Abstract
Multiple sclerosis is an autoimmune, inflammatory, and chronic neurodegenerative disease caused by myelin loss in the central nervous system. One strategy that shows evidence of numerous benefits is therapeutic exercise, but these therapies, based on repetitive physical actions, can sometimes be unmotivating for patients. Our proposal suggests that an exergame programme with immersive virtual reality (IVR) is feasible for people with multiple sclerosis (pwMS) and will improve their physical function through more motivational sessions. We present a protocol for a single-blind randomised controlled trial to assess the feasibility and impact on functional capacities of an 8-week IVR programme (ExeRVIEM protocol) in pwMS. Balance, gait, risk of falling, functional mobility and lower limb strength, fatigue, handgrip strength, and reaction times will be evaluated. The control group will maintain the usual activities scheduled in the centre, and the experimental group will add the ExeRVIEM protocol (two sessions per week). Therapies based on the combination of exercise and IVR explored in this study may offer new treatment approaches and open new lines of research in this field by improving the functionality of pwMS, as well as motivating patients and encouraging their adherence to treatment.
1. Introduction
Multiple sclerosis (MS) is an autoimmune, inflammatory, and chronic neurodegenerative disease caused by myelin loss in the central nervous system (CNS) [1]. MS usually begins in the third decade of life [2] and usually evolves in the form of unpredictable episodes [3] or outbreaks of neurological dysfunction [4,5] with a variety of motor and non-motor symptoms. The most common symptoms in people with multiple sclerosis (pwMS) are the following [4,6]: sensory disturbances (numbness and tingling), walking difficulties (due to fatigue, weakness, spasticity, imbalance, incoordination, and tremor) which increase fall risk, problems with vision, constipation, bladder dysfunction, learning difficulties, depression, dizziness and vertigo, and sexual problems.
MS is one of the most prevalent CNS pathologies, affecting an estimated 2.8 million people in 2020 (an incidence of 35.9 individuals per 100,000) [7], and is the most common non-traumatic pathology in young adults [8]. This updated prevalence data, gathered by Walton et al. [7], assumes an increase in the prevalence of 30% following the same methodology as was used for the 2013 data [5,9], and reflects the importance of health and healthcare costs that this increase may entail. Their study also points out that the countries with the highest proportion of pwMS are the United States of America, Canada, Iceland, Sweden, Finland, Denmark, Germany, and Italy, closely followed by several Western European countries and Australia. MS cases are twice as high in women as in men [1].
The lack of any treatment that provides a solution for such a prevalent health problem means that other non-pharmacological therapeutic strategies should be used to reduce the problems generating such symptoms as described above. One strategy that shows evidence of numerous benefits is therapeutic exercise [10,11], although this was not previously thought to be the case [12,13]. Therapeutic exercise can lead to benefits in gait [14], balance [14,15], cardiorespiratory performance [16], fatigue [17,18], musculoskeletal performance [19], blood levels of brain-derived neurotrophic factor [20], cognition [21], neurogenic bladder symptoms [22,23], symptoms of depression [24], and quality of life [25]. However, these benefits do not always occur, as reflected in the review by Malone et al. [19], where some of the reviewed studies demonstrated no significant benefits with regard to fatigue or symptoms of depression. There are also still gaps in our knowledge relating to the appropriate dose of therapeutic exercise [26].
Furthermore, exercise-based therapies, based on repetitive physical actions, can sometimes be unmotivating for patients [27]. This is why immersive virtual reality (IVR) may offer exercise-based programmes (exergames) that are both motivating and appropriate to the therapeutic objectives of the target group. This tool has already been successfully tested in other groups (post-stroke, Parkinson’s, etc.) with promising results [26,27,28,29,30,31].
