Abstract
Background: Atherosclerosis (AS) is an important pathological basis of many cardiovascular diseases. Canagliflozin and dapagliflozin have yielded impressive results in the treatment of cardiovascular disease in both diabetic and non-diabetic patients. In this study, we investigated their targets and mechanism involved in the treatment of atherosclerosis using network pharmacology. Methods: The potential targets of canagliflozin and dapagliflozin were gathered from the database PharmMapper. Targets associated with AS were derived from the GeneCards, Drugbank, DisGeNet, and therapeutic target databases (TTD) by searching for keywords on atherosclerosis and coronary artery disease. Overlap targets were collected by uploading drug and disease targets into jvenn. The cross-targets of the Venny plots were uploaded to the STRING database, and a protein–protein interaction (PPI) was constructed with their calculated features, aiming to reveal several key targets. Key targets were selected by using a plug-in of the Cytoscape software. Gene ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were performed using the database Metascape. Cytoscape was used to set up the pathways-genes network. Molecular docking with core targets and drugs was performed with AutoDock. Results: A total of 288 canagliflozin targets, 287 dapagliflozin targets and 4939 AS-related targets were obtained. A total of 191 overlapping targets were found after intersecting. Five core targets, including protein kinase B (Akt1), Mitogen-activated protein kinase 1 (MAPK1), Mitogen-activated protein kinase 14 (MAPK14), Proto-oncogene tyrosine-protein kinase SRC (SRC) and Epidermal growth factor receptor (EGFR) were collected. Pathways, biological processes, molecular functions and cellular components of canagliflozin and dapagliflozin were found. Conclusion: Canagliflozin and dapagliflozin play a role in atherosclerosis by regulating Akt1, MAPK1, MAPK14, SRC and EGFR. Our research provides further insights into the use of canagliflozin and dapagliflozin in the treatment of atherosclerosis.
1. Introduction
Atherosclerosis (AS) is a chronic inflammatory disease characterized by intense immune activity. Inflammation plays an important role in the occurrence and development of atherosclerotic lesions [1]. Atherosclerotic plaque formation is characterized by lipid accumulation, local inflammation, proliferation, apoptosis, necrosis and fibrosis of smooth muscle cells (SMCs) [2]. The main cause of atherosclerotic stenosis is the activation of inflammatory cells and a series of chronic inflammatory reactions after initial endothelial cell injury [3]. In the early stage of atherosclerosis, risk factors such as hyperlipidemia, diabetes, smoking and hypertension can induce an oxidative stress response, activate cytokines and increase LDL levels. Meanwhile, macrophages migrate to the vascular wall, and inflammatory stimulators promote endothelial dysfunction and initiate atherosclerotic lesions [4]. At present, the most effective therapeutic drugs for AS are statins, which reduce the levels of atherogenic lipoproteins and prevent major cardiovascular events. However, treatment with statins is ineffective in reducing cholesterol levels in a small proportion of users, and prolonged use of statins may increase the risk of side effects [5]. Therefore, it is urgent to find new anti-AS drugs of satisfactory therapeutic effect and suitable for long-term use.
Canagliflozin and dapagliflozin, known as sodium-glucose cotransporter-2 (SGLT2) inhibitors, are a class of anti-diabetic compounds that lower blood glucose by selectively blocking the reabsorption of glucose in the proximal convoluted tubule (PCT) [6]. Recently, a growing number of studies have shown that canagliflozin and dapagliflozin are surprisingly effective in improving cardiovascular and kidney disease in both diabetic and non-diabetic patients. New clinical outcome trials have demonstrated that glucose-lowering drugs, especially SGLT2 inhibitors, overall reduce the risk of fatal and non-fatal atherosclerotic cardiovascular events and all-cause mortality [7]. Similarly, in two trials involving patients with type 2 diabetes and an elevated risk of cardiovascular disease, canagliflozin reduced the risk for primary cardiovascular outcome compared with those who received placebos [8]. In patients with heart failure and reduced ejection fraction, regardless of diabetes, dapagliflozin reduced the number of hospitalizations for heart failure or cardiovascular death compared to those who received placebos [9]. Some evidence from animal models indicates that SGLT2 inhibitors could prevent AS. Canagliflozin attenuated the progression of AS in HFD-fed ApoE−/− mice [10]. In addition, dapagliflozin has been shown to inhibit AS in ApoE−/− mice [11].
Network pharmacology is based on the concept of a multilevel and multiangle interaction network of disease-gene-target-drug [12]. It is associated with multiple disciplines in systems biology, pharmacology, computational biology, and network analysis [13,14]. Professional databases, resources, and software were used to visualize the relationship between drug–drug interaction and drug–disease interaction. Network pharmacology broke the traditional concept of single drugs, single target and single disease, and proposes that drugs act on multiple targets and multiple pathways [15]. Thus, network pharmacology has been an effective way to search for new research drugs and discover their potential mechanisms.
In this article, we used network pharmacology to demonstrate the underlying mechanisms of canagliflozin and dapagliflozin in the management of atherosclerosis. Therefore, using a variety of databases, we systematically predicted and analyzed the complex relationship between the disease, drugs and targets. Meanwhile, we discussed the potential mechanisms of some important targets of canagliflozin and dapagliflozin in the treatment of atherosclerosis. This study provides a scientific basis for further research on SGLT2 inhibitors for cardiovascular diseases. The process for our study can be found in Figure S1.
2. Materials and Methods
2.1. Prediction of Targets for Canagliflozin and Dapagliflozin
Two-dimensional structures of canagliflozin and dapagliflozin were searched in the PubChem (https://pubchem.ncbi.nlm.nih.gov/, accessed on 11 August 2021) database. The potential targets of canagliflozin and dapagliflozin were gathered from the database PharmMapper (http://www.lilab-ecust.cn/pharmmapper/, accessed on 26 August 2021). Names of target proteins were translated into gene names in the UniProt (http://www.uniprot.org/, accessed on 26 August 2021) database [16]. Potential targets were deleted if their names were not found in the Uniprot database.
