Abstract
Lymphedema surgeries have been proven effective in treating lymphedema and are not considered experimental or unproven. The medical literature consistently supports the safe and successful use of physiologic drainage lymphedema surgeries such as lymphaticovenous anastomosis (LVA), vascularized lymph node transfer (VLNT), and reductive surgeries such as suction-assisted protein lipectomy (SAPL) when performed by an experienced lymphedema surgery team to treat properly selected patients. Proper integration of lymphedema therapy is critical to achieving successful outcomes. We review effective lymphedema surgeries, their indications, patient selection, and the proper application of surgical treatments to achieve optimal results.
1. Introduction
Lymphedema is a serious condition caused by dysfunction of the lymphatic system. Worldwide, most cases of lymphedema occur secondary to parasitic infections. In the developed world, most cases of lymphedema occur secondary to treatment for cancer. An estimated 250 million people suffer from lymphedema and its symptoms. The true incidence of lymphedema is difficult to determine as a result of significant differences in diagnostic criteria. Lymphedema incidences have been reported to be between 20% to 40% after breast cancer treatment, 25% after head and neck cancer treatment, 29% after prostate cancer treatment, and up to 60% after gynecological cancer treatment. Congenital lymphedema, or lymphedema without a known cause such as cancer treatment, is less common than secondary lymphedema. While the incidence of patients diagnosed with congenital lymphedema has been described as very low by some authors, we have noticed that the percentage of patients with congenital lymphedema has increased significantly in our Center and is likely higher than previously thought. This is consistent with previous studies that have found rates of congenital lymphedema in patients with leg lymphedema to be as high as 51% [1,2,3,4,5,6,7,8,9,10].
A multidisciplinary approach to lymphedema treatment is essential. The key member of the team is the Certified Lymphedema Therapist (CLT) who evaluates the patient, treats with conservative lymphedema therapy, and refers to specialists such as a lymphedema surgeon as needed. Conservative lymphedema therapy includes the use of manual lymphatic drainage (MLD), compression garments, pneumatic pumps, multi-layered compression bandaging, and other modalities such as topical lasers to increase lymph flow in affected limbs, manage symptoms, and decrease excess volume. Complete decongestive therapy (CDT) administered by a CLT is also a mainstay of treatment and consists of manual lymphatic drainage (MLD) and massage, compression bandaging, therapeutic exercises, and meticulous skin care. Non-surgical therapies, when administered correctly by an experienced CLT, can significantly improve symptoms and reduce excess fluid swelling [10,11,12,13]. However, such therapies cannot remove excess pathologic lymphedema solids found in stage 2 or greater lymphedema and must be continued indefinitely in order to remain effective. Continued use of compression garments, compression pumps, and other conservative treatments are time-consuming and uncomfortable, making them suboptimal for lymphedema patients. High ongoing treatment costs, variability in the quality of compression garments and treatment, and incomplete insurance coverage are common barriers to effective ongoing non-surgical treatment.
2. Fundamental Paradigm of Lymphedema Disease Progression
Experienced lymphedema surgeons and their care teams around the world have consistently demonstrated that lymphedema surgeries effectively treat patients at all stages who are properly selected for such surgeries [14]. Previously, differing approaches yielded variable outcomes. A critical advancement was the recognition that the lymphedema disease process is driven by inflammation caused by the accumulation of excess lymphatic fluid. The inflammatory lymphatic fluid causes progressive tissue damage and permanent accumulation of pathologic lymphedema solids in the later stages of the disease. The changes in the composition of the affected extremity directly dictate the correct treatment needed. This key concept of the Fundamental Paradigm of Lymphedema Disease Progression guides both successful conservative and also surgical lymphedema treatment and is critical for the overall success of treatment.
In the lymphedema disease process, impaired lymphatic drainage leads to the accumulation of excess lymphatic fluid. Initially, these fluid changes can only be seen on imaging such as lymphoscintigraphy or indocyanine green (ICG) mapping. This represents stage 0 lymphedema. Additional fluid accumulation leads to swelling and symptoms, but the affected arm or leg can return to the appearance of the unaffected side. This is stage 1, or fluid predominant, lymphedema. In the early stages of the lymphatic disease, such as stage 1 lymphedema, excess fluid can be treated effectively with conservative lymphedema therapy such as compression, MLD, CDT, or other non-surgical methods. Stage 1 lymphedema fluid excess also can be treated effectively with physiologic drainage surgeries such as lymphaticovenous anastomosis (LVA) surgery and vascularized lymph node transfer (VLNT) surgery.
If left undertreated, the excess lymphatic fluid causes progressive inflammation. The inflammation damages soft tissues such as skin and even the lymphatic drainage channels themselves, resulting in further inflammation and swelling. This chronic inflammation causes the accumulation of additional pathological lymphedema solids, such as excess fat and proteins, that accumulate permanently between the skin and underlying fascial layers. The accumulation of excess solids correlates with stage 2, or solid predominant lymphedema, and is characterized by large amounts of excess solids and severe soft tissue inflammation. These pathologic lymphedema solids cannot be removed with conservative lymphedema treatments. Instead, the solids that develop in stage 2 lymphedema must be removed with reductive surgeries specifically designed to treat lymphedema patients, such as suction-assisted protein lipectomy (SAPL) surgery. Once the pathologic solids have been removed, inflammation will decrease and healing can occur. Even more severe disease progression can occur, with continued inflammation causing irreversible thickening and damage to the skin and deeper tissues. This most severe form is stage 3 lymphedema and requires extreme excision of the affected tissues with procedures, such as the Charles Procedure, in addition to aggressive conservative management. This Fundamental Paradigm of Lymphedema Disease Progression and its importance in patient selection for surgical treatment was conceptualized by Granzow and first presented in 2010.
The understanding of this Fundamental Paradigm of Lymphedema Disease Progression has improved both patient selection and expected outcomes. We use a surgical staging system similar to the International Society of Lymphology (ISL) system [15]. Patients with stage 0 lymphedema, or lymphedema that is asymptomatic but clearly visible on imaging, may be treated with careful observation, conservative therapy, or LVA surgery depending on a patient’s given situation. Patients with stage 1 lymphedema disease will respond best to lymphedema therapy and benefit from physiologic drainage surgeries such as VLNT and/or LVA surgeries. Patients with stage 2 lymphedema disease require both lymphedema therapy to address the fluid still present and also reductive surgery such as SAPL surgery to remove the solids and return the patient’s arm or leg to a condition more closely resembling stage 1, fluid predominant only lymphedema. Physiologic drainage surgeries, such as LVA or VLNT, will produce limited volume reductions in stage 2 lymphedema patients [13,16]. Patients with advanced severe stage 3 lymphedema have such advanced disease that the irreversibly damaged skin and soft tissues require direct surgical excision as a component of treatment (Table 1).

Table 1.
Surgical Lymphedema Staging System [17].