Our proposal based on exergaming with IVR in MS is a strategy designed to improve the functionality of pwMS, where patients will carry out an exercise programme using virtual reality glasses. The use of IVR in this case is based on the fact—as other studies have shown [32,33,34,35,36]—that in addition to improving functionality, it can also motivate patients and encourage their adherence to the treatment programme. With the support of IVR, positive effects could be achieved on the motor functionality (coordination, balance, strength, or speed) of pwMS [37,38], as has been possibly found in Parkinson’s patients [32,39] or in older people [40,41,42,43]. Furthermore, we hope that not only will IVR bring health benefits for pwMS but also that its controlled use will prevent cybersickness and, therefore, avoid dizziness and vertigo [6], both common symptoms in pwMS.
Therefore, the aim of this study is to assess whether an exercise-based treatment intervention with IVR is feasible in pwMS and whether it improves their physical function by reducing their number of falls and increasing their personal autonomy.
2. Materials and Methods
2.1. Study Design and Participants
During the single-blind randomised controlled trial, the evaluators will not know to which group the pwMS being evaluated belong. The study has been registered at clinical-trials.gov (NCT05870254). People diagnosed with MS who regularly attend the AVEMPO Center in Vigo, Spain, will be invited to participate in the study. Once it has been determined that they meet the selection criteria, they will be randomly assigned (computer-generated random sequence) to an experimental group and a control group.
The inclusion criteria include the following: people diagnosed with MS (“relapsing-remitting” MS sub-type) belonging to the AVEMPO Vigo Association; age between 18 and 65 years; and ability to stand and follow the intervention protocol and scheduled assessments.
The exclusion criteria include the following: medical report advising against physical activity and exercise; uncontrolled outbreak of the disease (patients suffering from relapses in the last 3 months); receiving a cycle of steroids, either intravenously or orally; and dizziness, vertigo or severe visual or auditive impairment.
Each participant will sign an informed consent form. The study will be carried out in accordance with the Declaration of Helsinki and will be subject to evaluation by the Ethics Committee of the Galician Health Service (Servizo Galego de Saúde).
2.2. Sample Size Calculation
The sample size was calculated using the G*Power 3.1.9.6 programme (Mac OS). The sample size calculation was based on the results obtained by Ozkul et al. [38] in the Timed Up and Go test with an effect size of 0.58 (Cohen’s d). We have considered a power (1-β) of 0.80 and a significance level alpha (α) of 0.95. The estimated sample size was 14 participants for each group, of which 9 will be women and 5 men, taking into account the incidence of multiple sclerosis based on gender [3].
2.3. Intervention Protocol
There will be two groups: the experimental group and the control group. The experimental group will perform the ExeRVIEM protocol sessions (6 min) focused on the upper and lower limbs (2 sessions per week for 8 weeks). The exergame used will be Les Milles (available in the Oculus.com library) which simulates a gym where patients will perform a virtual exercise session based on boxing (Figure 1). This exergame was selected because it will require the patients to make decisions based on the stimuli presented to them to move their upper and lower limbs, trunks, and heads to coordinate their movements. It is a simple programme with music that helps to establish a rhythm and stimuli that appear at a speed and frequency appropriate for the group and without sudden accelerations or changes in the players’ point of view in order to minimise the occurrence of the negative symptoms that are linked with exposure to virtual environments. This research group has already verified the absence of such symptoms in these IVR environments after the application of similar interventions, both in healthy elderly people and in people with pathologies such as Parkinson’s disease [35,36].
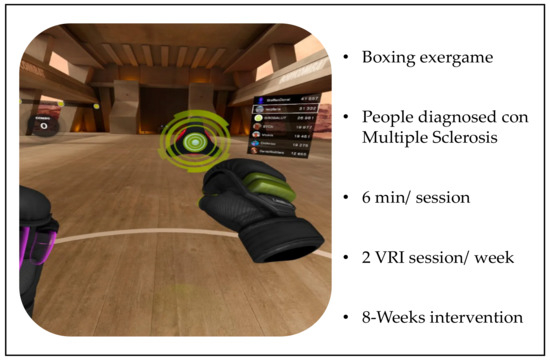
Figure 1.
Summary of the ExeRVIEM protocol.