2.2. Collection of Disease Targets of Atherosclerosis
The AS-related genes were derived from the GeneCards (https://www.GeneCards.org/, accessed on 11 August 2021) [17], Drugbank [18], DisGeNet [19] and TTD [20] databases by searching with the key words of Atherosclerosis and Coronary artery disease. Duplicate genes were deleted after putting all target genes together.
2.3. Venn Diagram Plotting
The website of jvenn (http://jvenn.toulouse.inra.fr/app/index.html, accessed on 26 August 2021) was used to plot Venn diagram by uploading both drug targets and disease targets. Intersecting genes were the potential targets of canagliflozin and dapagliflozin, and they overlapped with Atherosclerosis [21].
2.4. Protein-Protein Interaction (PPI)
Intersecting genes from Venny plots were uploaded to the STRING database (https://string-db.org/, accessed on 27 August 2021), and Homo sapiens was selected for species [22]. The protein–protein interaction (PPI) networks of 191 genes were created with a high confidence level (interaction score > 0.700), and irrelevant targets were concealed. The result from STRING database was downloaded and imported into Cytoscape3.7.2 U.S. (https://cytoscape.org/, accessed on 27 August 2021) [23], then the topology parameters (degrees) of the nodes were calculated, and core targets were defined based on the degree. The potential targets of canagliflozin and dapagliflozin were imported into Cytoscape3.7.2 software to construct networks.
2.5. Gene Functions and Pathway Enrichment Analysis with Potential Targets
Gene ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were carried out using the database Metascape (https://metascape.org/gp/index.html#/main/step1, accessed on 30 August 2021) [24]. The top 20 results from the GO enrichment and KEGG pathway enrichment analyses were presented. GO enrichment analysis mainly covers three aspects of biology, namely biological process, molecular function and cellular component. It is widely used in the field of gene function classification and function distribution prediction of targets.
2.6. Construction of Target Gene-Drug Network
Target genes and the top 20 results of KEGG pathway enrichment analyses were analyzed by Cytoscape3.7.2 software for visual network analysis. In the network, nodes represented genes and pathways, and edges represented interactions between the nodes [21].
The importance of the gene or pathway is assessed by the topology parameter (degree). The degree of a node is the number of edges connected to the node, and the greater the degree, the more important the node is in the network.
2.7. Molecular Docking of the Target Gene
The rigid docking analysis was performed by AutoDock 4.2 with MGL tools 1.5.6 (The Scripps Research Institutes, San Diego, CA, USA). The PDB files of canagliflozin and dapagliflozin were produced using Chem3D Pro software. The crystal structures of AKT1 (PDBID: 6HHG), MAPK1 (PDBID: 4XJ0), MAPK14 (PDBID: 4L8M), RHOA (PDBID: 1A2B), SRC (PDBID: 2H8H) and EGFR (PDBID: 5UG9) were downloaded from RCSB Protein Data Bank. Following the requirement of the docking study, ions, water molecules and non-standard amino acid residues were removed from the proteins. For the docking case, the model with the lowest energy was selected as the binding mode for analysis. The output from AutoDock was rendered by the PyMol program [25].
3. Results
3.1. Network Construction of Drugs and Targets
A total of 288 canagliflozin targets and 287 dapagliflozin targets were identified by using the PharmMapper database. Target names were translated into gene names from the UniProt database. Details of these targets are listed in Tables S1 and S2, and the maps of the drug-target networks are shown in Figure 1. The hexagons represent canagliflozin and dapagliflozin, and the squares represent the targets.
Figure 1.
The targets of canagliflozin (A) and dapagliflozin (B). Note: hexagons represent drugs, squares represent targets.
3.2. Targets of Atherosclerosis
By searching for Atherosclerosis and Coronary artery disease in the database, 92 targets were found in the Drugbank database, 1061 targets were found in the DisGeNet database after screening for scores above the median, 4708 targets were found in the GeneCards database after screening for scores above the median, and 26 targets were found in the TTD database. A total of 4939 AS-related targets were found after the duplicates were deleted.
3.3. Prediction of Canagliflozin and Dapagliflozin Targets in Atherosclerosis
After uploading targets of drugs and disease to jvenn, 191 overlapping targets were found, as shown in Figure 2. These targets might be the key genes of canagliflozin and dapagliflozin in the treatment of atherosclerosis.
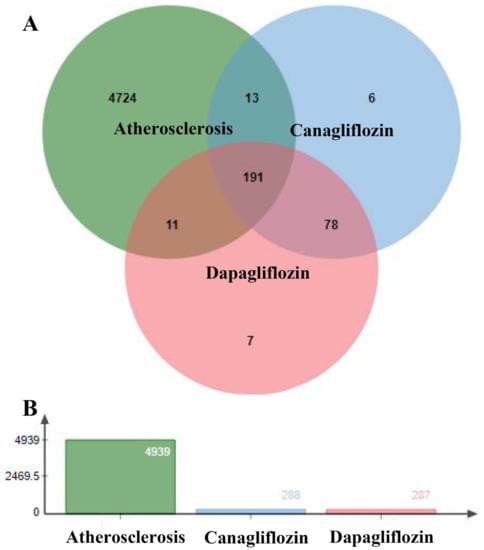
Figure 2.
Intersection targets of drugs and atherosclerosis. (A) Venn diagram of drug targets and atherosclerosis targets. (B) Histograms of atherosclerosis targets and drug targets.
