In Vitro Study of the Effects of Five Chemically Modified Tetracycline (CMT) Analogs on Human Epidermal Melanogenesis: Potential as Novel Anti-Melanogenic Agents
Abstract
1. Introduction
2. Results
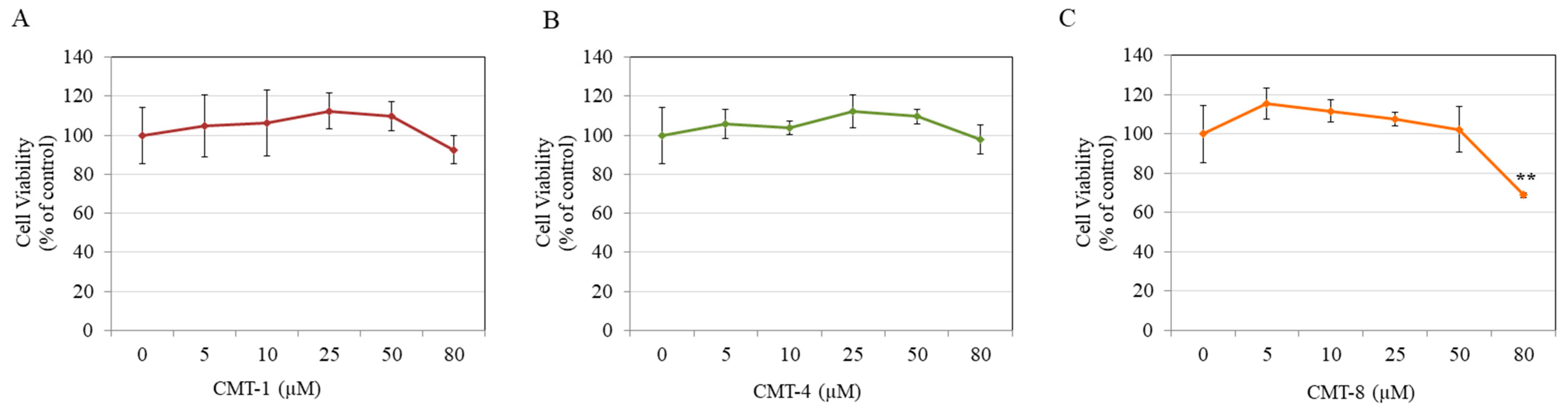
2.1. Cytotoxicity
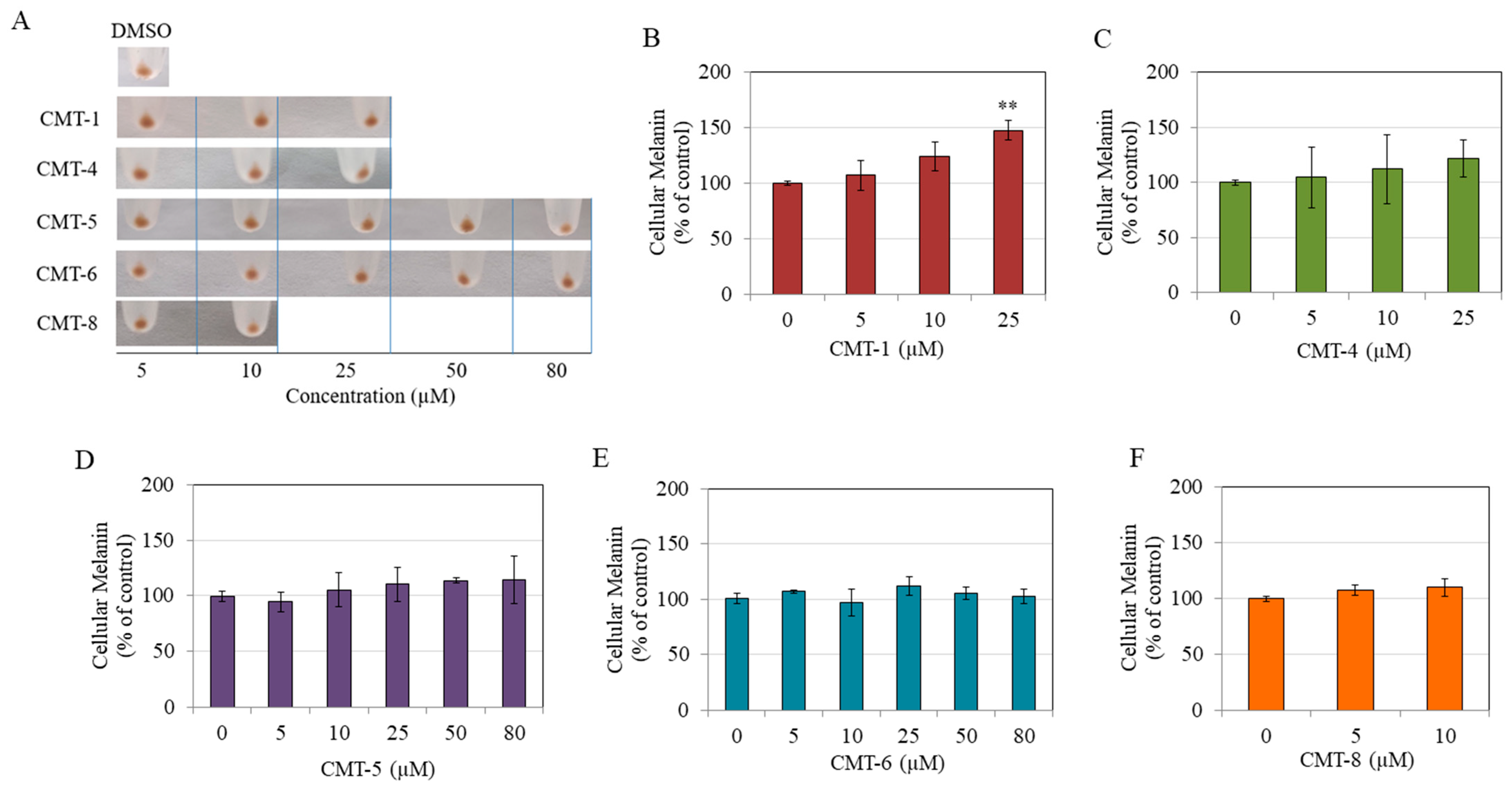
2.2. Effects on Intracellular Melanin
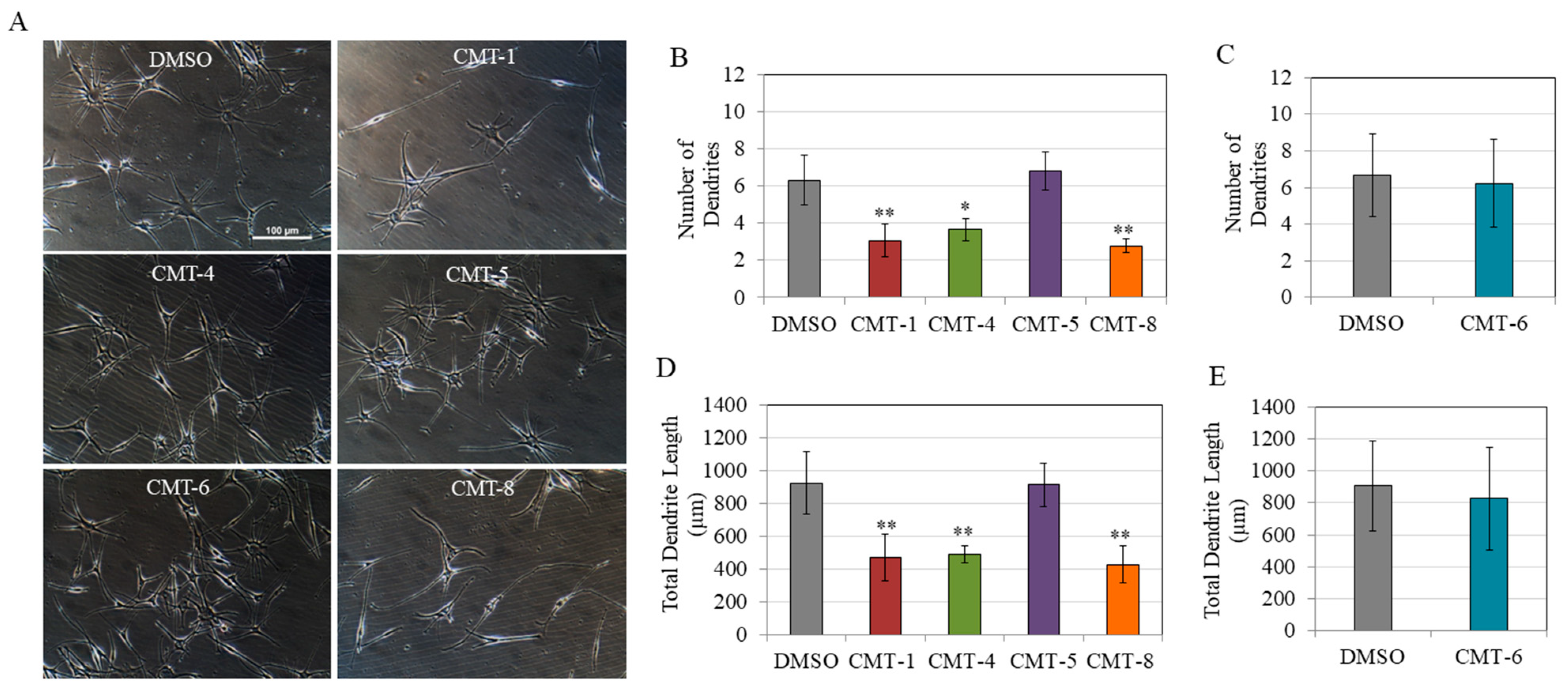
2.3. Melanocyte Dendricity
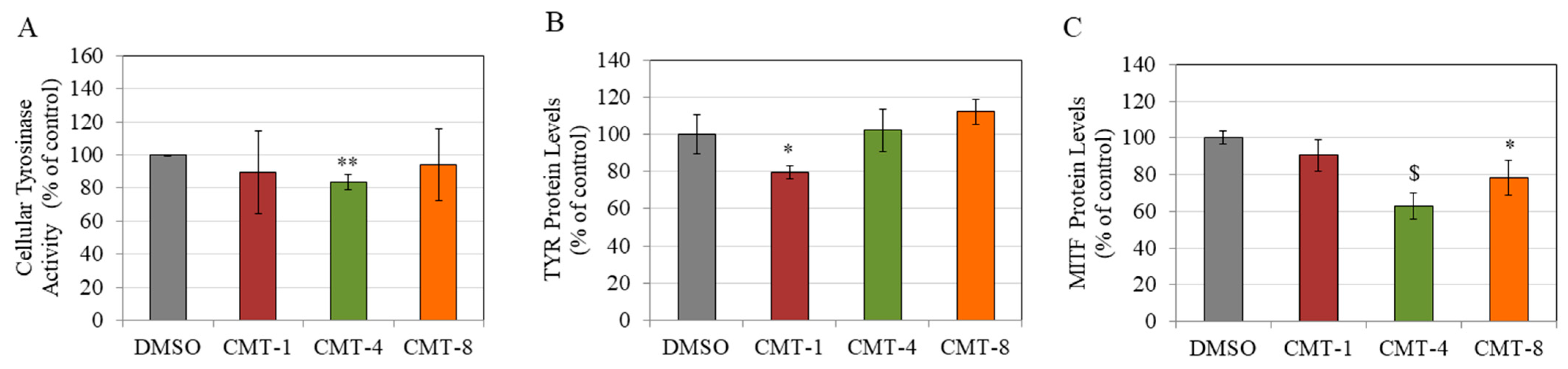
2.4. TYR Activity
2.5. TYR and MITF Protein Levels
2.6. Keratinocyte Viability
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Intracellular Melanin Assay
4.5. Quantitation of Dendricity
4.6. Intracellular TYR Activity
4.7. Protein Levels of TYR and MITF
4.8. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Hammer, J.A. Melanosome transfer: It is best to give and receive. Curr. Opin. Cell Biol. 2014, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Investig. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef]
- Park, H.; Kosmadaki, M.; Yaar, M.; Gilchrest, B. Cellular mechanisms regulating human melanogenesis. Cell. Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M. MITF: A stream flowing for pigment cells. Pigment Cell Res. 2000, 13, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Lacour, J.P.; Gordon, P.R.; Eller, M.; Bhawan, J.; Gilchrest, B.A. Cytoskeletal events underlying dendrite formation by cultured pigment cells. J. Cell. Physiol. 1992, 151, 287–299. [Google Scholar] [CrossRef]
- Kippenberger, S.; Bernd, A.; Bereiter-Hahn, J.; Ramirez-Bosca, A.; Kaufmann, R. The mechanism of melanocyte dendrite formation: The impact of differentiating keratinocytes. Pigment Cell Res. 1998, 11, 34–37. [Google Scholar] [CrossRef]
- Tada, A.; Kanamaru, A.; Ito, Y. Control of melanosome transfer by promoting shrinkage or expansion of melanocyte dendrites. Int. J. Cosmet. Sci. 2006, 28, 148. [Google Scholar] [CrossRef]
- Mattos, K.; Cintra, M.; Gouvêa, I.; Ferreira, L.; Velho, P.; Moriel, P. Skin hyperpigmentation following intravenous polymyxin B treatment associated with melanocyte activation and inflammatory process. J. Clin. Pharm. Ther. 2017, 42, 573–578. [Google Scholar] [CrossRef]
- Espósito, A.C.C.; Cassiano, D.P.; da Silva, C.N.; Lima, P.B.; Dias, J.A.; Hassun, K.; Bagatin, E.; Miot, L.D.; Miot, H.A. Update on Melasma—Part I: Pathogenesis. Dermatol. Ther. 2022, 12, 1967–1988. [Google Scholar] [CrossRef]
