Abstract
We reviewed the patentability of rosemary-derived drugs and bioactive compounds over the last 20 years and analyzed patent documents related to the rosemary (Rosmarinus officinalis Linné) plant using patent analysis techniques. A total of 12,320 patent documents (patent applications and granted patents) were identified, with China having the highest number of patent documents at 4384. The year 2017 had the most patent documents (1122). Patent classification codes indicate that most inventions are for medicinal preparations and cosmetics characterized by their composition. Further, expert driving forces and knowledge clusters showed that research and development has focused on methods to bring pharmaceutical products into specific physical or administering forms, which are described in the majority of patents. To demonstrate the innovation trends in rosemary-derived drugs and bioactive compounds, a selection of relevant patent documents, with publication dates between 2002 and 2022, is described at the end of the study. This selection contains a total of 13 patent documents, including six patent applications and seven granted patents, and deals with pharmaceutical and biomedical applications, processes for extracting rosemary-derived biomolecules (e.g., rosmarinic acid, carnosic acid, and carnosol), and cosmetic and food applications.
1. Introduction
Medicinal plants are widely employed in the treatment of human and animal diseases all over the world. Based on their ethnopharmacological uses and applications, the majority of current medications are developed from isolated compounds of medicinal plants [1]. Rosemary, one of these medicinal plants, is utilized in medicine due to its analgesic and antibacterial properties. Additionally, it serves as an antioxidant, carminative, and analgesic for muscles and joints, and is employed for the treatment of minor wounds, rashes, headaches, and circulation problems [2,3,4,5,6]. Furthermore, an ethanolic extract of rosemary has been shown to have antidiabetic activity [7]. Regarding other health pathologies, rosemary has revealed its protective action against types of cancer and cardiovascular diseases [8,9].
Historically, botanists identified the species of rosemary as Rosmarinus hortensis angustiore folium. However, in 1753, Carl Linnaeus (or Carl von Linné) formally classified it as Rosmarinus officinalis. As a result, Rosmarinus officinalis Linné (L.) has been widely accepted as a synonym for this plant species [10]. Rosmarinus officinalis L. (R. officinalis) is a fragrant, evergreen, perennial shrub with needle-like leaves that can be found growing wild in temperate regions of the Mediterranean such as Morocco, Spain, Portugal, Turkey, and others [11]. It is a versatile herb used both in culinary and medicinal practices, and its flowers, stems, and leaves can be extracted to produce essential oils, aromas, and fragrances [12,13,14]. The most commonly used extraction methods to obtain the bioactive compounds from R. officinalis are distillation, hydrodistillation, and supercritical fluid extraction [15]. Figure 1 shows R. officinalis’s broad properties regarding parts, extraction methods, bioactive compounds, and applications, as well as some examples of flowers and leaves of rosemary, dried rosemary leaves, and rosemary oil.
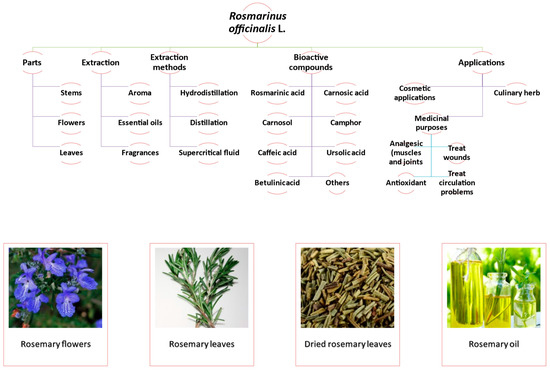
Figure 1.
Properties of R. officinalis with respect to plant parts, extraction methods, bioactive compounds, and applications. Frames show examples of flowers [16] and leaves [17] of rosemary, as well as dried rosemary leaves [18] and rosemary oil [19]. Photos in frames were obtained from https://pixabay.com, (accessed on 10 March 2023) with no attribution required, and free use under a Pixabay license.
Rosemary contains carnosic acid and carnosol as the main antioxidant components [20,21]. In addition, this medicinal plant is rich in rosmarinic acid, which is a potent radical scavenger and antioxidant [22,23]. Accordingly, the antioxidants in rosemary are widely recognized and have received European Union approval for use in food. This includes the food additive E-392, also known as extract of rosemary. In this respect, it is used as a food preservative to prevent oxidation and spoilage, particularly in oils and fats, and it is considered safe for consumption by regulatory agencies (i.e., European Union directives 2010/67/EU and 2010/69/EU) [24]. Moreover, rosemary extracts are generally recognized as safe for human consumption by the United States Food and Drug Administration. Therefore, they are allowable as excipients in the Welchol® chewable bar formulation which is an oral drug delivery system to deliver a bile acid sequestrant (i.e., a dosage form of colesevelam hydrochloride) [25,26].
The first patent application concerning R. officinalis was filed in 1897 and then published in 1898 [27]. Through this application, Feeny invented a process of hand-coloring photographs that involved the use of a mixture of different plant extracts, among them rosemary oil [27]. This application was never patented, and since then a number of other applications have been published without a patent agreement. The first patent relating to R. officinalis was not issued until 1917 [28]. Through this first granted patent, which was filed in 1916, Moore proposed a hair wash and restorer formula. The formula comprised a mixture of soap and different oil plant extracts, among which was rosemary oil [28].
The field of medicinal plant-based research, especially R. officinalis-based research, is rapidly advancing, with a focus on the development of natural products and drugs. Research, development, and innovation in raw material sourcing, extract preparation processes, methods for extraction, formulations, production technology, and applications are contributing to this progress [29]. This is supported by the increasing number of patent applications filed each year worldwide in this area of R. officinalis research and development. Notably, approximately 94% of patent applications in this field have been filed within the past two decades [30]. Presently, over 500 organizations are engaged in patent activity and filing for rosemary-based products [31].