3.4. Construction of PPI Networks
In order to discover the possible mechanisms of canagliflozin and dapagliflozin in treating atherosclerosis, the STRING database was used to construct the PPI network of 191 targets shared by drugs and disease as shown in Figure 3A. The information derived from the STRING database was imported into the Cytoscape3.7.2 software for further analysis, and a visualized PPI network was constructed, with the dot size and color reflecting the degree of freedom. The higher the degree of freedom, the more biological functions were involved. The intensity increased from outside to inside, which was 1–1, 2–3, 4–6, 7–11, 12–16, 17–46, respectively. The results are shown in Figure 3B. Then, three algorithms (degree of freedom, maximum neighborhood component (MNC), and maximal clique centrality (MCC)) in CytoHubba plug-in were used to analyze each node in the PPI network, and six genes (RHOA, AKT1, EGFR, MAPK1, MAPK14, and SRC) were screened out by taking the intersection of three results. Their network of interactions are shown in Figure 3C,D.
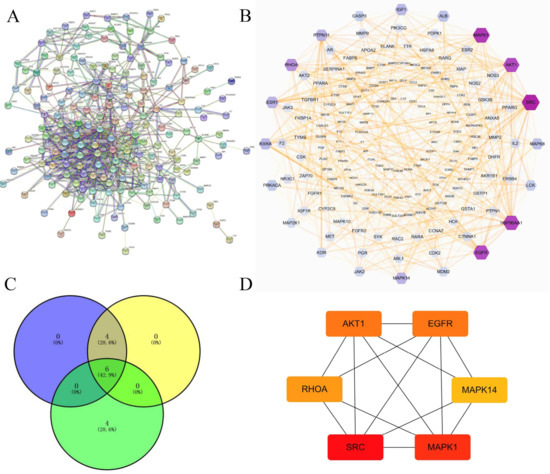
Figure 3.
The protein–protein interaction (PPI) network of intersection targets of drugs and disease. (A) The PPI network of 191 intersection targets for canagliflozin and dapagliflozin in treating atherosclerosis. Note: In the network diagram, the nodes represent each protein, and the node label is the name of the represented protein. The pattern in the node represents the three-dimensional structure of the protein. If it is empty, the structure is currently unknown. If there is an interaction between the two proteins, it is connected by a connecting line. The color of the line reflects the type of interaction, including experimentally verified or predicted, and also includes direct physical interaction, co-expressed gene fusion and other relationships. (B) The PPI network showing detailed interactions of the targets constructed by Cytoscape. Note: The result from STRING database was downloaded and imported into Cytoscape, then the topology parameters (degrees) of the nodes were calculated, and core targets were defined based on the degree. The larger the volume of the hexagon and the darker the color, the more important the target is. (C) The top 10 genes were calculated from the PPI network by the degree of freedom, MNC, and MCC, and the overlapping genes were then screened by Venn diagrams. (D) The PPI network of six overlapping genes.
3.5. GO Enrichment Analysis and KEGG Pathway Enrichment Analysis
To explore the underlying drug mechanism in managing AS, 191 predicted targets were entered into the Metascape database for GO enrichment analysis and KEGG pathway analysis.
3.5.1. KEGG Pathway Enrichment Analysis
The 20 main pathways were found following the KEGG pathway enrichment analysis (Figure 4 and Supplementary Figure S2). They were mainly involved in pathways in cancer (hsa05200), proteoglycans in cancer (hsa05205), endocrine resistance (hsa01522), fluid shear stress and atherosclerosis (hsa05418), Epstein–Barr virus infection (hsa05169), th17 cell differentiation (hsa04659), transcriptional misregulation in cancer (hsa05202), adherens junction (hsa04520), microRNAs in cancer (hsa05206), PPAR signaling pathway (hsa03320), IL-17 signaling pathway (hsa04657), epithelial cell signaling in helicobacter pylori infection (hsa05120), viral carcinogenesis (hsa05203), platinum drug resistance (hsa01524), chemical carcinogenesis (hsa05204), longevity regulating pathway (hsa04211), natural killer cell-mediated cytotoxicity (hsa04650), arachidonic acid metabolism (hsa00590), complement and coagulation cascades (hsa04610), and apoptosis-multiple species (hsa04215). Details on these pathways are listed in Table 1.
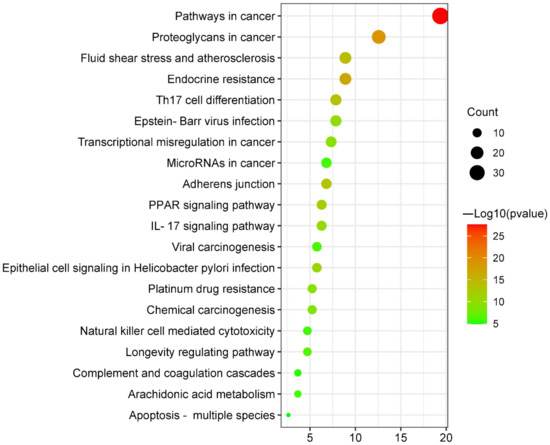
Figure 4.
Bubble plot of KEGG pathway analysis of targets shared by canagliflozin, dapagliflozin and atherosclerosis.

Table 1.
KEGG pathway analysis of targets shared by canagliflozin, dapagliflozin and Atherosclerosis.
3.5.2. Biological Process Enrichment Analysis
After the biological process enrichment analysis in the GO enrichment analysis, 20 results were selected based on their p values and number of enrichments (Figure 5 and Supplementary Figure S3). The predicted targets were included in cellular response to hormone stimulus (GO:0032870), cellular response to lipid (GO:0071396), muscle cell proliferation (GO:0033002), response to lipopolysaccharide (GO:0032496), wound healing (GO:0042060), positive regulation of cell migration (GO:0030335), organic hydroxy compound metabolic process (GO:1901615), regulation of kinase activity (GO:0043549), regulation of inflammatory response (GO:0050727), response to nutrient levels (GO:0031667), cellular response to chemical stress (GO:0062197), epithelial cell differentiation (GO:0030855), regulation of cell activation (GO:0050865), positive regulation of cell death (GO:0010942), leukocyte migration (GO:0050900), response to decreased oxygen levels (GO:0036293), response to drug (GO:0042493), regulation of establishment of protein localization (GO:0070201), reactive oxygen species metabolic process (GO:0072593), and negative regulation of intracellular signal transduction (GO:1902532). Details about these biological processes are listed in Table 2.