All sessions will begin with a warm-up focused on stimulating coordination and joint mobilisation so that the body is prepared both centrally and peripherally for the session and will end with a stretching routine accompanied by cycles of calm and controlled breathing. In addition, acclimatisation sessions aimed at getting to know the VR device will be scheduled in the week prior to the start of the study. The session will be supervised by the centre’s physiotherapist or occupational therapist, and data will be collected for each session (attendance and score achieved by each participant). Both groups will continue with the usual activities scheduled by the centre’s team.
2.4. Outcomes Measurement
After screening, selection, and randomisation of the sample, an initial assessment will be carried out. There will also be another one at the end of the intervention (after 8 weeks) and a third follow-up (1 month after the end of the programme).
In the initial assessment, in addition to the tests indicated below, information will be collected on the socio-demographic characteristics, and a clinical history will be taken of the participants in order to ascertain their age, sex, years since diagnosis, sub-type of MS, degree of disability (assessed with the Expanded Disability Status Scale (EDSS) [44]), and pharmacological treatment. Throughout the intervention, each participant’s degree of compliance with the assigned sessions will be recorded, as well as the reason for any absences.
2.4.1. Primary Outcome Measures
A functional assessment of each patient will be carried out evaluating the balance, gait, and risk of falling with the Tinetti test [45], functional mobility and lower limb strength with the five times sit-to-stand test [46,47], degree of functional autonomy using the simple and cognitive Timed Up and Go test [48], fatigue with the Fatigue Severity Scale (FSS) [49], handgrip with a hydraulic dynamometer [50], and the reaction time measured using the Rezzil (an immersive virtual reality software).
2.4.2. Secondary Outcome Measures
As secondary measures, the safety of the IVR intervention will be analysed with the Spanish version of the Simulator Sickness Questionnaire (SSQ) [51,52,53], its usability with the System Usability Scale (SUS) [54], and the patient’s experiences and satisfaction with the received treatment will be analysed with the post-game module of the Game Experience Questionnaire (GEQ-post-game) [55], an interview booklet, and an ad hoc questionnaire.
The SSQ consists of 16 items grouped into 3 subscales: oculomotor symptoms, disorientation, and nausea. Each item is assessed on a 4-point scale (0 = do not feel anything, 1 = a little, 2 = medium, and 3 = a lot), and the total score is the sum of the scores of the 3 subscales. The lower the total score, the safer the immersive experience is. The version translated and adapted to Spanish will be used [53].
The SUS, on the other hand, allows the usability of a product or service to be assessed. It is made up of 10 items, each of which is rated between 1 and 5 depending on the degree of agreement or disagreement. The maximum value that can be reached is 100 points; the higher the score, the greater the usability. The version validated in Spanish will be used [56].
The GEQ is a questionnaire consisting of 3 modules: main, social, and post-game, of which the latter will be used to find out how the patients felt after the IVR intervention. This post-game module consists of 17 questions relating to the intensity generated in relation to the same number of feelings. These are rated between 0, not at all, and 4, extremely. These items are divided into 4 components (positive experiences, negative experiences, tiredness, and return to reality). A Spanish version used in previous studies will be used [43,57].
Finally, patients will also be given an ad hoc satisfaction questionnaire consisting of 5 questions, which has been used in previous studies [42,43].
2.5. Statistical Analysis
A descriptive analysis of the sample and the main variables defining the characteristics of the disease in the patients under study will be carried out, and this analysis will be divided into the two groups to which the participants were assigned. The analysis will also be stratified according to gender.
Subsequently, a comparative study will be made of the functional parameters of both groups during the performance of the intervention programme and its subsequent follow-up. In order to analyse the influence of the two programmes on the physical and functional variables, an intra-group statistical inference will be carried out using the Student’s t-test for related data. In order to determine the differential effect of the programmes and the moments of information collection, an intergroup analysis will be carried out through Analysis of Variance (ANOVA 2 × 2; group and time) with the Bonferroni test.
Prior to this, the normality of the variables under study will be checked using the Shapiro–Wilk test (p > 0.05). To carry out the different analyses, the statistical package IMB SPSS Statistics for MAC version 25.0 (Armonk, NY, USA: IBM Corporation) will be used, and a confidence interval (95% CI) and a level of statistical significance will be considered for those values of p < 0.05.
