Recent Developments in Electrospun Nanofibers as Delivery of Phytoconstituents for Wound Healing
Abstract
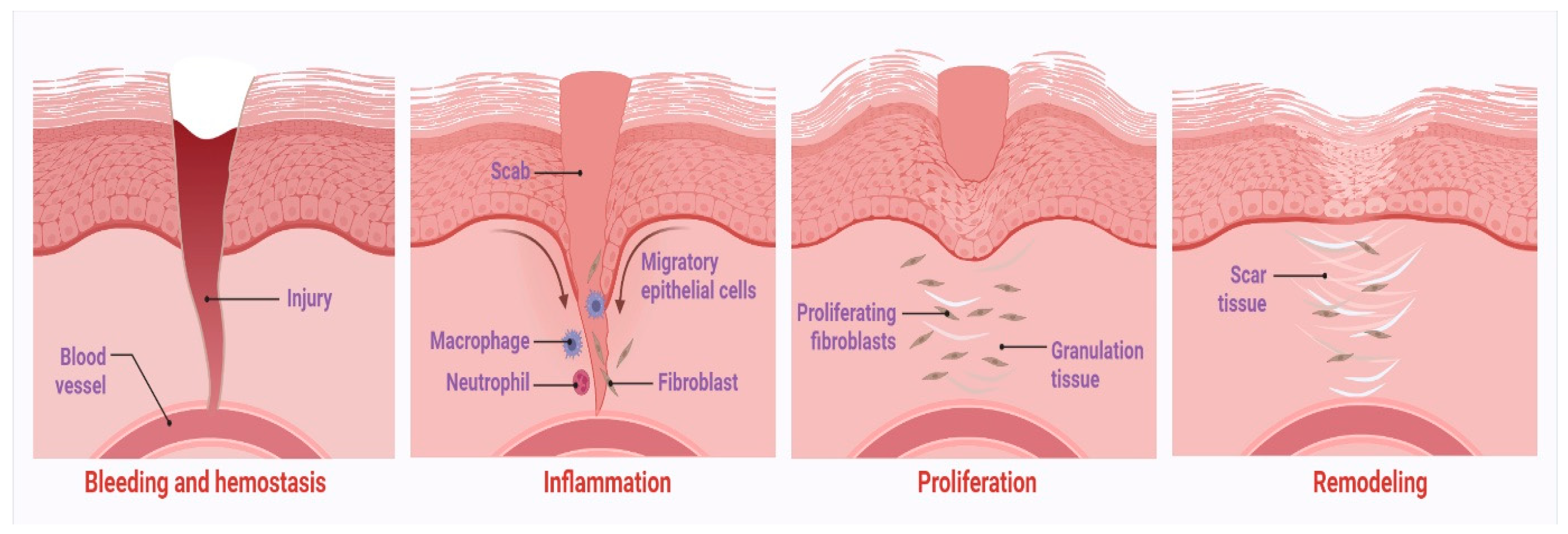
1. Introduction
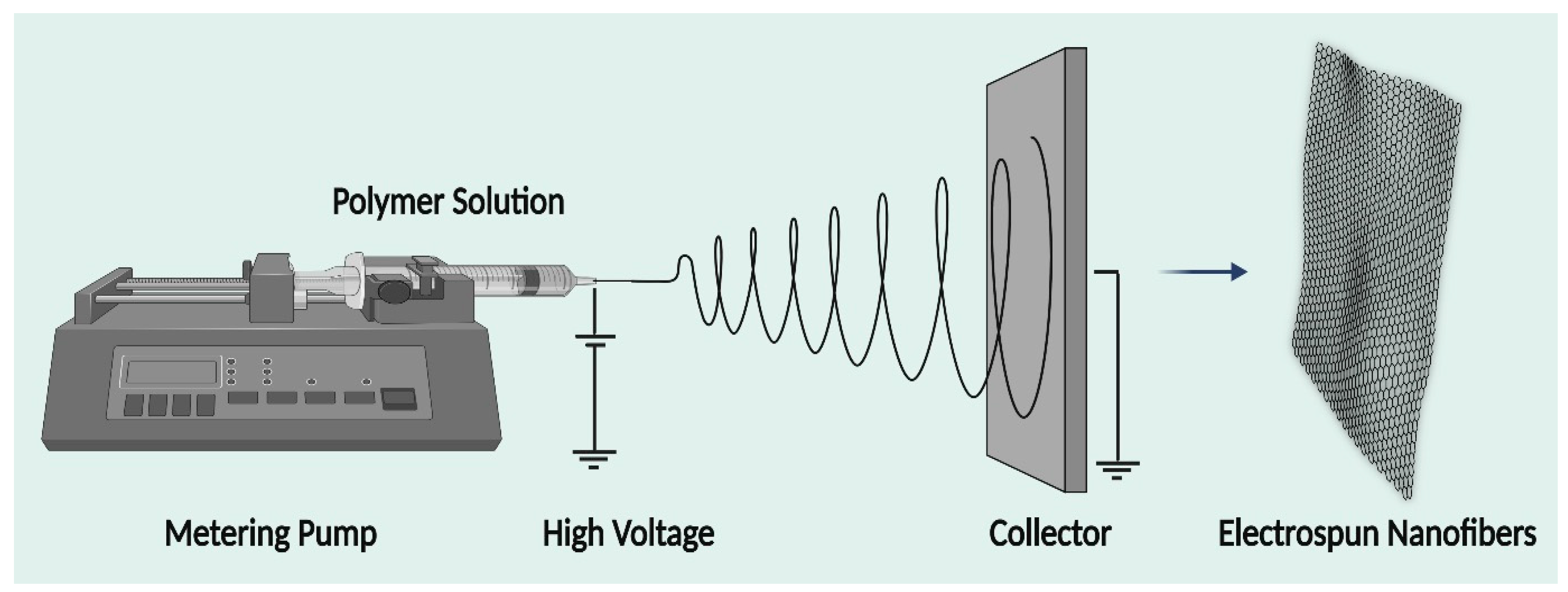
2. Electrospinning Technique
3. Factors Affecting Electrospinning
3.1. Solution-Related Parameters
3.2. Polymer Concentration
3.3. Processing Conditions
3.4. Effect of Voltage
