Macular Sensitivity after Intravitreal Ranibizumab Injection for Macular Edema in Central Retinal Vein Occlusion: One versus Three Initial Monthly Injections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment for Macular Edema Associated with Central Retinal Vein Occlusion
2.3. Clinical Parameters
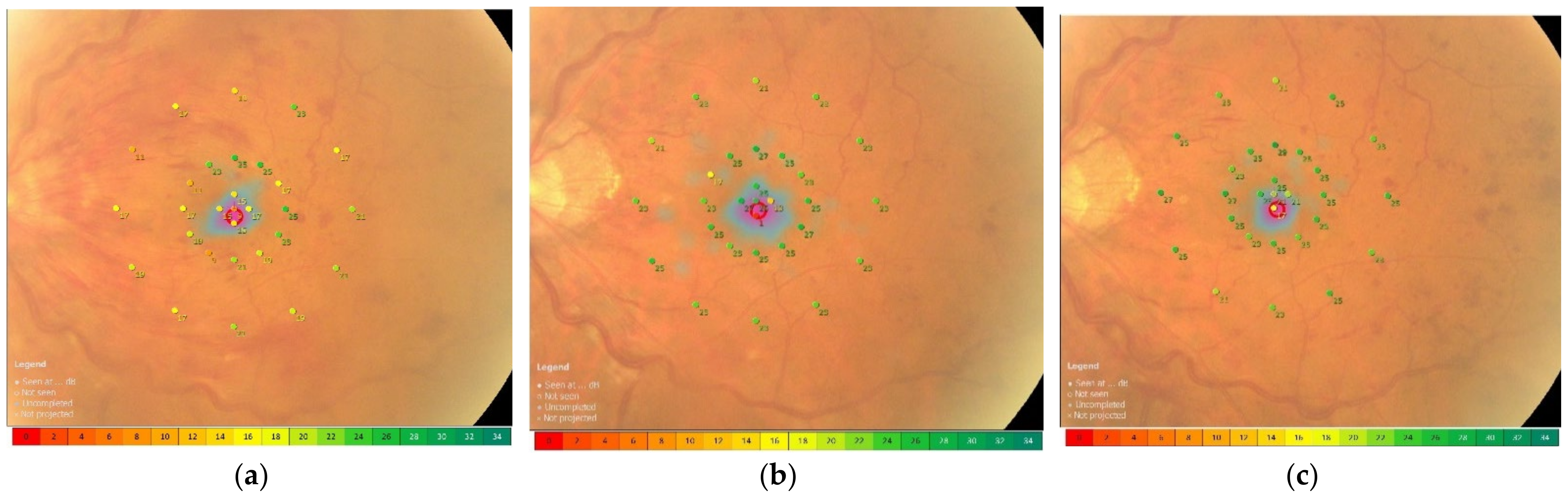
2.4. Functional Mapping by Microperimetry
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McIntosh, R.L.; Rogers, S.L.; Lim, L.; Cheung, N.; Wang, J.J.; Mitchell, P.; Kowalski, J.W.; Nguyen, H.P.; Wong, T.Y. Natural history of central retinal vein occlusion: An evidence-based systematic review. Ophthalmology 2010, 117, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Ocular vascular occlusive disorders: Natural history of visual outcome. Prog. Retin. Eye Res. 2014, 41, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Funatsu, H.; Mimura, T.; Harino, S.; Hori, S. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology 2009, 116, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Yasuda, K.; Shimura, M. Cytokines and Pathogenesis of Central Retinal Vein Occlusion. J. Clin. Med. 2020, 9, 3457. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Brown, D.M.; Awh, C.C.; Lee, S.Y.; Gray, S.; Saroj, N.; Murahashi, W.Y.; Rubio, R.G. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: Twelve-month outcomes of a phase III study. Ophthalmology 2011, 118, 2041–2049. [Google Scholar] [CrossRef]
- Heier, J.S.; Campochiaro, P.A.; Yau, L.; Li, Z.; Saroj, N.; Rubio, R.G.; Lai, P. Ranibizumab for macular edema due to retinal vein occlusions: Long-term follow-up in the HORIZON trial. Ophthalmology 2012, 119, 802–809. [Google Scholar] [CrossRef]
- Heier, J.S.; Clark, W.L.; Boyer, D.S.; Brown, D.M.; Vitti, R.; Berliner, A.J.; Kazmi, H.; Ma, Y.; Stemper, B.; Zeitz, O.; et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: Two-year results from the COPERNICUS study. Ophthalmology 2014, 121, 1414–1420.e1411. [Google Scholar] [CrossRef]
- Larsen, M.; Waldstein, S.M.; Priglinger, S.; Hykin, P.; Barnes, E.; Gekkieva, M.; Das Gupta, A.; Wenzel, A.; Monés, J. Sustained Benefits from Ranibizumab for Central Retinal Vein Occlusion with Macular Edema: 24-Month Results of the CRYSTAL Study. Ophthalmol. Retin. 2018, 2, 134–142. [Google Scholar] [CrossRef]
