Abstract
Treatment of hyperpigmented skin disorders by novel drug candidates without side effects remains an ongoing area of research. Chemically modified tetracyclines (CMTs) are a group of nonantimicrobial tetracycline drugs that have been shown to possess multiple pharmacological activities. We have previously documented the anti-melanogenic effects of CMT-3 and its 9-amino derivative, CMT-308. Herein, we have extended our analysis to evaluate other CMT analogs, namely CMT-1, CMT-4, CMT-5, CMT-6, and CMT-8, for their impact on melanogenesis using primary human epidermal melanocytes (HEMn-DP cells). CMT analogs were screened using a tetrazolium-based assay to identify nontoxic concentration ranges that were further used to analyze the effects of CMTs on cellular melanin content and morphology (via quantitation of dendricity). Cellular tyrosinase (TYR) activity and levels of melanogenesis proteins, TYR, and microphthalmia transcription factor (MITF) were also evaluated to elucidate the mechanisms underlying their effects on melanogenesis. The findings demonstrated that exposure to CMT-8 resulted in notable cytotoxic effects at concentrations >10 µM; hence, all five analogs were further evaluated and compared at 10 µM. None of the five CMT analogs exhibited any impact on intracellular melanin in HEMn-DP cells at the concentration of 10 µM. However, CMT-1, CMT-4, and CMT-8 robustly suppressed dendricity parameters in HEMn-DP cells, while CMT-5 and CMT-6 showed no effect, suggesting that only a subset of CMT analogs can attenuate melanocyte dendricity. Moreover, the analog CMT-5, which has β-diketone blocked, was ineffective, thus confirming the role of this moiety in suppressing dendrite formation. CMT-1 and CMT-8 did not affect cellular tyrosinase activity, while CMT-4 suppressed TYR activity at 10 µM. The capacity of CMT-4 and CMT-8 to suppress dendricity was partly associated with their ability to downregulate MITF protein levels, while CMT-1 had no effect on MITF but suppressed TYR protein levels. The results of this study indicate that CMT-1, CMT-4, and CMT-8 merit further investigation using in vivo studies as potential drug candidates for the treatment of hyperpigmentation disorders.
Keywords:
CMT-1; CMT-4; CMT-5; CMT-6; CMT-8; melanogenesis; HEMn-DP cells; dendricity; tyrosinase; MITF 1. Introduction
Melanocytes are specialized cells in the epidermis that contribute to skin coloration through their organelles, melanosomes, that possess the unique feature of synthesizing, storing, and exporting the pigment melanin to keratinocytes via dendrites [1,2]. Microphthalmia transcription factor (MITF), the central orchestrator of melanogenesis, is an essential part of all signal transduction pathways and exerts its influence by enhancing the expression of key enzymes involved in melanogenesis, namely tyrosinase (TYR), tyrosinase-related proteins TRP-1, and TRP-2 [3,4]. Melanocyte activation is characterized by several highly significant characteristics, one of which is called dendricity, which refers to the outgrowth of dendrites [5,6]. Dendrite outgrowth is also a vital mechanism for ensuring that melanosomes are transferred to keratinocytes [7]. The process of hyperpigmentation, or hypermelanosis, is expedited by the activation of melanocytes as a result of inflammation [8]. Post-inflammatory hyperpigmentation (PIH), melasma, and solar lentigines (or age spots) are the most prevalent disorders of hyperpigmentation. Melasma, a prevalent and persistent condition, is characterized by the localized accumulation of excessive melanin in the epidermis. This hypermelanosis primarily manifests in regions of the epidermis that are frequently exposed to sunlight and is characterized by increased melanocyte dendricity [9,10]. PIH is a form of hypermelanosis that is acquired and often occurs as a consequence of different inflammatory conditions. The incidence of PIH is observed to be 2.8 times higher in individuals diagnosed with melasma [11]. While PIH may occur in individuals with various skin types, it primarily impacts those with darker skin tones, namely those classified as Fitzpatrick types III-VI. Arachidonate-derived chemical mediators may also cause PIH [12]. Chemotherapeutic drugs have been shown to induce changes in pigmentation in the skin, nails, or hair, causing gradual changes that may present as either a widespread increase in skin pigmentation or localized hyperpigmentation in regions of skin that have experienced damage, such as contact or occlusion [13]. For instance, the administration of paclitaxel, a chemotherapeutic drug, has been observed to result in an elevation in skin pigmentation in individuals undergoing oncologic treatment [14]. Malignant melanoma, a neoplastic condition originating from melanocytes, manifests as a form of cancer. Notably, individuals with fair complexions that have lower levels of melanin are particularly susceptible to this malignancy, with ultraviolet (UV) radiation serving as the primary etiological factor [15]. Under the influence of UV radiation and inflammation-induced hyperpigmentation, a cascade of events occurs within the process of melanogenesis. This includes an upregulation in the production of melanin, an enhancement in the proliferation and dendricity of melanocytes, and an augmented migration of melanocytes toward the superficial layers of the skin [16,17]. During prolonged exposure to UV radiation, there is an increase in the concentrations of matrix metalloproteinases (MMPs), namely MMP-2 and MMP-9, that degrade type IV and type VI collagen within the skin, resulting in basement membrane destruction [18]. These are characteristics of skin aging-induced pigmentation.
The use of monotherapy with topical hydroquinone (HQ) or a combined treatment regime involving corticosteroids and retinoids has been the gold standard strategy for melasma and PIH treatment. Nevertheless, the efficacy of topical therapy may be suboptimal for patients due to adverse effects such as cutaneous irritation and erythema [19,20]. In particular, the extended use of HQ has been associated with the development of exogenous ochronosis and increased susceptibility to cancer [21]. To combat these skin disorders, novel drug candidates with minimal or no side effects are increasingly sought. Inhibition of TYR activity, one of the primary rate-limiting enzymes that convert L-tyrosine to L-dopaquinone (DQ) and its subsequent oxidation in melanosomes [22,23], has remained a conventional approach to treating hyperpigmentation. However, there has been an increasing focus on exploiting other targets within the complex route of melanogenesis, such as inhibitors that target dendritic or export processes.
Chemically modified tetracyclines (CMTs) represent a distinct group within the broader category of tetracycline antibiotics that have lost antimicrobial activity owing to the elimination of the 4-dimethylamino group on the C-4 position but still retain inhibitory efficacy towards MMPs, which is linked to the β-diketone structure on a different part of the four-ringed structure [24]. Several CMT analogs have been synthesized and have shown biological efficacy in previous studies. For example, CMT-1 and CMT-8 suppressed glomerular inflammation by inhibiting inducible nitric oxide synthase (iNOS), with CMT-8 showing a higher efficacy [25]. CMT-8 suppressed bone resorption in a rat model [26], was the most potent suppressor of periodontal bone loss in rats as compared with CMT-1 or CMT-4 [27], and also demonstrated therapeutic effects on pathological characteristics related to human aortic valve stenosis [28]. Recently, CMT-8 was shown to have good tolerability with limited side effects in dogs [29] and also to have a favorable pharmacological profile [30]. CMT-6 enhanced diabetic wound healing [31], and in another report, CMT-6 and CMT-1 suppressed the invasion of highly metastatic human melanoma cells [32]. CMT-5 is a nonantimicrobial pyrazole derivative of tetracycline that has the β-diketone moiety blocked by its replacement with nitrogen atoms; this leads to the abrogation of metal chelation capacity and, thereby, the loss of anti-MMP activity [33,34].
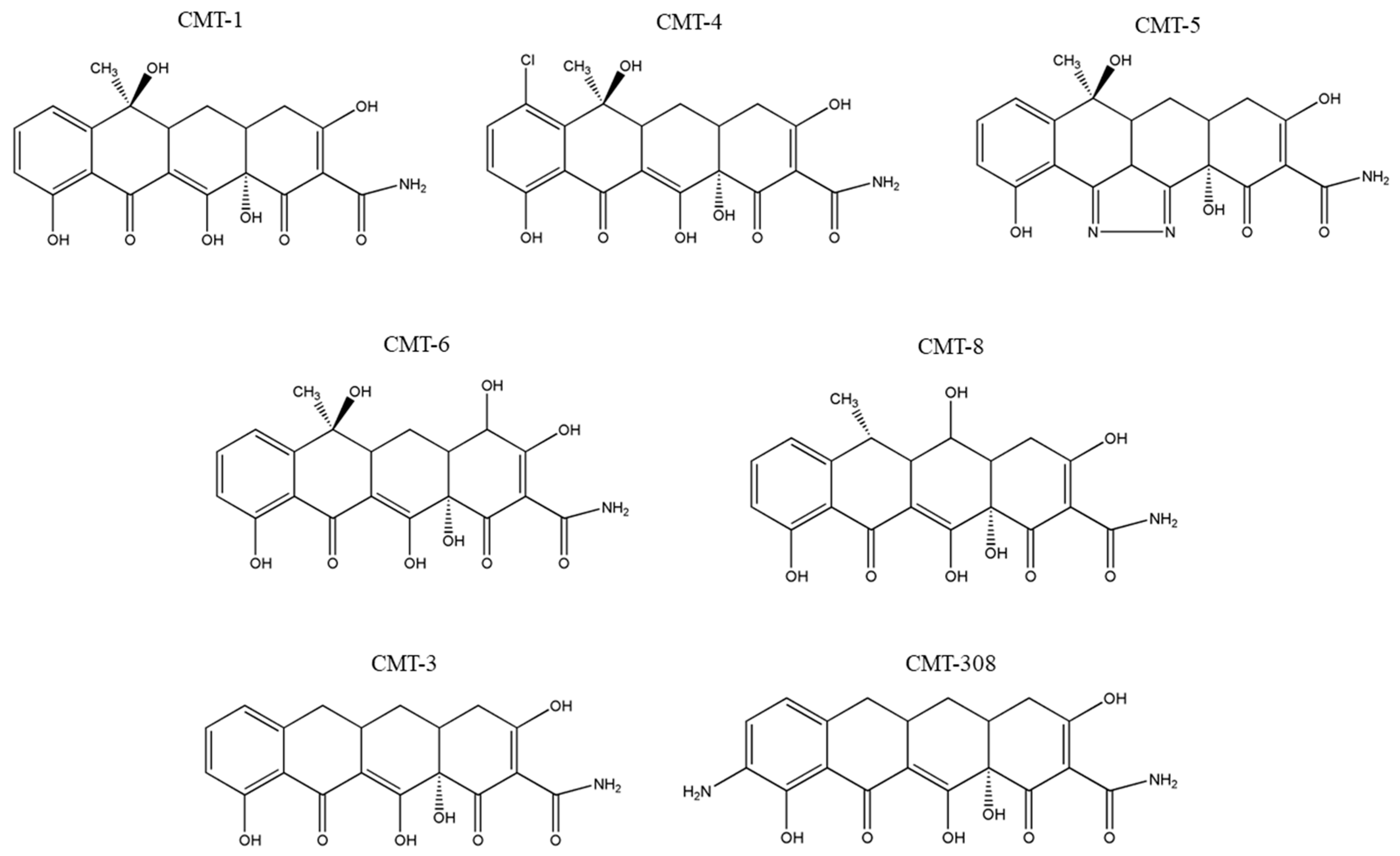
We previously demonstrated that two CMT analogs, namely, CMT-3 [35] and CMT-308 [36], suppressed melanocyte dendricity and that the removal of the 4-dimethylamino group (a modification of all CMTs) is necessary to suppress the melanocyte dendricity [35]. The chemical structures of CMT-3 (6-demethyl-6-deoxy-4-dedimethylamino-tetracycline) and CMT-308 (9-amino-6-demethyl-6-deoxy-4-dedimethylamino-tetracycline) are shown in Figure 1. To validate whether other CMTs in the library might also retain this effect, in this study we have selected five structurally related tetracycline analogs: CMT-1 (4-dedimethylamino-tetracycline), CMT-4 (7-chloro-4-dedimethylamino-tetracycline), CMT-5 (4-dedimethylamino-tetracycline-pyrazole), CMT-6 (4-hydroxy-4-dedimethylamino-tetracycline), and CMT-8 (6α-deoxy-5-hydroxy 4-dedimethylamino-tetracycline) (the structures depicted in Figure 1) and evaluated their effects on melanogenesis utilizing primary human melanocytes from darkly pigmented skin. We show that a subset of these analogs demonstrates the capacity to suppress melanogenesis by hindering melanosome export (quantitated via dendricity).