Volume reductions achieved by physiologic drainage surgeries are often reported in the medical literature. Such volume reductions represent improvements in fluid drainage of the treated extremities. However, we feel that this excess fluid should instead first be treated and removed with conservative lymphedema therapy and compression therapy before any type of surgical intervention is attempted. This allows for improved patient selection, significant reductions in inflammation at the delicate surgical sites, and better overall outcomes. Only after healing has occurred should physiologic procedures be used to maintain these fluid volume reductions achieved with conservative therapy. This is why we do not report volume reductions after physiologic surgeries such as VLNT and LVA surgeries. We also feel strongly that volumes can and should be reported as calculated from circumferential measurements made at 4 cm intervals, laser plethysmography, or similar technologies. This produces more accurate values that are more comparable across different centers [18,19].
In recent years, the Fundamental Paradigm of Lymphedema Disease Progression has been increasingly accepted around the world and used to improve patient selection for lymphedema surgeries. Some authors appropriately hesitate to perform physiologic VLNT or LVA surgery in patients with such advanced disease, citing increasing sclerosis in lymphatic vessels in these patients. We agree that physiologic procedures should not be used as a first-line treatment for such patients with stage 2 solid predominant and more advanced lymphedema [20,21,22,23].
3. Surgical Techniques: Reduction in Lymphedema Fluids—Physiologic Procedures
Physiologic drainage surgeries such as VLNT and LVA surgeries have been proven effective in treating lymphedema patients in numerous studies. Variations in these techniques such as lymphaticolymphatic bypass and lymph node-to-vein anastomosis have also been described [24,25,26,27,28]. As is the case with many other surgical treatments and specialties, different variations are employed successfully according to the specific surgeon’s training and experience. Common among these physiologic procedures is that they have been shown by the medical literature to improve lymphatic drainage. This leads to significant improvements in quality of life, reduced risk of infection, and reductions in the need for compression garments and lymphedema therapy greater than can be achieved with lymphedema therapy alone [13,29,30,31,32,33,34].
Since all patients are evaluated and treated by a CLT before consideration for any lymphedema surgery, all patients have a compression garment regimen in place prior to any LVA and/or VLNT surgery. During the postoperative healing process after VLNT or LVA surgery, we progressively decrease the use of the compression garments to the extent that the patient’s physiology allows and have been able to completely eliminate the need for daily compression garment use in some patients. However, we feel strongly that the patient expectation should be that the use of custom compression garments will be required to some extent as dictated by each patient’s individual situation.
We find that physiologic surgeries produce long-term improvements (Figure 1). Our patients have reported less swelling in their surgically treated lymphedema-affected limbs than their other limbs when confronted by unrelated systemic conditions, such as pregnancy, that produce fluid swelling in the other unaffected arms and legs. In our experience, in patients with congenital lymphedema, lymphatic vessels tend to be fewer in number, are more difficult to locate, and are of poorer quality with more sclerosis in patients with congenital lymphedema. This makes patients with congenital lymphedema more difficult to treat successfully with physiologic drainage lymphedema surgeries, consistent with previously published studies [35,36,37].
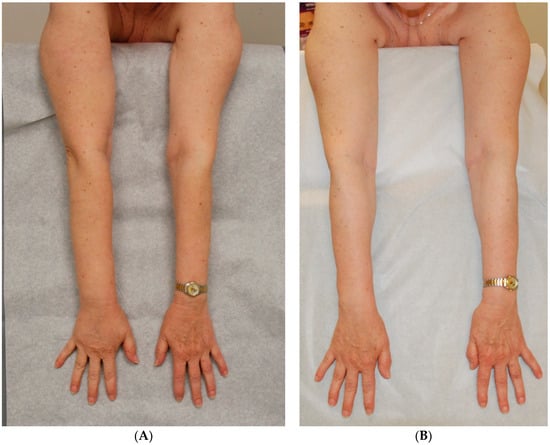
Figure 1.
Patient with right arm lymphedema following treatment for breast cancer with bilateral mastectomy, right lymph node dissection and radiation therapy [38]. (A) Arms prior to surgery; (B) Stable result 4½-years after VLNT to right axilla performed together with a DIEP flap for breast reconstruction. She requires no daily garment or therapy. Reproduced with permission from [38], published by Thieme, 2018.
4. Vascularized Lymph Node Transfer (VLNT) Surgery
VLNT surgery was first introduced in the early 1990s and has been used extensively to treat lymphedema successfully [39,40,41]. This microsurgical procedure involves the transfer of lymph nodes and surrounding soft tissues as a free flap from a donor site that contains excess lymph nodes to a lymphedema-affected area. Once in place, the blood vessels and lymphatics at the recipient site grow into the flap during the healing process and create new pathways for the removal of excess lymphatic fluid from the affected area. Different authors have described variations in the VLNT surgery. Donor sites include the groin, lateral torso, neck, or abdomen [42,43,44,45]. Lymph nodes containing flaps may be placed at proximal recipient locations such as the groin, proximal thigh or axilla, or distal locations such as the ankle or wrist [46,47,48].
If used to treat lymphedema after treatment with axillary lymph node dissection for cancer, VLNT surgery allows for the division of the constricting scar in the axilla and can significantly reduce new scar bands from forming again. We feel this is a significant advantage of VLNT surgery over LVA surgery when used to treat arm lymphedema after axillary node dissection. We only use proximal recipient sites for multiple reasons, the most important of which is the need to continue the use of flat-knit custom compression garments in all lymphedema patients as an essential part of treatment. This includes compression on possible distal recipient sites to prevent lymphatic fluid reaccumulation and inflammation as discussed previously.
The main disadvantage of the VLNT procedure is a possible but low risk of the development of lymphedema at or around the flap donor site [49,50,51,52]. However, this possible low risk is further reduced by the use of reverse lymphatic mapping [53].
5. Lymphaticovenous Anastomosis (LVA) Surgery
Also called lymphovenous anastomosis and lymphaticovenous bypass (LVB), LVA surgeries were first performed and described in the 1970s but were initially found to have limited success [54,55]. Later refinements in surgical techniques, most notably by Koshima, led to improved outcomes and greater adoption [32,56,57].
LVA surgery involves the connection of lymphatic vessels to adjacent venules at sites distal to lymphatic obstruction. This allows the excess lymphatic fluid to bypass the obstruction and drain directly into the venous system. The excess lymphatic fluid is cleared and the inflammation in the affected area improves. Supermicrosurgery is required to connect the lymphatic vessels that typically range in diameter from 0.1 mm to 0.9 mm [58]. The small size of the lymphatic vessels and the technical difficulty of LVA surgical techniques mandates the use of specialized supermicrosurgery and superfine instruments specifically suited for the detailed work required. The large number of variations in LVA techniques that continue to be published in the medical literature underscores the steep learning curve for this delicate surgery. Superfine instruments and microscopes capable of magnifications greater than that needed for standard microsurgery procedures are required to produce the best results.
The advantages of LVA surgeries include the fact that they are very effective, have no risk of donor site flap or lymphedema morbidity, are minimally invasive, and have the lowest pain and recovery of the lymphedema surgeries [59]. As also reported by other authors, we have found that increasing numbers of anastomoses appear to provide improved outcomes [60,61].
LVA surgeries can be performed even in patients with poor imaging in ICG and advanced-stage lymphedema [62,63,64]. However, as described previously, we feel strongly that patients with advanced stage 2 lymphedema should have solids removed first with SAPL surgery to reduce inflammation and volume. This will improve LVA outcomes in such patients.
