Abstract
Plants belonging to the genus Irvingia are widespread across the African and Southeast Asian regions of the world. Irvingia gabonensis, Irvingia malayana, and Irvingia grandifolia are among the commonly used species in ethnomedicine, especially in Africa. Fever, scabies, toothache, inflammation, and liver and gastrointestinal disorders are among the pathological conditions that are reverted by Irvingia plants upon traditional preparations. Modern pharmacological investigations have substantiated the ethnomedicinal uses of Irvingia spp. Reports on the phytochemical analysis of Irvingia plants have revealed the presence of a number of secondary metabolites such as flavonoids, phenolic compounds, tannins, saponins, and alkaloids. Based on the foregoing, the present study provides a comprehensive evaluation of reports on the ethnomedicinal use, phytochemistry, pharmacology, and toxicity of plants from the genus Irvingia. Relevant information on Irvingia plants was mostly obtained from major scientific databases from their inception until July 2023. As a result, more than forty compounds have been identified in Irvingia spp., proving the abundance of secondary metabolites in these plants. Reports have pointed out modern pharmacological activities such as antiprotozoal, antimicrobial, antioxidant, antidiabetic, anti-inflammatory, and hepatoprotective activities. The present study provides more insights for the successful utilization of Irvingia plants and may guide further research on their therapeutic potential in the treatment of various diseases.
1. Introduction
From the Irvingiaceae family, Irvingia (commonly called wild mango, bush mango, or ogbono) is a genus that is mainly found in African and Southeast Asian regions [1]. Taxonomically, World Flora Online has published seven accepted species of Irvingia that include Irvingia malayana, Irvingia excelsa, Irvingia fusca, Irvingia gabonensis, Irvingia wombolu, Irvingia smithii, and Irvingia grandifolia [2]. Also called bush mango or African mango, Irvingia gabonensis (bark) is used for the traditional treatment of dysentery, scabies, toothache, and skin diseases [3]. In combination with palm oil, the leaves of I. gabonensis are used to stop hemorrhage in pregnant women [4]. Irvingia gabonensis bark is very effective for treating skin bruises, toothaches, dysentery, and hernia [3]. The hepatoprotective activity of Irvingia gabonensis has also been reported by several authors [5,6]. The bark decoction of Irvingia grandifolia is used to relieve pain and for bathing to treat fever in children [7]. Various parts of Irvingia grandifolia and Irvingia malayana are used to treat a number of diseases such as diabetes, asthenia, icterus, dysentery, toothache, diarrhea, scabies, inflammation, and yellow fever [8,9,10]. Modern pharmacological properties of Irvingia spp. include antidiarrheal [11], antimicrobial [12,13,14], cytotoxic [15], antioxidant, and antidiabetic [16,17] activities, among others. The phytochemical screening of Irvingia spp. revealed the presence of saponins, tannins, alkaloids, flavonoids, cardiac glycosides, steroids, carbohydrates, volatile oils, and terpenoids [3] as well as phenolic acids such as 2,3,8-tri-O-methylellagic acid [18]. Regarding the phytochemistry and pharmacological studies on Irvingia spp., a number of studies are available across the literature. However, few reviews have been reported on Irvingia plants. In fact, emphasis has been placed mostly on food applications [19] and the cardiovascular disease outcomes [20,21] of Irvingia species. Thus, comprehensive reviews and/or monographs on the application of Irvingia species as ethnomedicinal plants are needed. In this line, the present work aims to summarize existing information regarding the phytochemistry, pharmacological activities, and ethnomedicinal uses of Irvingia plants.
2. Research Methods
The present study aims to substantially review the phytochemistry, pharmacological activity, and traditional uses of Irvinvia plants.
2.1. Literature Search
Information related to the pharmacological activity of chemical constituents from Irvingia plants was obtained from published and unpublished materials across the literature. Relevant data were obtained from databases such as PubMed (National Library of Medicine), American Chemical Society (ACS), Science Direct, SciFinder, Web of Science, Scopus, Wiley, Google Scholar, and Springer from their respective inception until August 2023. Furthermore, theses, dissertations, and textbooks were searched to obtain the relevant data. The search terms included “Irvingia”; OR “Irvingia spp.” AND “Traditional uses”; “Irvingia”; OR “Irvingia spp.” AND “Phytochemistry”; “Irvingia” OR “Irvingia spp.” AND “Pharmacology”; “Irvingia” OR “Irvingia spp.” AND “Pharmacological activity”; “Irvinbgia” OR “Irvingia spp.” AND “Toxicity”. In addition, books, scientific reports, dissertations, theses, and articles published in peer-reviewed journals were also scrutinized and searched. References obtained from various searches were also examined to obtain further additional information.
2.2. Data Extraction and Synthesis
Data extraction and synthesis were conducted by the first and second authors and confirmed by the other authors. Figures or tables were used to gather appropriate data that were further summarized and analyzed. In addition, a descriptive narration was used to provide a summary of the results. The chemical structures of the pharmacologically active compounds are provided using graphical representations.
2.3. Results of the Literature Search
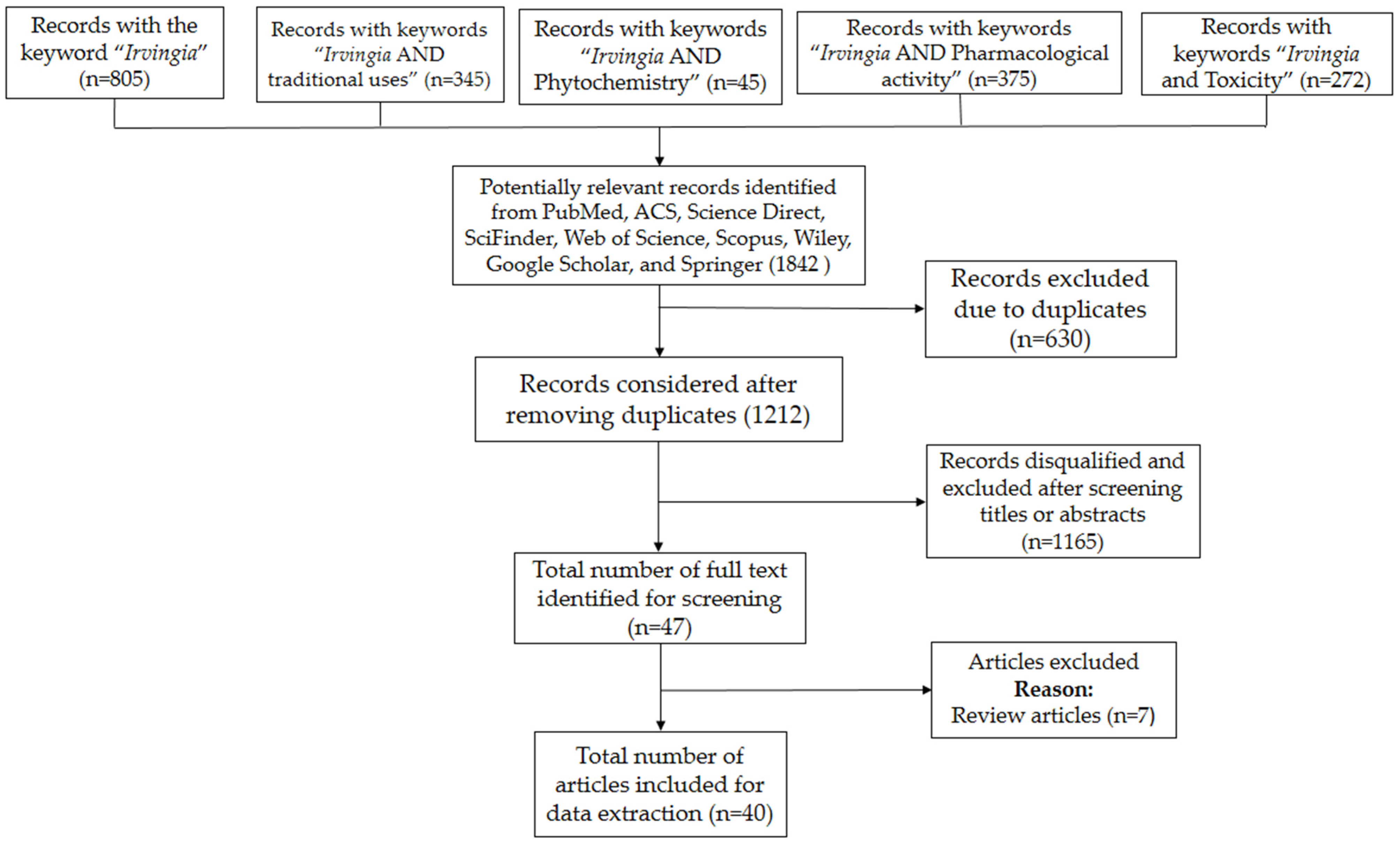
A total of 1842 (“Irvingia”: 805; “Irvingia and traditional uses”: 345; Irvingia and Phytochemistry”: 45; Irvingia and Pharmacological activity”: 193; “Irvingia and Pharmacology”: 182; “Irvingia and Toxicity”: 272) significant records were obtained, of which 630 were excluded for data duplicates. Among the 1212 records obtained after removing the duplicates, 1165 (Figure 1) were disqualified and omitted after screening the titles or abstracts. Thus, forty seven (47) full-length research articles were identified, of which seven were excepted as they were review articles. Finally, a total of forty (40) full text articles were included and exploited to collect relevant information. In addition, important facts obtained from unpublished materials were also included.
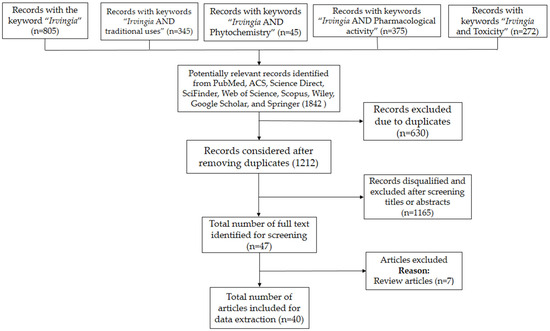
Figure 1.