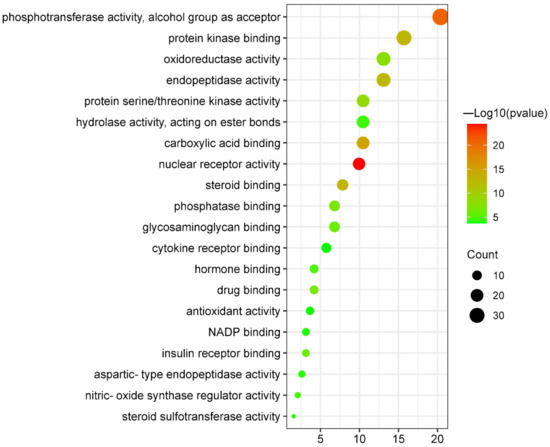
Figure 5.
Bubble plot of biological process enrichment analysis of targets shared by canagliflozin, dapagliflozin and atherosclerosis.

Table 2.
Biological process enrichment analysis of targets shared by canagliflozin, dapagliflozin and atherosclerosis.
3.5.3. Molecular Functions Enrichment Analysis
Canagliflozin and dapagliflozin might affect the following molecular functions (Figure 6 and Supplementary Figure S4) to improve AS: nuclear receptor activity (GO:0004879), phosphotransferase activity, alcohol group as acceptor (GO:0016773), carboxylic acid binding (GO:0031406), steroid binding (GO:0005496), protein kinase binding (GO:0019901), endopeptidase activity (GO:0004175), protein serine/threonine kinase activity (GO:0004674), oxidoreductase activity (GO:0016491), phosphatase binding (GO:0019902), drug binding (GO:0008144), glycosaminoglycan binding (GO:0005539), insulin receptor binding (GO:0005158), hormone binding (GO:0042562), nitric-oxide synthase regulator activity (GO:0030235), hydrolase activity, acting on ester bonds (GO:0016788), aspartic-type endopeptidase activity (GO:0004190), steroid sulfotransferase activity (GO:0050294), NADP binding (GO:0050661), antioxidant activity (GO:0016209), and cytokine receptor binding (GO:0005126). Details about these molecular functions are listed in Table 3.
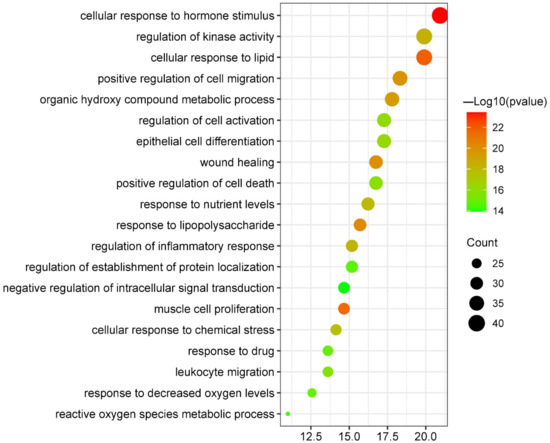
Figure 6.
Bubble plot of molecular functions enrichment analysis of targets shared by canagliflozin, dapagliflozin and atherosclerosis.

Table 3.
Molecular functions enrichment analysis of targets shared by canagliflozin, dapagliflozin and Atherosclerosis.
3.5.4. Cellular Components Enrichment Analysis
After cellular components enrichment analysis, the top 20 results were collected (Figure 7 and Supplementary Figure S5). They were primarily involved in vesicle lumen (GO:0031983), membrane raft (GO:0045121), extracellular matrix (GO:0031012), ficolin-1-rich granule (GO:0101002), focal adhesion (GO:0005925), side of membrane (GO:0098552), receptor complex (GO:0043235), tertiary granule lumen (GO:1904724), endoplasmic reticulum lumen (GO:0005788), endolysosome lumen (GO:0036021), extrinsic component of cytoplasmic side of plasma membrane (GO:0031234), postsynapse (GO:0098794), protein kinase complex (GO:1902911), endocytic vesicle (GO:0030139), late endosome (GO:0005770), calcium channel complex (GO:0034704), dendrite (GO:0030425), perinuclear region of cytoplasm (GO:0048471), actin cytoskeleton (GO:0015629), and platelet alpha granule (GO:0031091). Details about these cellular components are listed in Table 4.
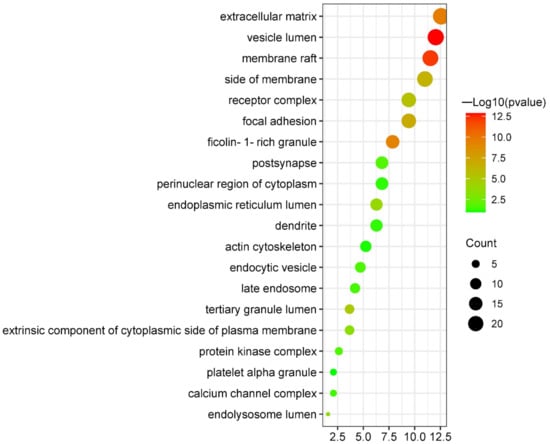
Figure 7.
Bubble plot of cellular components enrichment analysis of targets shared by canagliflozin, dapagliflozin and atherosclerosis.

Table 4.
Cellular components enrichment analysis of targets shared by canagliflozin, dapagliflozin and atherosclerosis.
3.6. Network Construction of Targets-Pathways
The relationship between KEGG pathways and pathway-related genes is shown in Figure 8. There were 125 nodes and 270 edges in this network. The hexagon represented the genes, and V represented the pathways. These results further suggested that Akt1, AKT2, MMP9, MDM2, CASP3, MAPK1, MAPK10, MAPK8, MAPK14, RHOA, SRC, MET, and EGFR were key targets for canagliflozin and dapagliflozin to improve AS. In addition, Akt1, MAPK1, MAPK14, RHOA, SRC and EGFR were six hub targets in the PPI network of 191 targets shared by drugs and disease.