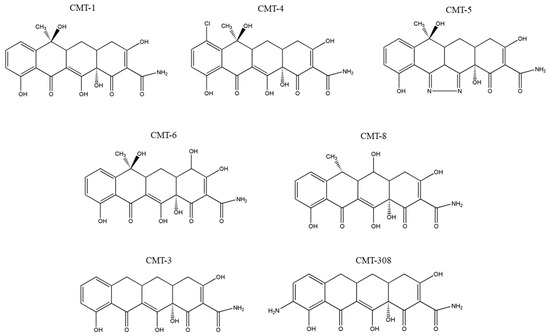
Figure 1.
Chemical structures of chemically modified tetracycline (CMT) analogs, CMT-1 (4-dedimethylamino-tetracycline), CMT-4 (7-chloro-4-dedimethylamino-tetracycline), CMT-5 (4-dedimethylamino-tetracycline-pyrazole), CMT-6 (4-hydroxy-4-dedimethylamino-tetracycline), CMT-8 (6α-deoxy-5-hydroxy 4-dedimethylamino-tetracycline), CMT-3 (6-demethyl-6-deoxy-4-dedimethylamino-tetracycline), and CMT-308 (9-amino-6-demethyl-6-deoxy-4-dedimethylamino-tetracycline). The structures were drawn using ChemDraw Pro 8.0.
2. Results
2.1. Cytotoxicity
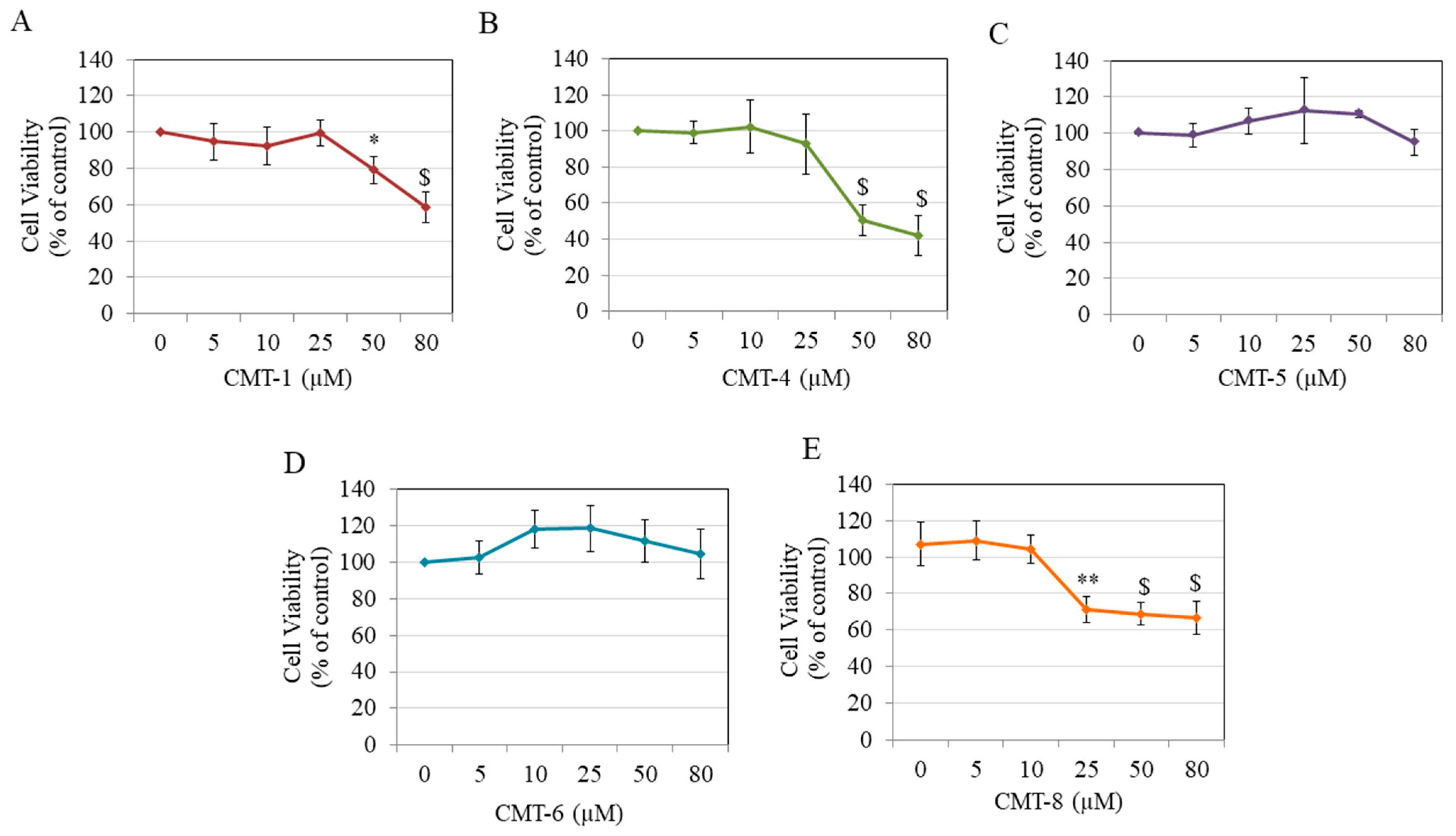
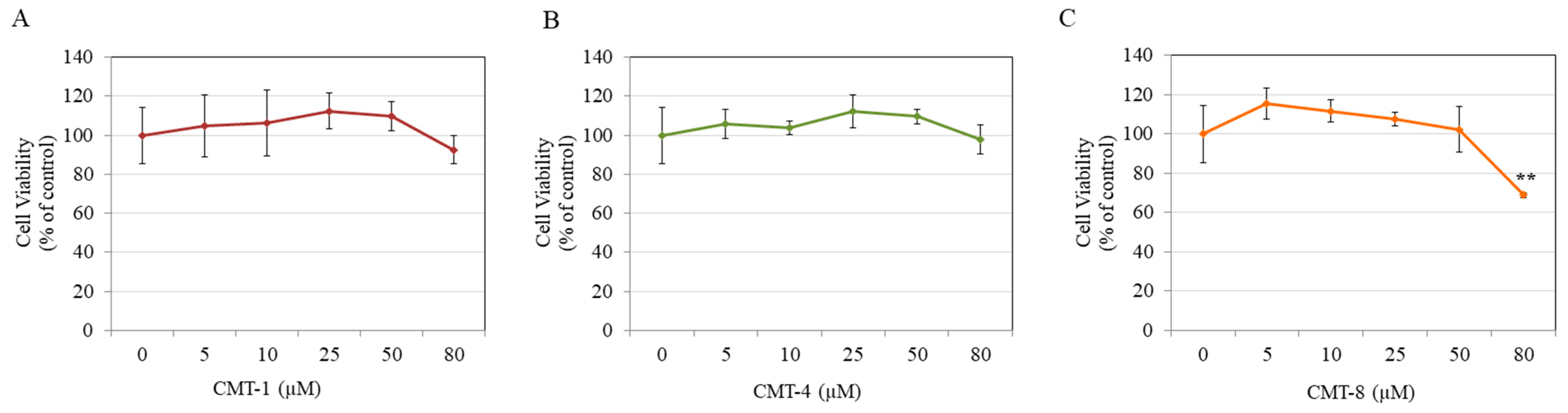
Results of cytotoxicity studies showed that CMT-1 significantly diminished HEMn-DP cell viability by 20.81% and 41.65% at 50 µM and 80 µM, respectively, with no effect at concentrations lower than 50 µM (Figure 2A). CMT-4 significantly diminished cell viability by 49.80% and 58.14% only at higher concentrations of 50 µM and 80 µM, respectively (Figure 2B). Both CMT-5 (Figure 2C) and CMT-6 (Figure 2D) showed no cytotoxicity at any concentration, while CMT-8 significantly diminished viability by 35.63%, 38.27%, and 40.46% at 25 µM, 50 µM, and 80 µM, respectively (Figure 2E).
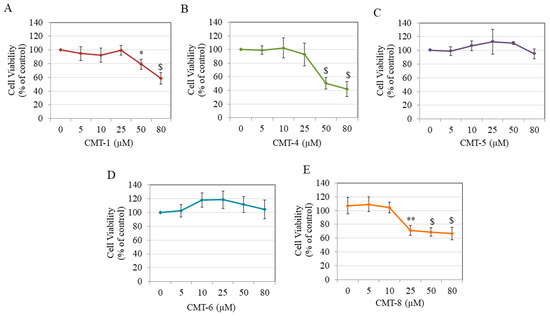
Figure 2.
Viability of human epidermal melanocytes-darkly pigmented (HEMn-DP) cells treated with different concentrations of (A) CMT-1, (B) CMT-4, (C) CMT-5, (D) CMT-6, and (E) CMT-8 as evaluated by MTS assay; (* p < 0.05, ** p < 0.01, and $ p < 0.001 vs. Ctrl, one-way analysis of variance (ANOVA) followed by Dunnett’s test); All data are mean ± SD of at least three independent experiments.