This study, presented as a patentability review and analysis, employed various techniques to examine the information contained in and related to patents, with a focus on R. officinalis applications. It outlines the current state of patented inventions in this field, providing valuable information for researchers in the domains of medicinal plants and medication development, as well as those interested in natural products. Furthermore, detailed sections are provided related to patent families, publication dates, jurisdictions, inventors, applicants, owners, and patent classifications. Finally, a selection of relevant patent applications and granted patents during the last 20 years is discussed to demonstrate the innovation trends in rosemary-derived drugs and bioactive compounds. This is examined by considering what has been patented in terms of extraction methods, processes, formulation of biomolecules, and applications.
2. Resources and Research Methods
In this study, four databases were utilized, including the “Patentscope search service” from the World Intellectual Property Organization (WIPO), the “Espacenet patent search” from the European Patent Office (EPO), the “PatFT/AppFT databases” from the United States Patent and Trademark Office (USPTO), and the “Lens patent data set” from the Cambia Institute [31,32,33,34]. Different keywords, such as rosemary, Rosmarinus officinalis, rosmarinic acid, carnosol, and carnosic acid, were utilized, and patents were searched by title, abstract, and claim [35,36,37]. To include more matches, a stemmed search was disabled (e.g., searching for “rosmarinus” or “rosmarinic” stemmed to the root word “rosmarin”). The search was then filtered to include only patent documents for the period between 2002 and 2022.
In this study, only patent applications and granted patents with publication dates between 2002 and 2022 is considered for analysis to highlight the 20-year study of the patentability of rosemary-derived drugs and bioactive compounds. According to standard recommendations for efficient patent analysis concerning R. officinalis, a schematic tree of the steps is shown in Figure 2 [35,36,37].
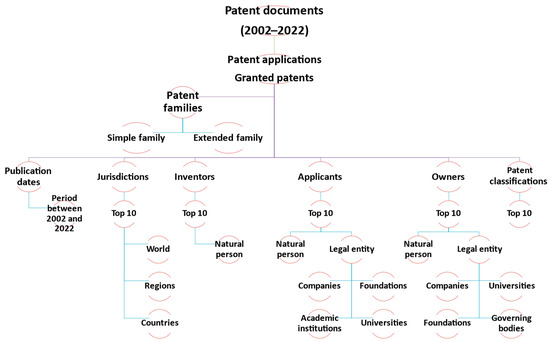
Figure 2.
Schematic tree of patent analysis concerning R. officinalis.
3. Analysis of Patent Documents
During a search of patents from 1897 to 2022, 14,062 patent documents were found. However, it should be noted that not only granted patents are published in patent databases. Thus, different documents for rosemary-related patents are available. Generally, these encompass patent applications (11,070), granted patents (2412), limited patents (303), other patent documents (100), search reports (95), patents of addition (55), amended applications (20), and amended patents (7).
Through the four databases, 12,320 patent documents (i.e., patent applications and granted patents) were found. The identified patent documents in relation to R. officinalis were divided into two categories: 10,360 patent applications and 1960 granted patents during the last 20 years.
Next, we will review the state of the art by examining what has been patented related to R. officinalis. A detailed analysis of the patentability is provided according to raw materials, extraction methods, processes, formulations, and applications, following the patent families, publication dates, jurisdictions, inventors, applicants, owners, and patent classifications (Figure 2).
3.1. Patent Families
A patent family is a group of related patent applications filed in one or more jurisdictions (countries or regions) to protect the same or similar invention, which is associated with a common inventor and referred to as a common priority (or priorities). To put it another way, it refers to a variety of distinct patent documents whose technical content is regarded as distinctive. A simple family and an extended family are typically the two sorts of families taken into account. The first one consists of a number of patent applications with the same technical subject matter. The second is a compilation of patent applications with related technical content [37,38,39,40].
For rosemary-related patents with publication dates between 2002 and 2022, a total of 12,320 patent documents were found, with 8497 simple families and 8267 extended families. These findings indicate that 8497 patent documents relate to the same technical content as the 8267 patent documents, which pertain to similar technical content but were published at different times in the same or different countries or regions.
The most representative simple family with a high score of 100 patent records concerns an invention of methods and compositions for affecting the flavor and aroma profiles of consumables [41]. In 2021, Fraser et al. proposed different food additive compositions that included one or more flavor precursor molecules, such as rosemary extract [41]. The patent application was filed in 2020, with the earliest priority in 2013 through 21 jurisdictions. However, the most representative extended family, with a higher score of 554 patent records, concerns the invention of a high-potency sweetener composition with antioxidant properties [42]. In 2007, Prakash and Dubois proposed functional sweetener compositions and methods to provide a sugar-like temporal profile, including antioxidants such as rosmarinic acid [42]. The patent application was filed in 2006, with the earliest priority in 2005, in 22 jurisdictions.
3.2. Publication Dates
The publication date is the date on which a patent document is published with an assigned publication number by a patent authority, thereby making it part of the state of the art [35,38,43,44]. Figure 3 presents the year in which a number of patent documents related to R. officinalis were published. For R. officinalis, 12,320 patent documents were found for the period between 2002 and 2022. The year 2002 saw the registration of only 211 patent documents. In 2019, 150 granted patents were delivered, which is more than all previous years combined. Furthermore, 2017 was the year with the highest number of patent documents (1122), including 1001 patent applications. Finally, in the year 2022, 500 patent documents were recorded.
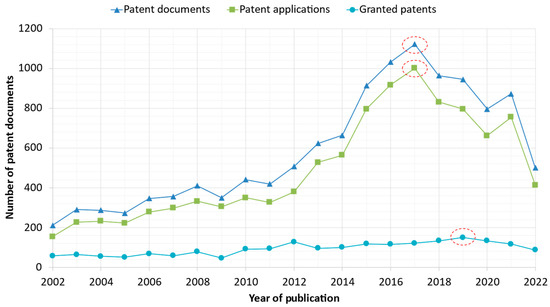
Figure 3.
Evolution of R. officinalis-related patent documents (patent applications and granted patents) as a function of the publication year. The dotted line/circles in the figure represent the highest number of patent documents, patent applications, and granted patents over 20 years.
3.3. Jurisdictions
Jurisdiction relates to the country or region where applicants can file a patent application through the appropriate patent office (e.g., EPO, USPTO, China National Intellectual Property Administration (CNIPA), etc.) [35,43,44,45,46,47]. For R. officinalis, the jurisdictions (top 10) of published patent documents between 2002 and 2022 are presented in Figure 4.
