers; (ii) evaluate the plasma and CSF A42/40 for early AD detection; and (iii) assess how AI/ML methods and tools can enhance pre-diagnostic performance (AUC, sensitivity, specificity).
As illustrated in Figure 1, a total of 6594 papers were initially retrieved from the four selected databases. After eliminating 2490 duplicates, 4104 articles proceeded to the title and abstract screening, with 171 advancing to the second stage of screening.
Following this process, 25 studies were selected for inclusion in the final analysis. One example of an excluded study is “A Possible Blood Plasma Biomarker for Early-Stage Alzheimer’s Disease” [31], the exclusion criterion applied in this case was criterion 4. Although the study addresses early detection of AD and involves a biomarker, it does not employ fluorescence techniques. The biomarker used is not a dye, and the detection method is based on mass spectrometry rather than optical fluorescence. Additionally, 3 external papers identified from the references of the final 25 studies were included, bringing the total number of studies considered to 28.
These studies were categorized into three main groups: (1) studies focused on comparing plasma A42/40 between PET-positive (PET+) and PET-negative (PET-) individuals; (2) studies investigating fluorescent biomarkers for the detection of amyloid aggregates, including oligomers and fibers; (3) studies employing ML and AI techniques on databases of spectral data to enhance the diagnosis of AD. The relevant information extracted from the papers of each group is collected in Table 1, Table 2 and Table 3. Also, the cohorts used in each of the studies in Table 1 are collected in Table 4.

Table 1.
Summary of papers with analysis of plasma Aβ42/40.

Table 2.
Summary of fluorescence-based biomarker studies for Aβ detection.

Table 3.
Summary of application of AI and ML in Aβ detection.

Table 4.
Cohorts comparation of studies on A42/40 summarized in Table 1.
This classification enabled a structured analysis of the current landscape of biomarker-based detection methods and the potential of AI-driven tools to facilitate early diagnosis.
4. Discussion
4.1. Analysis of Plasma and CSF A42/40 Across Studies and Its Relationship to PET Diagnosis
The studies reviewed collectively highlight the potential of plasma A42/40 as a non-invasive biomarker for identifying amyloid pathology in AD, though with varying degrees of sensitivity, specificity, and correlation with standard amyloid PET and CSF biomarkers.
Across all studies, plasma A42/40 consistently showed a significant reduction in amyloid PET+ individuals compared to PET- individuals, with differences typically ranging from to lower in PET+ participants. However, the degree of separation varied depending on the analytical platform used. Studies that employed high-precision mass spectrometry (MS)-based assays consistently achieved higher diagnostic accuracy compared to studies using immunoassay-based techniques (mean AUC of 0.80 and 0.71 respectively), see Table 1. The most significant reduction observed in A42/40 of PET+ individuals with respect to PET- ones was 18% in the work of Pascual et al. [13] and Weber et al. [16] using a novel antibody-free mass spectrometry (MS) and an immunoprecipitation (IP)-LC-MS/MS respectively.
Genetic factors influencing biomarker performance. Importantly, genetic predisposition significantly modulates plasma biomarker accuracy. Some studies analyzed the presence of Apolipoprotein (APoE). The APOE gene plays a role in lipid transport and is closely linked to AD risk. It has several alleles, with APOE 2 potentially offering some protection and delaying onset, found in approximately 5–10% of individuals. APOE 3, the most common form, is considered neutral, neither increasing nor decreasing risk. In contrast, APOE 4 is associated with a higher risk and earlier onset of Alzheimer’s, present in 15–25% of people, with 2–5% carrying two copies [42].The presence of APOE improved the AUC value in all studies in which it was used (see Table 1), reinforcing the role of genetic predisposition in modifying plasma biomarker performance. Some of these studies also used the age and/or sex for diagnostic.
It is important to note that a small subset of AD cases (<1%) follows an autosomal-dominant inheritance pattern and has an earlier age at onset than sporadic AD. These familial forms are driven by pathogenic mutations in the presenilin 1/2 (PSEN1/PSEN2) or amyloid precursor protein (APP) genes, which shift -secretase cleavage toward the more aggregation-prone A42 species [43,44]. Among the studies we reviewed, only Zhou et al. (2019) [38] stratified their experiments using APP/PSEN1 transgenic mice to validate probe 18, directly demonstrating how these mutations affect assay sensitivity. The remaining studies, while critical for evaluating amyloid detection technologies, assess biomarkers in AD cohorts without distinguishing etiology.
Analytical variability and calibration challenges. Cut-off values for plasma A42/40 varied dramatically across studies (0.06–0.303, Table 5), reflecting methodological differences that limit direct comparison and clinical standardization.

Table 5.
Reported mean and cut-off values of Aβ42/40 in plasma.
The observed cut-off variability reflects differences in analytical techniques, including detection of full-length versus truncated A species, sample preparation methods, and calibration approaches [13].
Calibration approaches varied between synthetic solutions [16,17,22] and human plasma matrices [12,13,19], with isotope-labeled internal standards providing the most accurate quantification.
These methodological variations highlight a critical barrier to clinical translation: the absence of standardized protocols prevents the establishment of universal diagnostic thresholds, limiting immediate clinical utility despite promising individual study results.
Together, these factors underscore the need for caution when interpreting and comparing cut-off values from different studies.