Based on these results, the concentration range of 5–25 µM was chosen for CMT-1 and CMT-4, the concentration range of 5–80 µM was selected for CMT-5 and CMT-6, and the concentrations of 5–10 µM were selected for CMT-8 in further experiments.
2.2. Effects on Intracellular Melanin
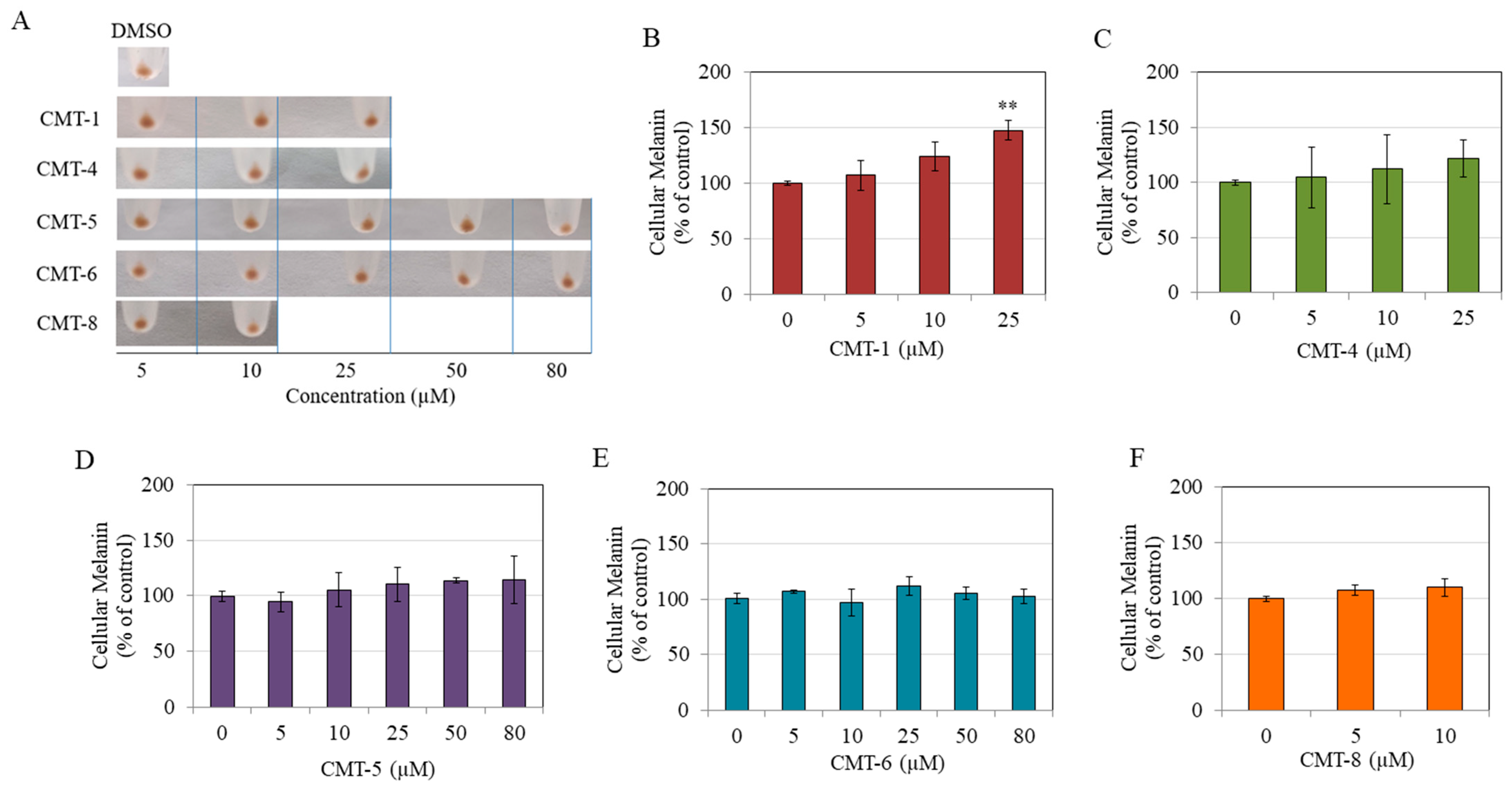
The pellets of HEMn-DP cells were visually inspected after exposure to non-toxic concentrations of CMT-1, CMT-4, CMT-5, CMT-6, and CMT-8. No significant alterations were seen in comparison to the control group, except for the CMT-1 group, which had somewhat of a darker appearance (Figure 3A). Quantitation of melanin contents confirmed qualitative observations. CMT-1 showed a concentration-dependent increase in the melanin content of HEMn-DP cells, with a significantly higher increase at 25 µM (Figure 3B). In contrast, CMT-4 (Figure 3C), CMT-5 (Figure 3D), CMT-6 (Figure 3E), and CMT-8 (Figure 3F) showed no change in the levels of intracellular melanin at any concentration. These results indicate that of all CMTs, only CMT-1 resulted in a higher level of melanin within HEMn-DP cells at the highest concentration.
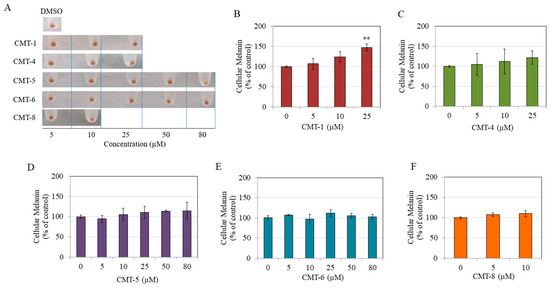
Figure 3.
(A) Representative photos of pellets of HEMn-DP cells after a 72 h treatment with five CMTs at various concentrations, with 0.4% dimethyl sulfoxide (DMSO) group; Intracellular melanin levels in HEMn-DP cells after treatment with different concentrations of (B) CMT-1, (C) CMT-4, (D) CMT-5, (E) CMT-6, and (F) CMT-8; (** p < 0.01 vs. Ctrl, one-way ANOVA followed by Dunnett’s test); All data are mean ± SD of at least three independent experiments, except for (C,D) which are average of values combined from two separate experiments (n = 4).
2.3. Melanocyte Dendricity
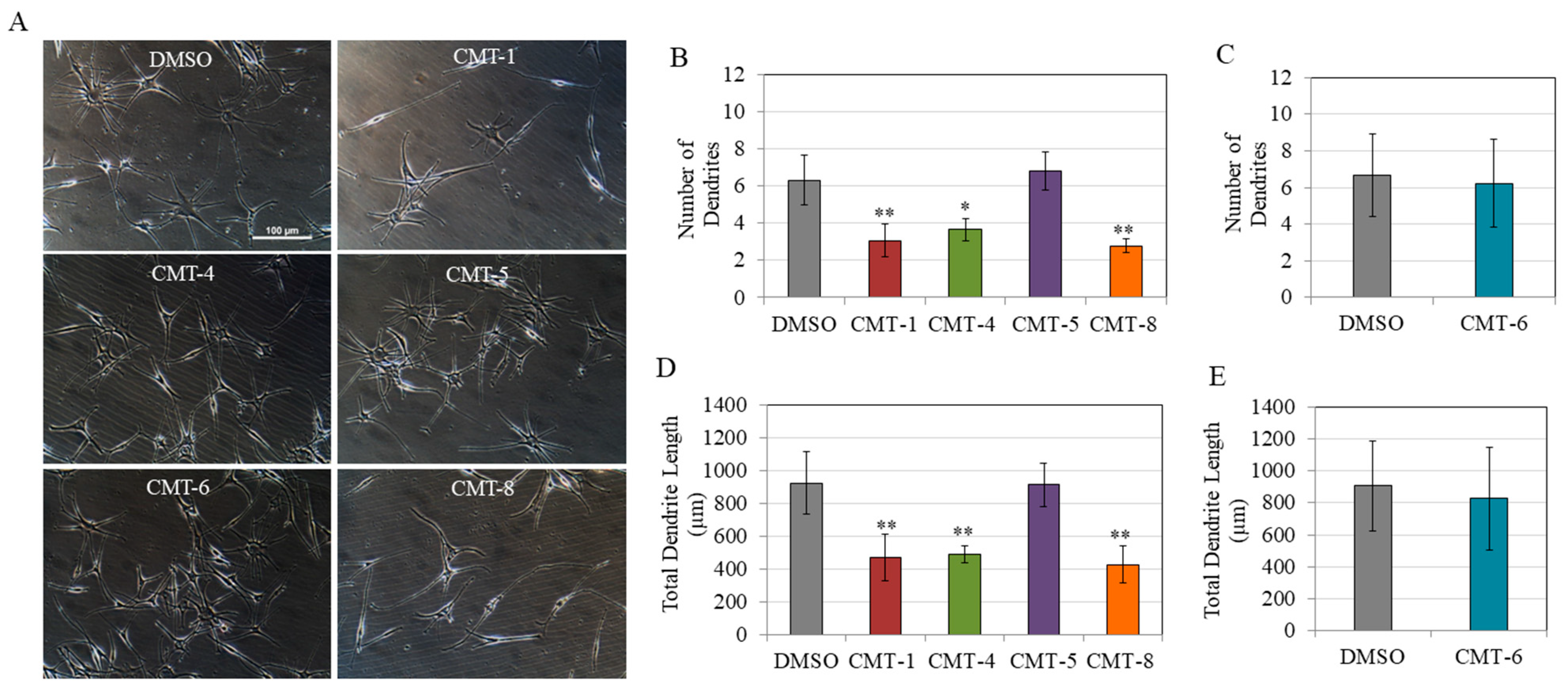
Next, we evaluated all CMTs at a concentration of 10 µM to compare their effects on the morphology of melanocytes. The morphological examination of HEMn-DP cells showed a dramatic decrease in the number of dendrites in cells treated with CMT-1, CMT-4, and CMT-8. In contrast, the number of dendrites in cells treated with CMT-5 and CMT-6 was unaltered (Figure 4A). Interestingly, when these CMTs were qualitatively compared at the higher concentration of 25 µM, a similar trend was noticeable, with lower dendricity in the cases of CMT-1 and CMT-4 (with CMT-8 not tested due to cytotoxicity), while there was no change in CMT-6 or CMT-5 (Figure S1).
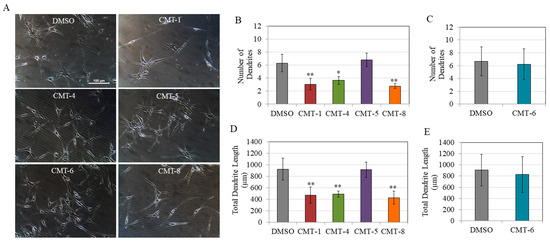
Figure 4.