3.5. Volumetric Flow Rate
3.6. Distance of Collector
3.7. Effect of Conductivity
3.8. Effect of Solvent
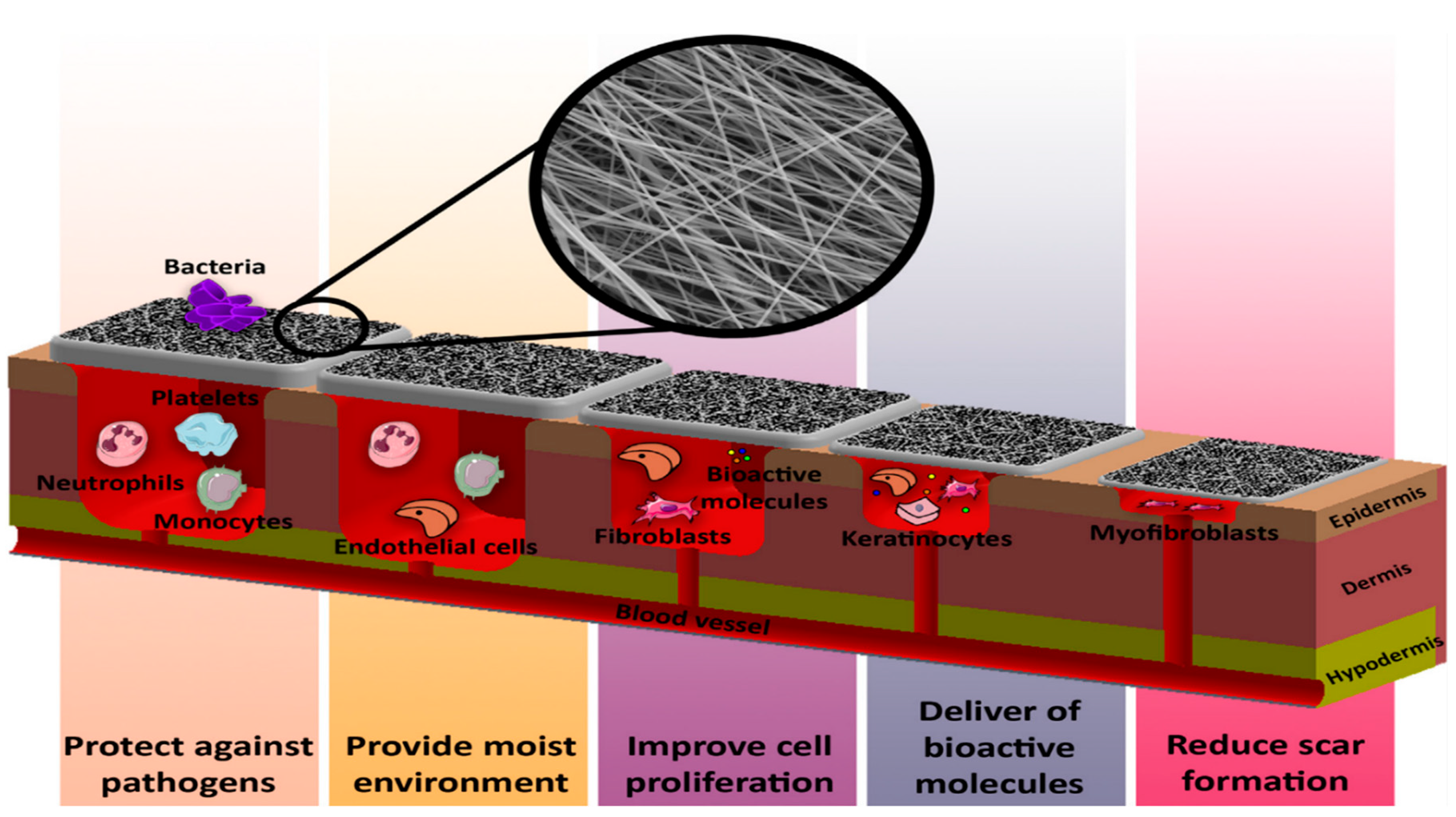
4. Advantages of Electrospun Nanofibers
5. Polymers
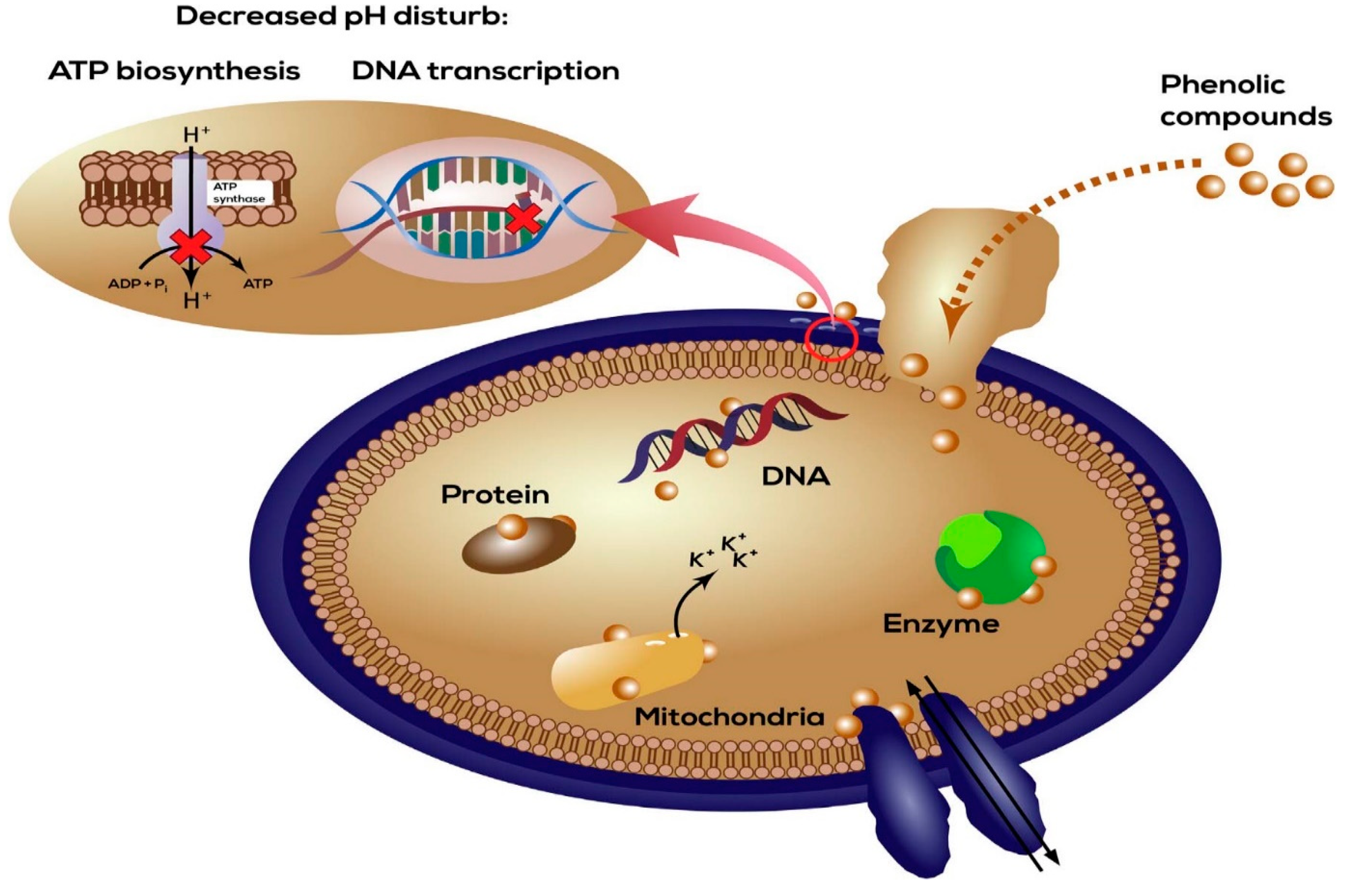
6. Antibacterial Mechanism of Phytoconstituents
7. Phytoextract-Based Electrospun Nanofiber Wound Dressings
7.1. Curcuma Longa