3. Discussion
The main objective of this study is to explore whether an exercise-based treatment intervention with IVR is feasible in pwMS and whether it can improve their functionality, reduce their risk of falls, and increase their personal autonomy in line with what was found by Ozkul et al. [38]. Similar programmes have been feasible in other neurogenerative pathologies such as Parkinson’s disease and have been successful in improving functional aspects in these types of patients [42], as well as in institutionalized older adults [43]. So far, the experiences of IVR-based therapeutic exercise are specific, focused on specific aspects (upper limb functionality [58], kinematic analysis of the “hand-to-mouth” movement [59], or postural stability [38]). The use of therapeutic exercise programmes not based on the use of IVR are much more common [10,14,15,18,19,23,24], and in the cases where virtual reality is used, it is usually non-immersive or semi-immersive [14,15,19,24,60,61,62,63,64,65]. Therefore, this study aims to provide a broader view of the possibilities of virtual reality, especially IVR in pwMS.
In addition, our proposal aims to evaluate commercially available hardware and software so that if the results are as expected, they could be more accessible to patient associations, which often have limited resources.
The analysed variables will allow us to understand the impact of a multicomponent exercise programme with IVR on general mobility, strength, fatigue, reaction times, and variables closely linked to functional independence and disability in pwMS, and to know if, for example, reaction times may be related to the risk of falls, as we have recently verified in patients with Parkinson’s disease [66]. We believe that this will not only lead to improvements in functional independence and disability but will also facilitate better adherence to an active treatment such as the one designed here, as it is motivating for pwMS.
Despite the limitations that our design may present (lack of double-blinding or representation of the different profiles and sub-types of MS), the expected results will provide evidence of the use of immersive virtual exergames and portable devices, bringing these new therapeutic possibilities closer to the reality of pwMS in patient associations and removing the need to travel to specialised centres.
4. Conclusions
Therapies based on the combination of exercise and IVR explored in this study may offer new treatment approaches and open new lines of research in this field by improving the functionality of pwMS, as well as motivating patients and encouraging their adherence to treatment.
Author Contributions
Conceptualization and methodology: G.R.-F., P.C.-P. and J.M.C.-C. (all authors); resources, G.R.-F.; writing—original draft preparation, review, and editing: G.R.-F., P.C.-P. and J.M.C.-C. (all authors); supervision, project administration, and funding acquisition: G.R.-F. and P.C.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and will be submitted for approval by the Ethics Committee of Servizo Galego de Saúde (Santiago de Compostela, Spain).
Informed Consent Statement
Informed consent will be obtained from all subjects involved in the study.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
Thanks to the AVEMPO Vigo Center (Vigo, Pontevedra, Spain) for the support of this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goldenberg, M.M. Multiple sclerosis review. Pharm. Ther. 2012, 37, 175–184. [Google Scholar]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Amatya, B.; Khan, F.; Galea, M. Rehabilitation for people with multiple sclerosis: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2019, 1, CD012732. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, J.M. Multiple sclerosis. Handb. Clin. Neurol. 2014, 122, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M.; et al. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2018, 26, 27–40. [Google Scholar] [CrossRef]