LVA surgery can be used at the time of cancer surgery to prevent the occurrence of lymphedema in a method known as Immediate Lymphatic Reconstruction (ILR). This is an important area but lies beyond the scope of this manuscript [65,66].
6. Surgical Techniques: Reductive Removal of Lymphedema Solids
The treatment of patients with late-stage lymphedema has always been challenging. The current use of SAPL surgery for properly selected patients can now consistently normalize the volume of an affected arm or leg. Previously, initial surgical attempts at volume reduction were developed over a century ago. The Charles procedure, first reported in 1912, involved the aggressive resection of the skin and soft tissues down to the deep fascia followed by skin grafting of the open areas [67]. Subsequent attempts to preserve lymphatic function following soft tissue excisions were described by Sistrunk and later, Thompson. However, the results of these early procedures were often ineffective and disfiguring. These procedures have largely been abandoned except in extreme cases of stage 3 lymphedema (elephantiasis), where massive skin thickening and swelling may necessitate direct tissue excision [68,69,70]. The morbidity of such procedures caused lymphedema therapists to be skeptical of lymphedema surgeons and discourage the referral of a patient to any surgeon for lymphedema surgery. However, current surgical approaches such as SAPL surgery are far less invasive surgeries, produce much better outcomes, and have brought lymphedema surgery back into the mainstream.
7. Suction Assisted Protein Lipectomy (SAPL) Surgery
SAPL effectively addresses the remaining pathologic excess solids in patients with advanced stage 2 lymphedema after the lymphedema fluid excess has been addressed first with conservative lymphedema therapy methods. The procedure has been shown to be consistently effective in removing excess lymphedema solids in hundreds of patients over many years [71,72,73,74,75].
SAPL surgery is derived from the procedure first described by Brorson in 1997 [76]. It is an intense procedure in which the dense lymphedema solids are aspirated through small incisions with specialized cannulas under general anesthesia in the operating room. A team-based approach is essential for good results since the intensity of the procedure and postoperative care required are high. Not only are the pathologic solids much thicker, dense, and adherent than the fat otherwise aspirated with cosmetic suction-assisted lipectomy (SAL), but also, the ability of patients with advanced-stage lymphedema to drain postoperative swelling is greatly impaired and requires tremendously more effort and care by the team than SAL. The volumes of pathologic solids that require removal are also much greater than most SAL procedures. In our experience, typical average volumes aspirated in one surgery approximate 4 L per leg [17].
The involvement of a lymphedema therapist specially trained to care for SAPL surgery patients before, during, and after surgery is essential. The surgery usually takes 4 to 5 h and the entire arm or leg is treated as one unit. Much of the surgery is performed under surgical tourniquet. The application of intraoperative short-stretch lymphedema compression bandages by a trained CLT is essential. Custom flat knit compression garments must be used to control postoperative swelling, and close postoperative monitoring and staged down-sizing of custom garments is required for a good outcome. SAPL surgery is always performed as an inpatient surgery and patients remain in the hospital for multiple days until they can ambulate and remove and replace compression garments independently. The procedure can effectively treat both congenital and secondary advanced stage 2 lymphedema. In our experience and that of other surgeons, the lymphedema inflamed skin retracts significantly more after SAPL surgery in comparison to patients who have undergone SAL or other procedures. Consistent with many previously published reports, we find that the resection of excess skin is rarely required in SAPL surgery [9,77] (Figure 2 and Figure 3).
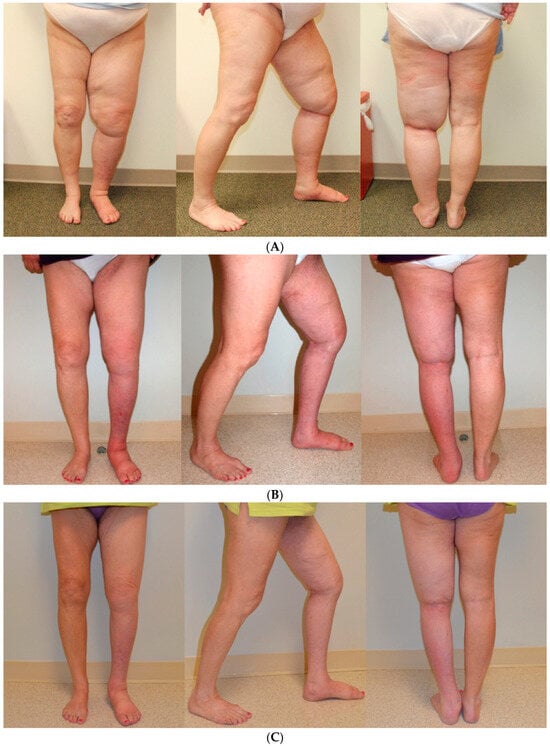
Figure 2.
Patient with a 46-year history of congenital lymphedema of the left leg prior to the SAPL procedure. The scar from her previous excisional operation, attempted elsewhere, is visible on the medial aspect of her left leg. An overall volume reduction of over 5000 cc was achieved with significant improvement in mobility of the knee joint. The excess loose skin present after surgery contracted completely, as typically occurs with the SAPL procedure. Since the procedure, she has had no further episodes of cellulitis [75]. (A) Before SAPL Surgery. (B) Nine months following SAPL. (C) Twenty-one months following SAPL. Reproduced with permission from [75], published by the International Society of Lymphology and the University of Arizona Libraries, 2017.
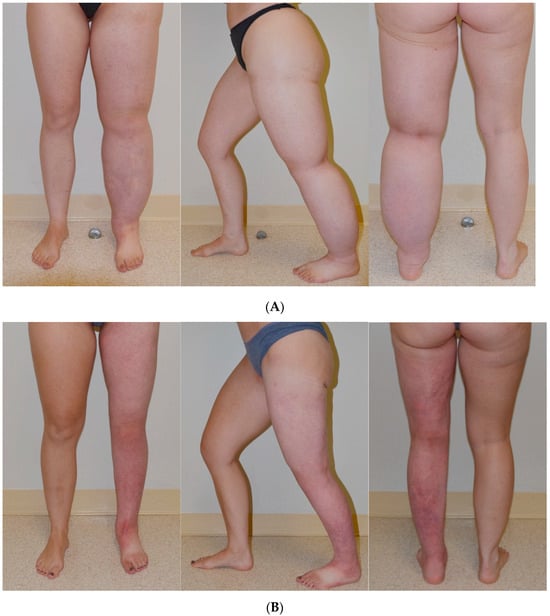
Figure 3.
Patient with a history of congenital lymphedema of the left leg for 17 years. Patient maintains a stable reduction in excess volume of 123% (affected left leg smaller than right leg) following SAPL surgery [38]. (A) Patient before SAPL surgery; (B) Patient 10 months after surgery. Reproduced with permission from [38], published by Thieme, 2018.
The improvements achieved through a team-based comprehensive approach for SAPL surgery can be tremendous. Solids that otherwise cannot be addressed by any other methods can be removed on a consistent basis. We have previously reported average excess volume reductions of 86% in legs and 101% in arms, and our current average volume reductions for both arms and legs are over 100%, meaning that the final volume of the treated limb is less than that of the unaffected side [17].