- Grimes, P.E.; Yamada, N.; Bhawan, J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am. J. Dermatopathol. 2005, 27, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Adalatkhah, H.; Bazargani, H.S. The Association Between Melasma and Postinflammatory Hyperpigmentation in Acne Patients. Iran. Red. Crescent Med. J. 2013, 15, 400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomita, Y.; MAEDA, K.; TAGAMI, H. Melanocyte-stimulating properties of arachidonic acid metabolites: Possible role in postinflammatory pigmentation. Pigment Cell Res. 1992, 5, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Masson Regnault, M.; Gadaud, N.; Boulinguez, S.; Tournier, E.; Lamant, L.; Gladieff, L.; Roche, H.; Guenounou, S.; Recher, C.; Sibaud, V. Chemotherapy-related reticulate hyperpigmentation: A case series and review of the literature. Dermatology 2015, 231, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.; Sanz, C.; Pérez-Fidalgo, J.A.; Pérez-Leal, M.; Milara, J.; Cortijo, J. Paclitaxel alters melanogenesis and causes pigmentation in the skin of gynecological cancer patients. Fundam. Clin. Pharmacol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Kanavy, H.E.; Gerstenblith, M.R. Ultraviolet radiation and melanoma. In Seminars in Cutaneous Medicine and Surgery; WB Saunders: Philadelphia, PA, USA, 2011; pp. 222–228. [Google Scholar]
- Gilchrest, B.A.; Park, H.-Y.; Eller, M.S.; Yaar, M. Mechanisms of ultraviolet light-induced pigmentation. Photochem. Photobiol. 1996, 63, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.H. Postinflammatory hyperpigmentation. Clin. Dermatol. 1989, 7, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Inomata, S.; Takada, K.; Tsunenaga, M.; Fukuda, M.; Matsunaga, Y.; Amano, S.; Kobayashi, K.; Nishiyama, T.; Kohno, Y. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Investig. Dermatol. 2003, 120, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, V.N.; Verma, P.; Srivastava, G.; Aggarwal, A.K.; Verma, S. Melasma: Treatment strategy. J. Cosmet. Laser Ther. 2011, 13, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.F.; Lee, T.L.; Oyerinde, O.; Desai, S.R.; Aljabban, A.; Bay, C.P.; Bain, P.A.; Chung, H.J. Efficacy and safety of topical agents in the treatment of melasma: What’s evidence? A systematic review and meta-analysis. J. Cosmet. Dermatol. 2023, 22, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Charoo, N.A. Hyperpigmentation: Looking beyond hydroquinone. J. Cosmet. Dermatol. 2022, 21, 4133–4145. [Google Scholar] [CrossRef]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef]