7.2. Aloe barbadensis Miller
7.3. Lawsonia inermis
7.4. Zataria multiflora Boiss
7.5. Matricaria chamomilla L.
7.6. Isatis tinctoria
7.7. Trigonella foenum-graecum
7.8. Melilotus officinalis
7.9. Achillea lycaonica
7.10. Zea mays
7.11. Lepidium sativum
7.12. Syzygium aromaticum
7.13. Garcinia mangostana Linn
7.14. Gymnema sylvestre
7.15. Carica papaya
7.16. Centella asiatica
7.17. Sorghum bicolor
7.18. Camellia sinensis
7.19. Hypericum perforatum
7.20. Nepeta dschuparensis Bornm
7.21. Annona muricata
7.22. Tridax procumbens
7.23. Vitis vinifera
7.24. Moringa oleifera
7.25. Querqus infectoria
7.26. Juniperus chinensis
7.27. Beta vulgaris
7.28. Biophytum sensitivum
7.29. Lavandula
7.30. Boraginaceae
7.31. Astragalus membranaceus
8. Conclusions
9. Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Wynn, T.A.; Barron, L. Macrophages: Master Regulators of Inflammation and Fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V. Wound Healing and Its Impairment in the Diabetic Foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Hajipour, M.J.; Gould, L.; Mahmoudi, M. Nanomedicine in Healing Chronic Wounds: Opportunities and Challenges. Mol. Pharm. 2021, 18, 550–575. [Google Scholar] [CrossRef]
- Giancotti, F.G.; Ruoslahti, E. Integrin Signaling. Science 1999, 285, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Hyldig, K.; Riis, S.; Pennisi, C.P.; Zachar, V.; Fink, T. Implications of Extracellular Matrix Production by Adipose Tissue-Derived Stem Cells for Development of Wound Healing Therapies. Int. J. Mol. Sci. 2017, 18, 1167. [Google Scholar] [CrossRef]
- Haukipuro, K.; Melkko, J.; Risteli, L.; Kairaluoma, M.I.; Risteli, J. Synthesis of Type I Collagen in Healing Wounds in Humans. Ann. Surg. 1991, 213, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.J. Tetracycline Hydrochloride-Loaded Electrospun Nanofibers Mats Based on PVA and Chitosan for Wound Dressing. Mater. Sci. Eng. C 2017, 77, 271–281. [Google Scholar] [CrossRef]
- Vilchez, A.; Acevedo, F.; Cea, M.; Seeger, M.; Navia, R. Applications of Electrospun Nanofibers with Antioxidant Properties: A Review. Nanomaterials 2020, 10, 175. [Google Scholar] [CrossRef]
- Ajazuddin; Saraf, S. Applications of Novel Drug Delivery System for Herbal Formulations. Fitoterapia 2010, 81, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.; Jain, N.; Valli, K. Importance of Novel Drug Delivery Systems in Herbal Medicines. Pharmacogn. Rev. 2010, 4, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy: PERSPECTIVE ARTICLE. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for Wound Healing: Scope and Advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A Review on Wound Dressings with an Emphasis on Electrospun Nanofibrous Polymeric Bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Kenry; Lim, C.T. Nanofiber Technology: Current Status and Emerging Developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, X.; Lu, T.J.; Xu, F. Recent Advances in Electrospun Nanofibrous Scaffolds for Cardiac Tissue Engineering. Adv. Funct. Mater. 2015, 25, 5726–5738. [Google Scholar] [CrossRef]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as New-Generation Materials: From Spinning and Nano-Spinning Fabrication Techniques to Emerging Applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.J. Current Progresses of 3D Bioprinting Based Tissue Engineering. Quant. Biol. 2017, 5, 136–142. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun Polymeric Nanofibres as Wound Dressings: A Review. Colloids Surf. B Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun Nanofibers for Wound Healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chemie Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Kadavil, H.; Zagho, M.; Elzatahry, A.; Altahtamouni, T. Sputtering of Electrospun Polymer-Based Nanofibers for Biomedical Applications: A Perspective. Nanomaterials 2019, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Reagan, M.R.; Kaplan, D.L. Electrospun Silk Biomaterial Scaffolds for Regenerative Medicine. Adv. Drug Deliv. Rev. 2009, 61, 988–1006. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The Effect of Processing Variables on the Morphology of Electrospun Nanofibers and Textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of Polymeric and Ceramic Nanofibers as Uniaxially Aligned Arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Dersch, R.; Liu, T.; Schaper, A.K.; Greiner, A.; Wendorff, J.H. Electrospun Nanofibers: Internal Structure and Intrinsic Orientation. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 545–553. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded Nanofibers Formed during Electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Chronakis, I.S. Novel Nanocomposites and Nanoceramics Based on Polymer Nanofibers Using Electrospinning Process—A Review. J. Mater. Process. Technol. 2005, 167, 283–293. [Google Scholar] [CrossRef]
- Macossay, J.; Marruffo, A.; Rincon, R.; Eubanks, T.; Kuang, A. Effect of Needle Diameter on Nanofiber Diameter and Thermal Properties of Electrospun Poly(Methyl Methacrylate). Polym. Adv. Technol. 2007, 18, 180–183. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Tong, H.W.; Wang, M. Effects of Processing Parameters on the Morphology and Size of Electrospun PHBV Micro- and Nano-Fibers. Key Eng. Mater. 2007, 334–335, 1233–1236. [Google Scholar]
- Baumgarten, P.K. Electrostatic Spinning of Acrylic Microfibers. J. Colloid Interface Sci. 1971, 36, 71–79. [Google Scholar] [CrossRef]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and Process Relationship of Electrospun Bioabsorbable Nanofiber Membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Huang, L.; Nagapudi, K.; Apkarian, P.R.; Chaikof, E.L. Engineered Collagen—PEO Nanofibers and Fabrics. J. Biomater. Sci. Polym. Ed. 2001, 12, 979–993. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in Drug Delivery and Tissue Engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; Du Toit, L.C.; Ndesendo, V.M.K. A Review of the Effect of Processing Variables on the Fabrication of Electrospun Nanofibers for Drug Delivery Applications. J. Nanomater. 2013, 2013, 1–22. [Google Scholar] [CrossRef]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for Tissue Engineering Scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Memic, A.; Abudula, T.; Mohammed, H.S.; Joshi Navare, K.; Colombani, T.; Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl. Bio Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef] [PubMed]
- Sabarees, G.; Velmurugan, V.; Tamilarasi, G.P.; Alagarsamy, V.; Raja Solomon, V. Recent Advances in Silver Nanoparticles Containing Nanofibers for Chronic Wound Management. Polymers 2022, 14, 3994. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.H.; Yeh, J.Y.; Chen, Y.S.; Li, M.H.; Huang, C.H. Wound-Healing Effect of Electrospun Gelatin Nanofibres Containing Centella Asiatica Extract in a Rat Model. J. Tissue Eng. Regen. Med. 2017, 11, 905–915. [Google Scholar] [CrossRef]