Like a sponge, the pathological solids of late-stage lymphedema retain the inflammatory lymphatic fluids, making compression and treatment increasingly difficult. By safely removing these excess solids, SAPL surgery effectively removes a large reservoir for inflammation and allows healing to occur. Custom compression garments are initially downsized every few months to capture volume improvements, residual fluid swelling decreases, and skin quality improvements. The risks of severe cellulitis are significantly reduced after SAPL surgery treatment on the order of 75% [78].
To be a candidate for SAPL surgery, a patient must have advanced stage 2 (solid predominant) lymphedema. They must have undergone therapy and compression by a trained CLT prior to consideration for surgery and must be wearing custom-fit flat knit compression garments full time to achieve maximum reduction in the excess lymphedema fluid and prevent further progression of the lymphedema disease process. By reducing the limb volume and inflammation, SAPL surgery makes conservative therapy, including compression, easier and more effective. Some patients find that they can slightly reduce the amount of compression needed to maintain their new reduced postoperative volume. However, unlike LVA surgery, SAPL surgery does not improve drainage directly and patients must continue to employ postoperative compression to prevent lymph reaccumulation.
When performed correctly by a trained and experienced surgeon, SAPL surgery has long been known not to further damage the already decreased lymphatic function in the affected limb. In fact, we find that the reduction in inflammation allows the underlying lymphatics to recover to varying degrees, which is seen in follow-up imaging such as ICG mapping [79,80]. At our Center, we have seen no instances of recurrence or the reaccumulation of stage 2 lymphedema solids in patients who have followed the proper postoperative compression protocols. This is similar to results seen by Brorson and others at their centers [72,81].
SAPL surgery is not a cosmetic surgery and must not be confused with cosmetic liposuction, which uses different equipment and techniques. Prior to the surgeries described by Brorson, liposuction-type procedures had been described with limited results [82,83,84,85]. Pathologic lymphedema solids are much more inflamed, dense, and difficult to remove than the fat aspirated in a cosmetic liposuction surgery. The severely compromised lymphatic drainage systems in advanced-stage lymphedema patients do not allow for proper drainage of postoperative swelling. As described previously, successful outcomes after SAPL surgery require considerable complex and detailed postoperative care and follow including the application of specialized compression bandaging, custom-fit flat-knit compression garments, and lymphedema therapy that differ greatly when compared to cosmetic procedures. Without such extensive postoperative care, risks of incomplete or overaggressive treatment, worsening of lymphedema swelling, and continued progression of disease otherwise may result.
SAPL surgery may be performed successfully to treat patients with stage 2 lymphedema even after unsuccessful LVA or VLNT surgeries have been performed in patients elsewhere. We find that patients with congenital lymphedema have successful SAPL surgery outcomes similar to those of patients with secondary lymphedema [86,87].
8. Importance of Lymphedema Therapy for Surgical Outcomes
We feel that the best results from lymphedema surgery are achieved when the surgery is performed as part of a comprehensive system with a team-centered approach. Lymphedema therapy and/or compression is a key component of such a system, ensures the control of the excess fluid that accumulates at all stages of the lymphedema disease process, and prevents the inflammation and continued progression of the disease.
We created the integrated treatment system, Functional Lymphatic Operations (FLO) System, which combines lymphedema therapy performed by trained CLTs and individualized VLNT, LVA, and SAPL surgery plans for patients. This is the first comprehensive treatment system, to our knowledge, to integrate conservative therapy and contemporary physiologic and reductive surgical techniques in order to specifically address the fluid and solid components of lymphedema. Patient selection is a critical component for our system, with only a small fraction of our prospective patients receiving a recommendation for surgery. Each individual patient’s lymphedema pathology is evaluated by the treatment team using the Fundamental Paradigm of Lymphedema Disease Progression to best select patients for the appropriate conservative and surgical treatments as described previously. We feel that morbidly obese patients have worse outcomes and first recommend weight loss in such cases [17].
Since our system was first reported over a decade ago, we have continued to improve our treatments and surgeries such that we achieve significant improvement in 99% of patients within one year after surgery. Our expectation is that we can reduce the volume of the affected arm or leg of our patients to approximate the volume of an unaffected arm or leg. Over the last 10 years, we have achieved average volume reductions of over 100% in both arms and legs, with postop volumes within 10% of the unaffected limb in over 90% of patients. In addition, almost all patients achieve significant improvements in symptoms and reductions in infection rates and the need for therapy and compression [88]. Similar to results found by other surgeons, we have also found that some of our patients also have consistent long-term improvements in their mental capacities, well-being, and ability to concentrate following lymphedema surgery [13,89,90].
9. Two-Phase Treatment Algorithms
Multiple descriptions of two-phase approaches to lymphedema surgery have appeared in the medical literature. For patients with advanced stage 2 lymphedema, we first treat the affected arm or leg with SAPL surgery. This normalizes limb volume, greatly reduces inflammation, and allows healing to occur and lymphatic vessels to recover. The treated arm or leg at this point typically looks similar to the unaffected side and, to the untrained observer, may even have a normal appearance. The symptoms and function greatly improve, as seen subjectively by patients and objectively on physical exams as well as in data such as preop vs postop Lymphedema Life Impact Scale (LLIS) scores. The incidence of cellulitis decreases significantly. After healing is complete, typically after one year or more, and LVA and/or VLNT surgeries are then used to reduce the conservative treatments needed to maintain the volume reduction long term (Figure 4). Since we first described this two-stage treatment to effectively treat both the solid and fluid components of lymphedema, [91,92], other authors have also found success with this type of staged treatment [93,94] (Table 2).
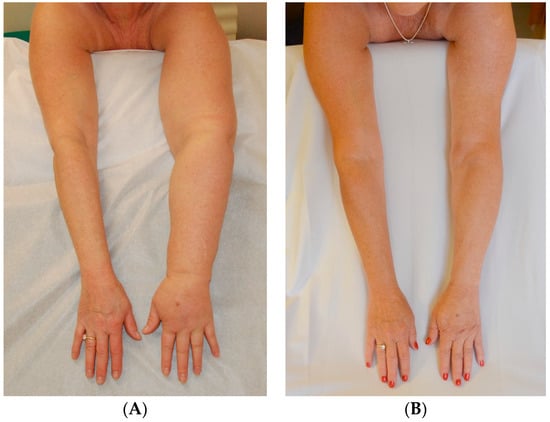
Figure 4.
(A) Before Surgery: Lymphedema of the left arm following treatment for breast cancer; (B) After SAPL and subsequent staged VLNT surgery: excess volume reduction of approx. 105% (affected arms slightly smaller than opposite side) and no garment required approx. 8–12 h each day [14]. Reproduced with permission from [14], published by Springer Nature B.V., 2022.

Table 2.
Lymphedema Life Impact Scale (LLIS), a validated instrument of physical, psychosocial, and functional impairments caused by lymphedema. Average LLIS Calculated % Impairment [95].
We do not agree with other authors that a physiologic lymphedema surgery should be performed for all patients first, and if not successful, additional volume reduction surgery be performed later [96]. We have found that experienced providers are able to find clear differentiation between patients with stage 1 lymphedema (only fluid excess) and stage 2 lymphedema (excess lymphedema solids also present) in almost all patients. A course of CDT performed by an experienced CLT will remove excess fluids present and further aid in diagnosis for those few cases where differentiation between stages 1 and 2 is in doubt.