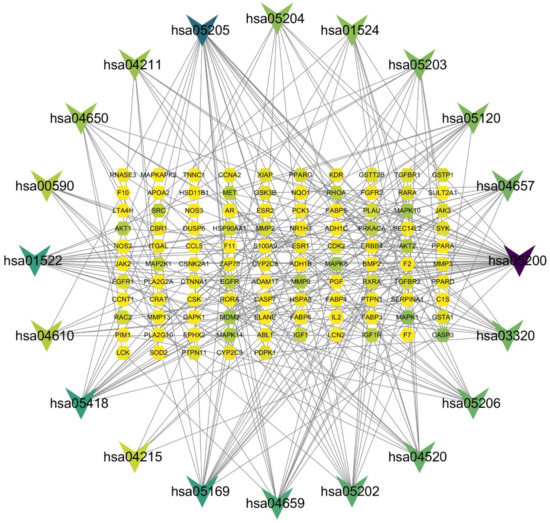
Figure 8.
The network of pathways and pathway-related targets. Note: Hexagons represent the 105 targets, inverted triangles represent the top 20 pathways. The color of a node represents its degree, and the darker the color, the more important the node.
3.7. Molecular Docking
Canagliflozin and dapagliflozin were molecularly docked with six potential targets including Akt1 (PDBID: 6HHG), MAPK1 (PDBID: 4XJ0), MAPK14 (PDBID: 4L8M), RHOA (PDBID: 1A2B), SRC (PDBID: 2H8H) and EGFR (PDBID: 5UG9). A total of 12 pairs of receptor–ligand combinations were obtained (Figure 9), and details about these combinations are listed in Table 5 and Table 6. The bindings in RHOA-canagliflozin (−5.59 kcal/mol) and RHOA-dapagliflozin (−6.90 kcal/mol) were weak and thus cannot be considered core targets. The average value of the other 10 combinations was −8.897 kcal/mol, suggesting that the binding between the core targets and the drugs was strong.
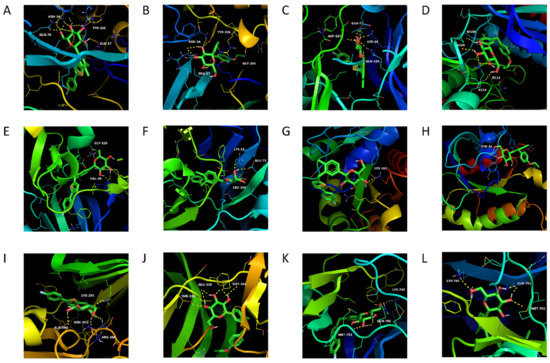
Figure 9.
Molecular docking of canagliflozin and dapagliflozin with six overlapping targets. (A) Akt1-Canagliflozin; (B) AKT1-Dapagliflozin; (C) MAPK1-Canagliflozin; (D) MAPK1-Dapagliflozin; (E) MAPK14-Canagliflozin; (F) MAPK14-Dapagliflozin; (G) RHOA-Canagliflozin; (H) RHOA-Dapagliflozin; (I) SRC-Canagliflozin; (J) SRC-Dapagliflozin; (K) EGFR-Canagliflozin; (L) EGFR-Dapagliflozin.

Table 5.
Molecular docking of canagliflozin with six overlapping targets.

Table 6.
Molecular docking of dapagliflozin with six overlapping targets.
4. Discussion
In our study, we collected the relevant targets of drugs and diseases separately, took the intersection of the targets of drugs and diseases, and focused on analyzing the 191 targets that they had intersections with in the following study. The purpose of our study was to discover whether Akt1, MAPK1, MAPK14, RHOA, SRC and EGFR are central genes in different networks. These genes are both drug targets and disease-related targets. These genes, which are both drug- and disease-related targets, are six potential central targets for canagliflozin or dapagliflozin to improve AS. After verification of molecular docking, all five targets except RHOA were found to bind well to canagliflozin or dapagliflozin, so that Akt1, MAPK1, MAPK14, SRC and EGFR were determined as the final core targets.
Akt/PKB (protein kinase B) is very important for cell survival induced by growth factor. Active Akt can suppress apoptosis independently of transcription through phosphorylation and inactivation of apoptotic machine components. Akt1, Akt2 and Akt3 are three Akt isoforms in macrophages. In the mammalian genome, the major Akt isoform is encoded by Akt1, which modulates apoptosis [26]. The PI3K/Akt pathway is a classic signaling pathway that plays a crucial role in cell survival and apoptosis. Akt activated m-TOR inhibits autophagy of macrophages in the inflammatory response [27]. The change of Akt subtype or the regulation of Akt activity level significantly affects the polarized phenotype of macrophages, which may impact the progression of atherosclerosis [28]. Canagliflozin can stimulate AMPK, Akt and eNOS and inhibits iNOS and NADPH oxidase isoform 4 (NOX4), all of which are associated with antioxidant and anti-inflammatory signaling pathways [29,30]. The combined treatment with dapagliflozin and rosuvastatin can synergistically inhibit apoptosis by activating the PI3K/AKt/mTOR signaling pathway in rats with myocardial ischemia [31].
Mitogen-activated protein kinase (MAPK), a kind of serine-threonine protein kinase, plays a more important role in many important physiological and pathological processes such as cell proliferation, differentiation and apoptosis [32]. There are four main subfamilies of the MAPK pathway: ERK1/2, c-Jun N-terminal kinase (JNK), P38/MAPK and ERK5 [33]. MAPK1 (MAP kinase ERK2) is a subfamily of MAPK, form ERK1/2 [34]. MAPK14, also named as p38α, is an isoform of the p38 MAPK family, and it is ubiquitously expressed in the family [35]. The MAPK/ERK pathway can be activated with proatherogenic stimuli in vitro and in vivo [36]. p38 MAPK is an important component of inflammatory signaling that can be activated by various stimuli such as oxidative stress, cytokines, and growth factors, all of which are involved in the formation of atherosclerosis [35,37,38].