Pre-analytical variables can markedly affect A42/40 stability, yet only six studies [12,13,17,19,21,22] report collection and processing details. All used EDTA tubes for plasma—with some directly comparing EDTA versus heparin—and Li et al. additionally examined differences in collection sites and centrifugation speed/time. None specified processing delays, but these omissions did not appear to impact performance: all studies achieved AUCs > 0.75, and the percent differences between amyloid positive and amyloid negative groups remained consistent (see Table 1). Nonetheless, standardizing and reporting these pre-analytical factors is important to improve assay reproducibility and cross-study comparisons.
Recent evidence indicates that a subset of extracellular A—particularly A42—is selectively packaged into exosomes, the small vesicles derived from endosomes involved in intercellular communication [45,46]. None of the studies included in this review have assessed how exosome-associated influences A measured A42/40 in plasma or CSF. We therefore recommend that future biomarker investigations incorporate an EV-fractionation step prior to A quantification to fully capture both free and vesicle-associated species.
Comparative performance with emerging biomarkers. CSF A42/40 consistently outperformed plasma measures, showing larger reductions in PET+ individuals (≈50% vs. 7–18%) and superior diagnostic accuracy [15,19,20,47].
One work also mentions the high concordance of A42/40 in CSF with tTau and pTau measurements, rather than just using A42 concentration [48]. Wisch et al. [20] demonstrates that CSF A42/40 correlates more strongly with amyloid PET centiloid values ( = −0.73) than plasma A42/40 ( = −0.56), suggesting that plasma biomarkers capture amyloid pathology less precisely.
Temporal dynamics and early detection potential. Some analyses [1,7,12,13,19,21,22,49,50] suggest that plasma A42/40 may become abnormal before amyloid PET detects amyloid aggregates (see Table 1). Several studies further support this, showing that plasma A42/40 declines before amyloid PET positivity, a pattern also observed with p-tau231, reinforcing the role of plasma A42/40 in detecting early amyloid pathology. Janelidze et al. [49] found that plasma A42/40 has comparable diagnostic accuracy to p-tau231 in cognitively unimpaired (CU) individuals (AUC 0.79 for A42/40 and AUC 0.71–0.83 for p-tau231), underscoring its potential in early detection. However, both A42/40 and p-tau231 exhibit early cross-sectional changes in response to amyloid pathology, but eventually reach a plateau, limiting their utility in monitoring disease progression over time. This suggests that plasma A42/40 and p-tau231 function primarily as state markers, effectively distinguishing between amyloid-positive and amyloid-negative individuals, but lacking the capacity to determine the stage or rate of disease progression [7,49,50].
Several other minimally invasive biomarkers also show promise, such as Glial Fibrillary Acidic Protein (GFAP), Neurofilament Light Chain (NfL), and Phosphorylated-tau-181 (pTau 181); these biomarkers provide complementary information on astrocytic activation, tau pathology, and neuroaxonal injury as the disease progresses [51,52].
4.2. Fluorescence-Based Amyloid Detection Methods
The studies reviewed employ a variety of fluorescence-based strategies to detect and characterize amyloid fibrils, oligomers, and other aggregated protein species relevant to neurodegenerative diseases such as AD. Approaches include classical dyes (e.g., Thioflavin T, ThT), ratiometric and near-infrared (NIR) probes, sensor arrays, and ML-assisted detection systems. Table 2 compares methodologies, excitation/emission wavelengths, binding affinities, changes in quantum yield, and other key performance metrics.
Classical markers such as ThT (see [40,41]) show a marked fluorescence enhancement on binding to -sheet-rich fibrils and thus remain useful indicators of fibrillar amyloid. However, ThT is insensitive to oligomeric species, can self-aggregate at high concentrations, and is subject to experimental variability [40,41]; these limitations have driven the development of probes with greater selectivity for oligomers and improved photophysical properties.
Evolution from traditional markers to advanced probe design. As noted above, several fluorescent probes are discussed in the studies reviewed here. Telpoukhovskaia et al. [32] investigated the compound HL22 and reported a 50% increase in fluorescence intensity on binding to amyloid fibrils, with an emission maximum at 420 nm. Mora et al. [36] examined 2Me-DABT and demonstrated ratiometric fluorescence detection with a pronounced blue shift (500–445 nm) on fibril binding, making this probe more reliable than ThT for quantitative applications. A 2019 study described an NIR probe that exhibited a marked shift in emission from 762 nm to 650 nm upon addition of A; this probe may be particularly useful for distinguishing A plaques from tau neurofibrillary tangles (NFTs), an important diagnostic distinction in AD [38].
Other probes have been applied to labeled brain sections and compared with ThT. CRANAD-28 was used to stain mounted brain sections to assess plaque-labeling quality and robustness [37]; an antibody (3D6) served as the control. Compared with ThT, CRANAD-28 labeled more plaques: the mean diameter of the labeled zone was 14, 18, and 20 μm for ThT, CRANAD-28 and 3D6, respectively. One plausible explanation for the larger labelled area with CRANAD-28 is that it can bind smaller A species, including soluble monomers and oligomers, thereby producing a wider staining halo.