Flowchart of the literature search and selection procedure.
3. Phytochemistry of Irvingia spp.
Previous phytochemical screening of Irvingia gabonensis and Irvingia wombolu peels, seed coats, leaves, and seeds revealed the presence of flavonoids, alkaloids, phenolic compounds, tannins, steroids, and saponins [13,22]. More importantly, previous reports pointed out the isolation and identification of the following classes of compounds: terpenoids, flavonoids, phenolic compounds, and miscellaneous compounds.
3.1. Terpenoids
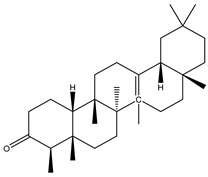
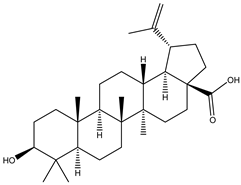
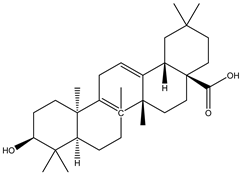
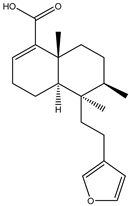
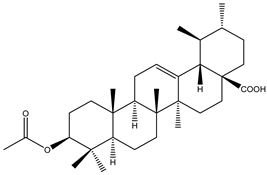
A couple of research groups [23,24] isolated and characterized several terpenoids from Irvingia gabonensis. These include Kuete et al. [23], who separated four terpenoids [friedelanone (138.88 µg/g of extract) (1), betulinic acid (3.31 mg/g of extract) (2), oleanolic acid (5.09 mg/g of extract) (3), and hardwickiic acid (11.95 mg/g of extract) (4)] from the stem bark of Irvingia gabonensis. In addition to the diterpenoid hardwickiic acid (4), Donfack et al. [24] isolated a triterpenoid, namely, 3-β-acetoxyursolic acid (5) (Table 1). From Irvingia malayana, Ng et al. [25] isolated and characterized betulinic acid (2), a compound that was already identified in Irvingia gabonensis by Kuete et al. [23] and Donfack et al. [24].

Table 1.
Terpenoids isolated thus far from Irvingia spp.
3.2. Flavonoids
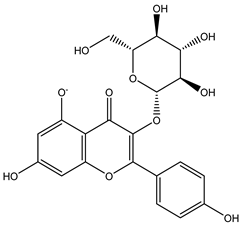
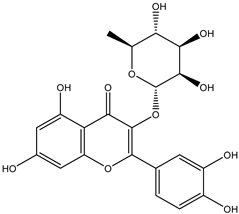
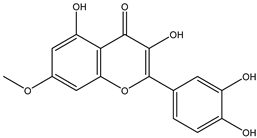
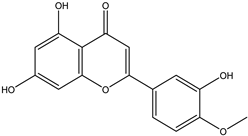
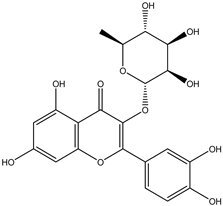
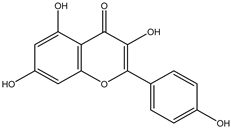
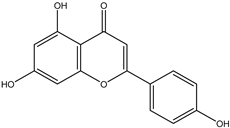
Regarding advances on the isolation of flavonoids from Irvingia species, a few flavonoids have been previously separated and characterized. These includes five flavonols [kaempferol 3-O-glucoside (6), quercetin 3-O-rhamnoside (7), rhamnetin (8) (from seeds) [26], quercetrin (9), kaempferol (10) (from stem bark) [27]] and two flavones [diosmetin (11) [28] and apigenin (12)] [27] from the seeds and stem bark, respectively (Table 2).

Table 2.
Flavonoids isolated thus far from Irvingia spp.
3.3. Phenolic Compounds
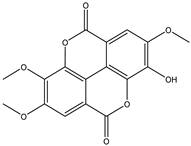
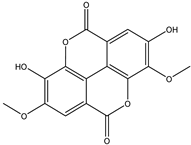
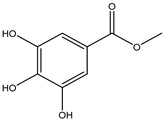
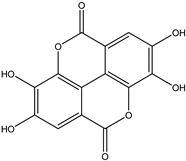
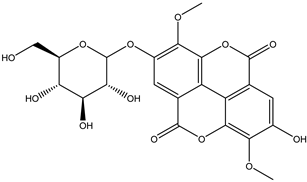
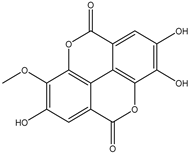
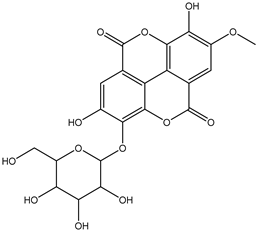
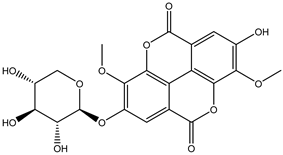
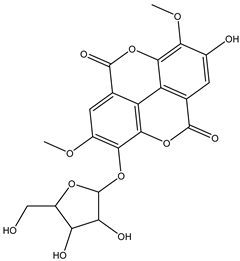
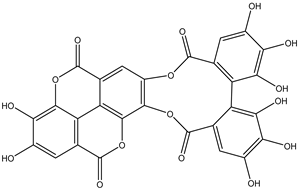
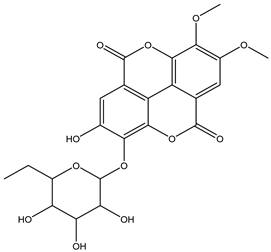
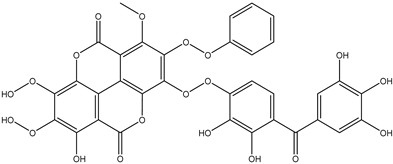
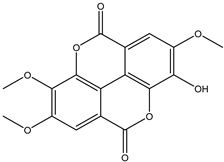
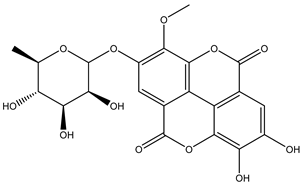
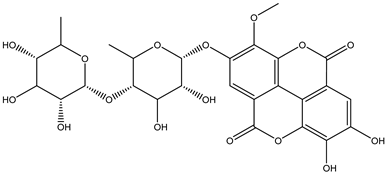
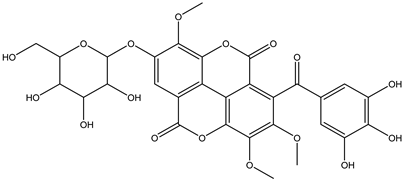
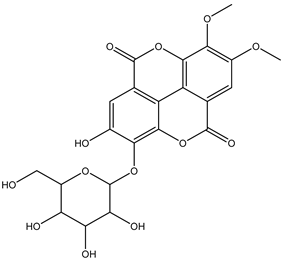
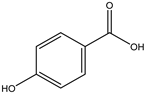
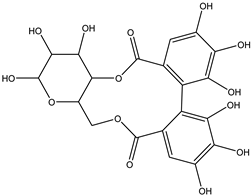
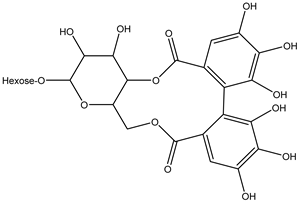
Various separation techniques (column chromatography, ultra-high-performance liquid chromatography, etc.) have been used to isolate or identify phenolic compounds from plants of the genus Irvingia. In 2012, Sun and Chen [26] used UHPLC/HRMS (ultra-high-performance liquid chromatography/high resolution mass spectrometry) analysis to identify the chemical constituents of Irvingia gabonensis seeds. Among the compounds identified, phenolics comprised: 3,3′,4′-tri-O-methylellagic acid (4.04 mg/g of extract) (13); 3,4-di-O-methylellagic acid (0.58 mg/g of extract) (14); methyl gallate (15); ellagic acid (16), di-O-methyl-ellagic acid hexoside (17); methyl-ellagic acid (18); mono-O-methyl ellagic acid deoxyhexoside (19); di-O-methyl ellagic acid (20); di-O-methyl ellagic acid-O-pentoside (21); di-hexahydroxydiphenoyl-ellagic acid (22); di-O-methyl-ellagic acid deoxyhexoside (23); galloyl-hexahydroxydiphenoyl-methyl-ellagic acid (24); tri-O-methyl-ellagic acid (25); mono-O-methyl-ellagic acid rhamnoside (26); mono-O-methyl-ellagic acid rhamnosyl-rhamnoside (27); galloyl-tri-O-methyl-ellagic acid hexoside (28); galloyl-HHDP-ellagic acid (29); di-O-methyl-ellagic acid deoxyhexide (30) [26] (Table 3). From the powdered seeds of African mango (I. gabonensis), 4-hydroxybenzoic acid (31) was also isolated and characterized. Furthermore, ellagic acid (16) was also identified in Irvingia gabonensis stem bark by Ojo et al. [27]. More recently, Yoon et al. [29] isolated the phenolic compound (tannin) terminalin (32) from Irvingia gabonensis seeds.

Table 3.
Phenolic compounds isolated or identified thus far from Irvingia plants.