A recent study suggests that canagliflozin has atheroprotective effects against atherosclerosis by promoting the Akt-eNOS pathway and inhibiting the activation of p38 MAPK [39]. Dapagliflozin shows a protective effect in complicated T2DM with CVD via the MAPK signaling pathway [40]. Similarly, dapagliflozin alleviates diabetic cardiomyopathy by upregulating the AKT/JAK/MAPK pathway via erythropoietin in diabetic rats [41].
Proto-oncogene tyrosine-protein kinase SRC (SRC), as other members of the SRC family kinases (SFK), plays an important role in the regulation of cellular metabolism, survival, and proliferation [42,43]. Many studies have shown that SRC plays an important role in the functional activation of macrophages and the regulation of cholesterol levels, which are involved in atherosclerosis [44,45]. A study demonstrated that canagliflozin and dapagliflozin protect endothelial cells from glucose-induced oxidative stress by blocking ROS-activated SRC, EGF receptor, protein kinase C and Rho kinase [46].
Epidermal growth factor receptor (EGFR) is a member of the ERBB family of tyrosine kinase receptors. EGFR plays a very important role in cell survival, proliferation, migration, differentiation and division [47,48]. Recently, several studies have indicated that EGFR is involved in the regulation of inflammation and oxidative stress in macrophages [49]. As we all know, inflammation and oxidative stress are significant manifestations of atherosclerosis development. Moreover, blocking EGFR induced anergia of T cells in vitro and in vivo and reduced atherosclerosis development [48]. These results suggest that EGFR plays an important role in the pathogenesis of atherosclerosis. There is currently little research on SGLT2 inhibitors and EGFR. Only one of the studies we mentioned above showed that SGLT2 inhibitors can block EGFR-related signaling pathways and play a role in protecting the vascular endothelium [46]. In our research, we found a very high degree of binding of EGFR to both canagliflozin and dapagliflozin, so we believe that EGFR may be a key target for canagliflozin and dapagliflozin in the treatment of AS.
Arterial bifurcations and intra-arterial curves are the most common sites of atherosclerotic plaque formation [50]. The same related experimental studies have found that the endothelium of vessels in these two locations is often affected by disorder or low blood flow rate. Contrarily, vascular areas exposed to high-speed blood flow within the same vessel are less prone to form plaques [51]. Numerous experimental studies have shown that low shear stress (LSS) on the surface of vascular endothelial cells is an important factor for the occurrence and development of atherosclerosis [52]. However, there is no research on SGLT2 inhibitors to improve atherosclerosis by adjusting the shear stress. In our study, KEGG pathway analyses showed that the fluid shear stress was closely related to atherosclerosis; our research found that canagliflozin and dapagliflozin may alter fluid shear force-related pathways to improve atherosclerosis. In detail, we found that the genes AKT1, MAPK14 and SRC are involved in fluid shear stress and the atherosclerosis pathway. In the results of the analysis of biological processes, the five core targets were mainly involved in biological processes, including cellular response to hormone stimulus, cellular response to lipid, muscle cell proliferation, a response to lipopolysaccharide, wound healing and positive regulation of cell migration. These biological processes are critical to the development of atherosclerosis. In addition, the five targets also affected molecular functions (phosphotransferase activity, protein kinase binding, protein serine/threonine kinase activity and phosphatase binding) and cellular composition (membrane raft, focal adhesion, postsynapse and postsynapse) related to atherosclerosis. Therefore, through our analysis, we speculate that canagliflozin and dapagliflozin may affect the fluid shear stress and then regulate these target genes, leading to further improvement of AS.
However, this study also has some limitations. Drug and disease targets were collected through databases with limited numbers, so some data may be biased. At the same time, since our current research results were only based on the analysis of the collected data, we need to further confirm our conclusions in future experiments.
5. Conclusions
Canagliflozin and dapagliflozin, potential targets, and underlying mechanisms of canagliflozin and dapagliflozin were examined using network pharmacology methods. In our study, we collected five core targets, Akt1, MAPK1, MAPK14, SRC and EGFR, for canagliflozin and dapagliflozin in the treatment of AS. In addition, analysis of the KEGG pathway showed that fluid shear stress and the atherosclerosis pathway were the key targets for AS treatment.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jvd1010007/s1, Figure S1: The framework of the present study. Figure S2: Column chart of KEGG pathway analysis of shared targets by canagliflozin, dapagliflozin and Atherosclerosis. Figure S3: Column chart of biological process enrichment analysis of shared targets by canagliflozin, dapagliflozin and Atherosclerosis. Figure S4: Column chart of molecular functions enrichment analysis of shared targets by canagliflozin, dapagliflozin and Atherosclerosis. Figure S5: Column chart of cellular components enrichment analysis of shared targets by canagliflozin, dapagliflozin and Atherosclerosis. Table S1: Targets of canagliflozin. Table S2: Targets of dapagliflozin.
Author Contributions
Conceptualization, D.L.; methodology, J.W. and H.W.; software, J.W. and D.L.; validation, D.L.; formal analysis, J.W.; investigation, J.W. and W.J.; resources, W.J.; data curation, J.W.; writing—original draft preparation, W.J. and H.W.; writing—review and editing, W.J. and H.W.; visualization, W.J. and H.W.; supervision, D.L.; project administration, J.W., D.L. and H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used and/or analyzed during this study are included within the article.