- Rayess, N.; Rahimy, E.; Shah, C.P.; Wolfe, J.D.; Chen, E.; DeCroos, F.C.; Storey, P.; Garg, S.J.; Hsu, J. Incidence and clinical features of post-injection endophthalmitis according to diagnosis. Br. J. Ophthalmol. 2016, 100, 1058–1061. [Google Scholar] [CrossRef]
- Kida, T.; Tsujikawa, A.; Muraoka, Y.; Harino, S.; Osaka, R.; Murakami, T.; Ooto, S.; Suzuma, K.; Morishita, S.; Fukumoto, M.; et al. Cotton Wool Spots after Anti-Vascular Endothelial Growth Factor Therapy for Macular Edema Associated with Central Retinal Vein Occlusion. Ophthalmologica 2016, 235, 106–113. [Google Scholar] [CrossRef]
- Osaka, R.; Muraoka, Y.; Miwa, Y.; Manabe, K.; Kobayashi, M.; Takasago, Y.; Ooto, S.; Murakami, T.; Suzuma, K.; Iida, Y.; et al. Anti-Vascular Endothelial Growth Factor Therapy for Macular Edema following Central Retinal Vein Occlusion: 1 Initial Injection versus 3 Monthly Injections. Ophthalmologica 2018, 239, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.R.; Bex, P.J.; Bauer, C.M.; Merabet, L.B. The Assessment of Visual Function and Functional Vision. Semin. Pediatr. Neurol. 2019, 31, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Mimura, T. Macular sensitivity and morphology after intravitreal injection of triamcinolone acetonide for macular edema secondary to central retinal vein occlusion. Clin. Ophthalmol. 2012, 6, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Mimura, T.; Shimada, K. Retinal function and morphology in central retinal vein occlusion with macular edema. Curr. Eye Res. 2013, 38, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Yamaike, N.; Tsujikawa, A.; Sakamoto, A.; Ota, M.; Kotera, Y.; Miyamoto, K.; Kita, M.; Yoshimura, N. Retinal sensitivity after intravitreal injection of bevacizumab for the treatment of macular edema secondary to retinal vein occlusion. Retina 2009, 29, 757–767. [Google Scholar] [CrossRef]
- Papadia, M.; Misteli, M.; Jeannin, B.; Herbort, C.P. The influence of anti-VEGF therapy on present day management of macular edema due to BRVO and CRVO: A longitudinal analysis on visual function, injection time interval and complications. Int. Ophthalmol. 2014, 34, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- The Central Vein Occlusion Study. Baseline and early natural history report. Arch. Ophthalmol. 1993, 111, 1087–1095. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Uji, A.; Lei, J.; Velaga, S.; Nittala, M.; Sadda, S. Interdevice comparison of retinal sensitivity assessments in a healthy population: The CenterVue MAIA and the Nidek MP-3 microperimeters. Br. J. Ophthalmol. 2018, 102, 109–113. [Google Scholar] [CrossRef]