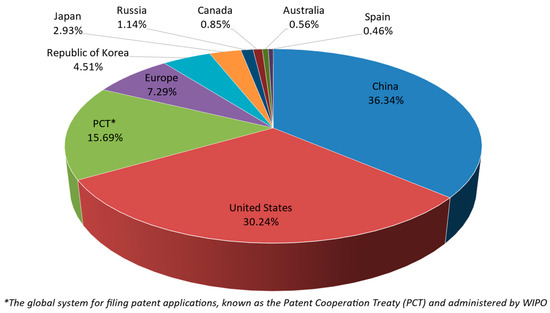
Figure 4.
Share of filed patent applications and granted patents of R. officinalis until 2022 per jurisdiction (top 10).
These findings indicate that in the first place, the CNIPA in China has the highest number of patent documents, with 4384 patents, a proportion of approximately 36% of the total. Secondly, the USPTO in the United States has 3648 patent documents and a proportion of approximately 30%. Then, the Patent Cooperation Treaty (PCT), which is managed by WIPO and allows for global patent applications, has 1893 patent documents, a proportion of approximately 16%. Next, the EPO, which enables regional patent filings in Europe, has 880 patent documents, a proportion of approximately 7% of the total.
3.4. Inventors, Applicants, and Owners
A patent inventor is an individual or group of individuals who create or conceive an idea, invention, or innovation that is novel and non-obvious and meets the requirements for patentability. The inventor(s) may or may not be the same as the applicant for the patent [35,38,43,44,47]. For R. officinalis, the inventors (top 10) of published patent documents between 2002 and 2022 are presented in Figure 5a. Yan Chao of Changsha Xiehaoji Biological Eng CO LTD (Changsha, China) is the first inventor, with 92 patent documents. In second place, the inventor Schulick Paul from New Chapter INC (Brattleboro, VT, United States) has recorded 62 patent documents. In third place, inventors with waivers of the right to be mentioned as inventors have recorded 48 patent documents. The waiver of the right to be mentioned as an inventor, in which case the inventor’s name is not mentioned in the publication, is a declaration signed and filed by the inventor with the relevant patent authority.
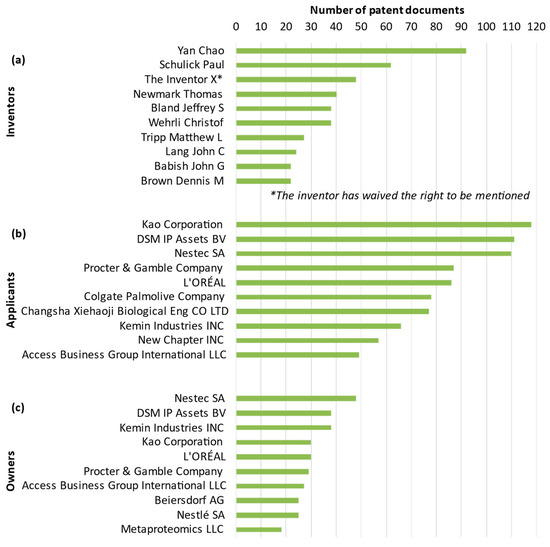
Figure 5.
Top 10 ranking of inventors (a), applicants (b), and owners (c) of R. officinalis-related patents as a function of the patent documents between 2002 and 2022.
A patent applicant refers to either a natural person or a legal entity that submits a patent application. In some cases the applicant can also be the inventor, and there may be more than one applicant for a single patent application [35,38,43,44,47]. For R. officinalis, the applicants (top 10) of published patent documents between 2002 and 2022 are presented in Figure 5b. As a legal entity, Kao Corporation (Tokyo, Japan) is ranked as the first applicant with 118 patent documents. In the second and third places, the applicants, DSM IP Assets BV (Heerlen, The Netherlands) and Nestec SA (Vevey, Switzerland), as legal entities, have 111 and 110 patent documents, respectively.
An owner is a natural person or a legal entity to whom the inventor or applicant has assigned the right to a patent [38,44,45,47,48]. For R. officinalis, the owners (top 10) of published patent documents between 2002 and 2022 are presented in Figure 5c. Nestec SA (Vevey, Switzerland), as a legal entity, is ranked as the first owner, recording 48 patent documents. With 38 patent documents, the second place is shared by two legal entities: DSM IP Assets BV (Heerlen, The Netherlands) and Kemin Industries INC (Plymouth, MI, United States). In third place, the legal entity Kao Corporation (Tokyo, Japan) has been recorded as the owner of 30 patent documents.
3.5. Patent Classifications
The International Patent Classification (IPC) is a code-based hierarchical system that separates all technological domains into sections, classes, subclasses, groups, and subgroups [45,49,50]. For R. officinalis, the IPC codes (top 10) of published patent documents between 2002 and 2022 are presented in Figure 6. For more details concerning this top 10, a description of each IPC code is shown in Table 1.
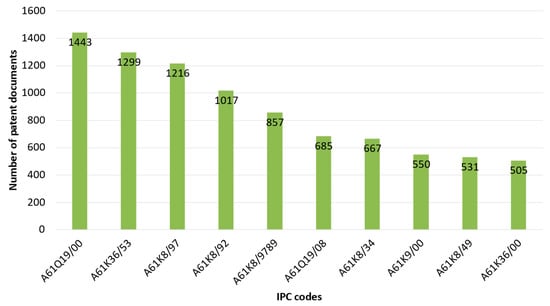
Figure 6.
IPC codes (top 10) of R. officinalis-related patents as a function of the patent documents.

Table 1.
Analysis of the patent classification codes (i.e., the top 10 IPC codes) related to the R. officinalis-related patents.
The most common IPC code is A61Q19/00, which is a subgroup of particular applications of cosmetics or similar toilet preparations for skin care. This group recorded, alone, 1443 patent documents. Furthermore, the subgroups A61K36/53 (i.e., medicinal preparations containing material from Lamiaceae or Labiatae, such as thyme, rosemary, or lavender) and A61K8/97 (i.e., cosmetics or similar toilet preparations that are characterized by their composition and contain materials from algae, fungi, lichens, or plants) have 1299 and 1216 patent documents, respectively. All three IPC codes are presented as the most comprehensive patent classification for the top ten jurisdictional rankings (Figure 7).