3.4. Miscellaneous Compounds
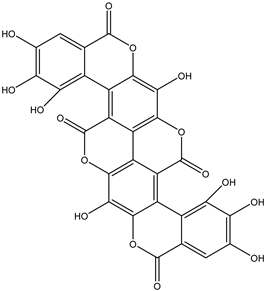
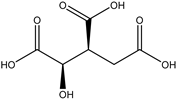
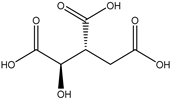
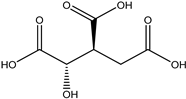
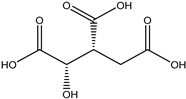
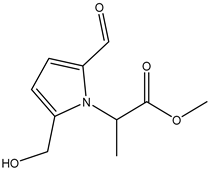
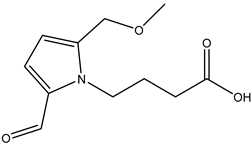
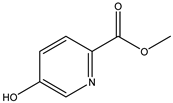
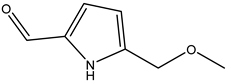
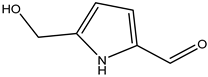
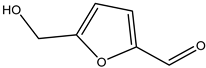
A total of thirteen miscellaneous compounds were identified from Irvingia species in former studies [26,31]. These include a series of organic acids [citric acid (1R, 2S) (33), citric acid (1R, 2R) (34), citric acid (1S, 2S) (35), and citric acid (1S, 2R) (36)] that were isolated from Irvingia gabonensis seeds [28]. In addition to these compounds, two sugars [hexahydroxydiphenoyl(HHDP)-hexose (37) and di-hexahydroxydiphenoyl-hexose (38)] were identified [28]. Moreover, three carboxylic acids [methyl 2-[2-formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl]-propanoate (39), 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl]butanoic acid (40), and methyl-5-hydroxy-2-pyridinecarboxylate (41)], three aldehydes [5-(methoxymethyl)-1H-pyrrole-2-carbaldehyde (42), 5-(hydroxymethyl)-1H-pyrrole-2-carbaldehyde (43), and 5-hydroxymethyl-2-furancarbaldehyde (44)], and one ketone [5-hydroxy-2-pyridyl methyl ketone (45)] (Table 4) were identified from Irvingia gabonensis seeds by Li et al. [31].

Table 4.
Miscellaneous compounds identified thus far from plants of the genus Irvingia.
4. Pharmacological Activity of Irvingia Plants
4.1. Antiprotozoal Activity
As protozoan diseases are a major threat to human health, medicinal plants have long been used to cure such ailments. It has also been reported that such plants possess a number of antiprotozoal hit compounds. Various organs of Irvingia gabonensis (antiplasmodial and anti-Trypanosoma brucei), Irvingia malayana (antiplasmodial), and Irvingia grandifolia (antileishmanial) have been reported to exhibit antiprotozoal activity (Table 5). In fact, studies by Atindehou et al. [28] have demonstrated the effectiveness of the crude ethanol extract of Irvingia gabonensis stem bark against Plasmodium falciparum strain K1 (resistant to chloroquine and pyrimethamine) and Trypanosoma brucei rhodesiense with IC50 values of 8 and >5 µg/mL vs. suramin (IC50: 0.010 µg/mL) and chloroquine (IC50: 0.064 µg/mL), respectively [28]. In 2007, Nguyen-Pouplin et al. [32] obtained IC50 values of 5.0 µg/mL and 10.5 µg/mL for methanol and ethanol extracts of Irvingia malayana, respectively (vs. chloroquine; IC50: 0.1 µM), upon testing against the chloroquine-resistant FcB1/Colombia strain of Plasmodium falciparum. Regarding cytotoxicity experiments, methanol and ethanol extracts were not cytotoxic toward HeLa cells (IC50: 14.8 and 11.7 µg/mL; SI: 2.9 and 1.1, respectively). Against MRC5 cells, the methanol extract yielded an IC50 value of 50.5 µg/mL (SI: 10) [32].

Table 5.
Antiprotozoal activity of plants from the genus Irvingia.
In an in vivo study, Agubata et al. [33] revealed the antiplasmodial activity of Irvingia gabonensis fats and homolipid-based artemether microparticles combined with Irvingia gabonensis in Plasmodium berghei-infected mice. In fact, the oral administration of Irvingia gabonensis fat (AD) and microparticle capsules of artemether (4 mg/kg) combined with I. gabonensis lipid matrices (LM) and phospholipon1 90 G (P90G) [ADP3 (3:1), ADP4 (4:1), and ADP9 (9:1)] led to a significant inhibition of the parasite (percent inhibition: 83.84, 83.68, 82.63, and 87.37%, respectively) compared to the artemether treatment (percent inhibition: 56.32%) [33]. Furthermore, Lamidi et al. [34] demonstrated the in vitro antileishmanial activity of methanol, methanol/water (50:50), and dichloromethane extracts from the stem bark and leaves with a common IC50 value (100 µg/mL). However, the methanol, methanol/water (50:50), and dichloromethane extracts from the stem bark (IC50 values: 7.7, 8.4, and 8.3 µg/mL, respectively) and leaves (IC50 values: >100, 6.2 and 5.4 µg/mL, respectively) of I. grandifolia were cytotoxic when tested against Vero cells [34]. Previous studies have demonstrated the presence of steroids, flavonoids, alkaloids, cardiac glycosides, volatile oils, terpenoids, tannins, saponins, etc. in Irvingia plants [35].
Secondary metabolites such as flavonoids have been proven to inhibit the growth of several protozoan parasites including Trypanosoma spp. and Leishmania spp. [36]. In fact, flavonoids are capable of binding to the C-terminal nucleotide-binding domain of the P-glycoprotein-like transporter in Leishmania spp. (a transporter involved in parasite multidrug resistance). This class of secondary metabolites works as inhibitors of enzymes or proteins that are crucial for the survival and virulence of certain Trypanosomatidae. These enzymes include DNA topoisomerases, protein tyrosine kinase, and squalene synthase. Flavonoids have been shown to induce the apoptosis of host cells such as epithelial cells [37] or by the direct induction of the apoptosis of the parasite [38,39].
4.2. Antimicrobial Activity
According to the literature, numerous studies have demonstrated the antimicrobial activity of plants from the genus Irvingia (Table 6). One such study is that of Osadebe and Ukwueze [40], who reported the antibacterial activity (MIC values: 4.31, 3.53, 5.53, and 5.55 µg/mL, respectively) of a petroleum ether extract from Irvingia gabonensis leaves against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, and Salmonella typhi. Moreover, Kuete et al. [23] evaluated the in vitro antimicrobial activity of the methanol extract, fractions (A, B, and C), and compounds [3-friedelanone (1), betulinic acid (2), oleanolic acid (3), hardwickiic acid (4), 3,3′,4′-tri-O-methylellagic acid (13), and 3,4-di-O-methylellagic acid (14)] from the stem bark of Irvingia gabonensis on 22 species of microorganisms including bacteria [Escherichia coli, Klebsiella pneumoniae, Morganella morganii, Neisseria gonorrhoeae, Salmonella typhi, Streptococcus faecalis, Staphylococcus aureus, Citrobacter freundii, two Enterobacter species, three Proteus species, two Shigella species, and four Bacillus species (MIC range: 1.22–312.50 µg/mL), vs. gentamicin (MIC range: 0.61–9.76 µg/mL)] and fungi [Candida albicans, Candida krusei, and Candida glabrata (MIC range: 9.76–312.50 µg/mL), vs. nystatin (MIC range: 2.44–9.76 µg/mL)] [23]. Furthermore, Candida albicans was also inhibited (MIC value: 11.4 µg/mL) by the ethanol extract of Irvingia malayana. Interestingly, this extract showed an MIC value greater than 100 µg/mL when tested against Vero cells, attesting to its high selectivity (SI: >8.77) [41]. Two years later, Nworie et al. [42] evaluated the in vitro antibacterial activity of hot water, cold water, and ethanol extracts of Irvingia gabonensis leaves and stem bark on Staphylococcus aureus and Escherichia coli using agar-well diffusion and agar dilution methods. As a result, the diameter of inhibition ranged between 8 and 23 mm for the ethanol extract, 8–14 mm for the hot water extract, and 8–20 mm for the cold water extract. Moreover, the MIC values ranged between 6.25 and 50 mg/mL [42]. Aqueous, methanol, and hexane extracts extracted from Irvingia gabonensis leaves were administered to Escherichia coli-induced diarrheal mice at 100 and 200 mg/kg. As a result, the treatment significantly reduced the mouse pathogenic conditions by 80 and 60%, 80 and 80%, and 60 and 40% for the aqueous, methanol, and hexane extracts, respectively [43] (Table 6). Wamba et al. [44] demonstrated the anti-staphylococcal activity of the methanol extract of Irvingia gabonensis leaves against seven (7) clinical isolates, namely SA18, SA23, SA56, SA116, MRSA3, MRSA9, and MRSA11, with an MIC value of 1024 µg/mL [44]. In addition, Olanrewaju et al. [13] assessed the antibacterial activity of the chloroform extract of I. gabonensis leaves against a series of bacteria (Salmonella typhi, Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa) and fungi (Trichophyton rubrum and Candida albicans). Interestingly, the chloroform extract inhibited the growth of S. aureus and S. typhi (MIC: 0.625 mg/mL), K. pneumoniae and E. coli (MIC: 5 mg/mL), P. aeruginosa and S. paratyphi (MIC: 10 mg/mL), T. rubrum (MIC: 10 mg/mL) and C. albicans (MIC: 2.5 mg/mL) [13].

Table 6.
Antimicrobial activity of the extracts and compounds from plants of the genus Irvingia.
More recently, Wandji et al. [45] reported the inhibitory effect of the methanol extract of I. gabonensis seeds against Staphylococcus aureus and Fusarium oxysporum with MIC values of 2 and 6.5 mg/mL, respectively [45].
As reported by Kuete et al. [23], Irvingia spp. possess a variety of antimicrobial compounds such as the triterpenoid 3-friedelanone (1) and the phenolic acids betulinic acid (2), oleanolic acid (3), 3,3′,4′-tri-O-methylellagic acid (13), 3,4-di-O-methylellagic acid (14), and hardwickiic acid (4) (Table 6). Generally, triterpenoids are reported to exhibit a number of biological activities such as antibacterial and antifungal activities. Several reports have revealed the inhibitory potential of triterpenoids on the expression of bacterial genes that are associated with the formation of biofilms. Cell autolysis and peptidoglycan cell wall turnover are also affected by triterpenoids [46]. Other mechanisms of action of this class of compounds include DNA fragmentation, cell cycle arrest [47], and apoptosis [48]. Antimicrobial mechanistic studies revealed that phenolic compounds might induce cell membrane destruction or suppress the formation of biofilms. Phenolics are also known to inhibit the virulence factors in bacteria [49].