Novel fluorescent probes with enhanced specificity. All probes mentioned before, (HL22, 2Me-DABT, Probe 18 of Zhou, and CRANAD-28) offer significant improvements compared to ThT in binding specificity, fluorescence response, and clinical applicability, with several showing blood-brain barrier (BBB) penetration, making them potential candidates for in vivo imaging of amyloid plaques. Moreover, ThT cannot be employed for in vivo imaging due to its positive charge, which makes it highly hydrophilic and prevents it from crossing the BBB.
Oligomer-specific detection advances. While many studies focus on mature fibrils, the ability to detect A oligomers is increasingly recognized as a major advancement in amyloid research, given their higher neurotoxicity. Lv et al. [34] introduced AN-SP, a spiropyran-based probe that selectively binds to AO over fibrils. Unlike ThT and other fibril-preferring dyes, AN-SP exhibited a 9.4-fold fluorescence enhancement upon binding to AO within a detection range of 0–12 μM, making it a promising candidate for the early detection of AD.
In contrast, Li et al. [24] developed a BPNS-Zn2+ fluorescent complex, which shows a quenched fluorescence at 505 nm upon A binding that recovers upon dissociation of oligomers at 423 nm, allowing a ratiometric approach to track AO formation.
Chen et al. [39] introduced a fluorescent aptasensor, FAM-Apt, capable of detecting AO in a range of 1.7 to 85.1 ng/mL in plasma. The detection principle involves the presence of FAM-Apt in the reaction buffer, followed by the addition of AO and cDNA-modified magnetic beads (cDNA-MBs). The aptamer binds specifically to the oligomers, forming an Apt-AO complex. A magnetic field is then applied to extract the trapped complexes, and the fluorescence intensity of the buffer is measured before and after the procedure to quantify A O.
Furthermore, Li et al. [53] developed F-SLOH, an oligomer-selective cyanine dye. When compared to ThT, F-SLOH demonstrated a significant increase in fluorescence intensity in the presence of AO (10 μM, 2-hour incubation), with fluorescence decreasing as fibrils formed.
Advanced detection platforms. Some works, like Zhou et al. [38] look for dyes with emission in the near-infrared (NIR) to improve in vivo imaging, or use multiple excitation-emission channels [25,33] to enable more robust fluorescence-based differentiation of amyloid aggregates. Even ratiometric probes were tested with 2Me-DABT and BPNS-Zn2+ [24,36] to reduce false positives from background fluorescence.
Other dyes like, Congo Red (CR), 8-anilino-1-naphthalenesulfonic acid (ANS), Safranine T (ST), berberine (BBR), and coptisine in Du et al. [25] were tested for detection of amyloid fibrils and cationic PPE, Nile Red (NR) and Victoria Blue B (VBB) in Li et al. [33] for monomers, oligomers, fibrils.
In this review, no studies have been found that utilize the intrinsic fluorescence of amyloids for the detection of their aggregates. Only the work by dos Santos et al. [26] analyzed the fluorescence of blood plasma samples using chemometric methods as a diagnostic tool for Alzheimer’s disease (AD), although they did not specifically address the fluorescence associated with the amyloid proteins themselves or their potential aggregates. Our group has recently published the first observation of Ab40 oligomers through their autofluorescence, particularly marked by the appearance of an aggregation-induced emission (AIE) band in the visible region of the spectrum [54]. Such emission bands have previously been observed in solid-state or dispersed amyloid samples and appear to be associated with the presence of beta-sheet-rich structures formed by aggregation [55].
4.3. Analytical Comparison of AI-Based Methods for Detecting AD Biomarkers
Advances in ML and AI have substantially improved early detection of AD by enabling the identification and quantification of A aggregates and related biomarkers. The reviewed studies apply a range of ML methods across diagnostic platforms—including fluorescence spectroscopy, metabolomics, and blood-based biomarker analysis (see Table 3). Each method presents advantages and challenges, reflecting the diverse potential and current limitations of AI-driven diagnostic strategies in AD research.
Algorithm selection and performance optimization. A key distinction among the reviewed studies lies in the choice of AI/ML methodologies and the nature of the datasets analyzed. Notably, several works highlight the effectiveness of tree-based ensemble learning models—particularly Random Forest (RF) and XGBoost—for handling the complexity and heterogeneity of biological data.
For instance, Han et al. [27] and Karaglani et al. [28] employed these models to classify blood-based biomarkers, achieving impressive AUC values exceeding 0.90, underscoring their robustness and predictive accuracy. Similarly, Li et al. [33] and Du et al. [25] integrated multichannel fluorescence sensor arrays with analytical techniques such as Linear Discriminant Analysis (LDA), Principal Component Analysis (PCA), and RF, achieving classification accuracies greater than 96%. Furthermore, Xu et al. [29] utilized a Multilayer Perceptron (MLP) and RF for miRNA classification in serum and plasma samples, with models surpassing 90% accuracy.
These findings reinforce the suitability of tree-based models, particularly RF and XGBoost, for biomarker detection tasks in AD research, owing to their ability to manage high-dimensional, non-linear, and multivariate biological data effectively.