- Golub, L.M.; McNamara, T.F.; D’Angelo, G.; Greenwald, R.A.; Ramamurthy, N.S. A non-antibacterial chemically-modified tetracycline inhibits mammalian collagenase activity. J. Dent. Res. 1987, 66, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Trachtman, H.; Futterweit, S.; Greenwald, R.; Moak, S.; Singhal, P.; Franki, N.; Amin, A.R. Chemically modified tetracyclines inhibit inducible nitric oxide synthase expression and nitric oxide production in cultured rat mesangial cells. Biochem. Biophys. Res. Commun. 1996, 229, 243–248. [Google Scholar] [CrossRef]
- Sasaki, T.; Ohyori, N.; Debari, K.; Ramamurthy, N.S.; Golub, L.M. Effects of chemically modified tetracycline, CMT-8, on bone loss and osteoclast structure and function in osteoporotic states. Ann. N. Y. Acad. Sci. 1999, 878, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, N.S.; Rifkin, B.R.; Greenwald, R.A.; Xu, J.w.; Liu, Y.; Turner, G.; Golub, L.M.; Vernillo, A.T. Inhibition of matrix metalloproteinase-mediated periodontal bone loss in rats: A comparison of 6 chemically modified tetracyclines. J. Periodontol. 2002, 73, 726–734. [Google Scholar] [CrossRef]
- Salo, T.; Soini, Y.; Oiva, J.; Nissinen, A.; Biancari, F.; Juvonen, T.; Satta, J. Chemically modified tetracyclines (CMT-3 and CMT-8) enable control of the pathologic remodellation of human aortic valve stenosis via MMP-9 and VEGF inhibition. Int. J. Cardiol. 2006, 111, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Sainz, Á.; García-Sancho, M.; Rodríguez-Franco, F.; San Andrés, M.I.; Rodríguez, C.; de Lucas, J.J.; San Andrés, M.D.; Agulla, B.; Villaescusa, A. Effect of chemically modified tetracycline-8 (CMT-8) on hematology, blood chemistry, cytokines and peripheral blood lymphocyte subsets of healthy dogs. Res. Vet. Sci. 2021, 136, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.; Golub, L.; Ramamurthy, N.; Chowdhury, M.; Moak, S.; Sorsa, T. In vitro sensitivity of the three mammalian collagenases to tetracycline inhibition: Relationship to bone and cartilage degradation. Bone 1998, 22, 33–38. [Google Scholar] [CrossRef]
- Zhang, X.; Kucine, A.; Ramamurthy, N.; McClain, S.; Ryan, M.; McNamara, T.; Golub, L. Chemically modified tetracycline (CMT-6) applied topically enhances diabetic wound healing. J. Dent. Res. 1996, 75, 723. [Google Scholar]