4.3. Antioxidant Activity/Capacity
The majority of diseases are intricately related to redox imbalance and oxidative stress. The inhibition of this phenomenon by plant secondary metabolites has been proven to be efficient in reducing the pathogenesis of several diseases. As reported by many authors, the presence of a variety of compounds such as flavonoids, phenolic compounds, alkaloids, tannins, and saponins, among others, in medicinal plants has contributed to reverting oxidative stress in disease pathogenesis. One such plant includes Irvingia gabonensis, which has been reported to exhibit antioxidant activity or to provide protection against damage caused by free radicals (antioxidant capacity). In brief, Agbor et al. [50] used Folin–Ciocalteu reagent (Folin) and ferric reducing antioxidant power (FRAP) tests to evaluate the total antioxidant capacity of a methanol extract from Irvingia gabonensis seeds [50]. In the FRAP assay, the free and total antioxidant capacities were found to be 283.1 and 431.58 mg/g, respectively. In the Folin test, the free and total antioxidant capacities were found to be 7.26 and 10.74 mg/g, respectively. In another experiment, Arogba and Omede [51] described the antiradical scavenging activity of the methanol extract of Irvingia gabonensis seeds with an IC50 value of 177.22 µg/mL compared with the activity of vitamin C (IC50: 300.22 µg/mL) and quercetin (184.71 µg/mL) [51]. Three compounds [methyl 2-[2-formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl]-propanoate (39), 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl]butanoic acid (40), and 5-hydroxy-2-pyridyl methyl ketone (45)], isolated from the African mango (I. gabonensis) seeds showed hydroxyl radical scavenging activity with ED50 values of 16.7, 11.9, and >20 µM, respectively, vs. quercetin (ED50 value: 1.3 µM) [31]. Atawodi [30] used hypoxanthine/xanthine oxidase and 2-deoxyguanosine assays as models to assess the antioxidant activity (IC50 value: 28 µL) and radical scavenging capacity (IC50 value: 281 µL), respectively, of the methanol extract of Irvingia gabonensis seeds [30]. Moreover, Ewere et al. [5] described the DPPH scavenging power of the ethanol extract of Irvingia gabonensis leaves with inhibition percentages ranging from 40 to 95% at 20 to 100 µg/mL [5]. FRAP, DPPH, and ABTS tests were used to assess the antioxidant efficacy of the phenol-rich fraction of Irvingia gabonensis stem bark with IC50 values of 113.10, 18.98, and 18.25 µg/mL, respectively [27].
The antioxidant efficacy of the ethanol extract of Irvingia gabonensis seeds was also confirmed by Olorundare et al. [15] by using DPPH, FRAP, and NO scavenging tests. At 25, 50, 75, and 100 μg/mL, this extract inhibited DPPH radicals by 14.59, 43.53, 67.98, and 75.44%, respectively, vs. vitamin C (percent inhibition: 45.06, 56.55, 76.92, and 89.83%, respectively). In FRAP (extract: 0.08, 0.13, 0.28, and 0.48%, vs. vitamin C: 0.24, 0.38, 0.48, and 0.63% at 25, 50, 75, and 100 μg/mL, respectively) and NO (13.55, 39.98, 68.39, and 77.09%, vs. vitamin C: 47.89, 63.09 76.07, and 84.91%, respectively) tests, the plant extract also displayed significant antioxidant activity [15].
To confirm the antioxidant capacity of methanol, ethanol, and phenol-rich extracts from Irvingia gabonensis seeds, Nguyen et al. [52] evaluated ferric ion reducing antioxidant power (FRAP), Trolox equivalent antioxidant capacity (TEAC), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity and found that these extracts exhibited significant ferric ion reducing power (FRAP values: 0.18, 0.18, and 0.09 mM Fe2+/g for the extracts, respectively), TEAC (3597.11, 3046.60, and 21.38 mM Trolox/g for the extracts, respectively), and DPPH radical scavenging potential (EC50: 2.81 and 2.81 mg/mL for the methanol and ethanol extracts, respectively). More recently, the in vitro and in vivo antioxidant activity of the ethanol extract of the Irvinga gabonensis leaves was determined by Ewere et al. [53] (Table 7). In the in vitro tests, the extract presented IC50 values of 258.47 and 640.05 μg/mL for nitric oxide and hydrogen peroxide scavenging activities, respectively, vs. ascorbic acid (IC50: 91.95 and 109.72 μg/mL, respectively). Upon in vivo studies, the oral administration of the ethanol extract of Irvingia gabonensis leaves to arsenic (dose: 4.1 mg/kg)-induced hepatic oxidative stress rats decreased the serum ALT, ALP, CAT, MDA, and GGT activities and increased the SOD and GPx concentrations, suggesting that this extract possesses antioxidant activity [53]. Furthermore, Atanu et al. [16] confirmed the antioxidant activity of aqueous, ethanol, chloroform, and n-hexane extracts from Irvingia gabonensis leaves through DPPH (IC50: 30.74, 21.42, 36.62, and 31.41 μg/mL, respectively), FRAP (23.91, 22.25, 22.43, and 11.57 mM Fe2+ equivalent, vs. gallic acid: 28.08 mM Fe2+ equivalent), and hydroxyl radical (percentage of radical scavenging: 23.02, 81.43, 69.66 and 23.77%, respectively, vs. gallic acid: 100%) inhibition assays [16] (Table 7).

Table 7.
Antioxidant effects of the extracts and compounds from plants of the genus Irvingia.
The presence of several classes of compounds such as flavonoids, phenolic compounds, and terpenoids contributes to the antioxidant activity of medicinal plants including Irvingia plants. The ability of most of these compounds to interact with reactive oxygen species (ROS) by scavenging or reducing them can characterize their mechanism of action. Controlling the rates of formation and the removal of ROS is a dually essential function. In fact, the intracellular levels of ROS required to perform biological functions should be secured and exceeding ROS levels that reach cytotoxic concentrations should be prevented. For example, flavonoids can exert antioxidant activity by direct radical scavenging and by interacting with the activity of a number of enzymes including nitric oxide synthase and xanthine oxidase [54]. It has also been reported that some flavonoids (which are converted into pro-oxidants during the oxidation process) exert their antioxidant activity indirectly through Nrf2 activation [55,56]. The presence of the benzene ring and the number and position of OH groups in phenolic compounds increase their antioxidant roles. Upon reaction with free radicals, the benzene ring stabilizes the antioxidant compounds. Moreover, phenolics contain H-atoms that can be transferred to free radical substrates to turn them into non-radical substrates (RH, ROH, or ROOH). The latter can contribute to the transfer of electrons or the transition-metal-mediated chelation [57]. The antioxidant mechanism of action has been attributed to three factors including hydrogen transfer, quenching of singlet oxygen, or/and electron transfer [58].
4.4. Antidiabetic Activity
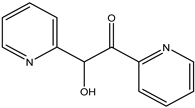
To evaluate the antidiabetic activity of aqueous, ethanol, chloroform, and n-hexane extracts from Irvingia gabonensis leaves, Atanu et al. [16] performed α-amylase and α-glucosidase inhibition tests. In the α-amylase test, the aqueous, ethanol, chloroform, and n-hexane extracts inhibited the tested enzyme with IC50 values of 30, 45, 130, and 75 µg/mL, respectively, vs. acarbose (IC50 value: 55 µg/mL). Moreover, these extracts afforded IC50 values of 10, 15, 18, and 60 µg/mL (vs acarbose: 35 µg/mL), respectively, in the α-glucosidase inhibition test. In another study, the antidiabetic activity of terminalin (32), a phenolic compound isolated from the aqueous extract of Irvingia gabonensis seeds, was evaluated through the inhibition of protein tyrosine phosphate enzymes (PTPs). Terminalin (32) demonstrated antidiabetic activity via in vitro inhibition (80%) of the catalytic sites of PTPN1, PTPN9, PTPN11, and PTPRS, which hampered the glucose uptake in differentiated C2C12 myocytes [29].
4.5. Other Biological Activities
In an in vitro study, Chung et al. [59] demonstrated the inhibitory effect of the methanol leaf extract of Irvingia malayana on the human receptor 5HT1a with an inhibition rate of 65%, inferring that this extract can overcome disorders of the central nervous system such as migraine, sleeping disorders, Alzheimer’s disease, epilepsy, and Parkinson’s disease [59]. Two fractions prepared from the methanol extract of Irvingia malayana inhibited the growth of the human leukemia cell-line HL60 by 73.9% and 46.3%, respectively [60]. Ng et al. [25] used the rat aortic ring assay to screen the antiangiogenic activity of the methanol extract of Irvingia malayana and its isolate betulinic acid. At 100 µg/mL, both the extract and betulinic acid inhibited the human umbilical vein endothelial cells by 46.5 and 45.5%, respectively, vs. suramin (percent inhibition: 55.5%). Furthermore, the studied extract revealed no cytotoxic activity against HepG2, HCT-116, T-47D, NCI-H23, and CCD-18Co cells, as the IC50 values were 88.23, 54.94, 64.32, 78.12, and 111.34 µg/mL, respectively [25]. In a randomized clinical trial, Méndez-Del Villar et al. [61] described the effect of I. gabonensis administration in reverting metabolic syndrome (seven patients out of 12 patients; 58.3%) compared with the group of patients who received placebo (two patients remitted out of 12 patients; 16.7%). These results attested to the effectiveness of Irvingia against metabolic diseases such as diabetes [61]. The ethanol extract of I. gabonensis leaves inhibited the growth of the worm Heligmosomoides bakeri with percent inhibition values of 71.43, 57.14, and 42.9% at concentrations of 500, 250, and 125 mg/mL, respectively, suggesting that the I. gabonensis extract possesses anthelminthic activity [62]. To evaluate the protective role of Irvingia gabonensis, the ethanol extract of I. gabonensis leaves (at 250 and 500 mg/kg) was administered to male Wistar rats with sodium arsenite (2.5 mg/kg)-induced toxicity. This extract decreased the activities of ALT (52.71, 57.24, 40.72, and 39.65 U/L), AST (9, 9.46, 9.23, and 8.92 U/L), and γGT (5.21, 3.47, 6.94, and 4.63 U/L), respectively, when compared to the group treated only with sodium arsenite [ALT (78.61 U/L), AST (22.99 U/L), and γGT (10.42 U/L)] [4]. Recently, Ugwu et al. [63] used carrageenan-induced edema to evaluate the anti-inflammatory activity of solid lipid microparticles prepared from unPEGylated lipid matrices of the Irvingia fat matrix. Irvingia-loaded microparticles significantly reduced the volume of edema in rats by 38, 40, 87, 65, and 67% after 0.5, 1, 2, 3, and 4 h, respectively [63] (Table 8).