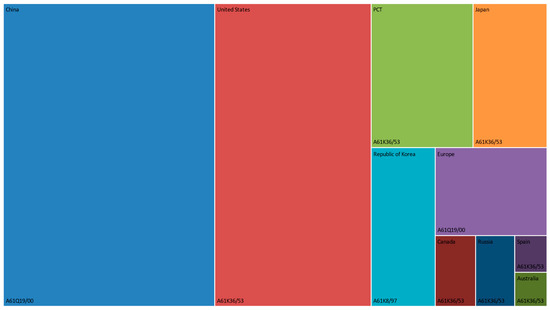
Figure 7.
Repartition of the most IPC code per jurisdiction (top 10) of R. officinalis-related patents.
4. Relevant Patents on Rosemary-Derived Drugs and Bioactive Compounds
4.1. Selection of Relevant Patents
This section presents examples of innovation and practical applications of R. officinalis, as evidenced by patents and inventions. The patents described in this section are the most pertinent and noteworthy patents pertaining to R. officinalis between 2002 and 2022, providing a clear understanding of the current status of patents in this field. Thirteen relevant patent documents, including six patent applications and seven granted patents, were selected according to the theory of relevance scoring. Briefly, relevance was based on the query matching score used in Elasticsearch (or Lucene), which uses a Boolean model to find matching documents and a formula called the practical scoring function to calculate relevance [55].
The different inventors have demonstrated, through this selection, different aspects related to R. officinalis. Some inventions dealt with processes for extracting rosemary-derived biomolecules, while others were related to pharmaceutical, biomedical, cosmetic, or food applications (Table 2).

Table 2.
Summary of relevant patents for R. officinalis between 2002 and 2022.
4.2. Review Based on Relevant Patents
In 2002, Kosaka et al. invented a drug-based rosemary extract useful for the treatment of senile dementia, in particular, Alzheimer’s-type dementia. They aimed to promote the synthesis of nerve growth factor (NGF), which is normally secreted from nerve cells and has an excellent effect on nerve-denaturing diseases. In their granted patent, the inventors claimed a method of promoting the synthesis of nerve growth factor in subjects who need it by using rosemary extract, sage extract, or a mixture of both. The embodiments must contain carnosic acid, carnosol, or both, obtained by extracting rosemary or sage with ethanol or a mixture of water and ethanol. Embodiments can be presented in the form of medicines or food preparations. The in vitro tests presented in the patent description demonstrate that the invented preparations, based on carnosic acid, carnosol, sage extract, or rosemary extract, effectively promote the synthesis of NGF and avoiding the adverse effects of previous drug solutions [56].
One year later, Miyazaki et al. invented a method of treating ulcers using rosemary-derived formulations. More specifically, in their granted patent, they described and claimed a method of treating and preventing ulcers (alcoholic ulcers and stress ulcers) by administering sufficient and effective doses of rosemary or sage extracts to a sick person, the active ingredients of which are carnosic acid and carnosol. They proposed using their invention as an oral-consumption drug (e.g., tablet, capsule, or syrup) or as a food supplement. They recommended, on the one hand, favoring carnosic acid and carnosol extracted from rosemary or sage over synthetic products, and, on the other hand, a concentration of active products between 0.1 and 500 mg per kilogram of body weight [57].
Another application of rosemary-based extracts concerns dentifrice compositions. For this purpose, Trivedi et al. (2006) proposed an invention for oral compositions containing rosemary extracts, which were added to different dentifrice compositions resulting in toothpaste, mouthwashes, and other compositions. The use of this preparation makes it possible to treat and prevent several oral diseases, such as gingivitis, dental plaque formation, and other similar oral problems. The inventors claimed that the active ingredients of their preparations are ursolic acid and carnosic acid extracted from rosemary. These agents had an antibacterial, antioxidant, and/or anti-inflammatory effect in the oral cavity. Based on other claims, these agents must be present in concentrations ranging from 0.01 to 5% w/w in a mixture containing a humectant, an abrasive material, a fluorine-releasing compound, and an anionic polycarboxylate polymer. To prove the concept, the inventors reported in their patent application the results of two compositions realized through artificial mouth tests. The two compositions tested, using 0.2% w/w rosemary extract, showed improved anti-plaque efficacy, respectively, by 53 and 54% compared to the negative control. The same proposed compositions, formulated with 0.3% w/w of rosemary extract, show improved antioxidant efficacy compared to a negative control in a lipid peroxide assay. The optical densities of the two embodiments were, respectively, about 0.72 and 0.74, compared to 0.81 for the control. It was shown that the anti-inflammatory efficacy and effect against gingivitis of certain embodiments based on rosemary extract were enhanced by the addition of an antibacterial agent such as triclosan [58].
In 2007, a patent application for pharmaceutical compositions for the treatment of cardiovascular and cerebrovascular diseases was filed. Through this application, Wei et al. described and claimed the development of a medicinal composition using plant extracts. The invention was based particularly on different plants used in traditional Chinese medicine. Among the claimed pharmaceutical compositions, an extract based on rosmarinic acid was used in the formulation, and in vivo tests in rats were conducted to prove the concept for the treatment of cardiovascular and cerebrovascular diseases. The proposed drug compositions showed significant therapeutic effects. They induced a significant decrease in neurological symptoms as well as a reduction in the area of cerebral infarction compared to the control rat group. The same compositions showed better effects than positive controls using commercial drugs [59].
Two years later, Wehrli developed a process for the preparation of carnosol from carnosic acid. The inventor reported that rosemary or sage extracts contain between 10 and 30% carnosic acid, which can be converted into carnosol, a biologically active form of polyphenol known for its antioxidant action and presumed anti-carcinogenicity. The invented process has the advantage of being easy and effective, in which the first oxidation transforms carnosic acid into quinone, which is then converted into carnosol. The catalyst can be iron or an iron salt, a small amount of water, rosemary needles, or mixtures thereof. The final product of the invented process was carnosol mixed with reaction by-products. The inventor finally described that embodiments may be nutritional as food supplements or pharmaceutical as different galenical preparations (i.e., solid or liquid) for human or animal use [60].