- Yang, S.B.; Yoo, S.H.; Rabbani, M.M.; Kim, I.K.; Oh, W.; Han, S.I.; Yeum, J.H. Incorporation of Sorghum Extract into Electrospun Zein Nanofibers and Their Characterization. J. Nanosci. Nanotechnol. 2017, 17, 9002–9008. [Google Scholar] [CrossRef]
- Choi, J.I.; Kim, M.S.; Chun, G.Y.; Shin, H.S. Spirulina Extract-Impregnated Alginate-PCL Nanofiber Wound Dressing for Skin Regeneration. Biotechnol. Bioprocess Eng. 2017, 22, 679–685. [Google Scholar] [CrossRef]
- Sadri, M.; Arab-Sorkhi, S.; Vatani, H.; Bagheri-Pebdeni, A. New Wound Dressing Polymeric Nanofiber Containing Green Tea Extract Prepared by Electrospinning Method. Fibers Polym. 2015, 16, 1742–1750. [Google Scholar]
- Yang, S.B.; Kim, E.H.; Kim, S.H.; Kim, Y.H.; Oh, W.; Lee, J.T.; Jang, Y.A.; Sabina, Y.; Ji, B.C.; Yeum, J.H. Electrospinning Fabrication of Poly(Vinyl Alcohol)/Coptis Chinensis Extract Nanofibers for Antimicrobial Exploits. Nanomaterials 2018, 8, 734. [Google Scholar] [CrossRef]
- Pourhojat, F.; Sohrabi, M.; Shariati, S.; Mahdavi, H.; Asadpour, L. Evaluation of Poly ε-Caprolactone Electrospun Nanofibers Loaded with Hypericum Perforatum Extract as a Wound Dressing. Res. Chem. Intermed. 2017, 43, 297–320. [Google Scholar] [CrossRef]
- Aruan, N.M.; Sriyanti, I.; Edikresnha, D.; Suciati, T.; Munir, M.M.; Khairurrijal, K. Polyvinyl Alcohol/Soursop Leaves Extract Composite Nanofibers Synthesized Using Electrospinning Technique and Their Potential as Antibacterial Wound Dressing. Procedia Eng. 2017, 170, 31–35. [Google Scholar] [CrossRef]
- Mirzaei, E.; Sarkar, S.; Rezayat, S.M.; Faridi-Majidi, R. Herbal Extract Loaded Chitosan-Based Nanofibers as a Potential Wound-Dressing. J. Adv. Med. Sci. Appl. Technol. 2016, 2, 141. [Google Scholar] [CrossRef]
- Ramalingam, R.; Dhand, C.; Leung, C.M.; Ong, S.T.; Annamalai, S.K.; Kamruddin, M.; Verma, N.K.; Ramakrishna, S.; Lakshminarayanan, R.; Arunachalam, K.D. Antimicrobial Properties and Biocompatibility of Electrospun Poly-ε-Caprolactone Fibrous Mats Containing Gymnema Sylvestre Leaf Extract. Mater. Sci. Eng. C 2019, 98, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Calendula Officinalis Extract/PCL/Zein/Gum Arabic Nanofibrous Bio-Composite Scaffolds via Suspension, Two-Nozzle and Multilayer Electrospinning for Skin Tissue Engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Avci, H.; Monticello, R.; Kotek, R. Preparation of Antibacterial PVA and PEO Nanofibers Containing Lawsonia Inermis (Henna) Leaf Extracts. J. Biomater. Sci. Polym. Ed. 2013, 24, 1815–1830. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Pradeepa, P. Development and Characterization of Nanofibrous Mat from PVA/Tridax Procumbens (TP) Leaves Extracts. Wound Med. 2017, 19, 15–22. [Google Scholar] [CrossRef]
- Lin, S.; Chen, M.; Jiang, H.; Fan, L.; Sun, B.; Yu, F.; Yang, X.; Lou, X.; He, C.; Wang, H. Green Electrospun Grape Seed Extract-Loaded Silk Fibroin Nanofibrous Mats with Excellent Cytocompatibility and Antioxidant Effect. Colloids Surf. B Biointerfaces 2016, 139, 156–163. [Google Scholar] [CrossRef]
- Khanzada, H.; Salam, A.; Qadir, M.B.; Phan, D.N.; Hassan, T.; Munir, M.U.; Pasha, K.; Hassan, N.; Khan, M.Q.; Kim, I.S. Fabrication of Promising Antimicrobial Aloe Vera/PVA Electrospun Nanofibers for Protective Clothing. Materials 2020, 13, 3884. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Ekennia, A.C.; Katata-Seru, L.; Ebokaiwe, A.P.; Ijomone, O.M.; Onwudiwe, D.C.; Ebenso, E.E. Antimicrobial and Wound Healing Properties of Polyacrylonitrile-Moringa Extract Nanofibers. ACS Omega 2018, 3, 4791–4797. [Google Scholar] [CrossRef]
- Sriyanti, I.; Edikresnha, D.; Rahma, A.; Munir, M.M.; Rachmawati, H.; Khairurrijal, K. Mangosteen Pericarp Extract Embedded in Electrospun PVP Nanofiber Mats: Physicochemical Properties and Release Mechanism of α-Mangostin. Int. J. Nanomed. 2018, 13, 4927–4941. [Google Scholar] [CrossRef]
- Koushki, P.; Bahrami, S.H.; Ranjbar-Mohammadi, M. Coaxial Nanofibers from Poly(Caprolactone)/Poly(Vinyl Alcohol)/Thyme and Their Antibacterial Properties. J. Ind. Text. 2018, 47, 834–852. [Google Scholar] [CrossRef]
- Ali, A.; Shahid, M.A.; Hossain, M.D.; Islam, M.N. Antibacterial Bi-Layered Polyvinyl Alcohol (PVA)-Chitosan Blend Nanofibrous Mat Loaded with Azadirachta Indica (Neem) Extract. Int. J. Biol. Macromol. 2019, 138, 13–20. [Google Scholar] [CrossRef]
- Motealleh, B.; Zahedi, P.; Rezaeian, I.; Moghimi, M.; Abdolghaffari, A.H.; Zarandi, M.A. Morphology, Drug Release, Antibacterial, Cell Proliferation, and Histology Studies of Chamomile-Loaded Wound Dressing Mats Based on Electrospun Nanofibrous Poly(ε-Caprolactone)/Polystyrene Blends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.H.; Cho, J.Y. Myocardial Tissue Engineering Using Electrospun Nanofiber Composites. BMB Rep. 2016, 49, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.A.T.; do Vale Baracho, N.C.; de Brito, J.; de Queiroz, A.A.A. Hyperbranched Polyglycerol Electrospun Nanofibers for Wound Dressing Applications. Acta Biomater. 2010, 6, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Soleimani, M.; Vossoughi, M.; Ranjbarvan, P.; Hamedi, S.; Zamanlui, S.; Mahmoudifard, M. Study of Epithelial Differentiation and Protein Expression of Keratinocyte-Mesenchyme Stem Cell Co-Cultivation on Electrospun Nylon/B. Vulgaris Extract Composite Scaffold. Mater. Sci. Eng. C 2017, 75, 653–662. [Google Scholar] [CrossRef]
- Namboodiri, A.G.; Parameswaran, R. Fibro-Porous Polycaprolactone Membrane Containing Extracts of Biophytum Sensitivum: A Prospective Antibacterial Wound Dressing. J. Appl. Polym. Sci. 2013, 129, 2280–2286. [Google Scholar] [CrossRef]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Annamalai, S.K.; Arunachalam, K.D.; Ramakrishna, S. Tissue Engineered Plant Extracts as Nanofibrous Wound Dressing. Biomaterials 2013, 34, 724–734. [Google Scholar] [CrossRef]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate-Lavender Nanofibers with Antibacterial and Anti-Inflammatory Activity to Effectively Promote Burn Healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar]
- Liakos, I.; Rizzello, L.; Hajiali, H.; Brunetti, V.; Carzino, R.; Pompa, P.P.; Athanassiou, A.; Mele, E. Fibrous Wound Dressings Encapsulating Essential Oils as Natural Antimicrobial Agents. J. Mater. Chem. B 2015, 3, 1583–1589. [Google Scholar] [CrossRef]
- Rafiq, M.; Hussain, T.; Abid, S.; Nazir, A.; Masood, R. Development of Sodium Alginate/PVA Antibacterial Nanofibers by the Incorporation of Essential Oils. Mater. Res. Express 2018, 5, 035007. [Google Scholar] [CrossRef]