Table 8.
Other pharmacological activities of the extracts and compounds from Irvingia plants.
5. Toxicity Profile of Irvingia spp.
A number of studies have reported on the toxicity profile of plants from the genus Irvingia. For example, in a study published by Kothari et al. [65], the oral administration of an Irvingia gabonensis extract at 0, 100, 1000, and 2500 mg/kg for 90 days did not induce any clinical signs or mortality in Sprague Dawley rats. Accordingly, the NOAEL (no observed adverse effect level) for the I. gabonensis extract was predicted to be more than the highest dose tested (i.e., 2500 mg/kg bw/day (LD50)).
Clinical symptoms, body weight, feed consumption, and mortality were not observed in the treated rats. Moreover, there were no significant changes in the hematological and biochemical parameters of rats [65]. In another study, the oral administration of aqueous extracts from I. gabonensis stem bark and leaves to male Wistar rats did not cause significant changes compared to the negative control (without treatment). In brief, the oral treatment of rats with three doses (100, 1000, and 2000 mg/kg) of an aqueous leaf extract of I. gabonensis did not affect the serum biochemical markers of toxicity such as ALT (63.30, 59.50, and 58.00 IU/L at 100, 1000, and 2000 mg/kg, respectively, vs. the untreated control: 66.50 IU/L), AST (158.33, 159.33, and 158.00 IU/L at 100, 1000, and 2000 mg/kg, respectively, vs. the untreated control, 159.16 IU/L) and ALP (127.90, 102.00, and 121.13 IU/L at 100, 1000, and 2000 mg/kg, respectively, vs. the untreated control, 103.50 IU/L), suggesting that these extracts might not affect the liver physiology [66]. In this study, the authors confirmed the NOAEL (2500 mg/kg) reported previously by Kothari et al. [65].
To evaluate the potential of an ethanol extract from Irvingia gabonensis seeds in reverting the cardiotoxicity caused by doxorubicin in mice, Olorundare et al. [15] administered 100, 200, and 400 mg/kg to doxorubicin (15 mg/kg)-mediated cardiotoxicity and determined the level of serum cardiac enzymes such as cardiac troponin I (cTI) and lactate dehydrogenase (LDH) as well as cardiac tissue oxidative stress markers [catalase (CAT), malonyldialdehyde (MDA), superoxide dismutase (SOD), glutathione-S-transferase (GST), glutathione peroxidase (GSH-Px), and reduced glutathione (GSH)]. The plant extract increased the levels of serum GSH [21.3, 17.0, 19.6, and 24.7 U/L at 20, 100, 200, and 400 mg/kg, respectively, vs. the Dox-treated group (14.8 U/L) and negative control (18.8 U/L)], GST [2.6, 2.0, 2.4, and 3.0 U/L at 20, 100, 200, and 400 mg/kg, respectively, vs. the doxorubicin -treated group (1.1 U/L) and negative control (1.6 U/L)], GPx [2.4, 1.3, 2.3, and 3.3 U/L at 20, 100, 200, 400 mg/kg, respectively, vs. the doxorubicin-treated group (1.0 U/L) and negative control (1.2 U/L)], SOD [11.5, 11.1, 12.8, and 15.4 at 20, 100, 200, and 400 mg/kg, respectively, vs. the doxorubicin-treated group (6.9 U/L) and negative control (9.5 U/L)], and CAT [51.4, 54.2, 56.6, and 78.7 U/L at 20, 100, 200, and 400 mg/kg, respectively vs. doxorubicin-treated group (16.7 U/L) and negative control (43.6 U/L)]. In contrast, there was a decrease in the levels of MDA [5.2, 5.3, 3.9, and 3.5 U/L at 20, 100, 200, and 400 mg/kg, respectively, vs. the doxorubicin-treated group (12.8 U/L) and negative control (4.3 U/L)] [15].
6. Traditional Uses of Irvingia spp.
The genus Irvingia encompasses seven species, among which six occur in tropical Africa and one in Southeast Asia.
Seven species of Irvingia are abundant in the African continent and include Irvingia gabonensis (syn. I. pauciflora Tiegh., I. platycarpa Tiegh., and I. tenuifolia Hook), I. robur, I. smithii, I. excels, I. grandiofolia, I. wombolu (syn. I. tenuinucleata), and I. malayana (syn. I. oliveri). Among these, I. gabonensis and I. wombulu contain sweet and bitter comestible pulps, respectively, which are used as traditional foods by many people, especially in the countryside [3]. The seeds of I. gabonensis are commercially available and are marketed across a number of African countries including Liberia, Nigeria, Sierra Leone, Cameroon, Gabon, and Côte d’Ivoire [3]. These plant species are used in traditional medicine to treat a number of disease conditions including diarrhea, scabies, toothache, yellow fever, inflammation, and liver and gastrointestinal disorders [53] (Table 9).
In Cameroon, the decoction of I. gabonensis and I. wombolu seeds is administered twice a day until body weight is reduced [8,67]. In Gabon, the bark decoction of I. grandifolia (common name: Mulenda) is used in a bath to treat asthenia; however, in children, the leaves are pounded and mixed with food to overcome this disease condition [68]. Commonly called Aslotin in Benin, Irvingia gabonensis is used to treat icterus [69]. In Nigeria, Irvingia tenuinucleata is also called Bush mango or Oro, and its fruits and bark have been used traditionally to treat diarrhea, scabies, toothache, yellow fever, inflammation, and diabetes. Irvingia gabonensis has also been indicated for the treatment of dysentery in this country [10]. In Laos, the bark or wood is grilled on fire, boiled in salty water, and drunk to obtain relief from various diseases [9,41] (Table 9).

Table 9.
Traditional uses of Irvingia spp.
Table 9.
Traditional uses of Irvingia spp.
| Plant Species | Common Names | Plant Organs Used/ Route of Administration | Traditional Uses | Country of Use | References |
|---|---|---|---|---|---|
| Irvingia gabonensis (Aubry-Lecomte ex O’Rorke) Baill. | Aslotin | Leaves | Used to treat icterus. | Benin | [69] |
| -Irvingia gabonensis (Aubry-Lecomte ex O’Rorke) Baill.; -Irvingia wombolu Vermoesen (Syn: Irvingia tenuinucleata Tiegh.). | bojep | Seed/oral administration | The seeds are used as condiments and the decoction is administered (a bowlful 2 times a day until recovery) to reduce body weight. | Cameroon | [8,67] |
| Irvingia grandtfolia (Engl.) Engl | Mulenda | Bark/leaves | The bark decoction is used in a bath to treat asthenia. For children, the leaves are pounded and mixed with food. | Gabon | [68] |
| Irvingia malayana Oliver ex Bennett | Bohk; Yarr/Niharr | Bark/wood | The bark or wood is grilled on fire and boiled with salt in water and administered orally to overcome tuberculosis related symptoms. | Laos | [9,41] |
| -Irvingia gabonensis (Aubry-Lecomte ex O’Rorke) Baill.; -Irvingia tenuinucleata Tiegh. Irvingiaceae. | -Bush mango, Oro, dika nut tree, Ugiri, Goron, Biri, and Apon -Bush mango, Oro | -Fruit, bark, seeds and roots -Fruit and bark | -These organs are used as food and to get relief from dysentery, diarrhea toothache and diabetes; -These organs are used to relieve dysentery, diarrhea toothache and diabetes. | Nigeria | [10] |
7. Critical Assessment and Discussion
The present work discusses recent developments in the traditional uses, phytochemical composition, and pharmacological and toxicological studies of plants from the genus Irvingia. Research gaps that have not been explored thus far are also presented and discussed.
At least six species (Irvingia gabonensis, I. grandifolia, I. tenuinucleata, I. malayana, I. wombolu, and I. midbr, among others) of Irvingia are traditionally used to relieve a number of diseases including diarrhea, scabies, toothache, yellow fever, inflammation, liver and gastrointestinal disorders, and others. In traditional medicine, the fruit, bark, leaves, seeds, and roots are the most commonly used organs of Irvingia plants. Decoction is the main mode of preparation, while the main mode of administration is via the oral route. Noteworthy, Irvingia gabonensis has been the most widely studied species of the genus Irvingia. Except for betulinic acid (2), which was also pinpointed to originate from Irvingia malayana, the remaining compounds were identified from various organs [stem bark (compounds 1–4, 13, 14, [23]), seeds (compounds 15, 16, and 33 [30]; compounds 6–9, 13, 16–30, and 33–38 [26]; compounds 31, 39–45, [31]) of Irvingia gabonensis. Most importantly, these compounds were found to exhibit antimicrobial (compounds 1–4, 13, and 14 [23]) and antioxidant (compounds 39, 40, and 45 [31]) activities. Nevertheless, a substantial amount of extracts were prepared from Irvingia plants and evaluated for a number of biological activities including antiprotozoal, antimicrobial, antioxidant, antidiabetic, antiangiogenic, and anthelmintic activities.