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef]
- Learmonth, Y.C.; Motl, R.W. Exercise Training for Multiple Sclerosis: A Narrative Review of History, Benefits, Safety, Guidelines, and Promotion. Int. J. Environ. Res. Public Health 2021, 18, 13245. [Google Scholar] [CrossRef]
- Motl, R.W.; Sandroff, B. Benefits of Exercise Training in Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2015, 15, 62. [Google Scholar] [CrossRef]
- Iodice, R.; Aceto, G.; Ruggiero, L.; Cassano, E.; Manganelli, F.; Dubbioso, R. A review of current rehabilitation practices and their benefits in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 69, 104460. [Google Scholar] [CrossRef] [PubMed]
- Giesser, B.S. Exercise in the management of persons with multiple sclerosis. Ther. Adv. Neurol. Disord. 2015, 8, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhang, X.; Chen, P. Effects of Different Exercise Therapies on Balance Function and Functional Walking Ability in Multiple Sclerosis Disease Patients—A Network Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 7175. [Google Scholar] [CrossRef] [PubMed]
- Corrini, C.; Gervasoni, E.; Perini, G.; Cosentino, C.; Putzolu, M.; Montesano, A.; Pelosin, E.; Prosperini, L.; Cattaneo, D. Mobility and balance rehabilitation in multiple sclerosis: A systematic review and dose-response meta-analysis. Mult. Scler. Relat. Disord. 2023, 69, 104424. [Google Scholar] [CrossRef]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef]
- Torres-Costoso, A.; Martínez-Vizcaíno, V.; Reina-Gutiérrez, S.; Álvarez-Bueno, C.; Guzmán-Pavón, M.J.; Pozuelo-Carrascosa, D.P.; Fernández-Rodríguez, R.; Sanchez-López, M.; Cavero-Redondo, I. Effect of Exercise on Fatigue in Multiple Sclerosis: A Network Meta-analysis Comparing Different Types of Exercise. Arch. Phys. Med. Rehabil. 2022, 103, 970–987.e18. [Google Scholar] [CrossRef]
- Harrison, A.M.; Safari, R.; Mercer, T.; Picariello, F.; van der Linden, M.L.; White, C.; Moss-Morris, R.; Norton, S. Which exercise and behavioural interventions show most promise for treating fatigue in multiple sclerosis? A network meta-analysis. Mult. Scler. 2021, 27, 1657–1678. [Google Scholar] [CrossRef]
- Malone, L.A.; Mendonca, C.J.; Kim, Y. Active Videogaming Interventions in Adults with Neuromuscular Conditions: A Scoping Review. Games Health J. 2022, 11, 141–156. [Google Scholar] [CrossRef]
- Shobeiri, P.; Karimi, A.; Momtazmanesh, S.; Teixeira, A.L.; Teunissen, C.E.; van Wegen, E.E.H.; Hirsch, M.A.; Yekaninejad, M.S.; Rezaei, N. Exercise-induced increase in blood-based brain-derived neurotrophic factor (BDNF) in people with multiple sclerosis: A systematic review and meta-analysis of exercise intervention trials. PLoS ONE 2022, 17, e0264557. [Google Scholar] [CrossRef]
- Li, G.; You, Q.; Hou, X.; Zhang, S.; Du, L.; Lv, Y.; Yu, L. The effect of exercise on cognitive function in people with multiple sclerosis: A systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2023, 270, 2908–2923. [Google Scholar] [CrossRef] [PubMed]
- Kajbafvala, M.; Ashnagar, Z.; Lucio, A.; Firoozeh, F.; Salehi, R.; Pashazadeh, F.; Dadgoo, M.; Jafari, H. Pelvic floor muscle training in multiple sclerosis patients with lower urinary tract dysfunction: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2022, 59, 103559. [Google Scholar] [CrossRef] [PubMed]