- Ardekani, N.T.; Khorram, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of Electrospun Poly (Vinyl Alcohol)-Based Nanofiber Mats Incorporated with Zataria Multiflora Essential Oil as Potential Wound Dressing. Int. J. Biol. Macromol. 2019, 125, 743–750. [Google Scholar] [CrossRef]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin-Loaded Poly(ε-Caprolactone) Nanofibres: Diabetic Wound Dressing with Anti-Oxidant and Anti-Inflammatory Properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannopoulos, K.N.; Assimopoulou, A.N.; Tsivintzelis, I.; Panayiotou, C.; Papageorgiou, V.P. Electrospun Fiber Mats Containing Shikonin and Derivatives with Potential Biomedical Applications. Int. J. Pharm. 2011, 409, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.H.; Peng, L.H.; Liu, X.; Chen, X.; Xiong, J.; Gao, J.Q. Silk Fibroin/Gelatin Electrospun Nanofibrous Dressing Functionalized with Astragaloside IV Induces Healing and Anti-Scar Effects on Burn Wound. Int. J. Pharm. 2015, 479, 291–301. [Google Scholar] [CrossRef]
- Zeng, J.; Ji, Q.; Liu, X.; Yuan, M.; Yuan, M.; Qin, Y. Electrospun Polylactic Acid /Poly (ε-Caprolactone) Fibrous Encapsulated Thymol/MIL-68(Al) as a Food Packaging Material. J. Mater. Res. Technol. 2022, 18, 5032–5044. [Google Scholar]
- Akhmetova, A.; Heinz, A. Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges. Pharmaceutics 2021, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- DeFrates, K.G.; Moore, R.; Borgesi, J.; Lin, G.; Mulderig, T.; Beachley, V.; Hu, X. Protein-Based Fiber Materials in Medicine: A Review. Nanomaterials 2018, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Orue, I.; Gainza, G.; Gutierrez, F.B.; Aguirre, J.J.; Evora, C.; Pedraz, J.L.; Hernandez, R.M.; Delgado, A.; Igartua, M. Novel Nanofibrous Dressings Containing RhEGF and Aloe Vera for Wound Healing Applications. Int. J. Pharm. 2017, 523, 556–566. [Google Scholar] [CrossRef]
- Iacob, A.T.; Drăgan, M.; Ionescu, O.M.; Profire, L.; Ficai, A.; Andronescu, E.; Confederat, L.G.; Lupașcu, D. An Overview of Biopolymeric Electrospun Nanofibers Based on Polysaccharides for Wound Healing Management. Pharmaceutics 2020, 12, 983. [Google Scholar] [CrossRef]
- Alturki, A.M. Rationally Design of Electrospun Polysaccharides Polymeric Nanofiber Webs by Various Tools for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2021, 184, 648–665. [Google Scholar] [CrossRef]
- Anaya Mancipe, J.M.; Boldrini Pereira, L.C.; de Miranda Borchio, P.G.; Dias, M.L.; da Silva Moreira Thiré, R.M. Novel Polycaprolactone (PCL)-Type I Collagen Core-Shell Electrospun Nanofibers for Wound Healing Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 111, 366–381. [Google Scholar] [CrossRef]
- Peng, Y.; Ma, Y.; Bao, Y.; Liu, Z.; Chen, L.; Dai, F.; Li, Z. Electrospun PLGA/SF/Artemisinin Composite Nanofibrous Membranes for Wound Dressing. Int. J. Biol. Macromol. 2021, 183, 68–78. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun Nanofibers of Natural and Synthetic Polymers as Artificial Extracellular Matrix for Tissue Engineering. Nanomaterials 2021, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rai, V.K.; Narang, R.K.; Markandeywar, T.S. Collagen-Based Formulations for Wound Healing: A Literature Review. Life Sci. 2022, 290, 120096. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun Chitosan Membranes Containing Bioactive and Therapeutic Agents for Enhanced Wound Healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Almasian, A.; Najafi, F.; Eftekhari, M.; Ardekani, M.R.S.; Sharifzadeh, M.; Khanavi, M. Polyurethane/Carboxymethylcellulose Nanofibers Containing Malva Sylvestris Extract for Healing Diabetic Wounds: Preparation, Characterization, In Vitro and in Vivo Studies. Mater. Sci. Eng. C 2020, 114, 111039. [Google Scholar] [CrossRef]
- Muhamed, I.; Sproul, E.P.; Ligler, F.S.; Brown, A.C. Fibrin Nanoparticles Coupled with Keratinocyte Growth Factor Enhance the Dermal Wound-Healing Rate. ACS Appl. Mater. Interfaces 2019, 11, 3771–3780. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Li, Y.; Wang, M.; Fan, T.; Liu, M.; Ke, Q.; Xu, H.; Yi, Z. An Aligned Porous Electrospun Fibrous Scaffold with Embedded Asiatic Acid for Accelerating Diabetic Wound Healing. J. Mater. Chem. B 2019, 7, 6125–6138. [Google Scholar] [CrossRef]
- Barbu, A.; Neamtu, B.; Zăhan, M.; Iancu, G.M.; Bacila, C.; Mireșan, V. Current Trends in Advanced Alginate-Based Wound Dressings for Chronic Wounds. J. Pers. Med. 2021, 11, 890. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, W.; Wang, C.; Zheng, H.; Liu, Z.; Chen, Z.; Gao, C. Methylcobalamin-Loaded PLCL Conduits Facilitate the Peripheral Nerve Regeneration. Macromol. Biosci. 2020, 20, 382. [Google Scholar] [CrossRef]
- Abdel Khalek, M.A.; Abdel Gaber, S.A.; El-Domany, R.A.; El-Kemary, M.A. Photoactive Electrospun Cellulose Acetate/Polyethylene Oxide/Methylene Blue and Trilayered Cellulose Acetate/Polyethylene Oxide/Silk Fibroin/Ciprofloxacin Nanofibers for Chronic Wound Healing. Int. J. Biol. Macromol. 2021, 193, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- Eren Boncu, T.; Ozdemir, N. Electrospinning of Ampicillin Trihydrate Loaded Electrospun PLA Nanofibers I: Effect of Polymer Concentration and PCL Addition on Its Morphology, Drug Delivery and Mechanical Properties. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 669–676. [Google Scholar] [CrossRef]
- Valachová, K.; El Meligy, M.A.; Šoltés, L. Hyaluronic Acid and Chitosan-Based Electrospun Wound Dressings: Problems and Solutions. Int. J. Biol. Macromol. 2022, 206, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.G.; Kim, I. Recent Advances in Poly(α-L-Glutamic Acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef]
- Sabarees, G.; Tamilarasi, G.P.; Velmurugan, V.; Alagarsamy, V.; Sibuh, B.Z.; Sikarwar, M.; Taneja, P.; Kumar, A.; Gupta, P.K. Emerging Trends in Silk Fibroin Based Nanofibers for Impaired Wound Healing. J. Drug Deliv. Sci. Technol. 2023, 79, 103994. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Cucchiarini, M. PEO-PPO-PEO Tri-Block Copolymers for Gene Delivery Applications in Human Regenerative Medicine—An Overview. Int. J. Mol. Sci. 2018, 19, 775. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.D.; Elmer, J.; Harris, D.R.; Huntley, J.; Palmer, A.F.; Nelson, T.; Johnson, J.K.; Xue, R.; Lannutti, J.J.; Viapiano, M.S. Hemoglobin Regulates the Migration of Glioma Cells along Poly(ε-Caprolactone)-Aligned Nanofibers. Biotechnol. Prog. 2014, 30, 1214–1220. [Google Scholar] [CrossRef]
- Rivera-Hernández, G.; Antunes-Ricardo, M.; Martínez-Morales, P.; Sánchez, M.L. Polyvinyl Alcohol Based-Drug Delivery Systems for Cancer Treatment. Int. J. Pharm. 2021, 600, 120478. [Google Scholar] [CrossRef]