- Seftor, R.E.; Seftor, E.A.; De Larco, J.E.; Kleiner, D.E.; Leferson, J.; Stetler-Stevenson, W.G.; McNamara, T.F.; Golub, L.M.; Hendrix, M.J. Chemically modified tetracyclines inhibit human melanoma cell invasion and metastasis. Clin. Exp. Metastasis 1998, 16, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.E.; Usman, A.; Ramamurthy, N.; Golub, L.M.; Greenwald, R.A. Excessive matrix metalloproteinase activity in diabetes: Inhibition by tetracycline analogues with zinc reactivity. Curr. Med. Chem. 2001, 8, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Ramamurthy, N.; McNamara, T.F.; Greenwald, R.A.; Rifkin, B.R. Tetracyclines inhibit connective tissue breakdown: New therapeutic implications for an old family of drugs. Crit. Rev. Oral. Biol. Med. 1991, 2, 297–321. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Comparative study of doxycycline, sancycline, and 4-dedimethylamino sancycline (CMT-3) on epidermal melanogenesis. Arch. Dermatol. Res. 2021, 315, 249–257. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Cmt-308, a nonantimicrobial chemically-modified tetracycline, exhibits anti-melanogenic activity by suppression of melanosome export. Biomedicines 2020, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.; Sorsa, T.; Uitto, V.-J.; Salo, T.; Teronen, O.; Larjava, H. The effects of chemically modified tetracyclines (CMTs) on human keratinocyte proliferation and migration. Adv. Dent. Res. 1998, 12, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Rok, J.; Rzepka, Z.; Respondek, M.; Beberok, A.; Wrześniok, D. Chlortetracycline and melanin biopolymer–The risk of accumulation and implications for phototoxicity: An in vitro study on normal human melanocytes. Chem.-Biol. Interact. 2019, 303, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ramamurthy, S.; Marecek, J.; Lee, M.; Chen, L.; Ryan, E.; Rifkin, R.; Golub, M. The lipophilicity, pharmacokinetics, and cellular uptake of different chemically-modified tetracyclines (CMTs). Curr. Med. Chem. 2001, 8, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S. Effects of a standardized hydrogenated extract of curcumin (curowhite™) on melanogenesis: A pilot study. Nutraceuticals 2023, 3, 421–437. [Google Scholar] [CrossRef]
- Oliveira, K.B.; Palú, É.; Weffort-Santos, A.M.; Oliveira, B.H. Influence of rosmarinic acid and Salvia officinalis extracts on melanogenesis of B16F10 cells. Rev. Bras. Farmacogn. 2013, 23, 249–258. [Google Scholar] [CrossRef]
- Liu, Y.; Ryan, M.E.; Lee, H.M.; Simon, S.; Tortora, G.; Lauzon, C.; Leung, M.K.; Golub, L.M. A chemically modified tetracycline (CMT-3) is a new antifungal agent. Antimicrob. Agents Chemother. 2002, 46, 1447–1454. [Google Scholar] [CrossRef]