Acknowledgments
We thank Weijing Yun for supporting this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kirichenko, T.V.; Sukhorukov, V.N.; Markin, A.M.; Nikiforov, N.G.; Liu, P.Y.; Sobenin, I.A.; Tarasov, V.V.; Orekhov, A.N.; Aliev, G. Medicinal Plants as a Potential and Successful Treatment Option in the Context of Atherosclerosis. Front. Pharmacol. 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Liu, N.; Yan, Y.; Liu, B. Apoptosis, autophagy and atherosclerosis: Relationships and the role of Hsp27. Pharmacol. Res. 2021, 166, 5169. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Wu, W.K.; Melnichenko, A.A.; Wetzker, R.; Sukhorukov, V.; Markin, A.M.; Khotina, V.A.; Orekhov, A.N. Signaling Pathways and Key Genes Involved in Regulation of foam Cell Formation in Atherosclerosis. Cells 2020, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 2011, 6, 23–44. [Google Scholar] [CrossRef]
- Moss, J.W.; Ramji, D.P. Nutraceutical therapies for atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 13–32. [Google Scholar] [CrossRef]
- Ghosh, R.K.; Ghosh, S.M.; Chawla, S.; Jasdanwala, S.A. SGLT2 inhibitors: A new emerging therapeutic class in the treatment of type 2 diabetes mellitus. J. Clin. Pharmacol. 2012, 52, 57–63. [Google Scholar] [CrossRef]
- Ghosh-Swaby, O.R.; Goodman, S.G.; Leiter, L.A.; Cheng, A.; Connelly, K.A.; Fitchett, D.; Jüni, P.; Farkouh, M.E.; Udell, J.A. Glucose-lowering drugs or strategies, atherosclerotic cardiovascular events, and heart failure in people with or at risk of type 2 diabetes: An updated systematic review and meta-analysis of randomised cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2020, 8, 18–35. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 44–57. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 995–2008. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, S.J.; Lee, J.J.; Kim, J.S.; Lee, O.H.; Kim, C.K.; Kim, D.; Lee, Y.H.; Oh, J.; Park, S.; et al. Anti-Inflammatory Effect for Atherosclerosis Progression by Sodium-Glucose Cotransporter 2 (SGLT-2) Inhibitor in a Normoglycemic Rabbit Model. Korean Circ. J. 2020, 50, 43–57. [Google Scholar] [CrossRef]
- Leng, W.; Ouyang, X.; Lei, X.; Wu, M.; Chen, L.; Wu, Q.; Deng, W.; Liang, Z. The SGLT-2 Inhibitor Dapagliflozin Has a Therapeutic Effect on Atherosclerosis in Diabetic ApoE(-/-) Mice. Mediat. Inflamm. 2016, 2016, 305735. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huai, Y.; Miao, Z.; Qian, A.; Wang, Y. Systems Pharmacology for Investigation of the Mechanisms of Action of Traditional Chinese Medicine in Drug Discovery. Front. Pharmacol. 2019, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.T.; Hajduk, P.J. Rational approaches to targeted polypharmacology: Creating and navigating protein-ligand interaction networks. Curr. Opin. Chem. Biol. 2010, 14, 98–504. [Google Scholar] [CrossRef]
- UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D89–D480. [CrossRef]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H. GeneCards Version 3: The human gene integrator. Database J. Biol. Databases Curation 2010, 2010, baq020. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Piñero, J.; Queralt-Rosinach, N.; Bravo, À.; Deu-Pons, J.; Bauer-Mehren, A.; Baron, M.; Sanz, F.; Furlong, L.I. DisGeNET: A discovery platform for the dynamical exploration of human diseases and their genes. Database J. Biol. Databases Curation 2015, 2015, bav028. [Google Scholar]
- Wang, Y.; Zhang, S.; Li, F.; Zhou, Y.; Zhang, Y.; Wang, Z.; Zhang, R.; Zhu, J.; Ren, Y.; Tan, Y.; et al. Therapeutic target database 2020: Enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020, 48, D1031–D1041. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Chen, J.; Wu, Y.; Cao, Y.; Zhong, P. A Network Pharmacology to Explore the Mechanism of Calculus Bovis in the Treatment of Ischemic Stroke. BioMed Res. Int. 2021, 2021, 611018. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Otasek, D.; Morris, J.H.; Bouças, J.; Pico, A.R.; Demchak, B. Cytoscape Automation: Empowering workflow-based network analysis. Genome Biol. 2019, 20, 85. [Google Scholar] [CrossRef]
- Tripathi, S.; Pohl, M.O.; Zhou, Y.; Rodriguez-Frandsen, A.; Wang, G.; Stein, D.A.; Moulton, H.M.; DeJesus, P.; Che, J.; Mulder, L.C.; et al. Meta- and Orthogonal Integration of Influenza “OMICs” Data Defines a Role for UBR4 in Virus Budding. Cell Host Microbe. 2015, 18, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Dan, W.; Liu, J.; Ha, P.; Zhou, T.; Guo, X.; Hou, W. The Use of Traditional Chinese Medicine in Relieving EGFR-TKI-Associated Diarrhea Based on Network Pharmacology and Data Mining. Evid.-Based Complementary Altern. Med. 2021, 2021, 530898. [Google Scholar] [CrossRef]
- Revathidevi, S.; Munirajan, A.K. Akt in cancer: Mediator and more. Semin. Cancer Biol. 2019, 59, 89–91. [Google Scholar] [CrossRef]
- Linton, M.F.; Moslehi, J.J.; Babaev, V.R. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 2703. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 93–604. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Lasker, S.; Hasan, A.; Zerin, F.; Zamila, M.; Parvez, F.; Rahman, M.M.; Khan, F.; Subhan, N.; Alam, M.A. Canagliflozin ameliorates renal oxidative stress and inflammation by stimulating AMPK-Akt-eNOS pathway in the isoprenaline-induced oxidative stress model. Sci. Rep. 2020, 10, 4659. [Google Scholar] [CrossRef]