- Mistry, P.; Chhabra, R.; Muke, S.; Narvekar, A.; Sathaye, S.; Jain, R.; Dandekar, P. Fabrication and Characterization of Starch-TPU Based Nanofibers for Wound Healing Applications. Mater. Sci. Eng. C 2021, 119, 111316. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, Y.; Zhang, L.; Zhang, Y.; Liu, J.; Yu, P. Poly Ethylene Glycol (PEG)-Related Controllable and Sustainable Antidiabetic Drug Delivery Systems. Eur. J. Med. Chem. 2021, 217, 113372. [Google Scholar] [CrossRef]
- Wen, Q.; Mithieux, S.M.; Weiss, A.S. Elastin Biomaterials in Dermal Repair. Trends Biotechnol. 2020, 38, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical Assessment of Polyvinylpyrrolidone (PVP): As Excipient from Conventional to Controlled Delivery Systems with a Spotlight on COVID-19 Inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Giacomazza, D.; Carzino, R.; Goldoni, L.; Scoponi, G.; et al. Electrospun Polyvinylpyrrolidone (PVP) Hydrogels Containing Hydroxycinnamic Acid Derivatives as Potential Wound Dressings. Chem. Eng. J. 2021, 409, 128144. [Google Scholar]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of Plant Alkaloids on Human Health: A Review of Biological Activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Tanase, C.; Cosarcă, S.; Muntean, D.L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential Use of Phenolic Acids as Anti-Candida Agents: A Review. Front. Microbiol. 2015, 6, 5377. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Chrzanowski, W.; Ostrikov, K. Anti-Bacterial Surfaces: Natural Agents, Mechanisms of Action, and Plasma Surface Modification. RSC Adv. 2015, 5, 48739–48759. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent Advances on Antimicrobial Wound Dressing: A Review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.; Gupta, B. Scar Free Healing Mediated by the Release of Aloe Vera and Manuka Honey from Dextran Bionanocomposite Wound Dressings. Int. J. Biol. Macromol. 2018, 120, 1581–1590. [Google Scholar] [CrossRef]
- Miguel, S.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and Characterization of Electrospun Silk Fibroin Based Asymmetric Membranes for Wound Dressing Applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef]
- Vakilian, S.; Norouzi, M.; Soufi-Zomorrod, M.; Shabani, I.; Hosseinzadeh, S.; Soleimani, M.L. Inermis-Loaded Nanofibrous Scaffolds for Wound Dressing Applications. Tissue Cell 2018, 51, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Ghosh, C.; Hwang, S.G.; Tran, L.D.; Park, J.S. Characteristics of Curcumin-Loaded Poly (Lactic Acid) Nanofibers for Wound Healing. J. Mater. Sci. 2013, 48, 7125–7133. [Google Scholar] [CrossRef]
- Liu, G.S.; Yan, X.; Yan, F.F.; Chen, F.X.; Hao, L.Y.; Chen, S.J.; Lou, T.; Ning, X.; Long, Y.Z. In Situ Electrospinning Iodine-Based Fibrous Meshes for Antibacterial Wound Dressing. Nanoscale Res. Lett. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Mahmud, M.M.; Zaman, S.; Perveen, A.; Jahan, R.A.; Islam, M.F.; Arafat, M.T. Controlled Release of Curcumin from Electrospun Fiber Mats with Antibacterial Activity. J. Drug Deliv. Sci. Technol. 2020, 55, 101386. [Google Scholar] [CrossRef]
- Bui, H.T.; Chung, O.H.; Dela Cruz, J.; Park, J.S. Fabrication and Characterization of Electrospun Curcumin-Loaded Polycaprolactone-Polyethylene Glycol Nanofibers for Enhanced Wound Healing. Macromol. Res. 2014, 22, 1288–1296. [Google Scholar] [CrossRef]
- Bakhshi, H.; Yeganeh, H.; Mehdipour-Ataei, S.; Shokrgozar, M.A.; Yari, A.; Saeedi-Eslami, S.N. Synthesis and Characterization of Antibacterial Polyurethane Coatings from Quaternary Ammonium Salts Functionalized Soybean Oil Based Polyols. Mater. Sci. Eng. C 2013, 33, 153–164. [Google Scholar] [CrossRef]
- Mutlu, G.; Calamak, S.; Ulubayram, K.; Guven, E. Curcumin-Loaded Electrospun PHBV Nanofibers as Potential Wound-Dressing Material. J. Drug Deliv. Sci. Technol. 2018, 43, 185–193. [Google Scholar] [CrossRef]
- Tamilarasi, G.P.; Krishnan, M.; Sabarees, G.; Gouthaman, S. Emerging Trends in Curcumin Embedded Electrospun Nanofibers for Impaired Diabetic Wound Healing. Appl. Nano 2022, 3, 202–232. [Google Scholar] [CrossRef]
- Urena-Saborio, H.; Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Gunasekaran, S. Electrospun Plant Mucilage Nanofibers as Biocompatible Scaffolds for Cell Proliferation. Int. J. Biol. Macromol. 2018, 115, 1218–1224. [Google Scholar] [CrossRef]
- Dong, W.H.; Liu, J.X.; Mou, X.J.; Liu, G.S.; Huang, X.W.; Yan, X.; Ning, X.; Russell, S.J.; Long, Y.Z. Performance of Polyvinyl Pyrrolidone-Isatis Root Antibacterial Wound Dressings Produced in Situ by Handheld Electrospinner. Colloids Surf. B Biointerfaces 2020, 188, 110766. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Gharakhloo, M.; Agheb, M.; Khodabakhshi, D.; Khorshidi, M.; Sheikhmoradi, V.; Rafienia, M.; Salehi, H. A Propolis Enriched Polyurethane-Hyaluronic Acid Nanofibrous Wound Dressing with Remarkable Antibacterial and Wound Healing Activities. Int. J. Biol. Macromol. 2020, 149, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Baghersad, S.; Hivechi, A.; Bahrami, S.H.; Brouki Milan, P.; Siegel, R.A.; Amoupour, M. Optimal Aloe Vera Encapsulated PCL/Gel Nanofiber Design for Skin Substitute Application and the Evaluation of Its in Vivo Implantation. J. Drug Deliv. Sci. Technol. 2022, 74, 103536. [Google Scholar]
- Farahani, H.; Barati, A.; Arjomandzadegan, M.; Vatankhah, E. Nanofibrous Cellulose Acetate/Gelatin Wound Dressing Endowed with Antibacterial and Healing Efficacy Using Nanoemulsion of Zataria Multiflora. Int. J. Biol. Macromol. 2020, 162, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Fabrication and Characterization of PCL/Zein/Gum Arabic Electrospun Nanocomposite Scaffold for Skin Tissue Engineering. Mater. Sci. Eng. C 2018, 93, 356–366. [Google Scholar] [CrossRef]
- Selvaraj, S.; Duraipandy, N.; Kiran, M.S.; Fathima, N.N. Anti-Oxidant Enriched Hybrid Nanofibers: Effect on Mechanical Stability and Biocompatibility. Int. J. Biol. Macromol. 2018, 117, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Abou Zekry, S.S.; Abdellatif, A.; Azzazy, H.M.E. Fabrication of Pomegranate/Honey Nanofibers for Use as Antibacterial Wound Dressings. Wound Med. 2020, 28, 100181. [Google Scholar] [CrossRef]
- Adeli-Sardou, M.; Yaghoobi, M.M.; Torkzadeh-Mahani, M.; Dodel, M. Controlled Release of Lawsone from Polycaprolactone/Gelatin Electrospun Nano Fibers for Skin Tissue Regeneration. Int. J. Biol. Macromol. 2019, 124, 478–491. [Google Scholar]
- Haghighi, Z.; Asadi, M. The Effects of Chitosan-Based Nanofibers/PEO/Henna Extract on Recovery of Superficial Second-Degree Burn in Rat. Mbtj 2019, 3, 26–28. [Google Scholar]
- Faraji, S.; Nowroozi, N.; Nouralishahi, A.; Shabani Shayeh, J. Electrospun Poly-Caprolactone/Graphene Oxide/Quercetin Nanofibrous Scaffold for Wound Dressing: Evaluation of Biological and Structural Properties. Life Sci. 2020, 257, 118062. [Google Scholar] [CrossRef]
- Selvaraj, S.; Fathima, N.N. Fenugreek Incorporated Silk Fibroin Nanofibers—A Potential Antioxidant Scaffold for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 5916–5926. [Google Scholar]