- Wang, W.Q.; Wu, J.F.; Xiao, X.Q.; Xiao, Q.; Wang, J.; Zuo, F.G. Narrow-band UVB radiation promotes dendrite formation by activating Rac1 in B16 melanoma cells. Mol. Clin. Oncol. 2013, 1, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Aimvijarn, P.; Payuhakrit, W.; Charoenchon, N.; Okada, S.; Suwannalert, P. Riceberry rice germination and UVB radiation enhance protocatechuic acid and vanillic acid to reduce cellular oxidative stress and suppress B16F10 melanogenesis relating to f-actin rearrangement. Plants 2023, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Aspengren, S.; Norström, E.; Wallin, M. Effects of hydroquinone on cytoskeletal organization and intracellular transport in cultured Xenopus laevis melanophores and fibroblasts. Int. Sch. Res. Netw. 2012, 2012, 524781. [Google Scholar] [CrossRef]
- Rifkin, B.R.; Vernillo, A.T.; Golub, L.M.; Ramamurthy, N.S. Modulation of Bone Resorption by Tetracyclines a. Ann. N. Y. Acad. Sci. 1994, 732, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Karg, E.; Rosengren, E.; Rorsman, H. Stimulation of tyrosinase by dihydroxy phenyl derivatives. Acta Derm.-Venereol. 1989, 69, 521–524. [Google Scholar]
- Brown, D.A.; Ren, W.-Y.; Khorlin, A.; Lesiak, K.; Conklin, D.; Watanabe, K.A.; Seidman, M.M.; George, J. Aliphatic and alicyclic diols induce melanogenesis in cultured cells and guinea pig skin. J. Investig. Dermatol. 1998, 110, 428–437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Motokawa, T.; Miwa, T.; Mochizuki, M.; Toritsuka, M.; Sakata, A.; Ito, M. Adrenomedullin: A novel melanocyte dendrite branching factor. J. Dermatol. Sci. 2015, 79, 307–310. [Google Scholar] [CrossRef]
- Hara, M.; Yaar, M.; Gilchrest, B.A. Endothelin-1 of keratinocyte origin is a mediator of melanocyte dendricity. J. Investig. Dermatol. 1995, 105, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Novel Chemically Modified Curcumin (CMC) analogs exhibit anti-melanogenic activity in primary human melanocytes. Int. J. Mol. Sci. 2021, 22, 6043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, Y.; Lee, H.-M.; Hambardjieva, E.; Vranková, K.; Golub, L.M.; Johnson, F. Design, synthesis and biological activity of new polyenolic inhibitors of matrix metalloproteinases: A focus on chemically-modified curcumins. Curr. Med. Chem. 2012, 19, 4348–4358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Golub, L.M.; Johnson, F.; Wishnia, A. pKa, zinc-and serum albumin-binding of curcumin and two novel biologically-active chemically-modified curcumins. Curr. Med. Chem. 2012, 19, 4367–4375. [Google Scholar] [CrossRef]