- Yavas, I.; Emuk, Y.; Kahraman, T. Pelvic floor muscle training on urinary incontinence and sexual function in people with multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2022, 58, 103538. [Google Scholar] [CrossRef] [PubMed]
- Kyriakatis, G.M.; Besios, T.; Lykou, P.M. The effect of therapeutic exercise on depressive symptoms in people with multiple sclerosis—A systematic review. Mult. Scler. Relat. Disord. 2022, 68, 104407. [Google Scholar] [CrossRef]
- Latimer-Cheung, A.E.; Pilutti, L.A.; Hicks, A.L.; Martin Ginis, K.A.; Fenuta, A.M.; MacKibbon, K.A.; Motl, R.W. Effects of Exercise Training on Fitness, Mobility, Fatigue, and Health-Related Quality of Life Among Adults With Multiple Sclerosis: A Systematic Review to Inform Guideline Development. Arch. Phys. Med. Rehabil. 2013, 94, 1800–1828.e3. [Google Scholar] [CrossRef]
- Brincks, J.; Dalgas, U.; Franzén, E.; Callesen, J.; Wallin, A.; Johansson, S. Unwrapping the “black box” of balance training in people with multiple sclerosis—A descriptive systematic review of intervention components, progression, and intensity. Mult. Scler. Relat. Disord. 2023, 69, 104412. [Google Scholar] [CrossRef]
- Jansen-Kosterink, S.M.; Huis in ’t Veld, R.M.H.A.; Schönauer, C.; Kaufmann, H.; Hermens, H.J.; Vollenbroek-Hutten, M.M.R. A Serious Exergame for Patients Suffering from Chronic Musculoskeletal Back and Neck Pain: A Pilot Study. Games Health J. 2013, 2, 299–307. [Google Scholar] [CrossRef]
- Hao, J.; He, Z.; Yu, X.; Remis, A. Comparison of immersive and non-immersive virtual reality for upper extremity functional recovery in patients with stroke: A systematic review and network meta-analysis. Neurol. Sci. 2023. [Google Scholar] [CrossRef]
- Demeco, A.; Zola, L.; Frizziero, A.; Martini, C.; Palumbo, A.; Foresti, R.; Buccino, G.; Costantino, C. Immersive Virtual Reality in Post-Stroke Rehabilitation: A Systematic Review. Sensors 2023, 23, 1712. [Google Scholar] [CrossRef]
- Martino Cinnera, A.; Bisirri, A.; Chioccia, I.; Leone, E.; Ciancarelli, I.; Iosa, M.; Morone, G.; Verna, V. Exploring the Potential of Immersive Virtual Reality in the Treatment of Unilateral Spatial Neglect Due to Stroke: A Comprehensive Systematic Review. Brain Sci. 2022, 12, 1589. [Google Scholar] [CrossRef]
- Lahude, A.B.; Souza Corrêa, P.; Cabeleira, M.E.P.; Cechetti, F. The impact of virtual reality on manual dexterity of Parkinson’s disease subjects: A systematic review. Disabil. Rehabil. Assist. Technol. 2022, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Campo-Prieto, P.; Santos-García, D.; Cancela-Carral, J.M.; Rodríguez-Fuentes, G. Current status of immersive virtual reality as a tool for physical and functional rehabilitation in patients with Parkinson’s disease: Systematic review. Rev. Neurol. 2021, 73, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Patsaki, I.; Dimitriadi, N.; Despoti, A.; Tzoumi, D.; Leventakis, N.; Roussou, G.; Papathanasiou, A.; Nanas, S.; Karatzanos, E. The effectiveness of immersive virtual reality in physical recovery of stroke patients: A systematic review. Front. Syst. Neurosci. 2022, 16, 880447. [Google Scholar] [CrossRef]
- Benham, S.; Kang, M.; Grampurohit, N. Immersive virtual reality for the management of pain in community-dwelling older adults. OTJR Occup. Particip. Health 2019, 39, 90–96. [Google Scholar] [CrossRef] [PubMed]
- De Keersmaecker, E.; Lefeber, N.; Geys, M.; Jespers, E.; Kerckhofs, E.; Swinnen, E. Virtual reality during gait training: Does it improve gait function in persons with central nervous system movement disorders? A systematic review and meta-analysis. NeuroRehabilitation 2019, 44, 43–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shen, M.; Wang, Y.X.; He, Z.W.; Chi, S.Q.; Yang, Z.H. Effect of virtual reality on balance and gait ability in patients with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 1130–1138. [Google Scholar] [CrossRef]