Feature extraction and dimensionality reduction strategies. Several studies enhanced classification performance by applying statistical decomposition techniques to spectroscopic data. For example, Dos Santos el al. [26] decomposed fluorescence emission spectra into distinct components to identify AD-related spectral variations, applying PARAFAC-QDA and Tucker3-QDA and reaching an accuracy of 94.12%. Similarly, Li et al. [33] and Du et al. [25] employed PCA to reduce the dimensionality of multichannel fluorescence sensor data, facilitating more efficient and accurate classification. In contrast, other studies focused on biological feature extraction, utilizing genomic and metabolic signatures to derive informative features. Han et al., Karaglani et al. and Xu et al. [27,28,29] exemplify this approach, integrating omics data into their ML models to improve diagnostic accuracy (see Table 3).
Validation methodologies and clinical translation challenges. To ensure analytical robustness, most studies employed cross-validation techniques, and several additionally incorporated independent test sets to validate performance. Han et al. [27] implemented a 10-fold cross-validation approach, achieving an AUC of 0.948 for Alzheimer’s diagnosis by integrating metabolic and clinical risk factors. Li et al. [33] reported perfect classification accuracy (100%) in PBS samples, which decreased to 91.7% in serum—highlighting the practical challenges of applying fluorescence-based methods to complex biological matrices. Meanwhile, Karaglani et al. [28] used an AutoML platform (JADBIO) to optimize biomarker selection, achieving an AUC of 0.975 for miRNA-based classification, representing the highest diagnostic performance reported in this review.
Despite the evident potential of AI-based approaches, their widespread application is constrained by limited access to large-scale, high-quality omics datasets, which significantly hinders real-world clinical integration [56]. This scarcity of publicly available data, coupled with the high computational demands of deep learning models, may explain why no such models were identified among the studies reviewed, despite their growing promise in biomedical applications [57]. Nevertheless, Du et al. demonstrated the power of fluorescence colorimetric sensing combined with hierarchical clustering, achieving 100% classification accuracy in differentiating amyloid fibrils, with high selectivity and sensitivity (LOD: 2.52 nM for insulin fibrils, 14.57 nM for (A) fibrils). Their work highlights the potential of low-cost, smartphone-compatible diagnostic platforms for accessible Alzheimer’s screening (see Table 3).
4.4. Limitations
This systematic review adhered strictly to the PRISMA guidelines, employing a rigorous methodology to ensure a comprehensive analysis of existing literature. Despite these efforts, certain limitations must be acknowledged. Firstly, while the search strategy involved multiple reputable databases (Scopus, PubMed, Web of Science, IEEE Xplore) and a well-constructed query string, the possibility of missing relevant studies cannot be entirely ruled out. Some articles may not have been indexed within the selected databases or may have used unconventional terminology, leading to potential gaps in the reviewed literature.
Secondly, a significant limitation arises from the novelty of the research area. Many of the included studies are in preliminary stages. This limitation affects the robustness of the data and the generalizability of the findings, as many studies are yet to undergo the rigorous scrutiny associated with established scientific publications. Additionally, the early developmental status of many diagnostic tools and AI methodologies for AD further constrains the ability to draw definitive conclusions.
Given the emerging nature of ML applications in AD biomarker detection, a key limitation of this review is the lack of availability of standardized, publicly accessible datasets, which currently hinders the feasibility of conducting a comprehensive meta-analysis. The heterogeneity of methods, models, and patient cohorts requires a further qualitative, scoping approach. While this offers valuable insights, it lacks the statistical rigor of meta-analyses and may carry a higher risk of bias. To address this, the review followed PRISMA guidelines, assessing risk of bias by comparing pre-specified outcomes with reported results, and documenting data availability across studies (see Table 1, Table 2 and Table 3).
Finally, this review identified other broad reviews in related fields, but none of them focused specifically on the research question addressed here. This underscores the originality of this study.
5. Conclusions
5.1. A42/40 Detection in Plasma
Plasma biomarkers are most useful as screening tools to reduce reliance on expensive and invasive PET scans, particularly in research settings and early clinical trials. Plasma A42/40 is a promising non-invasive biomarker for detecting amyloid pathology, with moderate to high accuracy depending on the assay used. However, CSF A42/40 remains the superior predictor of amyloid burden, demonstrating higher sensitivity, specificity, and stronger correlation with amyloid PET.
5.2. Fluorescent Probes
The detection of oligomers is crucial for the early diagnosis of AD. Although many dyes have been studied, most are selective for fibrils rather than oligomers. Only a few of the dyes cited in this work specifically target oligomers [24,34,39,53].
Beyond in vitro binding studies, several ratiometric and NIR probes demonstrate clear advantages over ThT in live models by reducing background fluorescence and improving signal specificity. CRANAD-28, Probe 18, HL22 and 2-Me-DABT have been validated for penetration of BBB and in vivo imaging [32,36,37,38].
5.3. AI for AD Detection
From an AI-analysis perspective, ML methods have demonstrated a strong potential for early AD detection, particularly through the use of tree-based models and statistical techniques for dimensionality reduction. Models like Random Forest and XGBoost performed robustly on various types of biological data, while PCA and LDA helped optimize the classification accuracy. Although deep learning was absent in the reviewed studies, probably due to limited access to large, high-quality omics datasets and computational demands, the overall findings highlight the promise of accessible, cost-effective diagnostic tools, such as smartphone-compatible fluorescence platforms, for future clinical applications.