The antioxidant activity of R. officinalis was highlighted in a patent application proposed by Xie et al. (2011) [61]. The invention focused on technology for producing two antioxidant agents from the branches and leaves of R. officinalis. More specifically, it concerned pharmacological data attesting to the multiple beneficial effects of the agents targeted by this new technique, namely, diterpene phenols (including carnosic acid), which have antioxidant, antitumor, and anti-HIV effects. Effects of rosmarinic acid, among which are its analgesic, anti-inflammatory, antioxidant, antithrombotic, and fibrinolytic activities, were reported. Furthermore, the innovation was characterized by the use of counter-current ultrasound as an extraction method. The extracted liquid, whose solid/liquid ratio was between 1:10 and 1:30, was separated for 30 min by a system of membrane sieves at a temperature ranging from 15 to 65 °C and with the addition of 30 to 85% ethanol, to obtain a fat-soluble component mainly formed by carnosic acid and a water-soluble component mainly formed by rosmarinic acid. This technology for the production of rosemary extracts has the advantages of simplicity, low energy consumption, production efficiency, and safety, due to the homogeneity of the extracted components [61].
Another medical application of rosemary extracts was published in 2012. The granted patent was for the use of extracts of aromatic and medicinal plants, including rosemary, for the health benefits of their constituents. The invention by Offord Cavin et al. was a pharmaceutical composition to promote bone growth and maintain bone health with rosemary or caraway extracts. The scientific background in the description of the invention reports that the bone renewal process, and thus bone density, are genetically regulated in humans. According to the inventors, the patent claims were based on the introduction of a phytochemical having the ability to induce genetic expression of a bone morphogenic protein using one or more polyphenols among feruloylnepitrin, foumaroylnepitrin, dehydroxyrosmarinic acid, eupafoline, carnosol, scutellarin, gencwanine, kaempferol, and acacetin. The product may be presented in the form of a medicinal or food preparation, and can be administered orally and/or enterally. Active plant agents can be mixed with a protein source, a fat source, and/or a carbohydrate source. The invention also claims the ability to maintain bone health and prevent bone disorders, as well as their relief and/or treatment. The preparations based on the invented composition act through the inhibition of bone resorption, and thus participate in maintaining adequate bone density in the elderly and after the advent of menopause in women [62].
The antioxidant activity of R. officinalis was highlighted in another patent application published in 2014. The invention concerned an antioxidant composition that can be used as a food preservative of natural origin and is therefore a good substitute for poorly perceived chemical antioxidants. Indeed, the background reported in the description of the patent application insists on the importance of developing natural, economical, and effective food preservatives to meet the demand of the food industry. For this purpose, Torben et al. proposed a composition (e.g., an emulsion-based food) based on two plant extracts, one comprising carnosic acid and carnosol from a plant of the Lamiaceae family such as rosemary or sage, and the other comprising pregnane glycosides obtained from a plant of the genus Caralluma. These two compounds can be mixed beforehand for direct use or separated as a kit from the two components to be mixed when used with adequate dosage. The invented composition is intended to inhibit the oxidation of foodstuffs. Its proposed applications are multiple, including but not limited to the protection of raw or cooked foods such as meat, seafood, pasta sauces, pasteurized soups, mayonnaise, salad dressings, oil-in-water emulsions, and various dairy products. Furthermore, this antioxidant composition was also proposed as consumable by pets in the form of a supplement or food constituent [63].
As mentioned in the introduction, R. officinalis is able to produce bioactive compounds by extraction from flowers, stems, or leaves. In this regard, He et al. proposed, through a patent application published in 2015, an invention that highlighted R. officinalis leaf extracts for pharmaceutical applications. This invention exploits the properties of the active constituents of rosemary to reduce important health problems such as type II diabetes, obesity, and cardiovascular disease. More specifically, the claimed composition consists of about 40 to 65% w/w carnosic acid, about 2 to 10% w/w carnosol, and about 2 to 10% w/w 12-O-methylcarnosic acid. It allows the sick person to attain noticeable improvements. This pharmaceutical composition makes it possible to reduce the oxidation of low-density lipoproteins in cases of cholesterol-related disorders, increase the activity of a receptor activated by a peroxisome proliferator involved in lipid metabolism and energy homeostasis, inhibit the activity of pancreatic lipase, decrease fasting blood glucose, reduce fasting plasma insulin levels in diabetic people, and reduce weight and body fat in individuals suffering from or at risk of obesity. The results of in vivo experiments in mice were reported to confirm the efficacy of the invention. Mice fed for 16 weeks with a diet rich in fat and treated with the invented composition based on rosemary extract dosed with 50% w/w carnosic acid showed statistically lower blood glucose than a positive control of mice fed with a similar rich diet without administration of the invented composition. The sacrifice of these mice allowed the determination of their amount of fat, and a considerable decrease in fat mass was noted in treated mice compared to control mice. In vitro results were also reported. The inhibition effect of pancreatic lipase on the invented composition, dosed at 20% w/w carnosic acid, was compared with that of a commercially recognized drug. The assay revealed that the invented composition had significant pancreatic inhibitory activity, confirming its utility in the treatment of obesity [64].
As described above, several inventions have been concerned with extracting and purifying bioactive compounds from plants, including rosemary. In 2016, Lin et al. developed a novel process for extracting carnosic acid from R. officinalis. The description of the invention reports the benefits of carnosic acid, in particular the effect of reducing weight and body lipids, the prevention of cardiovascular disease, and an antitumor effect. In this patent application, the inventors claimed a method of extracting carnosic acid from dried and powdered rosemary leaves. The method described involved the filtration of rosemary powder in several steps with an ethanol solution of 85–95% v/v for a period of between 1 and 2 h at a temperature ranging from 78 to 80 °C. Carnosic acid was then obtained following the crystallization of the filtrate. The method invented aimed to overcome the problems found during the examination of the prior art. This invention has the advantage of reducing the cost of production through a simple and fast process that exploits a large part of the plant. This new process claims high product purity and adaptability to large-scale industrial production [65].