- Castellano-Aguilera, A.; Biviá-Roig, G.; Cuenca-Martínez, F.; Suso-Martí, L.; Calatayud, J.; Blanco-Díaz, M.; Casaña, J. Effectiveness of Virtual Reality on Balance and Risk of Falls in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 14192. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, C.; Guclu-Gunduz, A.; Yazici, G.; Atalay Guzel, N.; Irkec, C. Effect of immersive virtual reality on balance, mobility, and fatigue in patients with multiple sclerosis: A single-blinded randomized controlled trial. Eur. J. Integr. Med. 2020, 35, 101092. [Google Scholar] [CrossRef]
- Sánchez-Herrera-Baeza, P.; Cano-de-la-Cuerda, R.; Oña-Simbaña, E.D.; Palacios-Ceña, D.; Pérez-Corrales, J.; Cuenca-Zaldivar, J.N.; Gueita-Rodríguez, J.; Balaguer-Bernaldo de Quiros, C.; Jardon-Huete, J.; Cuesta-Gomez, A. The Impact of a Novel Immersive Virtual Reality Technology Associated with Serious Games in Parkinson’s Disease Patients on Upper Limb Rehabilitation: A Mixed Methods Intervention Study. Sensors 2020, 20, 2168. [Google Scholar] [CrossRef]
- Donath, L.; Rössler, R.; Faude, O. Effects of Virtual Reality Training (Exergaming) Compared to Alternative Exercise Training and Passive Control on Standing Balance and Functional Mobility in Healthy Community-Dwelling Seniors: A Meta-Analytical Review. Sports Med. 2016, 46, 1293–1309. [Google Scholar] [CrossRef]
- Campo-Prieto, P.; Cancela, J.M.C.; Rodríguez-Fuentes, G. Immersive virtual reality as physical therapy in older adults: Present or future (systematic review). Virtual Real. 2021, 25, 801–817. [Google Scholar] [CrossRef]
- Campo-Prieto, P.; Cancela-Carral, J.M.; Rodríguez-Fuentes, G. Wearable Immersive Virtual Reality Device for Promoting Physical Activity in Parkinson’s Disease Patients. Sensors 2022, 22, 3302. [Google Scholar] [CrossRef] [PubMed]
- Campo-Prieto, P.; Cancela-Carral, J.M.; Rodríguez-Fuentes, G. Feasibility and Effects of an Immersive Virtual Reality Exergame Program on Physical Functions in Institutionalized Older Adults: A Randomized Clinical Trial. Sensors 2022, 22, 6742. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Williams, T.F.; Mayewski, R. Fall risk index for elderly patients based on number of chronic disabilities. Am. J. Med. 1986, 80, 429–434. [Google Scholar] [CrossRef]
- Goldberg, A.; Chavis, M.; Watkins, J.; Wilson, T. The five-times-sit-to-stand test: Validity, reliability and detectable change in older females. Aging Clin. Exp. Res. 2012, 24, 339–344. [Google Scholar] [CrossRef]
- Bohannon, R.W. Reference Values for the Five-Repetition Sit-to-Stand Test: A Descriptive Meta-Analysis of Data from Elders. Percept. Mot. Skills 2006, 103, 215–222. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Krupp, L.B.; La Rocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The Fatigue Severity Scale: Application to Patients With Multiple Sclerosis and Systemic Lupus Erythematosus. Arch. Neurol. 1989, 46, 1121. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, L.-Y. Grip Strength in Older Adults: Test-Retest Reliability and Cutoff for Subjective Weakness of Using the Hands in Heavy Tasks. Arch. Phys. Med. Rehabil. 2010, 91, 1747–1751. [Google Scholar] [CrossRef]
- Kennedy, R.S.; Lane, N.E.; Berbaum, K.S.; Lilienthal, M.G. Simulator sickness questionnaire: An enhanced method for quantifying simulator sickness. Int. J. Aviat. Psychol. 1993, 3, 203–220. [Google Scholar] [CrossRef]