5.4. Answer to the Primary Research Question
This review directly addresses the three components of the primary question. First, fluorescence-based probes (for example, CRANAD-28, AN-SP and related benzothiazole derivatives) demonstrate sensitive detection of A oligomeric species in vitro and in early ex vivo or small clinical studies, indicating potential to identify aggregate forms that predate fibrillar deposition; however, most probe evidence remains preclinical or derived from small cohorts and therefore requires larger clinical validation. Second, plasma A42/40 consistently decreases in PET-positive individuals (reported reductions ≈ 7–18%) and shows assay-dependent discriminative ability (mean AUC ≈ 0.80 for mass-spectrometry versus ≈0.71 for immunoassays), indicating that the plasma ratio can approximate PET-confirmed amyloid status with reasonable accuracy depending on the assay. Third, AI/ML methods applied to spectral and multivariate plasma data report improved classification in single-study settings and can extract weak multivariate signals; nevertheless, few studies provide independent external validation or direct head-to-head comparisons of ratio alone versus ratio + AI, so firm conclusions that AI materially increases routine diagnostic performance beyond optimized assays are not yet supported.
5.5. Implications for Clinical Application
Taken together, the evidence suggests different levels of clinical readiness: plasma A42/40—especially when measured by mass spectrometry—currently presents the strongest evidence for identifying PET-confirmed amyloid pathology (AUCs approximately 0.8 in many studies), while fluorescent probes offer a complementary, mechanistic route to detect oligomeric species but mostly remain at proof-of-concept or small clinical scales. AI/ML approaches hold promise to enhance diagnostic algorithms and to combine spectral and fluid markers, but translation into clinical practice will require demonstration of consistent incremental gains in AUC/sensitivity/specificity on external validation cohorts.
5.6. Synthesis and Future Directions
This systematic review shows that plasma A42/40, particularly when measured by mass spectrometry, currently offers the most direct and reproducible approximation to amyloid PET (mean AUC ≈ 0.80 in published studies), and therefore represents the most immediately applicable fluid marker for early detection. Fluorescent probes are promising for detecting oligomeric aggregates but remain largely at proof-of-concept or small clinical stages. AI/ML analytical tools can improve signal extraction from complex spectral or multivariate data, but robust evidence of incremental diagnostic benefit (increased AUC, sensitivity, or specificity) over optimized assays is still limited pending externally validated comparisons. Overall, while each approach contributes to early detection in complementary ways, further standardized and externally validated studies are required before routine clinical adoption.
Author Contributions
Conceptualization, S.H., S.M.V.-R., M.N. and W.A.-S.; methodology, S.H. and S.M.V.-R.; formal analysis, S.H.; investigation, S.H. and W.A.-S.; data curation, S.H. and M.N.; writing—original draft preparation, S.H.; writing—review and editing, S.H., S.M.V.-R., M.N. and W.A.-S.; supervision, S.M.V.-R., M.N. and W.A.-S.; All authors have read and agreed to the published version of the manuscript.
Funding
S.H. thanks the Xunta de Galicia for her research scholarship (reference number ED481A and IN606A).
Data Availability Statement
This systematic review was prospectively registered on the Open Science Framework (OSF). The preregistration, including study design, inclusion criteria, and analysis plan, is available at: https://osf.io/4uxeb (accessed on 28 of November 2024) with a registration DOI https://doi.org/10.17605/OSF.IO/4UXEB.
Conflicts of Interest
None of the publications reported conflicts that would compromise the integrity or objectivity of their findings relevant to this review.
References
- Fandos, N.; Pérez-Grijalba, V.; Pesini, P.; Olmos, S.; Bossa, M.; Villemagne, V.L.; Doecke, J.; Fowler, C.; Masters, C.L.; Sarasa, M.; et al. Plasma amyloid β 42/40 ratios as biomarkers for amyloid β cerebral deposition in cognitively normal individuals. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 8, 179–187. [Google Scholar] [CrossRef]