In antiprotozoal assays, three plant species were involved including Irvingia gabonensis (antiplasmodial and anti-Trypanosoma brucei; IC50: <8 µg/mL), Irvingia malayana (antiplasmodial) and Irvingia grandifolia (antileishmanial activity; IC50 values: <8.4 µg/mL), whereas the solvents mostly used for extraction comprised ethanol, methanol, and dichloromethane. Stem bark and leaves were the plant organs tested. As already discussed, the seeds of Irvingia spp. contain a number of secondary metabolites (compounds 6–9, 17–31, 33–45) that can be further isolated and characterized as antiprotozoal compounds. In most of the reported studies, the cytotoxicity of the plant extracts was not investigated against human mammalian cells. The antiprotozoal mechanisms of action of the extracts and compounds from Irvingia spp. are still unexplored. Thus, research breaches to be filled in antiprotozoal drug screening include the following: (i) antiprotozoal guided-fractionation of the constituents from all known and available species (>5) of Irvingia should be performed; (ii) antiprotozoal experiments should be followed by cytotoxicity assays to depict the selectivity of test samples; (iii) the polarity of solvent used for extraction should be varied to extract both polar and nonpolar potential antiprotozoal compounds from Irvingia spp.; (iv) appropriate negative and positive controls should be considered in antiprotozoal assays; (v) in-depth toxicity studies and antiprotozoal mechanisms of action of the most promising compounds should be explored. In antimicrobial assays, numerous reports related to the screening of plant extracts are widespread across the literature; however, only a few authors [23] have isolated and characterized antimicrobial compounds from Irvingia gabonensis. Therefore, it will be of interest for researchers working on antimicrobial drug discovery, to (i) explore other Irvingia species and their constituents with respect to antimicrobial drug screening, (ii) screen extracts and compounds against dermatophytes (Epidermophyton, Microsporum, and Trichophyton spp.) and associated fungi that cause superficial infections of the skin, hair and nails, and (iii) perform toxicity studies and antimicrobial mechanisms of action of the most promising compounds.
Although several methods (FRAP, ABTS, DPPH, and NO scavenging tests, etc.) have been used to screen extracts and compounds from Irvingia plants, Irvingia gabonensis was the only plant species mostly involved in biological tests. In fact, the ability of DPPH and ABTS to eliminate hydroxyl and superoxide radicals is attributable to their high hydrogen-donating capacity [70]. Specifically, the ABTS test is based on the production of a blue/green ABTS•+, which is reduced by antioxidants; whereas the DPPH assay is established on the reduction of the purple DPPH• to 1,1-diphenyl-2-picryl hydrazine. In contrast, the FRAP test differs from the DPPH and ABT assays as there are no free radicals involved, but the reduction of ferric iron (Fe3+) to ferrous iron (Fe2+) by antioxidants has been examined [71]. Although there was an adequate correlation with results obtained from the antioxidant efficacy of Irvingia gabonensis extracts using the FRAP and DPPH methods [15,27,52], the presence of polyphenols in plants, in general, has explained the antioxidant nature of plants [72,73], and the use of several approaches to study the anti-radical scavenging activity might influence the outcome of the tests. On the other hand, studying the antioxidant activity of plants and their secondary metabolites might help to better elucidate the mechanisms of action of bioactive extracts or compounds. In the case of Irvingia, a study of the antioxidant activity of other Irvingia species (such as Irvingia malayana and Irvingia grandifolia) and compounds thereof is warranted. A couple of antidiabetic experiments were recently conducted by Atanu et al. [16] and Yoon et al. [29] using enzyme inhibition assays (inhibition of α-amylase, α-glucosidase, and protein tyrosine phosphatase). However, these experiments provide an idea about the in vitro antidiabetic effect of only one species of Irvingia (i.e., Irvingia gabonensis). Extending antidiabetic studies to other Irvingia species might afford more insights into the antidiabetic potential of Irvingia spp. and their secondary metabolites. Other biological tests on the extracts and compounds from Irvingia spp. included antiangiogenic (isolate of I. malayana: betulinic acid; [25]), anthelmintic (ethanol extract of I. gabonensis leaves; Heligmosomoides bakeri; percent inhibition: 42.9% at 125 mg/mL; [62], and hepatoprotective (sodium arsenite-induced toxicity in male Wistar rats; decrease in ALT and AST enzymes; I. gabonensis; [4]) activities. However, there is almost no information on the secondary metabolites (active principles) that are responsible for the observed biological activities. As Irvingia gabonensis is the most investigated plant of the genus Irvingia, phytochemical and biological studies of the other species (>5) of Irvingia are warranted. Overall, the genus Irvingia comprises at least six species (Irvingia gabonensis, I. grandifolia, I. tenuinucleata, I. malayana, I. wombolu, and I. midbr) with ethnomedicinal indications. Various organs (leaves, stem bark, seeds, among others) of these species are used traditionally to overcome a number of diseases such as diarrhea, yellow fever, scabies, toothache, inflammation, dysentery as well as liver and gastrointestinal disorders.
Modern pharmacological studies have revealed antiprotozoal, antimicrobial, antidiabetic, antioxidant, anti-inflammatory, and hepatoprotective activities, thus validating the ethnomedicinal uses of Irvingia plants in the treatment of fever and inflammation conditions and bacterial gastroenteritis (vomiting, diarrhea). Furthermore, several studies have revealed the presence of more than forty (40) compounds from Irvingia plants, mostly phenolic compounds, flavonoids, and terpenoids. These compounds might be responsible for the reported biological activities of Irvingia plants. Nevertheless, there are research gaps that need to be filled regarding the potential application of Irvingia spp., from their ethnopharmacological validation and phytochemical evaluation to preclinical and clinical investigations. These include: (i) the lack of in vitro and in vivo toxicity tests and mechanistic studies on the extracts and compounds from Irvingia spp. in reported works, (ii) a lack of appropriate controls in most of the experiments, (iii) only a few of the existing species of Irvingia have been studied up until now and most of the traditional indications (jaundice and related symptoms, scabies, asthenia, or respiratory ailments, and so on) have not been scrutinized using modern pharmacological experiments; (iv) compared to Irvingia crude extracts, less information is available regarding biological studies of pure compounds from Irvingia. Therefore, (a) more studies on in vitro and in vivo toxicity tests as well as mechanistic studies of the extracts and compounds from Irvingia spp. should be investigated; (b) robust and comprehensive phytochemical analyses such as LC-MS/MS, GC/MS, HPTLC, and UHPLC-ESI-Q-TOF-MS, in association with biological screening (bioassay-guided), should be performed to substantially identify the bioactive compounds from Irvingia species; (c) other Irvingia species and traditional indications should be considered in modern pharmacological studies. These studies might contribute to the potential therapeutic applications of Irvingia in the treatment of various diseases.
8. Conclusions and Perspectives
The present study provides a comprehensive analysis regarding the traditional uses, phytochemical analyses, and pharmacological and toxicity studies of plants from the genus Irvingia. Irvingia plants are used for the traditional treatment of a number of diseases such as diarrhea, yellow fever, scabies, toothache, inflammation, dysentery, and liver and gastrointestinal disorders. Previous reports have revealed a number of pharmacological activities including antiprotozoal, antimicrobial, antidiabetic, antioxidant, anti-inflammatory, and hepatoprotective activities. The reported activities have been attributed to the existence of a number of secondary metabolites (flavonoids, phenolic compounds, terpenoids, tannins, saponins, alkaloids, etc.) in Irvingia plants. On the other hand, Irvingia species such as Irvingia gabonensis, which is used as a food and as a weight loss supplement, can be a potential remedy in treating cardiovascular diseases. Nevertheless, a number of studies (in vitro and in vivo toxicity and in-depth mechanistic experiments, clinical trials, additional phytochemical and pharmacological studies of the uninvestigated species of Irvingia) are needed for the successful utilization of Irvingia plants in the treatment of various diseases.
Author Contributions
Conceptualization, B.P.K. and F.F.B.; Methodology, B.-N.N.-D. and B.P.K.; Software, B.P.K.; Validation, B.P.K.; Formal analysis, B.-N.N.-D. and B.P.K.; Investigation, B.-N.N.-D. and B.P.K.; Resources, B.P.K.; Data curation, B.-N.N.-D., B.P.K. and P.K.L.; Writing—original draft preparation, B.-N.N.-D. and B.P.K.; Writing—review and editing, B.-N.N.-D., B.P.K. and P.K.L.; Visualization, B.P.K.; Supervision, F.F.B. and B.P.K.; Project administration, F.F.B. and B.P.K.; Funding acquisition, F.F.B. and B.P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ABTS: 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); ADP: Arthemether-Dika (Irvingia) fat-Phospholipon; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CAT: catalase; cTI: cardiac troponin I; DNA: deoxyribonucleic acid; DPPH: 2,2-diphenyl-1-picrylhydrazyl; ED50: median effective dose; FRAP: ferric reducing antioxidant power; GC/MS: gas chromatography–mass spectrometry; GGT: gamma-glutamyl transferase; GPx: glutathione peroxidase; GSH: reduced glutathione; GSH-Px: glutathione peroxidase; γGT: gamma-glutamyl transferase; HCT-116: human colorectal carcinoma cell line; HeLa: human cervix carcinoma cells; HepG2: human liver cancer cell line; HPTLC: high-performance thin layer chromatography; IC50: half maximal inhibitory concentration; Irvingia spp.: Irvingia species; LC-MS/MS: liquid chromatography with tandem mass spectrometry; LD50: median lethal dose; LDH: lactate dehydrogenase; LM: lipid matrices; MDA: malondialdehyde; MIC: minimum inhibitory concentration; MRC5: human diploid embryonic lung cells; MRSA: Methicillin-resistant Staphylococcus aureus; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NCI-H23: human non-small cell lung carcinoma; human fibroblast cell line; NO: nitric oxide; NOAEL: no observed adverse effect level; Nrf2: nuclear factor erythroid 2–related factor 2; NS: not specified; NT: not tested; ORAC: oxygen radical absorbance capacity; P90G: Phospholipon 90 G containing about 94.0% of phosphatidylcholine with 0.1% ascorbyl palmitate; PEG: polyethylene glycol; PTPs: protein tyrosine phosphate enzymes; PTPN1: protein tyrosine phosphatase non-receptor type 1; PTPN9: PTPN type 9; PTPN11: PTPN type 11; PTPRS: protein tyrosine phosphatase receptor type S; ROS: reactive oxygen species; SA: Staphylococcus aureus; SI: Selectivity index; SOD: superoxide dismutase; T-47D: human epithelial cell line derived from a mammary ductal carcinoma; TEAC: Trolox equivalent antioxidant capacity; UHPLC-ESI-Q-TOF-MS: ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry; UHPLC/HRMS: ultra-high-performance liquid chromatography/high resolution mass spectrometry.