10. Combined Physiologic Surgeries
The use of both VLNT and LVA to treat a patient has also been described elsewhere [59,97,98]. We have long used both VLNT and LVA surgeries in combination to treat patients and feel that these procedures complement one another. Since both VLNT and LVA procedures address the pathologic fluid accumulation in stage 1 lymphedema, both can be used during the same surgery and do not require a staged approach (Figure 5). The indications for combined VLNT and LVA surgeries are similar to those for the individual procedures when used separately.
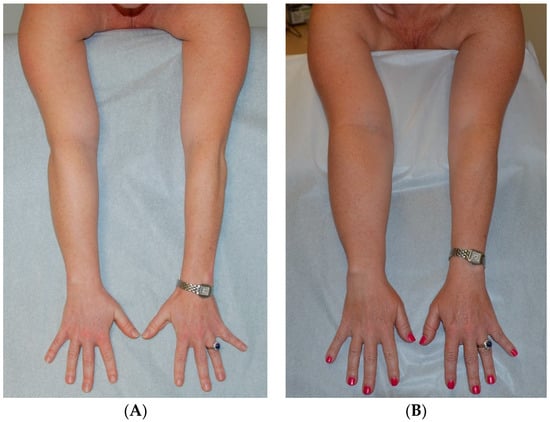
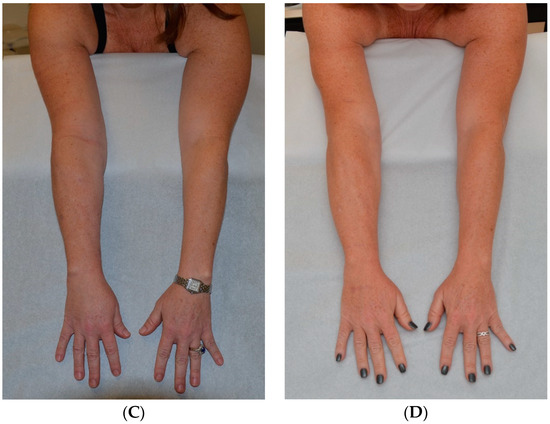
Figure 5.
Patient with lymphedema of right arm following right lumpectomy and axillary lymph node dissection and radiation therapy for breast cancer. (A) Patient at initial presentation with fluid predominant, compressible right arm lymphedema. Patient declined surgery at that time. (B) Patient 3 years later, lymphedema swelling progressed and volume increased despite conservative therapy to become solid predominant and nonpitting. (C) Patient 2 months following SAPL. (D) Patient 17 months after SAPL and 2 months after staged LVA and VLNT surgery. She has a stable excess volume reduction of 98% and wears no compression an average of 16 h per day [38]. Reproduced with permission from [38], published by Thieme, 2018.
11. Conclusions
In conclusion, surgical techniques such as VLNT, LVA, and SAPL surgery have repeatedly been shown to be effective in the treatment of lymphedema by the medical literature and are no longer considered to be experimental. As is the case with most types of surgery, different surgeons employ various techniques to varying degrees of effect. A comprehensive team-based treatment system with proper patient selection and integrated lymphedema therapy can achieve significant reductions in inflammation, volume, and infections, producing drastic improvements in symptoms, quality of life, and independence from the needs of therapy and compression.
Author Contributions
All authors contributed to background, writing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schulze, H.; Nacke, M.; Gutenbrunner, C.; Hadamitzky, C. World assessment of healthcare personnel dealing with lymphoedema. Health Econ. Rev. 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Janda, M.; Ward, L.C.; Reul-Hirche, H.; Steele, M.L.; Carter, J.; Quinn, M.; Cornish, B.; Obermair, A. Lymphedema following gynecological cancer: Results from a prospective, longitudinal cohort study on prevalence, incidence and risk factors. Gynecol. Oncol. 2017, 146, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.R. Breast cancer-related lymphedema: Symptoms, diagnosis, risk reduction, and management. World J. Clin. Oncol. 2014, 5, 241–247. [Google Scholar] [CrossRef] [PubMed]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- Tribius, S.; Pazdyka, H.; Tennstedt, P.; Busch, C.J.; Hanken, H.; Krull, A.; Peterson, C. Prognostic factors for lymphedema in patients with locally advanced head and neck cancer after combined radio(chemo)therapy—Results of a longitudinal study. Oral. Oncol. 2020, 109, 104856. [Google Scholar] [CrossRef]
- Clinckaert, A.; Callens, K.; Cooreman, A.; Bijnens, A.; Moris, L.; Van Calster, C.; Geraerts, I.; Joniau, S.; Everaerts, W. The Prevalence of Lower Limb and Genital Lymphedema after Prostate Cancer Treatment: A Systematic Review. Cancers 2022, 14, 5667. [Google Scholar] [CrossRef]
- Senger, J.B.; Kadle, R.L.; Skoracki, R.J. Current Concepts in the Management of Primary Lymphedema. Medicina 2023, 59, 894. [Google Scholar] [CrossRef]
- Goss, J.A.; Maclellan, R.A.; Greene, A.K. Adult-Onset Primary Lymphedema: A Clinical-Lymphoscintigraphic Study of 26 Patients. Lymphat. Res. Biol. 2019, 17, 620–623. [Google Scholar] [CrossRef]
- Karlsson, T.; Hoffner, M.; Brorson, H. Liposuction and Controlled Compression Therapy Reduce the Erysipelas Incidence in Primary and Secondary Lymphedema. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4314. [Google Scholar] [CrossRef]
- Ko, D.S.; Lerner, R.; Klose, G.; Cosimi, A.B. Effective treatment of lymphedema of the extremities. Arch. Surg. 1998, 133, 452–458. [Google Scholar] [CrossRef]
- Lasinski, B.B.; McKillip Thrift, K.; Squire, D.; Austin, M.K.; Smith, K.M.; Wanchai, A.; Green, J.M.; Stewart, B.R.; Cormier, J.N.; Armer, J.M. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM&R 2012, 4, 580–601. [Google Scholar]
- Hamner, J.B.; Fleming, M.D. Lymphedema therapy reduces the volume of edema and pain in patients with breast cancer. Ann. Surg. Oncol. 2007, 14, 1904–1908. [Google Scholar] [CrossRef] [PubMed]
- Jonis, Y.M.J.; Wolfs, J.A.G.N.; Hummelink, S.; Tielemans, H.J.P.; Keuter, X.H.A.; van Kuijk, S.; Ulrich, D.J.O.; van der Hulst, R.R.W.J.; Qiu, S.S. The 6 month interim analysis of a randomized controlled trial assessing the quality of life in patients with breast cancer related lymphedema undergoing lymphaticovenous anastomosis vs. conservative therapy. Sci. Rep. 2024, 14, 2238. [Google Scholar] [CrossRef] [PubMed]
- Bernas, M.; Thiadens, S.R.J.; Stewart, P.; Granzow, J. Secondary lymphedema from cancer therapy. Clin. Exp. Metastasis 2022, 39, 239–247. [Google Scholar] [CrossRef]
- Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Damstra, R.J.; Voesten, H.G.J.; van Schleven, W.D.; van der Lei, B. Lymphatic venous anastomosis (LVA) for treatment of secondary arm lymphedema. A prospective study of 11 LVA procedures in 10 patients with breast cancer related lymphedema and a critical review of the literature. Breast Cancer Res. Treat. 2009, 113, 199–206. [Google Scholar] [CrossRef]
- Granzow, J.W.; Soderberg, J.M.; Kaji, A.H.; Dauphine, C. An Effective System of Surgical Treatment of Lymphedema. J. Soc. Surg. Oncol. 2014, 21, 1189–1194. [Google Scholar] [CrossRef]