- Kennedy, R.S.; Drexler, J.; Kennedy, R.C. Research in visually induced motion sickness. Appl. Ergon. 2010, 41, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Campo-Prieto, P.; Rodríguez-Fuentes, G.; Cancela-Carral, J.M. Translation and cross-cultural adaptation to Spanish of the Simulator Sickness Questionnaire. Retos 2021, 43, 503–509. [Google Scholar] [CrossRef]
- Brooke, J. SUS: A quick and dirty usability scale. Usability Eval. Ind. 1995, 189, 4–7. [Google Scholar]
- Ijsselsteijn, W.A.; de Kort, Y.A.W.; Poels, K. The Game Experience Questionnaire; Eindhoven University of Technology: Eindhoven, The Netherlands, 2013. [Google Scholar]
- Hedlefs Aguilar, M.I.; Garza Villegas, A.A. Análisis comparativo de la Escala de Usabilidad del Sistema (EUS) en dos versiones. RECI 2016, 5, 44. [Google Scholar] [CrossRef]
- Campo-Prieto, P.; Rodríguez-Fuentes, G.; Cancela-Carral, J.M. Immersive Virtual Reality Exergame Promotes the Practice of Physical Activity in Older People: An Opportunity during COVID-19. Mutimodal. Technol. Interact. 2021, 5, 52. [Google Scholar] [CrossRef]
- Bertoni, R.; Mestanza Mattos, F.G.; Porta, M.; Arippa, F.; Cocco, E.; Pau, M.; Cattaneo, D. Effects of immersive virtual reality on upper limb function in subjects with multiple sclerosis: A cross-over study. Mult. Scler. Relat. Disord. 2022, 65, 104004. [Google Scholar] [CrossRef]
- Pau, M.; Porta, M.; Bertoni, R.; Mattos, F.G.M.; Cocco, E.; Cattaneo, D. Effect of immersive virtual reality training on hand-to-mouth task performance in people with Multiple Sclerosis: A quantitative kinematic study. Mult. Scler. Relat. Disord. 2023, 69, 104455. [Google Scholar] [CrossRef]
- Zanatta, F.; Farhane-Medina, N.Z.; Adorni, R.; Steca, P.; Giardini, A.; D’Addario, M.; Pierobon, A. Combining robot-assisted therapy with virtual reality or using it alone? A systematic review on health-related quality of life in neurological patients. Health Qual. Life Outcomes 2023, 21, 18. [Google Scholar] [CrossRef]
- Nascimento, A.S.; Fagundes, C.V.; Mendes, F.A.D.S.; Leal, J.C. Effectiveness of Virtual Reality Rehabilitation in Persons with Multiple Sclerosis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Mult. Scler. Relat. Disord. 2021, 54, 103128. [Google Scholar] [CrossRef]
- Casuso-Holgado, M.J.; Martín-Valero, R.; Carazo, A.F.; Medrano-Sánchez, E.M.; Cortés-Vega, M.D.; Montero-Bancalero, F.J. Effectiveness of virtual reality training for balance and gait rehabilitation in people with multiple sclerosis: A systematic review and meta-analysis. Clin. Rehabil. 2018, 32, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.; Poyade, M.; Rooney, S.; Paul, L. Upper limb rehabilitation interventions using virtual reality for people with multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2021, 47, 102610. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Pérez, I.; Sánchez-Alcalá, M.; Nieto-Escámez, F.A.; Castellote-Caballero, Y.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Virtual Reality-Based Therapy Improves Fatigue, Impact, and Quality of Life in Patients with Multiple Sclerosis. A Systematic Review with a Meta-Analysis. Sensors 2021, 21, 7389. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Gómez, A.; Sánchez-Herrera-Baeza, P.; Oña-Simbaña, E.D.; Martínez-Medina, A.; Ortiz-Comino, C.; Balaguer-Bernaldo-de-Quirós, C.; Jardón-Huete, A.; Cano-de-la-Cuerda, R. Effects of virtual reality associated with serious games for upper limb rehabilitation in patients with multiple sclerosis: Randomized controlled trial. J. NeuroEng. Rehabil. 2020, 17, 90. [Google Scholar] [CrossRef]
- Campo-Prieto, P.; Cancela-Carral, J.M.; Rodríguez-Fuentes, G. Immersive Virtual Reality Reaction Time Test and Relationship with the Risk of Falling in Parkinson’s Disease. Sensors 2023, 23, 4529. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).