(A) Representative phase-contrast images of cells treated with CMTs (CMT-1, CMT-4, CMT-5, CMT-6, and CMT-8) at 10 µM; quantitation of melanocyte dendricity by the number of dendrites for (B) CMT-1, CMT-4, CMT-5, CMT-8, and (C) CMT-6 group; and total dendrite length for (D) CMT-1, CMT-4, CMT-5, CMT-8, and (E) CMT-6 group; (* p < 0.05 vs. DMSO; ** p < 0.01 vs. DMSO; one-way ANOVA with Dunnett’s test); data for (B,D) is from a total of 50 cells for CMT-5 and 80 cells for DMSO, CMT-1, CMT-4, and CMT-groups from three independent experiments while data for (C,E) are from a total of 15 cells for the DMSO group and 18 cells for the CMT-6 group from one representative experiment.
The results of quantitation of dendritic parameters of cells corroborated the qualitative results, as only CMT-1, CMT-4, and CMT-8 at 10 µM significantly suppressed dendricity. Specifically, CMT-1, CMT-4, and CMT-8 significantly suppressed dendrite numbers by 51.37%, 42.09%, and 55.85%, respectively (Figure 4B). In contrast, CMT-5 (Figure 4B) and CMT-6 (Figure 4C) did not result in any significant change in the dendrite number. Next, CMT-1, CMT-4, and CMT-8 suppressed total dendrite lengths by 50.67%, 53.22%, and 46.28%, respectively (Figure 4D), while CMT-5 (Figure 4D) and CMT-6 (Figure 4E) did not affect the total dendrite lengths. Due to the marked suppression of dendricity by three CMT analogs (CMT-1, CMT-4, and CMT-8), we focused on these three analogs only in subsequent experiments.
2.4. TYR Activity
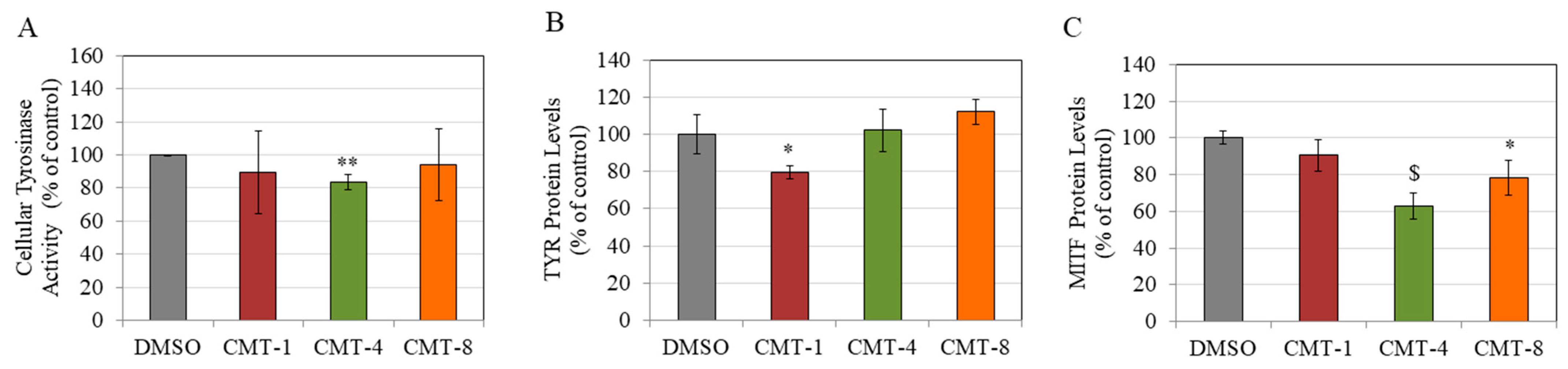
CMT-1 and CMT-8 did not significantly affect TYR activity in HEMn-DP cells at 10 µM, while CMT-4 significantly suppressed TYR activity by 16.60% (Figure 5A). Interestingly, at a higher concentration of 25 µM, CMT-1 and CMT-4 markedly suppressed the TYR activity by 49.03% and 44.47%, respectively (Figure S2).
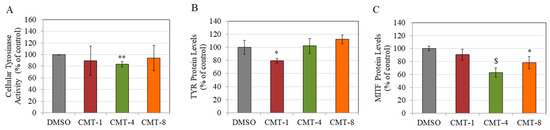
Figure 5.
(A) Cellular tyrosinase (TYR) activity in HEMn-DP cells treated with 0.4% DMSO and CMT-1, CMT-4, and CMT-8 at a concentration of 10 µM for 72 h. (* p < 0.05 vs. DMSO; ** p < 0.01 vs. DMSO, Students t-test); Data are mean ± SD of at least three independent experiments. Protein levels of (B) TYR and (C) Microphthalmia transcription factor (MITF) in HEMn-DP cells treated with CMT-1, CMT-4, and CMT-8 at a concentration of 10 µM for 72 h. (* p < 0.05, ** p < 0.01, and $ p < 0.001 vs. DMSO; one-way ANOVA with Dunnett’s test); Data are mean ± SD of triplicates for (B,C).
2.5. TYR and MITF Protein Levels
CMT-1 (10 µM) significantly attenuated the levels of TYR protein in HEMn-DP cells by 20.45%. In comparison, CMT-4 (10 µM) and CMT-8 (10 µM) had no effect (Figure 5B). Subsequently, our findings indicate that the presence of CMT-1 (10 µM) did not have a discernible impact on the levels of MITF protein in HEMn-DP cells. However, the protein levels were dramatically attenuated by the presence of other CMTs, namely CMT-4 (10 µM) and CMT-8 (10 µM), resulting in diminutions of 37.07% and 21.71%, respectively (Figure 5C).
2.6. Keratinocyte Viability
Treatment with CMT-1 did not alter HaCaT cell viability at concentrations of 5–80 µM (Figure 6A). Similarly, treatment with CMT-4 also showed no changes in keratinocyte viability (Figure 6B). However, CMT-8 significantly lowered HaCaT cell viability by 30.92% at 80 µM, with no effect at concentrations lower than 50 µM (Figure 6C).
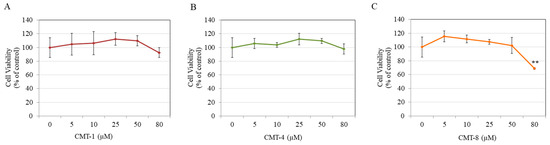
Figure 6.
Viability of human keratinocytes (HaCaT) treated with (A) CMT-1, (B) CMT-4, and C) CMT-8 for 72 h; one-way ANOVA with Dunnett’s, ** p < 0.01 vs. Ctrl; Data are mean ± SD (n = 3 per group).
3. Discussion
Our results of a greater inhibitory impact of CMT-8 than CMT-1 on HaCaT cell proliferation resemble the findings of a previous investigation that examined these analogs in a cell line of immortalized gingival keratinocytes and showed that at a concentration of 20 µM, CMT-8 lowered cell count by a greater amount (50%) than CMT-1, which lowered cell count by 20% [37]. CMT-1 and CMT-4 have similar structures, with the exception that CMT-4 has a chloro substituent. The higher cytotoxicity observed in CMT-4 (IC50: 54.63 ± 11.34 µM) compared with CMT-1 (IC50: 94.63 ± 2.77 µM) towards HEMn-DP cells can be attributed to the presence of the chloro substituent. This finding aligns with the results of a previous study [38], which demonstrated that chlortetracycline, differing from tetracycline by the addition of a chloro group, exhibited greater cytotoxicity towards HEMn-DP cells following a 24 h exposure. Furthermore, our results showed that CMT-8 at 25 µM diminished HEMn-DP cell viability by 35.63%, while the other CMTs did not affect viability at this concentration. Interestingly, these results show a comparable diminution as that of the analogs CMT-3 and CMT-308 (a derivative of CMT-3) in our previous studies [35,36], which suppressed the viability of HEMn-DP cells by 20% and 40% at 25 µM, respectively. According to a prior study [39], the lipophilicity as calculated from the octanol-water partition coefficient (logP) was in the order: CMT-8 > CMT-4 >> CMT-1 >> CMT-6 > CMT-5, suggesting that CMT-8 exhibits greater capability to permeate cells than CMT-4, while CMT-1, CMT-6, and CMT-5 exhibit the lowest permeation owing to the lowest lipophilicity. Our results of greater cytotoxicity of CMT-8 as compared with the other four analogs thus suggest that its high lipophilicity is correlated to its cellular uptake and cytotoxic action. The findings of elevated melanin levels within HEMn-DP cells after treatment with CMT-1 at a concentration of 25 µM did not correlate positively with enhanced TYR activity. In fact, the TYR activity was markedly suppressed by 49.03% at 25 µM (Figure S2). The observed inconsistency has been documented previously, where a hydrogenated curcumin extract suppressed TYR activity despite increasing cellular melanin content [40]. Additionally, another study [41] showed a similar occurrence wherein a sage fluid extract promoted melanin synthesis while concurrently impeding cellular TYR activity.
Our findings, which showed that only a subset of CMT analogs (CMT-1, CMT-4, and CMT-8) could suppress melanocyte dendricity, are in agreement with a prior study [42] that showed that only CMT-1 and CMT-8 exhibited antifungal activity against a Penicillium sp., while the analogs, CMT-5 and CMT-6, were ineffective. We showed that the three CMT analogs, CMT-1, CMT-4, and CMT-8, except CMT-5, significantly suppressed the dendrite number or length. In addition, the percentage of cells with > 2 dendrites was also quantitated as another parameter that confirmed no effect by CMT-5 (Figure S3A), while a decrease in multidendritic cells by CMT-1, CMT-4, and CMT-8 (Figure S3B). Based on the absence of any observable impact of CMT-6, as supported by microscopic imaging and quantitative analysis, it can be suggested that only a subset of CMT analogs can attenuate melanocyte dendricity. The observed outcomes of decreased dendricity of HEMn-DP cells by CMT-1, CMT-4, and CMT-8 are similar to the results of analog CMT-3 that were evaluated in our previous study [35], although CMT-3 robustly inhibited cellular TYR activity but had no effect on TYR or MITF protein levels. The capacity of CMT-4 and CMT-8 to suppress dendricity was associated, in part, with their ability to suppress MITF protein levels, while CMT-1 had no effect on MITF but suppressed TYR protein levels. The reorganization of filamentous actin (F-actin) and subsequent dendritic development plays a crucial role in cellular morphological alterations and the transfer of melanin [43,44]. Treatment of melanophores with the commercial anti-melanogenic drug HQ was shown to lower the formation of actin filaments [45]. Moreover, CMT-1 has been shown to lower F-actin filament amounts in osteoclasts, resulting in reduced podosome formation and adhesion of bone cell osteoclasts [46]. Although we did not specifically examine the effects of CMTs on F-actin amounts in HEMn-DP cells, we speculate that the reduction in dendricity might be linked, in part, to a decrease in F-actin amounts in cells.