- Granzow, J.W.; Brorson, H. Use Of Excess Volume As The Standard In Reporting Lymphedema Limb Size. In Proceedings of the National Lymphedema Network 2016 International Conference, Dallas, TX, USA, 2 September 2016. [Google Scholar]
- Brorson, H.; Höijer, P. Standardised measurements used to order compression garments can be used to calculate arm volumes to evaluate lymphoedema treatment. J. Plast. Surg. Hand Surg. 2012, 46, 410–415. [Google Scholar] [CrossRef]
- Blue Shield of California. Blue of California Medical Policy BCS7. 18 Immediate and Delayed Lymphatic Reconstruction Surgery, Policy Date 7/1/23; Blue Shield of California: Oakland, CA, USA, 2023. [Google Scholar]
- Beederman, M.; Garza, R.M.; Agarwal, S.; Chang, D.W. Outcomes for Physiologic Microsurgical Treatment of Secondary Lymphedema Involving the Extremity. Ann. Surg. 2022, 276, e255–e263. [Google Scholar] [CrossRef]
- Johnson, A.R.; Tsai, L.L.; Tran, B.N.N.; Lin, S.J.; Singhal, D. Reply: Technological Advances in Lymphatic Surgery: Bringing to Light the Invisible. Plast. Reconstr. Surg. 2019, 144, 43e. [Google Scholar] [CrossRef]
- Chang, D.W.; Dayan, J.; Greene, A.K.; MacDonald, J.K.; Masia, J.; Mehrara, B.; Neligan, P.C.; Nguyen, D. Surgical Treatment of Lymphedema: A Systematic Review and Meta-Analysis of Controlled Trials. Results of a Consensus Conference. Plast. Reconstr. Surg. 2021, 147, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Campisi, C.; Davini, D.; Bellini, C.; Taddei, G.; Villa, G.; Fulcheri, E.; Zilli, A.; Da Rin, E.; Eretta, C.; Boccardo, F. Lymphatic microsurgery for the treatment of lymphedema. Microsurgery 2006, 26, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Campisi, C.; Eretta, C.; Pertile, D.; Da Rin, E.; Campisi, C.; Macciò, A.; Campisi, M.; Accogli, S.; Bellini, C.; Bonioli, E.; et al. Microsurgery for treatment of peripheral lymphedema: Long-term outcome and future perspectives. Microsurgery 2007, 27, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, R.G.; Siuda, S.; Bohmert, H.; Moser, E. A microsurgical method for reconstruction of interrupted lymphatic pathways: Autologous lymph-vessel transplantation for treatment of lymphedemas. Scand. J. Plast. Reconstr. Surg. 1986, 20, 141–146. [Google Scholar] [PubMed]
- Jamal, S. Lymphovenous anastomosis in filarial lymphedema. Lymphology 1981, 14, 64–68. [Google Scholar] [PubMed]
- Pak, C.S.; Suh, H.P.; Kwon, J.G.; Cho, M.J.; Hong, J.P. Lymph Node to Vein Anastomosis (LNVA) for lower extremity lymphedema. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2059–2067. [Google Scholar] [CrossRef]
- Phillips, G.S.A.; Gore, S.; Ramsden, A.; Furniss, D. Lymphaticovenular Anastomosis Improves Quality of Life and Limb Volume in Patients with Secondary Lymphedema After Breast Cancer Treatment. Breast J. 2019, 25, 859–864. [Google Scholar] [CrossRef]
- Coriddi, M.; Wee, C.; Meyerson, J.; Eiferman, D.; Skoracki, R. Vascularized Jejunal Mesenteric Lymph Node Transfer: A Novel Surgical Treatment for Extremity Lymphedema. J. Am. Coll. Surg. 2017, 225, 650–657. [Google Scholar] [CrossRef]
- Mihara, M.; Hara, H.; Furniss, D.; Narushima, M.; Iida, T.; Kikuchi, K.; Ohtsu, H.; Gennaro, P.; Gabriele, G.; Murai, N. Lymphaticovenular anastomosis to prevent cellulitis associated with lymphoedema. Br. J. Surg. 2014, 101, 1391–1396. [Google Scholar] [CrossRef]
- Chang, D.W. Lymphaticovenular bypass for lymphedema management in breast cancer patients: A prospective study. Plast. Reconstr. Surg. 2010, 126, 752–758. [Google Scholar] [CrossRef]
- Bernas, M.; Thiadens, S.R.J.; Smoot, B.; Armer, J.M.; Stewart, P.; Granzow, J. Lymphedema following cancer therapy: Overview and options. Clin. Exp. Metastasis 2018, 35, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Granzow, J.W. Lymphedema surgery: The current state of the art. Clin. Exp. Metastasis 2018, 35, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yamamoto, N.; Hayashi, A.; Koshima, I. Supermicrosurgical deep lymphatic vessel-to-venous anastomosis for a breast cancer-related arm lymphedema with severe sclerosis of superficial lymphatic vessels. Microsurgery 2017, 37, 156–159. [Google Scholar] [CrossRef]
- Sun, J.M.; Yamamoto, T. Lymphovenous shunts in the treatment of lymphedema. J. Chin. Med. Assoc. 2024, 87, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.F.; Yamamoto, T.; Fisher, M.; Liao, J.; Carr, J. The “Octopus” lymphaticovenular anastomosis: Evolving beyond the standard supermicrosurgical technique. J. Reconstr. Microsurg. 2015, 31, 450–457. [Google Scholar] [PubMed]
- Granzow, J.W.; Soderberg, J.M. Lymphedema Surgery as Part of an Integrated Lymphedema Treatment System. In Lymphedema Management: The Comprehensive Guide for Practitioners, 4th ed.; Zuther, J.E., Norton, S., Eds.; Thieme: New York, NY, USA, 2018. [Google Scholar]
- Becker, C.; Hidden, G.; Godart, S.; Maurage, H.; Pecking, A. Free lymphatic transplant. Eur. J. Lymphol. Rel. Prob. 1991, 6, 25–77. [Google Scholar]
- Becker, C.; Assouad, J.; Riquet, M.; Hidden, G. Postmastectomy lymphedema: Long-term results following microsurgical lymph node transplantation. Ann. Surg. 2006, 243, 313–315. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Asaad, M.; Selber, J.C.; Liu, J.; Chen, D.N.; Hall, M.S.; Butler, C.E. Outcomes of Vascularized Lymph Node Transplantation for Treatment of Lymphedema. J. Am. Coll. Surg. 2021, 232, 982–994. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Badash, I.; Selber, J.C.; Cheng, M.-H.; Patel, K.M. Vascularized Lymph Node Transfer for Lymphedema. Semin. Plast. Surg. 2018, 32, 28–35. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Suami, H. Laparoscopic Free Omental Lymphatic Flap for the Treatment of Lymphedema. Plast. Reconstr. Surg. 2015, 136, 114–118. [Google Scholar] [CrossRef]