References
- Tobe, H.; Raven, P.H. Embryology of the Irvingiaceae, a family with uncertain relationships among the Malpighiales. J. Plant Res. 2011, 124, 577–591. [Google Scholar] [CrossRef] [PubMed]
- The World Flora Online (WFO). 2023. Available online: https://www.worldfloraonline.org/ (accessed on 17 August 2023).
- Mahunu, G.K.; Quansah, L.; Tahir, H.E.; Mariod, A.A. Irvingia gabonensis: Phytochemical constituents, bioactive compounds, traditional and medicinal uses. In Wild Fruits: Composition, Nutritional Value and Products; Springer: Cham, Swutzerland, 2019; pp. 253–270. [Google Scholar]
- Gbadegesin, M.A.; Degoke, A.M.; Ewere, E.G.; Odunola, O.A. Hepatoprotective and anticlastogenic effects of ethanol extract of Irvingia gabonensis (IG) leaves in sodium arsenite-induced toxicity in male Wistar rats. Niger. J. Physiol. Sci. 2014, 29, 29–36. [Google Scholar] [PubMed]
- Ewere, E.G.; Oyebadejo, S.A.; Peter, V.C. Ethanolic leaf extract of Irvingia gabonensis (O’Rorke) Baill protects against nephrotoxicity and hepatotoxocity in cadmium-induced Wistar albino rats. Int. J. Pharm. Toxicol. 2016, 4, 105–110. [Google Scholar] [CrossRef]
- Emejulu, A.; Alisi, C.; Asiwe, E.; Igwe, C.; Nwogu, L.; Onwuliri, V. Renal and hepato-protective effects of Irvingia gabonensis juice on sodium fluoride-induced toxicity in Wistar rats. J. Clin. Toxicol. 2016, 6, 296. [Google Scholar] [CrossRef]
- Oyen, L.P.A. Irvingia grandifolia (Engl.). In PROTA (Plant Resources of Tropical Africa/Ressources Végétales de l’Afrique Tropicale); van der Vossen, H.A.M., Mkamilo, G.S., Eds.; Wageningen University: Wageningen, The Netherlands, 2007. [Google Scholar]
- Dickel, M.L.; Rates, S.M.K.; Ritter, M.R. Plants popularly used for loosing weight purposes in Porto Alegre, South Brazil. J. Ethnopharmacol. 2007, 109, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Chassagne, F.; Hul, S.; Deharo, E.; Bourdy, G. Natural remedies used by Bunong people in Mondulkiri province (Northeast Cambodia) with special reference to the treatment of 11 most common ailments. J. Ethnopharmacol. 2016, 191, 41–70. [Google Scholar] [CrossRef]
- Olowo, S.F.; Omotayo, A.O.; Lawal, I.O.; Ndhlovu, P.T.; Aremu, A.O. Ethnobotanical use-pattern for indigenous fruits and vegetables among selected communities in Ondo State, Nigeria South African Journal of Botany Ethnobotanical use-pattern for indigenous fruits and vegetables among selected communities in Ondo State, Nigeria. S. Afr. J. Bot. 2022, 145, 501–511. [Google Scholar]
- Abdulrahman, F.; Inyang, I.S.; Abbah, J.; Binda, L.; Amos, S.; Gamaniel, K. Effect of aqueous leaf extract of Irvingia gabonensis on gastrointestinal tract in rodents. Indian J. Exp. Biol. 2004, 42, 787–791. [Google Scholar] [PubMed]
- Fadare, D.A.; Ajaiyeoba, E.O. Phytochemical and antimicrobial activities of the wild mango-Irvingia gabonensis extracts and fractions. Afr. J. Med. Med. Sci. 2008, 37, 119–124. [Google Scholar]
- Olanrewaju, I.O.; Mordi, R.C.; Echeme, J.O.; Owoeye, T.F.; Ejilude, O.; Aruwajoye, O.A. Anti-mycobacterial and GC-MS studies of Irvingia gabonensis Baill Ex. Lanen stem extracts. J. Phys. Conf. Ser. 2019, 1378, 042101. [Google Scholar] [CrossRef]
- Ibemologi, A.; Iope, A.T. Antimicrobial activities of Irvingia gabonensis leaf and Cyperus esculentus extracts on some selected isolates. Am. J. Biomed. Sci. Res. 2020, 7, 233–236. [Google Scholar] [CrossRef]
- Olorundare, O.; Adeneye, A.; Akinsolam, A.; Kolo, P.; Agede, O.; Soyemi, S.; Mgbehoma, A.; Okoye, I.; Albrecht, R.; Mukhtar, H. Irvingia gabonensis seed extract: An effective attenuator of doxorubicin-mediated cardiotoxicity in Wistar rats. Oxid. Med. Cell. Longev. 2020, 2020, 1602816. [Google Scholar] [CrossRef]
- Atanu, F.O.; Ikeojukwu, A.; Owolabi, P.A.; Avwioroko, O.J. Evaluation of chemical composition, in vitro antioxidant, and antidiabetic activities of solvent extracts of Irvingia gabonensis leaves. Heliyon 2022, 8, e09922. [Google Scholar] [CrossRef] [PubMed]
- Otitolaiye, C.; Omonkhua, A.; Oriakhi, K.; Okello, E.; Onoagbe, I.; Okonofua, F. Phytochemical analysis and in vitro antioxidant potential of aqueous and ethanol extracts of Irvingia gabonensis stem bark. Pharmacogn. Res. 2023, 15, 363–372. [Google Scholar] [CrossRef]
- George, N.I.; Zhao, Y. Pharmacological activity of 2,3,8-tri-O-methyl ellagic acid isolated from the stem bark of Irvingia gabonensis. Afr. J. Biotechnol. 2007, 6, 1910–1912. [Google Scholar] [CrossRef]
- Mateus-Reguengo, L.; Barbosa-Pereira, L.; Rembangouet, W.; Bertolino, M.; Giordano, M.; Rojo-Poveda, O.; Zeppa, G. Food applications of Irvingia gabonensis (Aubry-Lecomte ex. O’Rorke) Baill., the ‘bush mango’: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2446–2459. [Google Scholar] [CrossRef]
- Lee, J.; Chung, M.; Fu, Z.; Choi, J.; Lee, H.J. The Effects of Irvingia gabonensis seed extract supplementation on anthropometric and cardiovascular outcomes: A systematic review and meta-analysis. J. Am. Coll. Nutr. 2020, 39, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.; Davies, L.; Posadzki, P.; Ernst, E. The efficacy of Irvingia gabonensis supplementation in the management of overweight and obesity: A systematic review of randomized controlled trials. J. Diet. Suppl. 2013, 10, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Mgbemena, N.M.; Ilechukwu, I.; Okwunodolu, F.U.; Chukwurah, J.-V.O.; Lucky, I.B. Chemical composition, proximate and phytochemical analysis of Irvingia gabonensis and Irvingia wombolu peels, seed coat, leaves and seeds. Ovidius Univ. Ann. Chem. 2019, 30, 65–69. [Google Scholar] [CrossRef]
- Kuete, V.; Wabo, G.F.; Ngameni, B.; Mbaveng, A.T.; Metuno, R.; Etoam, F.X.; Ngadjui, B.T.; Beng, V.P.; Meyer, J.J.; Lall, N. Antimicrobial activity of the methanolic extract, fractions and compounds from the stem bark of Irvingia gabonensis (Ixonanthaceae). J. Ethnopharmacol. 2007, 114, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Donfack, J.H.; Fotso, W.G.; Ngameni, B.; Tsofack, F.N.; Tchoukoua, A.; Ambassa, P.; Abia, W.; Tchana, A.N.; Giardina, S.; Buonocore, D.; et al. In vitro hepatoprotective and antioxidant activities of the crude extract and isolated compounds from Irvingia gabonensis. Asian J. Tradit. Med. 2010, 5, 79–88. [Google Scholar]
- Ng, K.W.; Salhimi, S.M.; Majid, A.M.; Chan, K.L. Anti-Angiogenic and cytotoxicity studies of some Medicinal Plants. Planta Med. 2010, 76, 935–940. [Google Scholar] [CrossRef]
- Sun, J.; Chen, P. Ultra high-performance liquid chromatography with high-resolution mass spectrometry analysis of African mango (Irvingia gabonensis) seeds, extract, and related dietary supplements. J. Agric. Food Chem. 2012, 60, 8703–8709. [Google Scholar] [CrossRef]
- Ojo, O.A.; Ojo, A.B.; Ajiboye, B.O.; Oyinloye, B.E.; Akinyemi, A.J.; Okesola, M.A.; Boligon, A.A.; de Campos, M.M.A. Chromatographic fingerprint analysis, antioxidant properties, and inhibition of cholinergic enzymes (acetylcholinesterase and butyrylcholinesterase) of phenolic extracts from Irvingia gabonensis (Aubry-Lecomte ex O’Rorke) Baill bark. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Atindehou, K.K.; Schmid, C.; Brun, R.; Koné, M.W.; Traore, D. Antitrypanosomal and antiplasmodial activity of medicinal plants from Côte d’Ivoire. J. Ethnopharmacol. 2004, 90, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Kim, J.; Lee, B.S.; Baek, S.C.; Chung, S.J.; Kim, K.H. Terminalin from African Mango (Irvingia gabonensis) stimulates glucose uptake through inhibition of protein tyrosine phosphatases. Biomolecules 2022, 12, 321. [Google Scholar] [CrossRef]
- Atawodi, S.E. Polyphenol content and in vitro antioxidant activity of methanol extract of seeds of Irvingia gabonensis Baill of Nigerian origin. Electron. J. Environ. Agric. Food Chem. 2011, 10, 2314–2321. [Google Scholar]
- Li, J.; Pan, L.; Naman, C.B.; Deng, Y.; Chai, H.; Keller, W.J.; Kinghorn, A.D. Pyrrole alkaloids with potential cancer chemopreventive activity isolated from a goji berry-contaminated commercial sample of African mango. J. Agric. Food Chem. 2014, 62, 5054–5060. [Google Scholar] [CrossRef]
- Nguyen-Pouplin, J.; Tran, H.; Tran, H.; Phan, T.A.; Dolecek, C.; Farrar, J.; Tran, T.H.; Caron, P.; Bodo, B.; Grellier, P. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J. Ethnopharmacol. 2007, 109, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Agubata, C.O.; Nzekwe, I.T.; Attama, A.A.; Mueller-Goymann, C.C.; Onunkwo, G.C. Formulation, characterization and anti-malarial activity of homolipid-based artemether microparticles. Int. J. Pharm. 2015, 478, 202–222. [Google Scholar] [CrossRef] [PubMed]