CMT-5, a pyrazole derivative, has no inhibitory effect against MMPs due to the absence of the Ca2+ and Zn2+ binding sites at carbon 11 and carbon 12 [34]. Therefore, compared with the other nonantimicrobial CMTs that retain anti-MMP activity, CMT-5 is the only analog that lacks anti-MMP activity and was included as an internal control in this study. CMT-5 displayed no cytotoxicity to HEMn-DP cells (Figure 2C) as well as HaCaT cells (Figure S4) and did not affect dendricity. Moreover, CMT-5 (10 µM) did not affect either the TYR (Figure S5A) or the MITF protein levels (Figure S5B). In contrast, other analogs decreased dendrite outgrowth, indicating that the 11-oxy and 12-hydroxyl groups, which form the diketone structure in CMT, have a role. However, CMT-6 is an exception, as despite lacking the 4-dimethylamino group (a modification we have shown is critical to suppress dendricity [35]) and having the diketone group intact, it did not suppress dendricity. CMT-6 demonstrated a notable attribute in which the culture medium exhibited a dark coloration. Consequently, the absorbance of the culture medium was measured (Figure S6), and it was observed that melanin-like entities formed, as indicated by increased absorbance levels at 475 nm. These elevated absorbance levels correlated with concentration (Figure S6). This phenomenon is attributed to the presence of an OH group at the carbon-4 position of the A ring of the four-ringed structure, which is reminiscent of ortho-dihydroxy catechols and has been shown to stimulate the formation of melanin-like species or lead to toxicity [47]. Notably, we have also observed this phenomenon in an olive-based bioactive compound (unpublished results). Ortho-dihydroxy derivatives have been shown to stimulate melanogenesis in previous studies. For instance, dopac, a catechol compound, stimulated tyrosinase activity and led to quinone formation, which induced cytotoxicity [48], while in another study, aliphatic diols stimulated melanogenesis both in vitro and in vivo [49]. As we did not examine the effect of CMT-6 on tyrosinase activity in HEMn-DP, it is not possible to identify whether CMT-6, due to the presence of these ortho-dihydroxy catechol-like groups, might act as a substrate of tyrosinase and alter tyrosinase activity. The primary objective of this study was to identify potential drug candidates with anti-melanogenic properties. Consequently, we did not perform in-depth experiments on CMT-6 to investigate its apparent contradictory impact on melanogenesis. Further research is necessary to investigate if CMT-6 might be a pro-melanogenic agent. A comparative assessment of the various endpoints in HEMn-DP cells by the CMT analogs of the current study and those in our prior studies (Table 1) indicates that, with the exception of CMT-5, all CMTs exhibited a similar ability to suppress dendricity parameters at a concentration of 10 µM. These results suggest that the various substituents present in the CMTs did not significantly influence the observed effect. During our prior investigation [35], it was determined that eliminating the 4-dimethylamino moiety located in the upper region of the CMT structure was of utmost importance in achieving dendricity suppression. Notably, the current study has yielded additional insights, demonstrating that the presence of the β-diketone moiety in the lower region of the CMT structure also plays a crucial role in exerting the desired anti-dendritic effect. In relation to the process of melanogenesis, the examination of the impact of CMTs on cellular tyrosinase activity (Table 1) revealed that only CMT-3 (previously investigated) exhibited significant inhibition of cellular tyrosinase activity compared with CMT-1, CMT-4, and CMT-8 in this study. The observed higher inhibition may be ascribed to the lack of the dimethylamino group and other substituents (-OH, -CH3, -Cl) positioned on the top periphery of the CMT-3 molecule, resulting in a decrease in steric hindrance. This reduced steric hindrance facilitates the molecule’s binding to the active site of the enzyme and the formation of appropriate hydrogen bonds more readily in comparison to other CMTs. Additional investigation using in situ modeling of the hydrophobic interactions inside the binding pocket of the CMT-tyrosinase complex has the potential to provide conclusive findings. Dendrite outgrowth is a process regulated by multiple cytoskeletal proteins, although it is not directly associated with tyrosinase activity. It is postulated that the presence of the dimethylamino group in the upper periphery region could result in steric hindrance, potentially affecting the compound’s ability to inhibit dendrite outgrowth. Additional research is required to substantiate this hypothesis.

Table 1.
Comparison in HEMn-DP cells of the results of CMT analogs of this study (CMT-1, CMT-4, CMT-5, and CMT-8) and analogs of prior studies (CMT-3 and CMT-308), all at a concentration of 10 µM.
Keratinocytes produce and release vasoactive peptides, namely endothelin-1 (ET-1) and adrenomedullin (ADM), that are known to stimulate melanocyte dendricity [50,51]. We previously demonstrated that chemically modified curcumins (CMCs) inhibited melanin biosynthesis and suppressed dendricity and the expression of dendricity-associated proteins, ADM and ET-1 [52]. Notably, CMTs exhibit a structural resemblance to CMCs [53,54], which occurs from their shared presence of a β-diketone moiety, which imparts the capability to effectively bind Zn2+ ions. Therefore, we speculate that CMTs might also downregulate ET-1 and ADM protein levels, which might partly explain their capacity to suppress dendritic growth. The communication between melanocytes and keratinocytes is facilitated by the presence of the adhesion molecule known as E-cadherin [55]. The regulation of melanocyte growth rate and phenotype within the epidermis is controlled by keratinocytes, with a notable reliance on E-cadherin [56]. Keratinocyte-secreted ET-1 decreases E-cadherin expression in human melanocytes and melanoma cells [57]. Decreased E-cadherin expression is also a characteristic feature of melanoma cells [58]. A recent study [59] demonstrated that the epidermis of solar lentigines (age spots) had decreased E-cadherin expression, which resulted in increased melanogenesis-promoting factors and increased melanin uptake by keratinocytes. Moreover, the authors also showed that E-cadherin expression was enhanced in keratinocytes by a plant-based extract. Although we did not examine if any of the CMTs in this study might enhance E-cadherin levels in human melanocytes or epidermal keratinocytes, a prior study reported that CMT-8 and CMT-3 increased E-cadherin levels in human breast cancer cells [60]. Therefore, CMT-8 and, perhaps, CMT-4 and CMT-1 might enhance E-cadherin levels in keratinocytes, which might, in turn, decrease melanin uptake into keratinocytes, resulting in diminished pigmentation. Future research should rigorously examine this hypothesis. An elevation in intracellular calcium ion (Ca2+) concentration plays an essential role in the activation of melanin biosynthesis [61] and promotes dendrite formation in human melanocytes [62]. Additionally, primary human melanocytes have been shown to express the ryanodine receptor (RyR), which regulates intracellular calcium (Ca2+) responses that are closely linked with the proliferation and pigmentation of human melanocytes [63]. Elevated levels of intracellular calcium within keratinocytes enhanced melanin transfer from melanocytes, resulting in increased pigmentation. This phenomenon has been substantiated by a prior report wherein a calcium chelator suppressed pigment transfer within a melanocyte and keratinocyte coculture [64]. Another study further demonstrated that the inhibition of extracellular calcium concentrations resulted in a reduction in dendrite lengths and melanin transfer following exposure to H2O2 [65]. Interestingly, CMT-8 was shown to decrease the formation of ruffled borders in osteoclasts [26], which may be related to its binding to RyR on osteoclasts, which resulted in decreased intracellular calcium flux. Hence, the possibility that CMT-8 and other CMTs might similarly bind to RyR receptors on melanocytes and might chelate Ca2+ ions to suppress melanin transfer cannot be excluded. We conducted a preliminary experiment where HEMn-DP cells were cocultured with normal human epidermal keratinocytes (NHEK) and treated with CMT-1, CMT-4, and CMT-8 at a concentration of 10 µM (Figure S7). Our qualitative results showed that all analogs appeared to suppress melanin export from melanocytes to keratinocytes, with CMT-4 and CMT-1 showing much greater effects, while the effects of CMT-8 appeared weaker (Figure S7).
The current findings underscore the impact of pleiotropy in the context of CMTs and the influence of chemical modifications on substituents on the ring structure. This observation implies that different groups in the upper peripheral region of tetracyclines, as well as the presence of the β-diketone, may have an impact on their ability to prevent dendritic growth and export, providing valuable insights into the structure-activity relationship that might be used in the design of future drugs targeting melanosome export. It is noteworthy that another research team has also exploited the removal of the dimethylamino group of tetracycline to identify compounds that exhibit enhanced activity in the absence of any antimicrobial activity, although the application was directed at the treatment of neurodegenerative disorders. González et al. [66] showed that the presence of the dimethylamino group impeded the anti-aggregation characteristics, as both doxycycline and minocycline, which have one and two dimethylamino groups, respectively, did not inhibit the aggregation of α-synuclein amyloid, but CMT-3, which lacks this group, potently inhibited it. In a subsequent investigation [67], the same authors eliminated the dimethylamino group from the C-4 position of demeclocycline (DMC), a tetracycline antibiotic. The resulting derivative demonstrated notable effectiveness, surpassing that of CMT-3, in inhibiting α-synuclein amyloid aggregation. Premelanosome protein (PMEL) is expressed in melanocytes and is essential in the assembly of fibrillar sheets in the melanosome, which structurally support melanin polymerization during its synthesis [68]. Intriguingly, PMEL fibrils exhibit biophysical characteristics reminiscent of amyloid structures observed in neurodegenerative disorders [69]. The process of melanin polymer aggregation onto the network of PMEL fibrillar sheets may enhance the effective transfer of melanin from epidermal melanocytes to neighboring keratinocytes. This is because the aggregation of melanin into a singular, larger particle presents a more effective mechanism of phagocytic uptake in comparison to the endocytosis of numerous, irregularly-sized small aggregates [70]. Hence, as PMEL fibrils potentially serve as a facilitator for the transfer of melanin, suppression of PMEL fibrillation might hinder melanin export. For example, a decrease in pigmentation evidenced by diluted coat color that occurs due to disruption in the formation of PMEL fibril assembly has been noted in models of PMEL mutant silver mice or BACE2−/− mice [71,72], where the melanosomal morphology was altered with loss of fibrillar scaffold and shape without any alterations in the intracellular melanin contents. Our results showed that CMTs did not alter cellular pigmentation per se, while the cellular event of dendrite growth related to melanosome export was hindered. The possibility that CMTs modify PMEL fibril formation or impede melanin aggregation on these fibrils, resulting in changes in melanosome size or shape without impacting melanin synthesis, should be explored in future studies that examine the ultrastructural morphology of CMT-treated melanocytes.