- Lansbury, P.T.; Lashuel, H.A. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature 2006, 443, 774–779. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Bogdanovic, N.; Winblad, B.; Portelius, E.; Andreasen, N.; Cedazo-Minguez, A.; Zetterberg, H. Pathways to Alzheimer’s disease. J. Intern. Med. 2014, 275, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Kepp, K.P.; Robakis, N.K.; Høilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The amyloid cascade hypothesis: An updated critical review. Brain 2023, 146, 3969–3990. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Bilgel, M.; An, Y.; Walker, K.A.; Moghekar, A.R.; Ashton, N.J.; Kac, P.R.; Karikari, T.K.; Blennow, K.; Zetterberg, H.; Jedynak, B.M.; et al. Longitudinal changes in Alzheimer’s-related plasma biomarkers and brain amyloid. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2023, 19, 4335–4345. [Google Scholar] [CrossRef]
- Lee, S.J.C.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an understanding of amyloid-β oligomers: Characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Viola, K.L.; Bicca, M.A.; Bebenek, A.M.; Kranz, D.L.; Nandwana, V.; Waters, E.A.; Haney, C.R.; Lee, M.; Gupta, A.; Brahmbhatt, Z.; et al. The Therapeutic and Diagnostic Potential of Amyloid β Oligomers Selective Antibodies to Treat Alzheimer’s Disease. Front. Neurosci. 2022, 15, 768646. [Google Scholar] [CrossRef]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L.; Perry, G.; Avila, J.; Moreira, P.; Sorensen, A.; Tabaton, M. The Amyloid-β Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimer’s Dis. 2018, 64, S567–S610. [Google Scholar] [CrossRef] [PubMed]
- Allué, J.A.; Pascual-Lucas, M.; Sarasa, L.; Castillo, S.; Sarasa, M.; Sáez, M.E.; Abdel-Latif, S.; Rissman, R.A.; Terencio, J. Clinical utility of an antibody-free LC-MS method to detect brain amyloid deposition in cognitively unimpaired individuals from the screening visit of the A4 Study. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2023, 15, e12451. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Lucas, M.; Allué, J.A.; Sarasa, L.; Fandos, N.; Castillo, S.; Terencio, J.; Sarasa, M.; Tartari, J.P.; Sanabria, Á.; Tárraga, L.; et al. Clinical performance of an antibody-free assay for plasma Aβ42/Aβ40 to detect early alterations of Alzheimer’s disease in individuals with subjective cognitive decline. Alzheimer’s Res. Ther. 2023, 15, 2. [Google Scholar] [CrossRef]
- Udeh-Momoh, C.; Zheng, B.; Sandebring-Matton, A.; Novak, G.; Kivipelto, M.; Jönsson, L.; Middleton, L. Blood Derived Amyloid Biomarkers for Alzheimer’s Disease Prevention. J. Prev. Alzheimer’s Dis. 2022, 9, 12–21. [Google Scholar] [CrossRef]
- Gao, F.; Lv, X.; Dai, L.; Wang, Q.; Wang, P.; Cheng, Z.; Xie, Q.; Ni, M.; Wu, Y.; Chai, X.; et al. A combination model of AD biomarkers revealed by machine learning precisely predicts Alzheimer’s dementia: China Aging and Neurodegenerative Initiative (CANDI) study. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022, 19, 749–760. [Google Scholar] [CrossRef]
- Weber, D.M.; Taylor, S.W.; Lagier, R.J.; Kim, J.C.; Goldman, S.M.; Clarke, N.J.; Vaillancourt, D.E.; Duara, R.; McFarland, K.N.; Wang, W.E.; et al. Clinical utility of plasma Aβ42/40 ratio by LC-MS/MS in Alzheimer’s disease assessment. Front. Neurol. 2024, 15, 1364658. [Google Scholar] [CrossRef]
- Meyer, M.R.; Kirmess, K.M.; Eastwood, S.; Wente-Roth, T.; Irvin, F.; Holubasch, M.S.; Venkatesh, V.; Fogelman, I.; Monane, M.; Hanna, L.; et al. Clinical validation of the PrecivityAD2 blood test: A mass spectrometry-based test with algorithm combining %p-tau217 and Aβ42/40 ratio to identify presence of brain amyloid. Alzheimer’s Dement. 2024, 20, 3179–3192. [Google Scholar] [CrossRef]
- Zicha, S.; Bateman, R.J.; Shaw, L.M.; Zetterberg, H.; Bannon, A.W.; Horton, W.A.; Baratta, M.; Kolb, H.C.; Dobler, I.; Mordashova, Y.; et al. Comparative analytical performance of multiple plasma Aβ42 and Aβ40 assays and their ability to predict positron emission tomography amyloid positivity. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2022, 19, 956–966. [Google Scholar] [CrossRef]
- Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Li, Y.; Gordon, B.A.; Holtzman, D.M.; Morris, J.C.; Benzinger, T.L.S.; Xiong, C.; et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019, 93, E1647–E1659. [Google Scholar] [CrossRef]
- Wisch, J.K.; Gordon, B.A.; Boerwinkle, A.H.; Luckett, P.H.; Bollinger, J.G.; Ovod, V.; Li, Y.; Henson, R.L.; West, T.; Meyer, M.R.; et al. Predicting continuous amyloid PET values with CSF and plasma Aβ42/Aβ40. Alzheimer’s Dement. 2023, 15, e12405. [Google Scholar] [CrossRef]
- Li, Y.; Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Weiner, M.W.; Shaw, L.M.; Masters, C.L.; Fowler, C.J.; Trojanowski, J.Q.; et al. Validation of Plasma Amyloid-β 42/40 for Detecting Alzheimer Disease Amyloid Plaques. Neurology 2022, 98, E688–E699. [Google Scholar] [CrossRef]
- West, T.; Kirmess, K.M.; Meyer, M.R.; Holubasch, M.S.; Knapik, S.S.; Hu, Y.; Contois, J.H.; Jackson, E.N.; Harpstrite, S.E.; Bateman, R.J.; et al. A blood-based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: Findings from a multi cohort validity analysis. Mol. Neurodegener. 2021, 16, 30. [Google Scholar] [CrossRef]