In 2017, Yan developed a formulation for designing an anti-allergic day cream based on rosemary extract. This invention confirmed another pharmaceutical application of R. officinalis. The description provided in the application addressed the problem of aggression suffered by skin cells in the external environment. In some cases of allergies and weak immune reactions, damaged skin is not repaired, causing accelerated aging of the skin. According to patent claims, the invented rosemary-based cream offered a skin preparation using a formulation that incorporates 1.3% by weight of rosemary extract. In some embodiments, this preparation had a relaxing and moisturizing anti-inflammatory effect on the skin and limited problems of spots, eczema, wrinkles, and allergies [66].
The development of an antiviral drug against pneumonia with a composition based on rosemary extracts was confirmed through a patent application published in 2020. The active agents of this drug composition were rosmarinic acid, carnosic acid, and carnosol, with a mass ratio of 1:3:5, respectively. Although the production of antiviral drugs from plants is a promising research method, Hu et al. explained that the goal of the invention was to develop an antiviral against pneumonia using a simple method for the first time. Furthermore, the invention description reported test results of the invented composition that reduces the ratio of lung weight to wet weight of tissues, reduces the total content of proteins in the lungs, inhibits serum release, and regulates the release of cytokines involved in the immune response inflammation, which relieves damage due to infection. The results also showed a clear inhibition of H3N2 influenza virus replication in the lungs [67].
Carnosic acid can be extracted from rosemary and could play a key role as an antioxidant in food or animal foods, as well as in care products. However, wild-type rosemary contains only about 2 to 3% w/w of this acid. Following a long clonal breeding program, a patent application concerning rosemary’s high carnosic acid was published in 2022. Narasimhamoorthy et al. claimed a rosemary line rich in carnosic acid up to 9% w/w of its dry weight, obtained following a series of selective clonings made from existing rosemary by self-pollination or cross-pollination. The invention description provided in the patent stated that no line with such characteristics has ever been observed in nature. From the cultivar line, a node or cutting can be used to grow identical plants that have, in addition to the richness of carnosic acid, promising agronomic characteristics such as robustness, stability, and high production of biomass, which increase the yield of carnosic acid [68].
5. Conclusions
- This study concerns the innovation and improvement of rosemary-based products between 2002 and 2022. A detailed analysis of the patentability of rosemary-based products is provided.
- There were 12,320 patent documents, consisting of 10,360 patent applications and 1960 granted patents, belonging to 8497 simple families and 8267 extended families. China had the highest number of patent documents at 4384, and the most patent documents were published in 2017 at 1122.
- Raw materials, formulations, preparation processes, and applications are all part of the research and development projects around rosemary-based products.
- Analysis of the patent classification codes as a technology indicator revealed that the majority of filed patents and inventions are focused on medicinal preparations with unique physical properties and cosmetics/toiletry products with distinct compositions.
- Expert driving factors and knowledge clusters suggest that research and development efforts are primarily focused on developing methods to create pharmaceutical products with specific physical or chemical characteristics, as evidenced by the concentration of patents in this area.
- Thirteen patents in the area of R. officinalis are discussed. This constitutes a systematic patent review elucidating that the novelty of the major inventions during the last 20 years intended for processes for extracting rosemary-derived biomolecules as well as pharmaceutical, biomedical, cosmetic, and food applications.
- R. officinalis, through rosemary-based drugs and rosemary-derived biomolecules, has potential in the health care industry for its use as an antioxidant, and to prevent or treat inflammatory, metabolic, and neurodegenerative diseases. Innovation trends in this field include the optimization of the existing extracting process using green solvents, as well as development of new formulations and novel applications.
Author Contributions
Conceptualization, R.E.B. and A.F.; methodology, R.E.B. and A.F.; validation, A.F.; formal analysis, R.E.B. and A.F.; investigation, R.E.B. and A.F.; data curation, R.E.B. and A.F.; writing—original draft preparation, R.E.B.; writing—review and editing, A.F.; visualization, A.F.; supervision, A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within this article’s content.
Acknowledgments
The authors acknowledge the WIPO for the “Patentscope search service”, the EPO for the “Espacenet patent search”, the “USPTO for the PatFT/AppFT databases”, and the Cambia Institute for the “Lens patent data set” used in this study.
Conflicts of Interest
The authors declare that the content of this article has no conflict of interest. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this article.