The enzyme tyrosinase-related protein 1 (TRP-1) plays a crucial role in the subsequent stages of the melanogenesis pathway [73]. The crystallographic analysis of human TRP-1 has shown the existence of two Zn2+ ions within the active site [74]. Given the strong zinc-binding capability of these CMTs, it is reasonable to hypothesize that they may also have inhibitory effects on TYR-1. Additional future studies are warranted to examine this possibility. MITF has a crucial impact on several aspects of melanocyte function, including pigmentation, proliferation, and survival [75]. A prior study showed the inhibition of downstream melanogenic proteins and a diminution of skin pigmentation by an MITF-repressing drug in human melanoma cells [76]. A previous study showed that the plant bioactive Haginin A decreased MITF protein levels without affecting MITF gene expression, indicating that the observed phenomenon can be attributed to the degradation of MITF rather than the inhibition of MITF gene expression [77]. In this study, we did not examine the mRNA levels of MITF. Consequently, we cannot discern whether the results of decreased MITF protein levels by CMT-4 and CMT-8 may be related to MITF degradation or suppression of MITF gene expression. Besides, this study does not address the molecular pathways involved in melanocyte dendrite suppression by CMTs. Consequently, there is a need for additional research utilizing an in vivo model or, preferably, a 3D skin tissue equivalent to ascertain the efficacy of the three CMT analogs discovered in this research in terms of their capacity to inhibit exportation and attenuate observable pigmentation.
It is worth noting that among the CMT analogs examined, CMT-6 stands out due to its unique ortho-diphenolic structure and did not suppress dendricity, unlike CMT-1, CMT-4, and CMT-8. Therefore, this finding further substantiates that this phenomenon is limited to a specific subset of CMTs. It should be emphasized that originally ten CMTs were synthesized, of which CMT-2, CMT-7, CMT-9, and CMT-10 have not been studied in HEMn-DP cells. Nevertheless, because CMT-2 retains the dimethylamino group while CMT-10 is a derivative of minocycline with a second dimethylamino group, we believe neither might suppress dendricity. CMT-7 was not selected in this study owing to its solubility issues and instability in both in vitro and in vivo milieus [39], while due to limited biological studies on CMT-9, it was also excluded. There is an unmet need for the development of new drugs for the treatment of hyperpigmentation disorders. The advantages of CMTs include oral bioavailability and a lack of risk of antibiotic resistance after long-term administration, coupled with their anti-proteolytic and anti-inflammatory properties. Additionally, its ability to mitigate aging-associated symptoms, in conjunction with its anti-melanogenic effects, further enhances its therapeutic potential as a multifaceted intervention. Given that CMTs did not impact the process of melanin biosynthesis, it is plausible to consider their utilization in combination with other anti-melanogenic agents that impede melanin synthesis, as this could potentially result in a synergistic advantage. Moreover, some of the CMTs have demonstrated efficacy in human melanoma treatment. A previous study [32] reported that CMT-1 and CMT-4 inhibited MMP-2 and MMP-9 activities, while CMT-1 also potently suppressed the invasion and metastasis of human melanoma cells both in vitro and in vivo, although CMT-8 failed to inhibit MMP-2 or MMP-9 and was also ineffective at suppressing invasion or metastasis. CMT-1 and CMT-4 have yet to undergo comprehensive investigation, leaving their pharmacokinetics relatively unexplored. The journey towards utilizing CMT analogs is currently in its nascent stage. Nevertheless, a recent investigation successfully administered CMT-8 to a canine model [29]
4. Materials and Methods
4.1. Materials
The five CMT analogs, CMT-1, CMT-4, CMT-5, CMT-6, and CMT-8, were provided by Dr. Lorne M. Golub and Dr. Hsi-Ming Lee (Department of Oral Biology and Pathology, Stony Brook University, Stony Brook, NY, USA). Of these, CMT-4 was originally synthesized and supplied to the laboratory by Johnson and Johnson, Inc. (New Brunswick, NJ, USA). L-DOPA was purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). The cell proliferation agent MTS and the bicinchoninic acid (BCA) kit were acquired from Promega Corp. (Madison, WI, USA) and Thermo Fisher Scientific (Waltham, MA, USA), respectively. Cell-based tyrosinase (TYR) and cell-based microphthalmia transcription factor (MITF) kits were procured from LSBio (Seattle, WA, USA).
4.2. Cell Culture
Normal human epidermal melanocytes from a darkly pigmented neonatal donor (HEMn-DP) were procured from Cascade Biologics (Portland, OR, USA) and maintained in basal medium 254 (Cascade Biologics) supplemented with 1% human melanocyte growth supplement (HMGS; Cascade Biologics) and 1% penicillin (100 U/mL)-streptomycin (100 µg/mL) (Gibco Inc., Billings, MT, USA). The HaCaT cell line, derived from human keratinocytes, was procured from AddexBio (San Diego, CA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) medium with 10% fetal bovine serum and antibiotics. The cells were cultured at a temperature of 37 °C in an environment with 5% CO2 and controlled humidity.
4.3. Cell Viability Assay
HEMn-DP cells, at a seeding density of 2 × 104 cells per well, were cultivated in a 96-well plate for a duration of 72 h. Subsequently, the growth medium was substituted with compounds dissolved in dimethyl sulfoxide (DMSO), resulting in a final DMSO concentration of 0.4%. The cell cultures were then continued for an additional 72 h. At this stage, the existing culture medium was replaced with a volume of 100 μL of the newly prepared medium that contained 20 μL of MTS solution. Subsequently, the plate was subjected to incubation at a temperature of 37 °C for a duration of 90 min. The absorbances of 100 μL aliquots, which were transferred to a new 96-well plate, were measured at a wavelength of 490 nm using a Versamax® microplate reader. Cell viability was assessed by measuring the absorbance readings, which were then standardized against the control groups and presented as percentages.
To assess the viability of HaCaT cells, an initial population of 5 × 103 cells per well was introduced into a 96-well plate. Following a 24 h incubation period, the various compounds were introduced into the cultures, and the cells were allowed to grow undisturbed for a total of 72 h. Following this, the MTS assay was performed in a manner analogous to the procedure used for HEMn-DP cells.
4.4. Intracellular Melanin Assay
Melanin contents were determined in HEMn-DP based on the method employed in our previous investigations [35,36]. Briefly, 1.1 × 105 HEMn-DP cells per well were cultured in a 12-well plate for a duration of 72 h. Following this, varying concentrations of CMTs were introduced into the system, and the cultures were sustained for a duration of 72 h. Subsequently, cells were dislodged utilizing the TrypLE™ Express Enzyme solution, and cell pellets were washed with phosphate-buffered saline (PBS). To facilitate the dissolution of melanin, a volume of 250 µL of 1N sodium hydroxide (NaOH) was introduced, and the resulting mixture was subjected to a temperature of 70 °C. Next, a volume of 200 µL of lysates was dispensed into a 96-well plate, followed by the measurement of absorbance at a wavelength of 475 nm using a microplate reader. The absorbances corresponding to intracellular melanin were subjected to normalization with respect to the total protein concentrations and subsequently presented as relative melanin levels, expressed as a percentage in relation to the control.
4.5. Quantitation of Dendricity
Dendricity parameters were quantitated in HEMn-DP cells as a substitute metric for the export of melanosomes, in line with our earlier studies [36,78]. Cells at a density of 1 × 104 cells per well were cultured in 12-well plates for a period of 48 h. Subsequently, the cells were subjected to treatment with various compounds, and the cultures were then sustained for an additional 72 h. After a duration of 72 h, images were obtained from various arbitrary microscopic regions containing cells in each experimental cohort, utilizing the phase-contrast modality. Subsequently, the number of dendrites in each cell was enumerated through manual means. In contrast, the quantification of dendrite lengths was performed by subjecting the acquired images to analysis utilizing imaging software, specifically NIS Elements version 5.0 (Nikon Instruments Inc., Melville, NY, USA).
4.6. Intracellular TYR Activity
A total of 1.1 × 105 HEMn-DP cells were seeded in each well of a 12-well plate and grown for 72 h, after which the medium was renewed with compounds and cultures were incubated for 72 h. After this, cells were detached, washed, and lysed. Lysates (50 µL) were combined with 100 µL of 3 mM L-DOPA in a 96-well microplate; the absorbance was recorded at 475 nm in the kinetic mode for a period of 20 min using a microplate reader. The slope of the linear range of inhibitory velocities was used to quantify the percentage TYR activity and normalized to BCA kit protein content.
4.7. Protein Levels of TYR and MITF
HEMn-DP cells were cultured in a 96-well plate and treated with compounds for 72 h. After this step, cells were fixed and processed using a TYR cell-based Enzyme-Linked Immunosorbent Assay (ELISA) kit in accordance with the guidelines provided by the manufacturer. The recorded absorbance values were standardized based on the measurement of cell density using a crystal violet stain and expressed as a percentage relative to the untreated control.
To determine the quantities of MITF protein, HEMn-DP cells were cultivated and processed in a similar manner. The measurement was performed using an MITF cell-based ELISA kit, following the guidelines provided by the manufacturer.
4.8. Statistical Analysis
A student’s t-test was used for the comparison of the two groups. In contrast, for comparisons between multiple groups, a one-way analysis of variance (ANOVA) with Dunnett’s post hoc test was used. All the studies were conducted using GraphPad Prism software (version 9.0, San Diego, CA, USA), and the level of statistical significance was set at p < 0.05. All data are reported as the mean ± standard deviation (SD).
5. Conclusions
The results obtained from this investigation unveil the intriguing anti-melanogenic properties exhibited by this particular category of compounds. Our investigation highlights the noteworthy observation that the ability to suppress dendricity is restricted solely to a subset of CMT analogs, namely CMT-1, CMT-4, and CMT-8. Additional investigations are merited to ascertain the potential for in vivo recapitulation of these observed effects. Furthermore, they shed light on the prospective application of CMTs as adjuvants for melanomas when employed in conjunction with other depigmenting agents. The findings of the current investigation hold potential significance for the subsequent advancement of therapeutic drugs aimed at pigmented lesions and melanoma.