- Aliyan, A.; Cook, N.P.; Martí, A.A. Interrogating Amyloid Aggregates using Fluorescent Probes. Chem. Rev. 2019, 119, 11819–11856. [Google Scholar] [CrossRef]
- Li, C.; Yang, L.; Han, Y.; Wang, X. A simple approach to quantitative determination of soluble amyloid-β peptides using a ratiometric fluorescence probe. Biosens. Bioelectron. 2019, 142, 111518. [Google Scholar] [CrossRef]
- Du, J.Q.; Luo, W.C.; Zhang, J.T.; Li, Q.Y.; Bao, L.N.; Jiang, M.; Yu, X.; Xu, L. Machine learning-assisted fluorescence/fluorescence colorimetric sensor array for discriminating amyloid fibrils. Sens. Actuators B-Chem. 2024, 417, 136173. [Google Scholar] [CrossRef]
- Dos Santos, R.F.; Paraskevaidi, M.; Mann, D.M.A.; Allsop, D.; Santos, M.C.D.; Morais, C.L.M.; Lima, K.M.G. Alzheimer’s disease diagnosis by blood plasma molecular fluorescence spectroscopy (EEM). Sci. Rep. 2022, 12, 16199. [Google Scholar] [CrossRef] [PubMed]
- Han, L.Y.; Chen, X.; Wang, Y.; Zhang, R.J.; Zhao, T.; Pu, L.Y.; Huang, Y.; Sun, H.P. A machine learning algorithm based on circulating metabolic biomarkers offers improved predictions of neurological diseases. Clin. Chim. Acta 2024, 558, 119671. [Google Scholar] [CrossRef]
- Karaglani, M.; Gourlia, K.; Tsamardinos, I.; Chatzaki, E. Accurate Blood-Based Diagnostic Biosignatures for Alzheimer’s Disease via Automated Machine Learning. J. Clin. Med. 2020, 9, 3016. [Google Scholar] [CrossRef]
- Xu, A.; Kouznetsova, V.L.; Tsigelny, I.F. Alzheimer’s Disease Diagnostics Using miRNA Biomarkers and Machine Learning. J. Alzheimer’s Dis. JAD 2022, 86, 841–859. [Google Scholar] [CrossRef]
- Klunk, W.E.; Koeppe, R.A.; Price, J.C.; Benzinger, T.L.; Devous, M.D.; Jagust, W.J.; Johnson, K.A.; Mathis, C.A.; Minhas, D.; Pontecorvo, M.J.; et al. The Centiloid Project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimer’s Dement. 2015, 11, 1–15. [Google Scholar] [CrossRef]
- Banack, S.A.; Stark, A.C.; Cox, P.A. A possible blood plasma biomarker for early-stage Alzheimer’s disease. PLoS ONE 2022, 17, e0267407. [Google Scholar] [CrossRef]
- Telpoukhovskaia, M.A.; Cawthray, J.F.; Rodríguez-Rodríguez, C.; Scott, L.E.; Page, B.D.G.; Patrick, B.O.; Orvig, C. 3-Hydroxy-4-pyridinone derivatives designed for fluorescence studies to determine interaction with amyloid protein as well as cell permeability. Bioorg. Med. Chem. Lett. 2015, 25, 3654–3657. [Google Scholar] [CrossRef]
- Li, F.; Zhou, L.; Gao, X.; Ni, W.; Hu, J.; Wu, M.; Chen, S.; Han, J.; Wu, J. A Multichannel Fluorescent Tongue for Amyloid-β Aggregates Detection. Int. J. Mol. Sci. 2022, 23, 14562. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Sun, A.; Wei, P.; Zhang, N.; Lan, H.; Yi, T. A spiropyran-based fluorescent probe for the specific detection of β-amyloid peptide oligomers in Alzheimer’s disease. Chem. Commun. 2016, 52, 8865–8868. [Google Scholar] [CrossRef] [PubMed]
- Nabers, A.; Ollesch, J.; Schartner, J.; Kötting, C.; Genius, J.; Hafermann, H.; Klafki, H.; Gerwert, K.; Wiltfang, J. Amyloid-β-Secondary Structure Distribution in Cerebrospinal Fluid and Blood Measured by an Immuno-Infrared-Sensor: A Biomarker Candidate for Alzheimer’s Disease. Anal. Chem. 2016, 88, 2755–2762. [Google Scholar] [CrossRef]
- Mora, A.K.; Murudkar, S.; Alamelu, A.; Singh, P.K.; Chattopadhyay, S.; Nath, S. Benzothiazole-Based Neutral Ratiometric Fluorescence Sensor for Amyloid Fibrils. Chemistry 2016, 22, 16505–16512. [Google Scholar] [CrossRef]
- Ran, K.; Yang, J.; Nair, A.V.; Zhu, B.; Ran, C. CRANAD-28: A Robust Fluorescent Compound for Visualization of Amyloid Beta Plaques. Molecules 2020, 25, 863. [Google Scholar] [CrossRef]
- Zhou, K.; Yuan, C.; Dai, B.; Wang, K.; Chen, Y.; Ma, D.; Dai, J.; Liang, Y.; Tan, H.; Cui, M. Environment-Sensitive Near-Infrared Probe for Fluorescent Discrimination of Aβ and Tau Fibrils in AD Brain. J. Med. Chem. 2019, 62, 6694–6704. [Google Scholar] [CrossRef]
- Chen, C.H.; Jong, Y.J.; Chao, Y.Y.; Wang, C.C.; Chen, Y.L. Fluorescent aptasensor based on conformational switch-induced hybridization for facile detection of β-amyloid oligomers. Anal. Bioanal. Chem. 2022, 414, 8155–8165. [Google Scholar] [CrossRef]
- Freire, S.; De Araujo, M.H.; Al-Soufi, W.; Novo, M. Photophysical study of Thioflavin T as fluorescence marker of amyloid fibrils. Dye. Pigment. 2014, 110, 97–105. [Google Scholar] [CrossRef]
- Xue, C.; Lin, T.Y.; Chang, D.; Guo, Z. Thioflavin T as an amyloid dye: Fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017, 4, 160696. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Aging. Alzheimer’s Disease Genetics Fact Sheet. 2023. Available online: https://www.nia.nih.gov/health/alzheimers-causes-and-risk-factors/alzheimers-disease-genetics-fact-sheet (accessed on 15 April 2025).