- Cam, M.E.; Cesur, S.; Taskin, T.; Erdemir, G.; Kuruca, D.S.; Sahin, Y.M.; Kabasakal, L.; Gunduz, O. Fabrication, Characterization and Fibroblast Proliferative Activity of Electrospun Achillea Lycaonica-Loaded Nanofibrous Mats. Eur. Polym. J. 2019, 120, 109239. [Google Scholar] [CrossRef]
- Mouro, C.; Simões, M.; Gouveia, I.C.; Xu, B. Emulsion Electrospun Fiber Mats of PCL/PVA/Chitosan and Eugenol for Wound Dressing Applications. Adv. Polym. Technol. 2019, 2019, 9859506. [Google Scholar]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Sukma, M.; Opanasopit, P. Electrospun Chitosan-Based Nanofiber Mats Loaded with Garcinia Mangostana Extracts. Int. J. Pharm. 2013, 452, 333–343. [Google Scholar]
- Ahlawat, J.; Kumar, V.; Gopinath, P. Carica Papaya Loaded Poly (Vinyl Alcohol)-Gelatin Nanofibrous Scaffold for Potential Application in Wound Dressing. Mater. Sci. Eng. C 2019, 103, 109834. [Google Scholar]
- Manotham, S.; Pengpat, K.; Eitssayeam, S.; Rujijanagul, G.; Sweatman, D.R.; Tunkasiri, T. Fabrication of Polycaprolactone/Centella asIatica Extract Biopolymer Nanofiber by Electrospinning. Appl. Mech. Mater. 2015, 804, 151–154. [Google Scholar]
- Naeimi, A.; Payandeh, M.; Ghara, A.R.; Ghadi, F.E. In Vivo Evaluation of the Wound Healing Properties of Bio-Nanofiber Chitosan/Polyvinyl Alcohol Incorporating Honey and Nepeta Dschuparensis. Carbohydr. Polym. 2020, 240, 116315. [Google Scholar]
- Han, J.; Chen, T.X.; Branford-White, C.J.; Zhu, L.M. Electrospun Shikonin-Loaded PCL/PTMC Composite Fiber Mats with Potential Biomedical Applications. Int. J. Pharm. 2009, 382, 215–221. [Google Scholar]
- Safaee-Ardakani, M.R.; Hatamian-Zarmi, A.; Sadat, S.M.; Mokhtari-Hosseini, Z.B.; Ebrahimi-Hosseinzadeh, B.; Rashidiani, J.; Kooshki, H. Electrospun Schizophyllan/Polyvinyl Alcohol Blend Nanofibrous Scaffold as Potential Wound Healing. Int. J. Biol. Macromol. 2019, 127, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Kheradvar, S.A.; Nourmohammadi, J.; Tabesh, H.; Bagheri, B. Starch Nanoparticle as a Vitamin E-TPGS Carrier Loaded in Silk Fibroin-Poly(Vinyl Alcohol)-Aloe Vera Nanofibrous Dressing. Colloids Surf. B Biointerfaces 2018, 166, 9–16. [Google Scholar] [CrossRef]
- Cai, Z.X.; Mo, X.M.; Zhang, K.H.; Fan, L.P.; Yin, A.L.; He, C.L.; Wang, H.S. Fabrication of Chitosan/Silk Fibroin Composite Nanofibers for Wound-Dressing Applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial Performance and in Vivo Diabetic Wound Healing of Curcumin Loaded Gum Tragacanth/Poly(ε-Caprolactone) Electrospun Nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef]
- Xu, F.; Weng, B.; Gilkerson, R.; Materon, L.A.; Lozano, K. Development of Tannic Acid/Chitosan/Pullulan Composite Nanofibers from Aqueous Solution for Potential Applications as Wound Dressing. Carbohydr. Polym. 2015, 115, 16–24. [Google Scholar] [CrossRef]
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Amiri Darban, S.; Fazly Bazzaz, B.S.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-Loaded Chitosan-PEO Nanofibers for Local Antibiotic Delivery and Wound Healing. Int. J. Biol. Macromol. 2020, 162, 645–656. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Liu, X.; Mao, J.; Gu, S.; Xu, W. Electrospinning of Carboxyethyl Chitosan/Poly(Vinyl Alcohol)/Silk Fibroin Nanoparticles for Wound Dressings. Int. J. Biol. Macromol. 2013, 53, 88–92. [Google Scholar] [CrossRef]
| Herbal Product Extract | Polymers for Electrospinning | Type of Study | Biological Properties | Ref. |
|---|---|---|---|---|
| Centella asiatica | Gelatin, CA, PCL | In vitro | Wound healing, Burns, Skin lesions, Antibacterial | [43] |
| Sorghum bicolor | Zein | In vitro | Antioxidant, Antibacterial | [44] |
| Spirulina agilis | PCL, Alginate | In vitro, in vivo | Anti-inflammatory, Antibacterial, Wound healing | [45] |
| Camellia sinensis | CS, PEO | In vitro, in vivo | Anti-inflammatory, Antibacterial, Antioxidant | [46] |
| Coptis chinensis | PVA | In vitro | Antifungal, Anti-inflammatory, Antioxidant | [47] |
| Hypericum perforatum | PCL | In vitro | Wound healing burns, Antibacterial, Antioxidant | [48] |
| Sorghum bicolor | PVA | In vitro | Treatment of skin infection, Antibacterial | [49] |
| Melilotus officinalis | CS, PEO | In vitro | Antibacterial, Treatment of chronic wounds | [50] |
| Gymnema sylvestre | PCL | In vitro | Antibacterial, Anti-inflammatory, Wound healing | [51] |
| Senegalia senegal | PCL | In vitro | Antibacterial, Wound healing | [52] |
| Lawsonia inermis | CS, PEO, PVA, PEO | In vitro, in vivo | Antioxidant, Analgesic, Anti-inflammatory, Antibacterial | [53] |
| Tridax procumbens | PVA | In vitro | Antibacterial, Wound healing | [54] |
| Vitis vinifera | SF, PEO | In vitro, in vivo | Wound healing, Antioxidant | [55] |
| Aloe barbadensis miller | PLGA, CS, PVA | In vitro, in vivo | Antifungal, Anti-inflammatory, Antibacterial | [56] |
| Moringa oleifera | PAN | In vitro, in vivo | Antibacterial, Wound healing | [57] |
| Garcinia mangostana | PLA, CS, PVA, PVP | In vitro, in vivo | Antibacterial, Anti-inflammatory, Antioxidant | [58] |
| Zataria multiflora | CS, PVA, PCL, PVA | In vitro, in vitro | Antibacterial, Antifungal, Anti-inflammatory, Anticoagulant | [59] |
| Querqus infectoria | PVA | In vitro | Antioxidant, Antiseptic, Anti-diabetic, Antibacterial, Antifungal | [60] |
| Matricaria chamomilla | PCL, PS | In vitro, in vivo | Antibacterial, Wound healing | [61] |
| Juniperus chinensis | PVA | In vitro | Antibacterial, Wound healing | [62] |
| Calendula officinalis | PG, PCL | In vitro, in vivo | Anti-inflammatory, Wound healing, Antibacterial | [63] |
| Beta vulgaris | Nylon 66 | In vitro | Antimicrobial, Wound healing | [64] |
| Biophytum sensitivum | PCL | In vitro | Anti-inflammatory, Anti-diabetic, Antiseptic | [65] |
| Azadirachta indica | PCL | In vitro | Antimicrobial, Wound healing | [66] |
| Lavandula angustifolia | NaAlg, PAN, PVA | In vitro, in vivo | Antimicrobial, Anti-inflammatory, Pain relieving | [67] |
| Cinnamomum verum | PVA, NaAlg, CA | In vitro, In vitro | Antimicrobial, Wound healing | [68] |
| Syzygium aromaticum | PVA, NaAlg | In vitro | Antimicrobial, Antimicrobial | [69] |
| Zataria multiflora | Gelatin, PVP, CS | In vitro | Antibacterial, Antifungal, Anti-inflammatory, Anticoagulant | [70] |
| Curcuma longa | PLA, PCL, PEG, PU | In vitro, in vivo | Anticoagulant, Antioxidant, Antibacterial, Antifungal | [71] |
| Alkannin/shikonin | CA, PLA, PGA | In vitro | Antioxidant, Anti-inflammatory, Antibacterial, Wound healing | [72] |
| Astragaluspropinquus | SF, gelatin | In vitro | Antimicrobial, Wound healing | [73] |
| Zataria multiflora | PCL, PLA | In vitro, in vivo | Wound healing, Antibacterial | [74] |
| Biopolymers | Advantages | Ref. | Synthetic Polymers | Advantages | Ref. |