- Chu, Y.Y.; Allen, R.J., Jr.; Wu, T.J.; Cheng, M.-H. Greater Omental Lymph Node Flap for Upper Limb Lymphedema with Lymph Nodes-depleted Patient. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1288. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.J.; Mehrara, B.J.; Neligan, P.; Cheng, M.; Patel, K.M. Lymph node transplantation for the treatment of lymphedema. J. Surg. Oncol. 2018, 118, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Chen, S.C.; Henry, S.L.; Tan, B.K.; Lin, M.C.; Huang, J.J. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: Flap anatomy, recipient sites, and outcomes. Plast. Reconstr. Surg. 2013, 131, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Ali, R.; Chen, S.C.; Wallace, C.; Chang, Y.C.; Chen, H.C.; Cheng, M.H. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast. Reconstr. Surg. 2009, 123, 1265–1275. [Google Scholar] [CrossRef]
- Cheng, M.H.; Huang, J.J.; Wu, C.W.; Yang, C.Y.; Lin, C.Y.; Henry, S.L.; Kolios, L. The mechanism of vascularized lymph node transfer for lymphedema: Natural lymphaticovenous drainage. Plast. Reconstr. Surg. 2014, 133, 192e–198e. [Google Scholar] [CrossRef]
- Vignes, S.; Blanchard, M.; Yannoutsos, A.; Arrault, M. Complications of autologous lymph-node transplantation for limb lymphoedema. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 516–520. [Google Scholar] [CrossRef]
- Viitanen, T.P.; Maki, M.T.; Seppanen, M.P.; Suominen, E.A.; Saaristo, A.M. Donor site lymphatic function after microvascular lymph node transfer. Plast. Reconstr. Surg. 2012, 130, 1246–1253. [Google Scholar] [CrossRef]
- Pons, G.; Masia, J.; Loschi, P.; Nardulli, M.L.; Duch, J. A case of donor-site lymphoedema after lymph node-superficial circumflex iliac artery perforator flap transfer. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 119–123. [Google Scholar] [CrossRef]
- Lee, M.; McClure, E.; Reinertsen, E.; Granzow, J.W. Lymphedema of the Upper Extremity following Supraclavicular Lymph Node Harvest. Plast. Reconstr. Surg. 2015, 135, 1079e–1082e. [Google Scholar] [CrossRef]
- Dayan, J.H.; Dayan, E.; Smith, M.L. Reverse lymphatic mapping: A new technique for maximizing safety in vascularized lymph node transfer. Plast. Reconstr. Surg. 2015, 135, 277–285. [Google Scholar] [CrossRef]
- Gilbert, A.; O’Brien, B.M.; Vorrath, J.W.; Sykes, P.J. Lymphaticovenous anastomosis by microvascular technique. Br. J. Plast. Surg. 1977, 29, 355. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.M.; Shafiroff, B.B. Microlymphaticovenous and Resectional Surgery in Obstructive Lymphedema. World J. Surg. 1979, 3, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Forte, A.J.; Sisti, A.; Huayllani, M.T.; Boczar, D.; Cinotto, G.; Ciudad, P.; Manrique, O.J.; Lu, X.; McLaughlin, S. Lymphaticovenular Anastomosis for Breast Cancer-Related Upper Extremity Lymphedema: A Literature Review. Gland. Surg. 2020, 9, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Suami, H.; Chang, D.W. Overview of surgical treatments for breast cancer-related lymphedema (review). Plast Reconstr Surg 2010, 126, 1853–1863. [Google Scholar] [CrossRef]
- Badash, I.; Gould, D.J.; Patel, K.M. Supermicrosurgery: History, Applications, Training, and the Future. Front. Surg. 2018, 5, 23. [Google Scholar] [CrossRef]
- Akita, S.; Mitsukawa, N.; Kuriyama, M.; Kubota, Y.; Hasegawa, M.; Tokumoto, H.; Ishigaki, T.; Togawa, T.; Kuyama, J.; Satoh, K. Comparison of Vascularized Supraclavicular Lymph Node Transfer and Lymphaticovenular Anastomosis for Advanced State Lower Extremity Lymphedema. Ann. Plast. Surg. 2015, 74, 573–579. [Google Scholar] [CrossRef]
- Koshima, I.; Inagawa, K.; Urushibara, K.; Moriguchi, T. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J. Reconstr. Microsurg. 2000, 16, 437–442. [Google Scholar] [CrossRef]
- Mihara, M.; Hara, H.; Kawakami, Y.; Zhou, H.P.; Tange, S.; Kikuchi, K.; Iida, T. Multi-site lymphatic venous anastomosis using echography to detect suitable subcutaneous vein in severe lymphedema patients. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, e1–e7. [Google Scholar] [CrossRef]
- Cha, H.G.; Oh, T.M.; Cho, M.J.; Pak, C.S.J.; Suh, H.P.; Jeon, J.Y.; Hong, J.P. Changing the Paradigm: Lymphovenous Anastomosis in Advanced Stage Lower Extremity Lymphedema. Plast. Reconstr. Surg. 2021, 147, 199–207. [Google Scholar] [CrossRef]
- Kojimahara, T.; Tsukuura, R. Changing the Paradigm: Lymphovenous Anastomosis in Advanced Stage Lower Extremity Lymphedema. Plast. Reconstr. Surg. 2021, 148, 320e–321e. [Google Scholar] [CrossRef]
- Mo, Y.W.; Lee, S.J.; Lee, D.W.; Lee, W.J.; Im, S.H.; Suh, Y.C. Contrast-enhanced ultrasonography as an adjunctive method to ICG lymphography for functional lymphaticovenous anastomosis. J. Surg. Oncol. 2024, 129, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Singhal, D. Immediate lymphatic reconstruction. J. Surg. Oncol. 2018, 118, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Coriddi, M.; Mehrara, B.; Skoracki, R.; Singhal, D.; Dayan, J.H. Immediate Lymphatic Reconstruction: Technical Points and Literature Review. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3431. [Google Scholar] [CrossRef] [PubMed]
- Charles, H. Elephantiasis of the leg. In A System of Treatment; Latham, A., English, T.C., Eds.; Churchill: London, UK, 1912; Volume 3. [Google Scholar]
- Sistrunk, W.E. Contribution to plastic surgery: Removal of scars by stages; an open operation for extensive laceration of the anal sphincter; the Kondoleon operation for elephantiasis. Ann. Surg. 1927, 85, 185–193. [Google Scholar] [CrossRef]
- Thompson, N. The surgical treatment of chronic lymphedema of the extremities. Surg. Clin. N. Am. 1967, 47, 445–503. [Google Scholar] [CrossRef]
- Hassan, K.; Chang, D.W. The Charles Procedure as Part of the Modern Armamentarium Against Lymphedema. Ann. Plast. Surg. 2020, 85, e37–e43. [Google Scholar] [CrossRef]
- Brorson, H. Complete reduction of arm lymphedema following breast cancer—A prospective twenty-one years’ study. Plast. Reconstr. Surg. 2015, 136 (Suppl. S4), 134–135. [Google Scholar] [CrossRef]