6. Patents
L. M. Golub is listed as the inventor of several patents pertaining to the pharmaceutical compounds described in this article. These patents have been fully assigned to his institution, Stony Brook University, State University of New York.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ddc2040041/s1, References [35,36] are cited in the supplementary materials. Figure S1: Representative phase-contrast images of HEMn-DP cells treated for 72 h with CMT-1, CMT-4, CMT-5, and CMT-6 at a concentration of 25 µM; CMT-8 was not included due to cytotoxicity at 25 µM; DMSO group represents solvent-control (cells treated with 0.4% DMSO); Figure S2: Tyrosinase activity in HEMn-DP cells after a 72 h treatment with CMT-1 and CMT-4 at 25 µM for 72 h. Data is mean ± SD of three independent experiments; * p < 0.05 and ** p < 0.01 vs. Ctrl by one-way ANOVA with Tukey’s test; Figure S3: The percentage of cells with >2 dendrites were calculated for HEMn-DP cells treated with (A) CMT-5 (10 µM) and; (B) CMT-1, CMT-4, and CMT-8 all at a concentration of 10 µM for a duration of 72 h; a total of up to 50 cells were evaluated for each group from three independent experiments for (A), while a total of 60 cells were evaluated for each group from at least two independent experiments for (B); Figure S4: Viability of HaCaT cells after a 72 h treatment with varying concentrations of CMT-5; data are mean ± SD (n = 3 per group); Figure S5: Protein levels of (A) TYR and (B) MITF in HEMn-DP cells treated with CMT-5 at a concentration of 10 µM for 72 h; p > 0.05 vs. Ctrl by Student’s t-test; all data are mean ± SD of triplicates; Figure S6: Absorbance of the extracellular culture medium of HEMn-DP cells after a 72 h treatment with CMT-6 at various concentrations (0–80 µM); data are mean of duplicates; Figure S7: Bright-field micrographs (at 40× objective magnification) of HEMn-DP: NHEK cell cocultures that were stained with Fontana Masson (FM) after a 72 h treatment with various CMT compounds (CMT-1, CMT-4, CMT-5, and CMT-6) all a concentration of 10 µM.
Author Contributions
Conceptualization: S.G. and L.M.G.; methodology: S.G.; validation: S.G.; formal analysis: S.G.; investigation: S.G.; data curation: S.G.; writing—original draft preparation: S.G.; writing—review and editing: S.G. and L.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Stony Brook University Research Foundation #1050308-1-37298.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available from the corresponding author upon reasonable request.
Acknowledgments
The authors acknowledge Sanford R. Simon (Department of Biochemistry and Cell Biology, Stony Brook University) for facilities support and Hsi-Ming Lee (Department of Oral Biology and Pathology, Stony Brook University) for providing the five CMT samples for this study.
Conflicts of Interest
The author S.G. declares no conflict of interest. L.M. Golub is listed as the inventor of many patents pertaining to the pharmaceutical compounds described in this article. These patents have been duly assigned to his institution, State University of New York, Stony Brook. The funders had no role in the study’s design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wu, X.; Hammer, J.A. Melanosome transfer: It is best to give and receive. Curr. Opin. Cell Biol. 2014, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Investig. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef]
- Park, H.; Kosmadaki, M.; Yaar, M.; Gilchrest, B. Cellular mechanisms regulating human melanogenesis. Cell. Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M. MITF: A stream flowing for pigment cells. Pigment Cell Res. 2000, 13, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Lacour, J.P.; Gordon, P.R.; Eller, M.; Bhawan, J.; Gilchrest, B.A. Cytoskeletal events underlying dendrite formation by cultured pigment cells. J. Cell. Physiol. 1992, 151, 287–299. [Google Scholar] [CrossRef]
- Kippenberger, S.; Bernd, A.; Bereiter-Hahn, J.; Ramirez-Bosca, A.; Kaufmann, R. The mechanism of melanocyte dendrite formation: The impact of differentiating keratinocytes. Pigment Cell Res. 1998, 11, 34–37. [Google Scholar] [CrossRef]
- Tada, A.; Kanamaru, A.; Ito, Y. Control of melanosome transfer by promoting shrinkage or expansion of melanocyte dendrites. Int. J. Cosmet. Sci. 2006, 28, 148. [Google Scholar] [CrossRef]
- Mattos, K.; Cintra, M.; Gouvêa, I.; Ferreira, L.; Velho, P.; Moriel, P. Skin hyperpigmentation following intravenous polymyxin B treatment associated with melanocyte activation and inflammatory process. J. Clin. Pharm. Ther. 2017, 42, 573–578. [Google Scholar] [CrossRef]
- Espósito, A.C.C.; Cassiano, D.P.; da Silva, C.N.; Lima, P.B.; Dias, J.A.; Hassun, K.; Bagatin, E.; Miot, L.D.; Miot, H.A. Update on Melasma—Part I: Pathogenesis. Dermatol. Ther. 2022, 12, 1967–1988. [Google Scholar] [CrossRef]
- Grimes, P.E.; Yamada, N.; Bhawan, J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am. J. Dermatopathol. 2005, 27, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Adalatkhah, H.; Bazargani, H.S. The Association Between Melasma and Postinflammatory Hyperpigmentation in Acne Patients. Iran. Red. Crescent Med. J. 2013, 15, 400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomita, Y.; MAEDA, K.; TAGAMI, H. Melanocyte-stimulating properties of arachidonic acid metabolites: Possible role in postinflammatory pigmentation. Pigment Cell Res. 1992, 5, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Masson Regnault, M.; Gadaud, N.; Boulinguez, S.; Tournier, E.; Lamant, L.; Gladieff, L.; Roche, H.; Guenounou, S.; Recher, C.; Sibaud, V. Chemotherapy-related reticulate hyperpigmentation: A case series and review of the literature. Dermatology 2015, 231, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.; Sanz, C.; Pérez-Fidalgo, J.A.; Pérez-Leal, M.; Milara, J.; Cortijo, J. Paclitaxel alters melanogenesis and causes pigmentation in the skin of gynecological cancer patients. Fundam. Clin. Pharmacol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Kanavy, H.E.; Gerstenblith, M.R. Ultraviolet radiation and melanoma. In Seminars in Cutaneous Medicine and Surgery; WB Saunders: Philadelphia, PA, USA, 2011; pp. 222–228. [Google Scholar]
- Gilchrest, B.A.; Park, H.-Y.; Eller, M.S.; Yaar, M. Mechanisms of ultraviolet light-induced pigmentation. Photochem. Photobiol. 1996, 63, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.H. Postinflammatory hyperpigmentation. Clin. Dermatol. 1989, 7, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Inomata, S.; Takada, K.; Tsunenaga, M.; Fukuda, M.; Matsunaga, Y.; Amano, S.; Kobayashi, K.; Nishiyama, T.; Kohno, Y. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Investig. Dermatol. 2003, 120, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, V.N.; Verma, P.; Srivastava, G.; Aggarwal, A.K.; Verma, S. Melasma: Treatment strategy. J. Cosmet. Laser Ther. 2011, 13, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.F.; Lee, T.L.; Oyerinde, O.; Desai, S.R.; Aljabban, A.; Bay, C.P.; Bain, P.A.; Chung, H.J. Efficacy and safety of topical agents in the treatment of melasma: What’s evidence? A systematic review and meta-analysis. J. Cosmet. Dermatol. 2023, 22, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Charoo, N.A. Hyperpigmentation: Looking beyond hydroquinone. J. Cosmet. Dermatol. 2022, 21, 4133–4145. [Google Scholar] [CrossRef]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef]
- Golub, L.M.; McNamara, T.F.; D’Angelo, G.; Greenwald, R.A.; Ramamurthy, N.S. A non-antibacterial chemically-modified tetracycline inhibits mammalian collagenase activity. J. Dent. Res. 1987, 66, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Trachtman, H.; Futterweit, S.; Greenwald, R.; Moak, S.; Singhal, P.; Franki, N.; Amin, A.R. Chemically modified tetracyclines inhibit inducible nitric oxide synthase expression and nitric oxide production in cultured rat mesangial cells. Biochem. Biophys. Res. Commun. 1996, 229, 243–248. [Google Scholar] [CrossRef]
- Sasaki, T.; Ohyori, N.; Debari, K.; Ramamurthy, N.S.; Golub, L.M. Effects of chemically modified tetracycline, CMT-8, on bone loss and osteoclast structure and function in osteoporotic states. Ann. N. Y. Acad. Sci. 1999, 878, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, N.S.; Rifkin, B.R.; Greenwald, R.A.; Xu, J.w.; Liu, Y.; Turner, G.; Golub, L.M.; Vernillo, A.T. Inhibition of matrix metalloproteinase-mediated periodontal bone loss in rats: A comparison of 6 chemically modified tetracyclines. J. Periodontol. 2002, 73, 726–734. [Google Scholar] [CrossRef]
- Salo, T.; Soini, Y.; Oiva, J.; Nissinen, A.; Biancari, F.; Juvonen, T.; Satta, J. Chemically modified tetracyclines (CMT-3 and CMT-8) enable control of the pathologic remodellation of human aortic valve stenosis via MMP-9 and VEGF inhibition. Int. J. Cardiol. 2006, 111, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Sainz, Á.; García-Sancho, M.; Rodríguez-Franco, F.; San Andrés, M.I.; Rodríguez, C.; de Lucas, J.J.; San Andrés, M.D.; Agulla, B.; Villaescusa, A. Effect of chemically modified tetracycline-8 (CMT-8) on hematology, blood chemistry, cytokines and peripheral blood lymphocyte subsets of healthy dogs. Res. Vet. Sci. 2021, 136, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.; Golub, L.; Ramamurthy, N.; Chowdhury, M.; Moak, S.; Sorsa, T. In vitro sensitivity of the three mammalian collagenases to tetracycline inhibition: Relationship to bone and cartilage degradation. Bone 1998, 22, 33–38. [Google Scholar] [CrossRef]
- Zhang, X.; Kucine, A.; Ramamurthy, N.; McClain, S.; Ryan, M.; McNamara, T.; Golub, L. Chemically modified tetracycline (CMT-6) applied topically enhances diabetic wound healing. J. Dent. Res. 1996, 75, 723. [Google Scholar]