References
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, 283. [Google Scholar] [CrossRef] [PubMed]
- Sagor, A.T.M.; Reza, M.H.; Tabassum, N.; Sikder, B.; Ulla, A.; Subhan, N.; Hossain, H.M.; Alam, A.M. Supplementation of Rosemary Leaves (Rosmarinus officinalis) Powder Attenuates Oxidative Stress, Inflammation and Fibrosis in Carbon Tetrachloride (CCl4) Treated Rats. Curr. Nutr. Food Sci. 2016, 12, 288–295. [Google Scholar] [CrossRef]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. Antibacterial Activity and Anticancer Activity of Rosmarinus officinalis L. Essential Oil Compared to That of Its Main Components. Molecules 2012, 17, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, N.; Fu, Y.-J.; Wang, W.; Luo, M.; Zhao, C.-J.; Zu, Y.-G.; Liu, X.-L. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef]
- Peng, C.-H.; Su, J.-D.; Chyau, C.-C.; Sung, T.-Y.; Ho, S.-S.; Peng, C.-C.; Peng, R.Y. Supercritical fluid extracts of rosemary leaves exhibit potent anti-inflammation and anti-tumor effects. Biosci. Biotechnol. Biochem. 2007, 71, 2223–2232. [Google Scholar] [CrossRef]
- Malek, A.; Sadaka, W.M.M.; Hamo, S.; Al-Mahbashi, M.H. Evaluation of Antidiabetic Activity of Rosmarinus officinalis var. prostratus Growing in Syria in Alloxan Diabetic Rats. Curr. Bioact. Compd. 2021, 17, 187–193. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.d.P.; Herrero, M. Rosemary (Rosmarinus officinalis) as a functional ingredient: Recent scientific evidence. Curr. Opin. Food Sci. 2017, 14, 13–19. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Celotto, A.C.; Capellini, V.K.; Albuquerque, A.A.S.; Nadai, T.R.d.; Carvalho, M.T.M.d.; Evora, P.R.B. Does rosmarinic acid underestimate as an experimental cardiovascular drug? Acta Cirúrgica Bras. 2013, 28, 83–87. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L.; Ayala-Gómez, A. Rosmarinus officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetics. Cosmetics 2020, 7, 77. [Google Scholar] [CrossRef]
- Begum, A.; Sandhya, S.; Shaffath Ali, S.; Vinod, K.R.; Reddy, S.; Banji, D. An in-depth review on the medicinal flora Rosmarinus officinalis (Lamiaceae). Acta Sci. Pol. Technol. Aliment. 2013, 12, 61–73. [Google Scholar] [PubMed]
- del Baño, M.J.; Lorente, J.; Castillo, J.; Benavente-García, O.; del Río, J.A.; Ortuño, A.; Quirin, K.-W.; Gerard, D. Phenolic Diterpenes, Flavones, and Rosmarinic Acid Distribution during the Development of Leaves, Flowers, Stems, and Roots of Rosmarinus officinalis. Antioxidant Activity. J. Agric. Food Chem. 2003, 51, 4247–4253. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A. The Mediterranean aromatic plants and their culinary use. Nat. Prod. Res. 2015, 29, 201–206. [Google Scholar] [CrossRef]
- Oualdi, I.; Diass, K.; Azizi, S.-E.; Dalli, M.; Touzani, R.; Gseyra, N.; Yousfi, E.B. Rosmarinus officinalis essential oils from Morocco: New advances on extraction, GC/MS analysis, and antioxidant activity. Nat. Prod. Res. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern extraction methods for preparation of bioactive plant extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar] [CrossRef]
- Pixabay Free Images. Rosemary Flowers. Available online: https://pixabay.com/fr/photos/fleurs-de-romarin-fleurs-bleu-280976 (accessed on 10 March 2023).
- Pixabay Free Images. Rosemary Leaves. Available online: https://pixabay.com/fr/photos/romarin-%c3%a9pices-cuisson-ingr%c3%a9dient-674505 (accessed on 10 March 2023).
- Pixabay Free Images. Dried Rosemary Leaves. Available online: https://pixabay.com/fr/photos/sec-pimenter-parfum%c3%a9-assaisonnement-3331965 (accessed on 10 March 2023).
- Pixabay Free Images. Rosemary Oil. Available online: https://pixabay.com/fr/photos/un-verre-bouteilles-huile-5166844 (accessed on 10 March 2023).
- Oreopoulou, A.; Choulitoudi, E.; Tsimogiannis, D.; Oreopoulou, V. Six Common Herbs with Distinctive Bioactive, Antioxidant Components. A Review of Their Separation Techniques. Molecules 2021, 26, 2920. [Google Scholar] [CrossRef]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic acid and carnosol, two major antioxidants of rosemary, act through different mechanisms. Plant Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef]
- Psarrou, I.; Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction Kinetics of Phenolic Antioxidants from the Hydro Distillation Residues of Rosemary and Effect of Pretreatment and Extraction Parameters. Molecules 2020, 25, 4520. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef]
- Rocío Teruel, M.; Garrido, M.D.; Espinosa, M.C.; Linares, M.B. Effect of different format-solvent rosemary extracts (Rosmarinus officinalis) on frozen chicken nuggets quality. Food Chem. 2015, 172, 40–46. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Report. Available online: https://fda.report/DailyMed/4a06d3b2-7229-4398-baba-5d0a72f63821 (accessed on 4 March 2023).
- U.S. Food and Drug Administration. Pharmacology/Toxicology NDA Review and Evaluation. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/210895Orig1s000PharmR.pdf (accessed on 4 March 2023).
- Feeny, V.I. Improved Process of Applying Colours to Photographs. Patent Application GB189730115A, 12 February 1898. [Google Scholar]
- Moore, O.Z. Hair Restorer. Granted Patent GB104265A, 1 March 1917. [Google Scholar]
- Vasisht, K.; Sharma, N.; Karan, M. Current Perspective in the International Trade of Medicinal Plants Material: An Update. Curr. Pharm. Des. 2016, 22, 4288–4336. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A. Patent analysis of a medicinal plant as a source of natural products and drugs: Rosmarinus officinalis (Rosemary). In Proceedings of the 1st International Electronic Conference on Horticulturae (IECHO 2022), Basel, Switzerland, 16–30 April 2022. [Google Scholar]
- World Intellectual Property Organization. The Patentscope. Available online: https://patentscope.wipo.int (accessed on 25 January 2023).
- European Patent Office. Espacenet Patent Search. Available online: https://worldwide.espacenet.com (accessed on 25 January 2023).
- United States Patent and Trademark Office. USPTO Database (PatFT-AppFT). Available online: https://uspto.gov/patents/search (accessed on 25 January 2023).
- Cambia Institute. The Lens Patent Data Set. Available online: www.lens.org (accessed on 25 January 2023).
- Fatimi, A. Seaweed-based biofertilizers: A patent analysis. Recent Pat. Biotechnol. 2022, 16, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A. Exploring the patent landscape and innovation of hydrogel-based bioinks used for 3D bioprinting. Recent Adv. Drug Deliv. Formul. 2022, 16, 145–163. [Google Scholar] [CrossRef]
- Fatimi, A. A patent data analysis of the innovation trends in biological control agent formulations. Recent Adv. Food Nutr. Agric. 2022, 13, 59–69. [Google Scholar] [CrossRef] [PubMed]
- European Patent Office. Espacenet Glossary. Available online: https://worldwide.espacenet.com/patent/help/espacenet-glossary (accessed on 25 January 2023).