- Tang, A.; Eller, M.S.; Hara, M.; Yaar, M.; Hirohashi, S.; Gilchrest, B.A. E-cadherin is the major mediator of human melanocyte adhesion to keratinocytes in vitro. J. Cell Sci. 1994, 107, 983–992. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Meier, F.E.; Nesbit, M.; Hsu, J.-Y.; Van Belle, P.; Elder, D.E.; Herlyn, M. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am. J. Pathol. 2000, 156, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.; Schneider, R.J. UV-induction of keratinocyte endothelin-1 downregulates E-cadherin in melanocytes and melanoma cells. J. Clin. Investig. 2002, 110, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Poser, I.; Domınguez, D.; de Herreros, A.G.; Varnai, A.; Buettner, R.; Bosserhoff, A.K. Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. J. Biol. Chem. 2001, 276, 24661–24666. [Google Scholar] [CrossRef]
- Inoue, D.; Narita, T.; Ishikawa, K.; Maeno, K.; Motoyama, A.; Ono, T.; Aoki, H.; Shibata, T. A mechanism of melanogenesis mediated by E-cadherin downregulation and its involvement in solar lentigines. Int. J. Cosmet. Sci. 2023. [Google Scholar] [CrossRef]
- Meng, Q.; Xu, J.; Goldberg, I.D.; Rosen, E.M.; Greenwald, R.A.; Fan, S. Influence of chemically modified tetracyclines on proliferation, invasion and migration properties of MDA-MB-468 human breast cancer cells. Clin. Exp. Metastasis 2000, 18, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Carsberg, C.J.; Jones, K.T.; Sharpe, G.R.; Friedmann, P.S. Intracellular calcium modulates the responses of human melanocytes to melanogenic stimuli. J. Dermatol. Sci. 1995, 9, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naser, M.B.; Seltmann, H.; Zouboulis, C.C. SZ95 sebocytes induce epidermal melanocyte dendricity and proliferation in vitro. Exp. Dermatol. 2012, 21, 393–395. [Google Scholar] [CrossRef]
- Kang, H.Y.; Kim, N.S.; Lee, C.O.; Lee, J.Y.; Kang, W.H. Expression and function of ryanodine receptors in human melanocytes. J. Cell. Physiol. 2000, 185, 200–206. [Google Scholar] [CrossRef]
- Joshi, P.G.; Nair, N.; Begum, G.; Joshi, N.B.; Sinkar, V.P.; Vora, S. Melanocyte-keratinocyte interaction induces calcium signalling and melanin transfer to keratinocytes. Pigment Cell Res. 2007, 20, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, L.-Y.; Fu, W.-W.; Yuan, J.; Sheng, Y.-Y.; Yang, Q.-P. Low-concentration hydrogen peroxide can upregulate keratinocyte intracellular calcium and PAR-2 expression in a human keratinocyte–melanocyte co-culture system. Arch. Dermatol. Res. 2016, 308, 723–731. [Google Scholar] [CrossRef] [PubMed]