- Seftor, R.E.; Seftor, E.A.; De Larco, J.E.; Kleiner, D.E.; Leferson, J.; Stetler-Stevenson, W.G.; McNamara, T.F.; Golub, L.M.; Hendrix, M.J. Chemically modified tetracyclines inhibit human melanoma cell invasion and metastasis. Clin. Exp. Metastasis 1998, 16, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.E.; Usman, A.; Ramamurthy, N.; Golub, L.M.; Greenwald, R.A. Excessive matrix metalloproteinase activity in diabetes: Inhibition by tetracycline analogues with zinc reactivity. Curr. Med. Chem. 2001, 8, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Ramamurthy, N.; McNamara, T.F.; Greenwald, R.A.; Rifkin, B.R. Tetracyclines inhibit connective tissue breakdown: New therapeutic implications for an old family of drugs. Crit. Rev. Oral. Biol. Med. 1991, 2, 297–321. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Comparative study of doxycycline, sancycline, and 4-dedimethylamino sancycline (CMT-3) on epidermal melanogenesis. Arch. Dermatol. Res. 2021, 315, 249–257. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Cmt-308, a nonantimicrobial chemically-modified tetracycline, exhibits anti-melanogenic activity by suppression of melanosome export. Biomedicines 2020, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.; Sorsa, T.; Uitto, V.-J.; Salo, T.; Teronen, O.; Larjava, H. The effects of chemically modified tetracyclines (CMTs) on human keratinocyte proliferation and migration. Adv. Dent. Res. 1998, 12, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Rzepka, Z.; Respondek, M.; Beberok, A.; Wrześniok, D. Chlortetracycline and melanin biopolymer–The risk of accumulation and implications for phototoxicity: An in vitro study on normal human melanocytes. Chem.-Biol. Interact. 2019, 303, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ramamurthy, S.; Marecek, J.; Lee, M.; Chen, L.; Ryan, E.; Rifkin, R.; Golub, M. The lipophilicity, pharmacokinetics, and cellular uptake of different chemically-modified tetracyclines (CMTs). Curr. Med. Chem. 2001, 8, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S. Effects of a standardized hydrogenated extract of curcumin (curowhite™) on melanogenesis: A pilot study. Nutraceuticals 2023, 3, 421–437. [Google Scholar] [CrossRef]
- Oliveira, K.B.; Palú, É.; Weffort-Santos, A.M.; Oliveira, B.H. Influence of rosmarinic acid and Salvia officinalis extracts on melanogenesis of B16F10 cells. Rev. Bras. Farmacogn. 2013, 23, 249–258. [Google Scholar] [CrossRef]
- Liu, Y.; Ryan, M.E.; Lee, H.M.; Simon, S.; Tortora, G.; Lauzon, C.; Leung, M.K.; Golub, L.M. A chemically modified tetracycline (CMT-3) is a new antifungal agent. Antimicrob. Agents Chemother. 2002, 46, 1447–1454. [Google Scholar] [CrossRef]
- Wang, W.Q.; Wu, J.F.; Xiao, X.Q.; Xiao, Q.; Wang, J.; Zuo, F.G. Narrow-band UVB radiation promotes dendrite formation by activating Rac1 in B16 melanoma cells. Mol. Clin. Oncol. 2013, 1, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Aimvijarn, P.; Payuhakrit, W.; Charoenchon, N.; Okada, S.; Suwannalert, P. Riceberry rice germination and UVB radiation enhance protocatechuic acid and vanillic acid to reduce cellular oxidative stress and suppress B16F10 melanogenesis relating to f-actin rearrangement. Plants 2023, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Aspengren, S.; Norström, E.; Wallin, M. Effects of hydroquinone on cytoskeletal organization and intracellular transport in cultured Xenopus laevis melanophores and fibroblasts. Int. Sch. Res. Netw. 2012, 2012, 524781. [Google Scholar] [CrossRef]
- Rifkin, B.R.; Vernillo, A.T.; Golub, L.M.; Ramamurthy, N.S. Modulation of Bone Resorption by Tetracyclines a. Ann. N. Y. Acad. Sci. 1994, 732, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Karg, E.; Rosengren, E.; Rorsman, H. Stimulation of tyrosinase by dihydroxy phenyl derivatives. Acta Derm.-Venereol. 1989, 69, 521–524. [Google Scholar]
- Brown, D.A.; Ren, W.-Y.; Khorlin, A.; Lesiak, K.; Conklin, D.; Watanabe, K.A.; Seidman, M.M.; George, J. Aliphatic and alicyclic diols induce melanogenesis in cultured cells and guinea pig skin. J. Investig. Dermatol. 1998, 110, 428–437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Motokawa, T.; Miwa, T.; Mochizuki, M.; Toritsuka, M.; Sakata, A.; Ito, M. Adrenomedullin: A novel melanocyte dendrite branching factor. J. Dermatol. Sci. 2015, 79, 307–310. [Google Scholar] [CrossRef]
- Hara, M.; Yaar, M.; Gilchrest, B.A. Endothelin-1 of keratinocyte origin is a mediator of melanocyte dendricity. J. Investig. Dermatol. 1995, 105, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Novel Chemically Modified Curcumin (CMC) analogs exhibit anti-melanogenic activity in primary human melanocytes. Int. J. Mol. Sci. 2021, 22, 6043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, Y.; Lee, H.-M.; Hambardjieva, E.; Vranková, K.; Golub, L.M.; Johnson, F. Design, synthesis and biological activity of new polyenolic inhibitors of matrix metalloproteinases: A focus on chemically-modified curcumins. Curr. Med. Chem. 2012, 19, 4348–4358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Golub, L.M.; Johnson, F.; Wishnia, A. pKa, zinc-and serum albumin-binding of curcumin and two novel biologically-active chemically-modified curcumins. Curr. Med. Chem. 2012, 19, 4367–4375. [Google Scholar] [CrossRef]
- Tang, A.; Eller, M.S.; Hara, M.; Yaar, M.; Hirohashi, S.; Gilchrest, B.A. E-cadherin is the major mediator of human melanocyte adhesion to keratinocytes in vitro. J. Cell Sci. 1994, 107, 983–992. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Meier, F.E.; Nesbit, M.; Hsu, J.-Y.; Van Belle, P.; Elder, D.E.; Herlyn, M. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am. J. Pathol. 2000, 156, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.; Schneider, R.J. UV-induction of keratinocyte endothelin-1 downregulates E-cadherin in melanocytes and melanoma cells. J. Clin. Investig. 2002, 110, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Poser, I.; Domınguez, D.; de Herreros, A.G.; Varnai, A.; Buettner, R.; Bosserhoff, A.K. Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. J. Biol. Chem. 2001, 276, 24661–24666. [Google Scholar] [CrossRef]
- Inoue, D.; Narita, T.; Ishikawa, K.; Maeno, K.; Motoyama, A.; Ono, T.; Aoki, H.; Shibata, T. A mechanism of melanogenesis mediated by E-cadherin downregulation and its involvement in solar lentigines. Int. J. Cosmet. Sci. 2023. [Google Scholar] [CrossRef]
- Meng, Q.; Xu, J.; Goldberg, I.D.; Rosen, E.M.; Greenwald, R.A.; Fan, S. Influence of chemically modified tetracyclines on proliferation, invasion and migration properties of MDA-MB-468 human breast cancer cells. Clin. Exp. Metastasis 2000, 18, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Carsberg, C.J.; Jones, K.T.; Sharpe, G.R.; Friedmann, P.S. Intracellular calcium modulates the responses of human melanocytes to melanogenic stimuli. J. Dermatol. Sci. 1995, 9, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naser, M.B.; Seltmann, H.; Zouboulis, C.C. SZ95 sebocytes induce epidermal melanocyte dendricity and proliferation in vitro. Exp. Dermatol. 2012, 21, 393–395. [Google Scholar] [CrossRef]
- Kang, H.Y.; Kim, N.S.; Lee, C.O.; Lee, J.Y.; Kang, W.H. Expression and function of ryanodine receptors in human melanocytes. J. Cell. Physiol. 2000, 185, 200–206. [Google Scholar] [CrossRef]
- Joshi, P.G.; Nair, N.; Begum, G.; Joshi, N.B.; Sinkar, V.P.; Vora, S. Melanocyte-keratinocyte interaction induces calcium signalling and melanin transfer to keratinocytes. Pigment Cell Res. 2007, 20, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, L.-Y.; Fu, W.-W.; Yuan, J.; Sheng, Y.-Y.; Yang, Q.-P. Low-concentration hydrogen peroxide can upregulate keratinocyte intracellular calcium and PAR-2 expression in a human keratinocyte–melanocyte co-culture system. Arch. Dermatol. Res. 2016, 308, 723–731. [Google Scholar] [CrossRef] [PubMed]
- González-Lizárraga, F.; Ploper, D.; Ávila, C.L.; Socías, S.B.; dos-Santos-Pereira, M.; Machín, B.; Del-Bel, E.; Michel, P.P.; Pietrasanta, L.I.; Raisman-Vozari, R. CMT-3 targets different α-synuclein aggregates mitigating their toxic and inflammogenic effects. Sci. Rep. 2020, 10, 20258. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Grau, R.; González-Lizárraga, F.; Ploper, D.; Avila, C.L.; Socías, S.B.; Besnault, P.; Tourville, A.; Mella, R.M.; Villacé, P.; Salado, C. Neuroprotective effects of a novel demeclocycline derivative lacking antibiotic activity: From a hit to a promising lead compound. Cells 2022, 11, 2759. [Google Scholar] [CrossRef]
- Bissig, C.; Rochin, L.; Van Niel, G. PMEL amyloid fibril formation: The bright steps of pigmentation. Int. J. Mol. Sci. 2016, 17, 1438. [Google Scholar] [CrossRef] [PubMed]
- Watt, B.; van Niel, G.; Raposo, G.; Marks, M.S. PMEL: A pigment cell-specific model for functional amyloid formation. Pigment. Cell Melanoma Res. 2013, 26, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Watt, B.; Raposo, G.; Marks, M.S. Pmel17: An Amyloid Determinant of Organelle Structure. In Functional Amyloid Aggregation; Bucciantini, M., Ed.; Research Signpost: Trivandrum, India, 2010; pp. 89–113. [Google Scholar]
- Rochin, L.; Hurbain, I.; Serneels, L.; Fort, C.; Watt, B.; Leblanc, P.; Marks, M.S.; De Strooper, B.; Raposo, G.; Van Niel, G. BACE2 processes PMEL to form the melanosome amyloid matrix in pigment cells. Proc. Natl. Acad. Sci. USA 2013, 110, 10658–10663. [Google Scholar] [CrossRef] [PubMed]
- Theos, A.C.; Berson, J.F.; Theos, S.C.; Herman, K.E.; Harper, D.C.; Tenza, D.; Sviderskaya, E.V.; Lamoreux, M.L.; Bennett, D.C.; Raposo, G. Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17. Mol. Biol. Cell 2006, 17, 3598–3612. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Urabe, K.; Winder, A.; Jiménez-Cervantes, C.; Imokawa, G.; Brewington, T.; Solano, F.; García-Borrón, J.; Hearing, V. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994, 13, 5818–5825. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure of human tyrosinase related protein 1 reveals a binuclear zinc active site important for melanogenesis. Angew. Chem. Int. Ed. 2017, 56, 9812–9815. [Google Scholar] [CrossRef] [PubMed]
- Widlund, H.R.; Fisher, D.E. Microphthalamia-associated transcription factor: A critical regulator of pigment cell development and survival. Oncogene 2003, 22, 3035–3041. [Google Scholar] [CrossRef]
- Yi, X.; Zhao, G.; Zhang, H.; Guan, D.; Meng, R.; Zhang, Y.; Yang, Q.; Jia, H.; Dou, K.; Liu, C. MITF-siRNA formulation is a safe and effective therapy for human melasma. Mol. Ther. 2011, 19, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Baek, S.H.; Kim, D.H.; Choi, T.Y.; Yoon, T.J.; Hwang, J.S.; Kim, M.R.; Kwon, H.J.; Lee, C.H. Downregulation of melanin synthesis by haginin A and its application to in vivo lightening model. J. Investig. Dermatol. 2008, 128, 1227–1235. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Inhibitory effects of the bioactive thermorubin isolated from the fungus thermoactinomyces antibioticus on melanogenesis. Cosmetics 2020, 7, 61. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).