- World Intellectual Property Organization. Handbook on Industrial Property Information and Documentation; WIPO: Geneva, Switzerland, 2013; p. 43. [Google Scholar]
- Fatimi, A. Cellulose-based hydrogels: Patent analysis. J. Res. Updat. Polym. Sci. 2022, 11, 16–24. [Google Scholar] [CrossRef]
- Fraser, R.; Brown, P.O.R.; Karr, J.; Holz-Schietinger, C.; Cohn, E. Methods and Compositions for Affecting the Flavor and Aroma Profile of Consumables. Patent Application US20210037851A1, 11 February 2021. [Google Scholar]
- Prakash, I.; Dubois, G.E. High-potency sweetener composition with antioxidant and compositions sweetened therewith. Patent Application WO2007061900A1, 31 May 2007. [Google Scholar]
- Fatimi, A. Trends and recent patents on cellulose-based biosensors. Eng. Proc. 2022, 16, 12. [Google Scholar] [CrossRef]
- Fatimi, A. Hydrogel-based bioinks for three-dimensional bioprinting: Patent analysis. Mater. Proc. 2021, 7, 3. [Google Scholar] [CrossRef]
- World Intellectual Property Organization. What is Intellectual Property? Frequently Asked Questions: Patents, Publication No. 450E/20. Available online: https://www.wipo.int/patents/en/faq_patents.html (accessed on 10 October 2022).
- Intellectual Property India. Jurisdiction of Patent Offices. Available online: https://ipindia.gov.in/jurisdiction-of-patent-offices.htm (accessed on 10 December 2022).
- Fatimi, A. Patentability of biopolymer-based hydrogels. Chem. Proc. 2022, 8, 39. [Google Scholar] [CrossRef]
- United States Patent and Trademark Office. Manual of Patent Examining Procedure: Ownership/Assignability of Patents and Applications. Available online: https://www.uspto.gov/web/offices/pac/mpep/mpep-0300.html (accessed on 10 October 2022).
- World Intellectual Property Organization. IPC Publication. Available online: www.wipo.int/classifications/ipc/ipcpub (accessed on 25 January 2023).
- World Intellectual Property Organization. Guide to the International Patent Classification (IPC); WIPO: Geneva, Switzerland, 2020; p. 51. [Google Scholar]
- Kwasnicki, R.M.; Hughes-Hallett, A.; Marcus, H.J.; Yang, G.-Z.; Darzi, A.; Hettiaratchy, S. Fifty Years of Innovation in Plastic Surgery. Arch. Plast. Surg. 2016, 43, 145–152. [Google Scholar] [CrossRef]
- De Souza, L.M.; Oliveira, D.D.; Ribeiro, L.L.P.; de Paula Pereira, N.; Druzian, I.J. Nanoemulsions for Cosmetic Applications: What Innovation Status? Recent Pat. Nanotechnol. 2018, 12, 101–109. [Google Scholar] [CrossRef]
- Speziali, M.G. Cellulose technologies applied to biomedical purposes from the patentometric point of view. Cellulose 2020, 27, 10095–10117. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, A. Analysis of International Patent Applications for Inventions Like Traditional Herbal Medicines. In Research Anthology on Recent Advancements in Ethnopharmacology and Nutraceuticals; Khosrow-Pour, M., Ed.; IGI Global: Hershey, PA, USA, 2022. [Google Scholar]
- Hachimi Alaoui, C.; Fatimi, A. A 20-year patent review and innovation trends on hydrogel-based coatings used for medical device biofabrication. J. Biomater. Sci. Polym. Ed. 2023. In press. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, K.; Miyazaki, T.; Ito, H. Method of Promoting Synthesis of Nerve Growth Factor. Granted Patent US6391344B2, 21 May 2002. [Google Scholar]
- Miyazaki, T.; Kosaka, K.; Ito, H. Method of Treating Ulcers. Granted Patent US6638523B1, 28 October 2003. [Google Scholar]
- Trivedi, H.M.; Xu, T.; Worrell, C.L.; Panaligan, K. Oral Compositions Containing Extracts of Rosmarinus and Related Methods. Patent Application WO2006065522A2, 22 June 2006. [Google Scholar]
- Wei, F.; Li, D.; Luo, C.; Yue, H.; Chen, Q.; Huang, Z. Pharmaceutical Composition for the Treatment of Cardiovascular and Cerebrovascular Diseases. Patent Application US20070053999A1, 8 March 2007. [Google Scholar]
- Wehrli, C. Process for Producing Carnosol from Carnosic Acid. Patent Application EP2062899A1, 27 May 2009. [Google Scholar]
- Xie, K.; Zhang, M.; Fan, Y. Production Technology for Acquiring Two Antioxidant Agents from Rosmarinus officinalis L. Patent Application CN102199092A, 28 September 2011. [Google Scholar]
- Offord Cavin, E.; Williamson, G.; Courtois, D.; Lemaure, B.; Touche, A.; Soon Grace, I.N.G.; Ameye, L. Nutritional Compositions for Promotion of Bone Growth and Maintenance of Bone Health Comprising Extracts of for Example Rosemary or Caraway. Granted Patent US8299034B2, 30 October 2012. [Google Scholar]
- Torben, I.; Lars, M.; Jørn, M.; Niels, C. Antioxidant Composition. Patent Application WO2014195291A1, 11 December 2014. [Google Scholar]
- He, K.; Roller, M.; Ibarra, A.; Bai, N.; Dikansky, J. Extract of Rosmarinus officinalis L. leaves for Pharmaceutical Applications. Granted Patent US9011936B2, 21 April 2015. [Google Scholar]
- Lin, T.; Li, J. Method for Extracting Carnosic Acid from Rosmarinus Officinalis. Patent Application CN105777530A, 20 July 2016. [Google Scholar]
- Yan, C. Preparation Method of Enzyme Anti-Allergic Day Cream. Patent Application CN106860229A, 20 June 2017. [Google Scholar]
- Hu, W.; Zhang, R.; Yu, J.; Yu, H.; Zhang, L.; Zhao, R. Application of Rosemary Extract in Antiviral Pneumonia Medicine. Patent Application CN111202766A, 29 May 2020. [Google Scholar]
- Narasimhamoorthy, B.; Greaves John, A.; Zhao, L.; Qiu, Z.; Cox, J.; Baker, J. Rosemary (Rosmarinus officinalis L.) with High Carnosic Acid Denominated KI937. Granted Patent US11259488B2, 1 March 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).