- Sayour, A.A.; Korkmaz-Icöz, S.; Loganathan, S.; Ruppert, M.; Sayour, V.N.; Oláh, A.; Benke, K.; Brune, M.; Benkő, R.; Horváth, E.M.; et al. Acute canagliflozin treatment protects against in vivo myocardial ischemia-reperfusion injury in non-diabetic male rats and enhances endothelium-dependent vasorelaxation. J. Transl. Med. 2019, 17, 27. [Google Scholar] [CrossRef]
- Gong, L.; Wang, X.; Pan, J.; Zhang, M.; Liu, D.; Liu, M.; Li, L.; An, F. The co-treatment of rosuvastatin with dapagliflozin synergistically inhibited apoptosis via activating the PI3K/AKt/mTOR signaling pathway in myocardial ischemia/reperfusion injury rats. Open Med. 2021, 15, 7–57. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Richardson, B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005, 6, 22–27. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 997–2007. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.; Pannone, L.; Pantaleoni, F.; Bocchinfuso, G.; Radio, F.C.; Cecchetti, S.; Ciolfi, A.; Di Rocco, M.; Elting, M.W.; Brilstra, E.H.; et al. Enhanced MAPK1 Function Causes a Neurodevelopmental Disorder within the RASopathy Clinical Spectrum. Am. J. Hum. Genet. 2020, 107, 99–513. [Google Scholar] [CrossRef] [PubMed]
- Corre, I.; Paris, F.; Huot, J. The p38 pathway, a major pleiotropic cascade that transduces stress and metastatic signals in endothelial cells. Oncotarget 2017, 8, 5684–5714. [Google Scholar] [CrossRef]
- Cheng, M.; Yang, L.; Fan, M.; An, S.; Li, J. Proatherogenic stimuli induce HuR in atherosclerosis through MAPK/ErK pathway. Am. J. Transl. Res. 2019, 11, 317–327. [Google Scholar]
- Reustle, A.; Torzewski, M. Role of p38 MAPK in Atherosclerosis and Aortic Valve Sclerosis. Int. J. Mol. Sci. 2018, 19, 3761. [Google Scholar] [CrossRef]
- Madkour, M.M.; Anbar, H.S.; El-Gamal, M.I. Current status and future prospects of p38α/MAPK14 kinase and its inhibitors. Eur. J. Med. Chem. 2021, 213, 13216. [Google Scholar] [CrossRef]
- Rahadian, A.; Fukuda, D.; Salim, H.M.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Sata, M. Canagliflozin Prevents Diabetes-Induced Vascular Dysfunction in ApoE-Deficient Mice. J. Atheroscler. Thromb. 2020, 27, 141–151. [Google Scholar] [CrossRef]
- Yue, Z.; Li, L.; Fu, H.; Yin, Y.; Du, B.; Wang, F.; Ding, Y.; Liu, Y.; Zhao, R.; Zhang, Z.; et al. Effect of dapagliflozin on diabetic patients with cardiovascular disease via MAPK signalling pathway. J. Cell. Mol. Med. 2021, 25, 500–512. [Google Scholar] [CrossRef]
- El-Sayed, N.; Mostafa, Y.M.; AboGresha, N.M.; Ahmed, A.A.M.; Mahmoud, I.Z.; El-Sayed, N.M. Dapagliflozin attenuates diabetic cardiomyopathy through erythropoietin up-regulation of AKT/JAK/MAPK pathways in streptozotocin-induced diabetic rats. Chem.-Biol. Interact. 2021, 347, 09617. [Google Scholar] [CrossRef] [PubMed]
- De Kock, L.; Freson, K. The (Patho)Biology of SRC Kinase in Platelets and Megakaryocytes. Medicina 2020, 56, 633. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.E.; Yi, Y.S.; Oh, J.; Yoo, B.C.; Hong, S.; Cho, J.Y. The role of Src kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2012, 2012, 12926. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Jung, H.; Chen, J.; Kim, Y.C.; Kim, D.H.; Kong, B.; Guo, G.; Kemper, B.; Kemper, J.K. Phosphorylation of hepatic farnesoid X receptor by FGF19 signaling-activated Src maintains cholesterol levels and protects from atherosclerosis. J. Biol. Chem. 2019, 294, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Babaev, V.R.; Huang, J.; Linton, E.F.; Tao, H.; Yancey, P.G. Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis. Circ. J. 2016, 80, 259–268. [Google Scholar] [CrossRef] [PubMed]
- El-Daly, M.; Pulakazhi Venu, V.K.; Saifeddine, M.; Mihara, K.; Kang, S.; Fedak, P.W.M.; Alston, L.A.; Hirota, S.A.; Ding, H.; Triggle, C.R.; et al. Hyperglycaemic impairment of PAR2-mediated vasodilation: Prevention by inhibition of aortic endothelial sodium-glucose-co-Transporter-2 and minimizing oxidative stress. Vasc. Pharmacol. 2018, 109, 6–71. [Google Scholar] [CrossRef]
- Sabbah, D.A.; Hajjo, R.; Sweidan, K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr. Top. Med. Chenistry 2020, 20, 15–34. [Google Scholar] [CrossRef]
- Zeboudj, L.; Maître, M.; Guyonnet, L.; Laurans, L.; Joffre, J.; Lemarie, J.; Bourcier, S.; Nour-Eldine, W.; Guérin, C.; Friard, J.; et al. Selective EGF-Receptor Inhibition in CD4(+) T Cells Induces Anergy and Limits Atherosclerosis. J. Am. Coll. Cardiol. 2018, 71, 60–72. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Z.; Huang, W.; Chen, X.; Shan, P.; Zhong, P.; Khan, Z.; Wang, J.; Fang, Q.; Liang, G.; et al. Inhibition of epidermal growth factor receptor attenuates atherosclerosis via decreasing inflammation and oxidative stress. Sci. Rep. 2017, 8, 5917. [Google Scholar] [CrossRef]
- Hahn, C.; Schwartz, M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 3–62. [Google Scholar] [CrossRef]
- Chiu, J.J.; Chien, S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol. Rev. 2011, 91, 27–87. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.F.; Civelek, M.; Fang, Y.; Fleming, I. The atherosusceptible endothelium: Endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc. Res. 2013, 99, 15–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).