- Lamidi, M.; DiGiorgio, C.; Delmas, F.; Favel, A.; Eyele Mve-Mba, C.; Rondi, M.L.; Ollivier, E.; Nze-Ekekang, L.; Balansard, G. In vitro cytotoxic, antileishmanial and antifungal activities of ethnopharmacologically selected Gabonese plants. J. Ethnopharmacol. 2005, 102, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Obianime, A.W.; Uche, F.I. The phytoconstituents and the comparative effects of aqueous extract of Irvingia gabonensis seeds and proviron on the biochemical parameters of male guinea pigs. Asian Pac. J. Trop. Med. 2010, 3, 101–104. [Google Scholar] [CrossRef]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U.; Daniel, H. Early and late apoptosis events in human transformed and non-transformed colonocytes are independent on intracellular acidification. Cell. Physiol. Biochem. 2004, 14, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Mamani-Matsuda, M.; Rambert, J.; Malvy, D.; Lejoly-Boisseau, H.; Daulouede, S.; Thiolat, D.; Coves, S.; Courtois, P.; Vincendeau, P.; Mossalayi, M.D. Quercetin induces apoptosis of Trypanosoma brucei gambiense and decreases the proinflammatory response of human macrophages. Antimicrob. Agents Chemother. 2004, 48, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Mead, J.R.; McNair, N. Antiparasitic activity of flavonoids and isoflavones against Cryptosporidium parvum and Encephalitozoon intestinalis. FEMS Microbiol. Lett. 2006, 259, 153–157. [Google Scholar] [CrossRef]
- Osadebe, P.O.; Abba, C.C.; Agbo, M.O. Antimotility effects of extracts and fractions of Eastern Nigeria mistletoe (Loranthus micranthus Linn). Asian Pac. J. Trop. Med. 2012, 5, 556–560. [Google Scholar] [CrossRef]
- Elkington, B.G.; Sydara, K.; Newsome, A.; Hwang, C.H.; Lankin, D.C.; Simmler, C.; Napolitano, J.G.; Ree, R.; Graham, J.G.; Gyllenhaal, C.; et al. New finding of an anti-TB compound in the genus Marsypopetalum (Annonaceae) from a traditional herbal remedy of Laos. J. Ethnopharmacol. 2014, 151, 903–911. [Google Scholar] [CrossRef][Green Version]
- Nworie, O.; Orji, J.O.; Ekuma, U.O.; Agah, M.V.; Okoli, C.S.; Nweke, M.C. Antibacterial activity of the leaf and stem bark of Irvingia gabonensis (Bush Mango) against Escherichia coli and Staphylococcus aureus. Glob. J. Pharmacol. 2016, 10, 13–18. [Google Scholar]
- Unaeze, B.C.; Ilo, C.E.; Egwuatu, C.; Orabueze, I.; Obi, E. Anti-diarrhoeal effects of three Nigerian medicinal plant extracts on E. coli-induced diarrhea. Int. J. Biol. Chem. Sci. 2017, 11, 414. [Google Scholar] [CrossRef]
- Wamba, B.E.N.; Mbaveng, A.T.; Nayim, P.; Dzotam, J.K.; Ngalani, O.J.T.; Kuete, V. Antistaphylococcal and antibiotic resistance modulatory activities of thirteen Cameroonian edible plants against resistant phenotypes. Int. J. Microbiol. 2018, 2018, 1920198. [Google Scholar] [CrossRef] [PubMed]
- Wandji, F.N.B.; Achidi, A.U.; Ngemenya, M.N.; Nyongbela, K.D.; Tiencheu, B. In vitro antifungal, antibacterial activities and nutritional value of nine Cameroonian edible vegetables. Acta Sci. Pol. Technol. Aliment. 2019, 18, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Wrońska, N.; Szlaur, M.; Zawadzka, K.; Lisowska, K. The synergistic effect of triterpenoids and flavonoids-New approaches for treating bacterial infections? Molecules 2022, 27, 847. [Google Scholar] [CrossRef] [PubMed]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef]
- Chen, H.L.; Lin, K.W.; Huang, A.M.; Tu, H.Y.; Wei, B.L.; Hour, T.C.; Yen, M.H.; Pu, Y.S.; Lin, C.N. Terpenoids induce cell cycle arrest and apoptosis from the stems of Celastrus kusanoi associated with reactive oxygen species. J. Agric. Food Chem. 2010, 58, 3808–3812. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- Agbor, G.A.; Oben, J.E.; Ngogang, J.Y.; Cai, X.; Vinson, J.A. Antioxidant capacity of some herbs/spices from Cameroon: A comparative study of two methods. J. Agric. Food Chem. 2005, 53, 6819–6824. [Google Scholar] [CrossRef] [PubMed]
- Arogba, S.S.; Omede, A. Comparative antioxidant activity of processed Mango (Mangifera indica) and Bush Mango (Irvingia gabonensis) Kernels. Niger. Food J. 2012, 30, 17–21. [Google Scholar] [CrossRef]
- Nguyen, A.T.L.; Akanbi, T.O.; Tawiah, N.A.; Aryee, A.N.A. Valorization of seed and kernel marcs and evaluation of their antioxidant potential. Food Chem. 2022, 390, 133168. [Google Scholar] [CrossRef] [PubMed]
- Ewere, E.G.; Okolie, N.P.; Ndem, J.I.; Eze, G.I.; Oyebadejo, S.A. Irvingia gabonensis leaf extract scavenges nitric oxide and hydrogen peroxide in vitro and modulates arsenic-induced hepatic oxidative stress in Wistar rats. Clin. PhytoSci. 2022, 8, 15. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Speisk, H.; Shahidi, F.; de Camargo, A.C.; Fuentes, J. Revisiting the oxidation of flavonoids: Loss, conservation or enhancement of their antioxidant propertie. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef]
- Silvestro, S.; Mazzon, E. Nrf2 Activation: Involvement in central nervous system traumatic injuries. A promising therapeutic target of natural compounds. Int. J. Mol. Sci. 2023, 24, 199. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, J. Terpenoids as plant antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar] [PubMed]
- Chung, L.Y.; Goh, S.H.; Imiyabir, Z. Central nervous system receptor activities of some Malaysian plant species. Pharm. Biol. 2005, 43, 280–288. [Google Scholar] [CrossRef]
- Ong, C.Y.; Ling, S.K.; Ali, R.M.; Chee, C.F.; Samah, Z.A.; Ho, A.S.; Teo, S.H.; Lee, H.B. Systematic analysis of in vitro photo-cytotoxic activity in extracts from terrestrial plants in Peninsula Malaysia for photodynamic therapy. J. Photochem. Photobiol. B 2009, 96, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Del Villar, M.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; Cortez-Navarrete, M. Effect of Irvingia gabonensis on metabolic syndrome, insulin sensitivity, and insulin secretion. J. Med. Food. 2018, 21, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Nweze, N.E.; Ogidi, A.; Ngongeh, L.A. Anthelmintic potential of three plants used in Nigerian ethnoveterinary medicine. Pharm. Biol. 2013, 51, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, C.E.; Oraeluno, J.N.; Eze, K.C.; Ezenma, C.O.; Nwankwo, A.O. PEGylated aceclofenac solid lipid microparticles homolipid-based solidified reverse micellar solutions for drug delivery. Heliyon 2022, 8, e09247. [Google Scholar] [CrossRef] [PubMed]
- Okolo, C.O.; Johnson, P.B.; Abdurahman, E.M.; Abdu-Aguye, I.; Hussaini, I.M. Analgesic effect of Irvingia gabonensis stem bark extract. J. Ethnopharmacol. 1995, 45, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Kothari, S.C.; Shivarudraiah, P.; Venkataramaiah, S.B.; Gavara, S.; Soni, M.G. Subchronic toxicity and mutagenicity/genotoxicity studies of Irvingia gabonensis extract (IGOB131). Food Chem. Toxicol. 2012, 50, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Ezeasor, C.K.; Chukwuma, C.; Ekere, S.O.; Abah, P. Subchronic toxicity studies of aqueous leaf and stem bark extract of Irvingia gabonensis in male albino wistar rats. Comp. Clin. Pathol. 2017, 26, 553–559. [Google Scholar] [CrossRef]
- Ndah, N.; Egbe, A.; Bechem, E.; Asaha, S.; Yengo, T.; Chia, E.; Ngaiwi, M. Ethnobotanical study of commonly used medicinal plants of the Takamanda Rainforest South West, Cameroon. Afr. J. Plant Sci. 2013, 7, 21–34. [Google Scholar]
- Akendengué, B.; Louis, A.M. Medicinal plants used by the Masango people in Gabon. J. Ethnopharmacol. 1994, 41, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Allabi, A.C.; Busia, K.; Ekanmian, V.; Bakiono, F. The use of medicinal plants in self-care in the Agonlin region of Benin. J. Ethnopharmacol. 2011, 133, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and anti-Inflammatory activity determination of one hundred kinds of pure chemical compounds using offline and online screening HPLC assay. Evid.-Based Complement. Alternat. Med. 2015, 2015, 165457. [Google Scholar] [CrossRef] [PubMed]
- Sharopov, F.S.; Winka, M.; Setzer, W.N. Radical scavenging and antioxidant activities of essential oil components-An experimental and computational investigation. Nat. Prod. Commun. 2015, 10, 1934578X1501000135. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 283. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).