- González-Lizárraga, F.; Ploper, D.; Ávila, C.L.; Socías, S.B.; dos-Santos-Pereira, M.; Machín, B.; Del-Bel, E.; Michel, P.P.; Pietrasanta, L.I.; Raisman-Vozari, R. CMT-3 targets different α-synuclein aggregates mitigating their toxic and inflammogenic effects. Sci. Rep. 2020, 10, 20258. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Grau, R.; González-Lizárraga, F.; Ploper, D.; Avila, C.L.; Socías, S.B.; Besnault, P.; Tourville, A.; Mella, R.M.; Villacé, P.; Salado, C. Neuroprotective effects of a novel demeclocycline derivative lacking antibiotic activity: From a hit to a promising lead compound. Cells 2022, 11, 2759. [Google Scholar] [CrossRef]
- Bissig, C.; Rochin, L.; Van Niel, G. PMEL amyloid fibril formation: The bright steps of pigmentation. Int. J. Mol. Sci. 2016, 17, 1438. [Google Scholar] [CrossRef] [PubMed]
- Watt, B.; van Niel, G.; Raposo, G.; Marks, M.S. PMEL: A pigment cell-specific model for functional amyloid formation. Pigment. Cell Melanoma Res. 2013, 26, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Watt, B.; Raposo, G.; Marks, M.S. Pmel17: An Amyloid Determinant of Organelle Structure. In Functional Amyloid Aggregation; Bucciantini, M., Ed.; Research Signpost: Trivandrum, India, 2010; pp. 89–113. [Google Scholar]
- Rochin, L.; Hurbain, I.; Serneels, L.; Fort, C.; Watt, B.; Leblanc, P.; Marks, M.S.; De Strooper, B.; Raposo, G.; Van Niel, G. BACE2 processes PMEL to form the melanosome amyloid matrix in pigment cells. Proc. Natl. Acad. Sci. USA 2013, 110, 10658–10663. [Google Scholar] [CrossRef] [PubMed]
- Theos, A.C.; Berson, J.F.; Theos, S.C.; Herman, K.E.; Harper, D.C.; Tenza, D.; Sviderskaya, E.V.; Lamoreux, M.L.; Bennett, D.C.; Raposo, G. Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17. Mol. Biol. Cell 2006, 17, 3598–3612. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Urabe, K.; Winder, A.; Jiménez-Cervantes, C.; Imokawa, G.; Brewington, T.; Solano, F.; García-Borrón, J.; Hearing, V. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994, 13, 5818–5825. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure of human tyrosinase related protein 1 reveals a binuclear zinc active site important for melanogenesis. Angew. Chem. Int. Ed. 2017, 56, 9812–9815. [Google Scholar] [CrossRef] [PubMed]