- Hoffner, M.; Ohlin, K.; Svensson, B.; Manjer, J.; Hansson, E.; Troëng, T.; Brorson, H. Liposuction Gives Complete Reduction of Arm Lymphedema following Breast Cancer Treatment-A 5-year Prospective Study in 105 Patients without Recurrence. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1912. [Google Scholar] [CrossRef]
- Brorson, H.; Freccero, C.; Ohlin, K.; Svensson, B. Liposuction of postmastectomy arm lymphedema completely removes excess 206. volume: A 15 year study. Lymphology 2010, 43, 108–110. [Google Scholar]
- Karlsson, T.; Hoffner, M.; Ohlin, K.; Svensson, B.; Brorson, H. Complete Reduction of Leg Lymphedema after Liposuction: A 5-Year Prospective Study in 67 Patients without Recurrence. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5429. [Google Scholar] [CrossRef]
- Lee, M.; Perry, L.; Granzow, J. Suction Assisted Protein Lipectomy (SAPL) Even for the Treatment of Chronic Fibrotic and Scarified Lower Extremity Lymphedema. Lymphology 2016, 49, 36–41. [Google Scholar] [PubMed]
- Brorson, H.; Svensson, H. Complete reduction of lymphedema of the arm by liposuction after breast cancer. Scand. J. Plast. Surg. Hand Surg. 1997, 31, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Schaverien, M.V.; Munnoch, D.A.; Brorson, H. Liposuction Treatment of Lymphedema. Semin. Plast. Surg. 2018, 32, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Brorson, H.; Svensson, H. Skin blood flow of the lymphedematous arm before and after liposuction. Lymphology 1997, 30, 165–172. [Google Scholar]
- Brorson, H.; Svensson, H.; Norrgren, K.; Thorsson, O. Liposuction reduces arm lymphedema without significantly altering the already impaired lymph transport. Lymphology 1998, 31, 156–172. [Google Scholar]
- Chen, W.F.; Pandey, S.K.; Lensing, J.N. Does Liposuction for Lymphedema Worsen Lymphatic Injury? Lymphology 2023, 56, 3–12. [Google Scholar] [CrossRef]
- Karlsson, T.; Mackie, H.; Koelmeyer, L.; Heydon-White, A.; Ricketts, R.; Toyer, K.; Boyages, J.; Brorson, H.; Lam, T. Liposuction for Advanced Lymphedema in a Multidisciplinary Team Setting in Australia: 5-Year Follow-Up. Plast. Reconstr. Surg. 2024, 153, 482–491. [Google Scholar] [CrossRef]
- O’Brien, B.M.; Khazanchi, R.K.; Kumar, P.A.; Dvir, E.; Pederson, W. Liposuction in the treatment of lymphoedema; a preliminary report. Br. J. Plast. Surg. 1989, 42, 530–533. [Google Scholar] [CrossRef]
- Nava, V.M.; Lawrence, W.T. Liposuction on a lymphedematous arm. Ann. Plast. Surg. 1988, 21, 366–368. [Google Scholar] [CrossRef]
- Sando, W.C.; Nahai, F. Suction lipectomy in the management of limb lymphedema. Clin. Plast. Surg. 1989, 16, 369–373. [Google Scholar]
- Greene, A.K.; Slavin, S.A.; Borud, L. Treatment of lower extremity lymphedema with suction-assisted lipectomy. Plast. Reconstr. Surg. 2006, 118, 118e–121e. [Google Scholar] [CrossRef] [PubMed]
- Granzow, J.W.; Andersen, G.J.; Soderberg, J.M.; Kaji, A.H. Suction Assisted Protein Lipectomy (SAPL) Surgery Is Equally Effective in the Treatment of Chronic Congenital and Secondary Lymphedema Patients. In Proceedings of the National Lymphedema Network 2016 International Conference, Dallas, TX, USA, 3 September 2016. [Google Scholar]
- Granzow, J.W. Suction Assisted Protein Lipectomy (SAPL) Surgery---Where It Fits into Lymphedema Treatment. In Proceedings of the 2022 National Lymphedema Network Conference, Cleveland, OH, USA, 19 November 2022. [Google Scholar]
- Granzow, J.W. The Importance of Lymphedema Therapy for Excellent Lymphatic Surgery Results. In Proceedings of the International Society of Lymphology (ISL) World Congress of Lymphology, Genoa, Italy, 12 September 2023. [Google Scholar]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018, 560, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Louveau, A.; Pandey, S.; Zeng, W.; Chen, W.F. Rewiring the Brain: The Next Frontier in Supermicrosurgery. Plast. Reconstr. Surg. 2024, 153, 494e–495e. [Google Scholar] [CrossRef]
- Granzow, J.W.; Soderberg, J.M.; Dauphine, C. A Novel Two-Stage Surgical Approach to Treat Chronic Lymphedema. Breast J. 2014, 20, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Granzow, J.W.; Soderberg, J.M.; Kaji, A.H.; Dauphine, C. Review of Current Surgical Treatments for Lymphedema. J. Soc. Surg. Oncol. 2014, 21, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Leppäpuska, I.M.; Suominen, E.; Viitanen, T.; Rannikko, E.; Visuri, M.; Mäki, M.; Saarikko, A.; Hartiala, P. Combined Surgical Treatment for Chronic Upper Extremity Lymphedema Patients: Simultaneous Lymph Node Transfer and Liposuction. Microsurgery 2019, 83, 308–317. [Google Scholar] [CrossRef]
- Chang, K.; Xia, S.; Liang, C.; Sun, Y.; Xin, J.; Shen, W. A Clinical Study of Liposuction Followed by Lymphovenous Anastomosis for Treatment of Breast Cancer-Related Lymphedema. Front. Surg. 2023, 10, 1065733. [Google Scholar] [CrossRef]
- Granzow, J.W. Early Surgical Management of Arm and Leg Lymphedema. In Proceedings of the International Society of Lymphology (ISL) World Congress of Lymphology, Presentation of Preliminary Data, Genoa, Italy, 13 September 2023. [Google Scholar]
- Almadani, Y.; Davison, P.; Efanov, J.I.; Kokosis, G.; Vorstenbosch, J. Demystifying vascularized lymph node transfers and lymphatico-venous anastomoses. Ann. Transl. Med. 2024, 12, 8. [Google Scholar] [CrossRef]
- Cheng, M.H.; Tee, R.; Chen, C.; Lin, C.Y.; Pappalardo, M. Simultaneous Ipsilateral Vascularized Lymph Node Transplantation and Contralateral Lymphovenous Anastomosis in Bilateral Extremity Lymphedema with Different Severities. J. Soc. Surg. Oncol. 2020, 27, 5267–5276. [Google Scholar] [CrossRef]
- Raman, S.; Sanka, S.A.; Ji, J.; Yaeger, L.; Skolnick, G.B.; Christensen, J.M. Vascularized Lymph Node Transfer for the Treatment of Lymphedema: A systematic review and meta-analysis of clinical and patient-reported outcomes. Plast. Aesthetic Res. 2023, 10, 6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).