- Petit, D.; Fernández, S.G.; Zoltowska, K.M.; Enzlein, T.; Ryan, N.S.; O’Connor, A.; Szaruga, M.; Hill, E.; Vandenberghe, R.; Fox, N.C.; et al. Aβ profiles generated by Alzheimer’s disease causing PSEN1 variants determine the pathogenicity of the mutation and predict age at disease onset. Mol. Psychiatry 2022, 27, 2821–2832. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.R., III; Long, A.P.; Lichtenstein, M.L. Population prevalence of autosomal dominant Alzheimer’s disease: A systematic review. Alzheimer’s Dement. 2020, 16, e037129. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J. Biol. Chem. 2012, 287, 10977–10989. [Google Scholar] [CrossRef]
- Sardar Sinha, M.; Ansell-Schultz, A.; Civitelli, L.; Hildesjö, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef]
- Ovod, V.; Ramsey, K.N.; Mawuenyega, K.G.; Bollinger, J.G.; Hicks, T.; Schneider, T.; Sullivan, M.; Paumier, K.; Holtzman, D.M.; Morris, J.C.; et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2017, 13, 841–849. [Google Scholar] [CrossRef]
- Delaby, C.; Estellés, T.; Zhu, N.; Arranz, J.; Barroeta, I.; Carmona-Iragui, M.; Illán-Gala, I.; Santos-Santos, M.Á.; Altuna, M.; Sala, I.; et al. The Aβ1–42/Aβ1–40 ratio in CSF is more strongly associated to tau markers and clinical progression than Aβ1–42 alone. Alzheimer’s Res. Ther. 2022, 14, 20. [Google Scholar] [CrossRef]
- Janelidze, S.; Palmqvist, S.; Leuzy, A.; Stomrud, E.; Verberk, I.M.W.; Zetterberg, H.; Ashton, N.J.; Pesini, P.; Sarasa, L.; Allué, J.A.; et al. Detecting amyloid positivity in early Alzheimer’s disease using combinations of plasma Aβ42/Aβ40 and p-tau. Alzheimer’s Dement. 2022, 18, 283–293. [Google Scholar] [CrossRef]
- Ashton, N.J.; Janelidze, S.; Mattsson-Carlgren, N.; Binette, A.P.; Strandberg, O.; Brum, W.S.; Karikari, T.K.; González-Ortiz, F.; Di Molfetta, G.; Meda, F.J.; et al. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nat. Med. 2022, 28, 2555–2562. [Google Scholar] [CrossRef]
- Ingannato, A.; Bagnoli, S.; Mazzeo, S.; Giacomucci, G.; Bessi, V.; Ferrari, C.; Sorbi, S.; Nacmias, B. Plasma GFAP, NfL and pTau 181 detect preclinical stages of dementia. Front. Endocrinol. 2024, 15, 1375302. [Google Scholar] [CrossRef]
- Chatterjee, P.; Pedrini, S.; Stoops, E.; Goozee, K.; Villemagne, V.L.; Asih, P.R.; Verberk, I.M.W.; Dave, P.; Taddei, K.; Sohrabi, H.R.; et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl. Psychiatry 2021, 11, 27. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.; Sun, A.; Ho, S.L.; Poon, C.Y.; Chan, H.N.; Ng, O.T.W.; Yung, K.K.L.; Yan, H.; Li, H.W.; et al. Fluoro-substituted cyanine for reliable in vivo labelling of amyloid-β oligomers and neuroprotection against amyloid-β induced toxicity. Chem. Sci. 2017, 8, 8279–8284. [Google Scholar] [CrossRef]
- Novo, M.; Illodo, S.; Seijas, J.; Hernández, S.; Rodríguez-Prieto, F.; Al-Soufi, W. Intrinsic visible emission of amyloid-β oligomers: A potential tool for early alzheimer’s diagnosis. Phys. Chem. Chem. Phys. 2025, 27, 16733–16737. [Google Scholar] [CrossRef]
- Balasco, N.; Diaferia, C.; Rosa, E.; Monti, A.; Ruvo, M.; Doti, N.; Vitagliano, L. A Comprehensive Analysis of the Intrinsic Visible Fluorescence Emitted by Peptide/Protein Amyloid-like Assemblies. Int. J. Mol. Sci. 2023, 24, 8372. [Google Scholar] [CrossRef]
- Tran, K.A.; Kondrashova, O.; Bradley, A.; Williams, E.D.; Pearson, J.V.; Waddell, N. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021, 13, 152. [Google Scholar] [CrossRef]
- Alzubaidi, A.; Tepper, J.; Lotfi, A. A novel deep mining model for effective knowledge discovery from omics data. Artif. Intell. Med. 2020, 104, 101821. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).