- Widlund, H.R.; Fisher, D.E. Microphthalamia-associated transcription factor: A critical regulator of pigment cell development and survival. Oncogene 2003, 22, 3035–3041. [Google Scholar] [CrossRef]
- Yi, X.; Zhao, G.; Zhang, H.; Guan, D.; Meng, R.; Zhang, Y.; Yang, Q.; Jia, H.; Dou, K.; Liu, C. MITF-siRNA formulation is a safe and effective therapy for human melasma. Mol. Ther. 2011, 19, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Baek, S.H.; Kim, D.H.; Choi, T.Y.; Yoon, T.J.; Hwang, J.S.; Kim, M.R.; Kwon, H.J.; Lee, C.H. Downregulation of melanin synthesis by haginin A and its application to in vivo lightening model. J. Investig. Dermatol. 2008, 128, 1227–1235. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Inhibitory effects of the bioactive thermorubin isolated from the fungus thermoactinomyces antibioticus on melanogenesis. Cosmetics 2020, 7, 61. [Google Scholar] [CrossRef]


| Parameter | CMT-1 | CMT-4 | CMT-8 | CMT-5 | CMT-3 [35] | CMT-308 [36] |
|---|---|---|---|---|---|---|
| Melanin Content | × | × | × | × | × | × |
| TYR Activity | × | ↓ 16.60% | × | N.D. | ↓ 66% | × |
| TYR Protein | ↓ 20.45% | × | × | × | × | N.D. |
| MITF Protein | × | ↓ 37.07% | ↓ 21.71% | × | × | N.D. |
| Dendrite number | ↓ 50.67% | ↓ 53.22% | ↓ 46.28% | × | ↓ 47.63% | ↓ 43.80% |
| Total Dendrite Length | ↓ 51.37% | ↓ 42.09% | ↓ 55.85% | × | ↓ 46.20% | ↓ 46.74% |
| Recovery of Dendricity | N.D. | N.D. | N.D. | N.A. | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S.; Golub, L.M. In Vitro Study of the Effects of Five Chemically Modified Tetracycline (CMT) Analogs on Human Epidermal Melanogenesis: Potential as Novel Anti-Melanogenic Agents. Drugs Drug Candidates 2023, 2, 810-826. https://doi.org/10.3390/ddc2040041
Goenka S, Golub LM. In Vitro Study of the Effects of Five Chemically Modified Tetracycline (CMT) Analogs on Human Epidermal Melanogenesis: Potential as Novel Anti-Melanogenic Agents. Drugs and Drug Candidates. 2023; 2(4):810-826. https://doi.org/10.3390/ddc2040041
Chicago/Turabian StyleGoenka, Shilpi, and Lorne M. Golub. 2023. "In Vitro Study of the Effects of Five Chemically Modified Tetracycline (CMT) Analogs on Human Epidermal Melanogenesis: Potential as Novel Anti-Melanogenic Agents" Drugs and Drug Candidates 2, no. 4: 810-826. https://doi.org/10.3390/ddc2040041
APA StyleGoenka, S., & Golub, L. M. (2023). In Vitro Study of the Effects of Five Chemically Modified Tetracycline (CMT) Analogs on Human Epidermal Melanogenesis: Potential as Novel Anti-Melanogenic Agents. Drugs and Drug Candidates, 2(4), 810-826. https://doi.org/10.3390/ddc2040041