|---|---|---|---|---|---|
| Collagen | • Natural protein | [84] | PCL | • Good electrospinning properties | [81] |
| • Biocompatible | • Soluble in most the organic solvents | ||||
| • Biodegradable | • Biocompatible | ||||
| • Low antigenicity | • Biodegradable | ||||
| • Cheaper | • FDA approved | ||||
| • Antithrombogenic | • Good mechanical property | ||||
| • Mimics native ECM | |||||
| Gelatin | • Biocompatible | [85] | PLGA | • Cytocompatible | [82] |
| • Biodegradable | • FDA approved | ||||
| • Low antigenicity | • Soluble in most the organic solvents | ||||
| • Cheaper | • Excellent antiadhesive property | ||||
| • Antithrombogenic | |||||
| Chitosan | • Biocompatible | [86] | PU | • Creates a moist environment | [87] |
| • Bioactive | • Suitable coverage for burns | ||||
| • Biodegradable | • Good mechanical strength | ||||
| • Bactericidal material | |||||
| • Hydrophilic material | |||||
| • Nontoxic | |||||
| • Degradable by enzymes (chitosanase and lysozyme) | |||||
| Fibronectin/fibrin | • Adjustable mechanical properties | [88] | PLLA | • FDA approved | [89] |
| • Hemostatic properties | • Excellent cellular compatibility | ||||
| • Soluble in most the organic solvents | |||||
| • Suitable for drug delivery | |||||
| Alginate | • Biocompatible | [90] | PLCL | • FDA approved | [91] |
| • Biodegradable | • Suitable for drug delivery | ||||
| • Nontoxicity | • Good mechanical strength | ||||
| • Water soluble | |||||
| • Nonimmunogenic | |||||
| • Inexpensive | |||||
| • Simply cross-linked | |||||
| • High stability | |||||
| • Zero shear viscosity | |||||
| Cellulose | • Biocompatible | [92] | PLA | • Good ductility | [93] |
| • Biodegradable | • Good biocompatibility | ||||
| • Mechanical stability | • Good processability | ||||
| • Cost-effectiveness | • Biodegradability | ||||
| • Hydrophilic nature | • Bioresorbability | ||||
| • Purity | |||||
| Hyaluronic acid | • Biodegradable | [94] | PGA | • Supports various cell types | [95] |
| • Biocompatibility | • Good ductility | ||||
| • Biopolymers are present in the majority of living organisms | • Good processability | ||||
| • Bioresorbability | |||||
| • Biodegradability | |||||
| • Good biocompatibility | |||||
| Silk fibroin | • Biocompatibility | [96] | PEO | • Easy modified | [97] |
| • Water vapor transmission rate | • Biocompatible | ||||
| • Water retention capacity | • Hydrophilic | ||||
| • Elasticity | |||||
| Myoglobin/Hemoglobin | • Biocompatible | [98] | PVA | • High-temperature stability | [99] |
| • Excellent oxygen permeation | • Long-lasting durability | ||||
| • Alleviate wound hypoxia | • Relatively low-cost | ||||
| • Biodegradability | |||||
| • High solubility | |||||
| Starch | • Biodegradability | [100] | PEG | • Reasonable control over structural and compositional properties | [101] |
| • Low cost | |||||
| • Renewability | |||||
| • Hydrophilic | |||||
| Elastin | • High elasticity | [102] | PVP | • Low toxicity | [103] |
| • Half-life > 70 years, and the monomer can reversibly stretch up to eight times its resting length | • Excellent biocompatibility | ||||
| • Hydrophilic nature | |||||
| • Soluble in water/most organic solvents |
| Electrospun Nanofibrous Wound Dressings | Properties Investigated | Ref. |
|---|---|---|
| Chitosan/PEO/semelil (herbal extract drug) | release of semelil, high swelling | [134] |
| Chitosan/PVA/gelatin/Zataria multiflora | antibacterial properties, nontoxic and biocompatible | [70] |
| Cellulose acetate/gelatin/Zataria multiflora | antibacterial properties, wound healing, wound re-epithelialization, biocompatible | [138] |
| Vitamin E/starch nanoparticle/silk fibroin/PVA aloe Vera | nontoxic, biocompatible, release vitamin E, protect cells from toxic oxidation products | [139] |
| PVA/curcumin | nontoxic, biocompatible, antibacterial, release of curcumin | [140] |
| PVA/honey/curcumin | antibacterial activity, good moisture properties | [141] |
| PCL/gum tragacanth/curcumin | collagen deposition, regenerate epithelial layer, healing, fast wound closure | [142] |
| PCL/PVA/curcumin | antibacterial property, absorbable, biocompatible | [143] |
| Thiocarbohydrazide-modified gelatin/curcumin | nontoxic, antibacterial, release curcumin | [121] |
| PVA/gelatin/Carica papaya | hemocompatible, antibacterial, nontoxic, wound healing | [144] |
| PVA/mucilage | anti-inflammatory, promote cell growth and fibroblasts cells attachment, biocompatible | [119] |
| PLA/achillea lycaonica | compatible, nontoxic, release of achillea | [131] |
| PCL/gum arabic/Corn protein | antibacterial, biodegradable, porosity, good mechanical properties | [126] |
| Silk fibroin/Fenugreek (natural antioxidant) | antioxidant property, biocompatible, wound healing | [130] |
| Silk fibroin/soy protein isolate | nontoxic, biocompatible, biodegradable, wound healing activity | [124] |
| PCL/Gymnema sylvestre leave extract | antibacterial, biocompatible, mechanical properties, wettability | [51] |
| Polyvinyl pyrrolidone containing isatis root | Antibacterial, excellent wetting, permeable, active, wound closure | [120] |
| PCL/PVA/Chitosan/Eugenol | antibacterial action, biocompatible, nontoxic, release Eugenol | [132] |
| Silk fibroin-PCL/silk fibroin-hyaluronic acid, thymol | antioxidant and antibacterial properties, biocompatible, wound healing | [129] |
| Chitosan/PVA/honey/Nepeta dschuparensis | biodegradable, biocompatible, faster wound healing, tissue regeneration | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajith, G.; Tamilarasi, G.P.; Sabarees, G.; Gouthaman, S.; Manikandan, K.; Velmurugan, V.; Alagarsamy, V.; Solomon, V.R. Recent Developments in Electrospun Nanofibers as Delivery of Phytoconstituents for Wound Healing. Drugs Drug Candidates 2023, 2, 148-171. https://doi.org/10.3390/ddc2010010
Ajith G, Tamilarasi GP, Sabarees G, Gouthaman S, Manikandan K, Velmurugan V, Alagarsamy V, Solomon VR. Recent Developments in Electrospun Nanofibers as Delivery of Phytoconstituents for Wound Healing. Drugs and Drug Candidates. 2023; 2(1):148-171. https://doi.org/10.3390/ddc2010010
Chicago/Turabian StyleAjith, Govindaraj, Ganesan Padmini Tamilarasi, Govindaraj Sabarees, Siddan Gouthaman, Krishnan Manikandan, Vadivel Velmurugan, Veerachamy Alagarsamy, and Viswas Raja Solomon. 2023. "Recent Developments in Electrospun Nanofibers as Delivery of Phytoconstituents for Wound Healing" Drugs and Drug Candidates 2, no. 1: 148-171. https://doi.org/10.3390/ddc2010010
APA StyleAjith, G., Tamilarasi, G. P., Sabarees, G., Gouthaman, S., Manikandan, K., Velmurugan, V., Alagarsamy, V., & Solomon, V. R. (2023). Recent Developments in Electrospun Nanofibers as Delivery of Phytoconstituents for Wound Healing. Drugs and Drug Candidates, 2(1), 148-171. https://doi.org/10.3390/ddc2010010