- Ogura, Y.; Roider, J.; Korobelnik, J.F.; Holz, F.G.; Simader, C.; Schmidt-Erfurth, U.; Vitti, R.; Berliner, A.J.; Hiemeyer, F.; Stemper, B.; et al. Intravitreal aflibercept for macular edema secondary to central retinal vein occlusion: 18-month results of the phase 3 GALILEO study. Am. J. Ophthalmol. 2014, 158, 1032–1038. [Google Scholar] [CrossRef]
- Brown, D.M.; Heier, J.S.; Clark, W.L.; Boyer, D.S.; Vitti, R.; Berliner, A.J.; Zeitz, O.; Sandbrink, R.; Zhu, X.; Haller, J.A. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am. J. Ophthalmol. 2013, 155, 429–437.e427. [Google Scholar] [CrossRef]
- Fujino, R.; Asaoka, R.; Aoki, S.; Sugiura, A.; Kusakabe, M.; Asano-Shimizu, K.; Nomura, Y.; Aoki, A.; Hashimoto, Y.; Azuma, K.; et al. The usefulness of the retinal sensitivity measurement with a microperimetry for predicting the visual prognosis of branch retinal vein occlusion with macular edema. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Seeböck, P.; Vogl, W.D.; Waldstein, S.M.; Orlando, J.I.; Baratsits, M.; Alten, T.; Arikan, M.; Mylonas, G.; Bogunović, H.; Schmidt-Erfurth, U. Linking Function and Structure with ReSensNet: Predicting Retinal Sensitivity from OCT using Deep Learning. Ophthalmol. Retin. 2022, 6, 501–511. [Google Scholar] [CrossRef] [PubMed]


| Findings | Total | 1 + PRN Group (n = 11) | 3 + PRN Group (n = 9) | p Value |
|---|---|---|---|---|
| Baseline | ||||
| Age (years) | 62.2 ± 11.5 ‡ | 59.0 ± 13.5 ‡ | 66.1 ± 7.38 ‡ | 0.176 |
| Gender (female/male) | 9/11 | 3/8 | 6/3 | 0.078 |
| Duration of macular edema (days) | 30.8 ± 23.9 ‡ | 36.3 ± 24.6 ‡ | 24.1 ± 22.5 ‡ | 0.269 |
| Hypertension | 15 | 8 | 7 | 0.795 |
| Systolic blood pressure (mmHg) | 139 ± 19 ‡ | 140 ± 12 ‡ | 137 ± 26 ‡ | 0.723 |
| Diastolic blood pressure (mmHg) | 87 ± 12 ‡ | 88 ± 12 ‡ | 86 ± 13 ‡ | 0.667 |
| Hyperlipidemia | 9 | 5 | 4 | 0.964 |
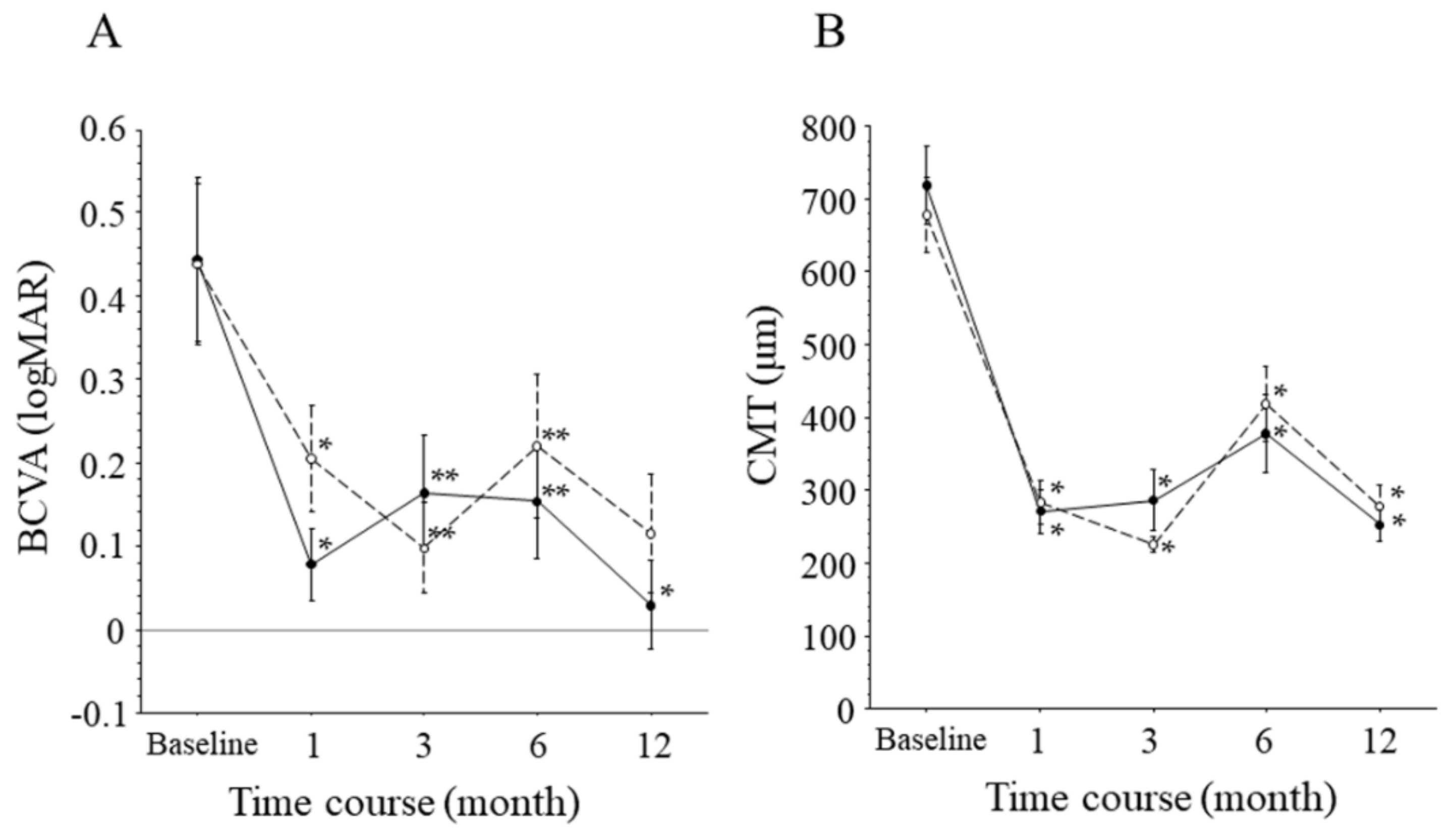
| BCVA (logMAR, ETDRS letters) | 0.44 ± 0.30, 62.7 ± 15.2 ‡ | 0.44 ± 0.33, 62.6 ± 16.4 ‡ | 0.44 ± 0.29, 62.7 ± 14.4 ‡ | 0.966 |
| CMT (μm) | 701 ± 168 ‡ | 718 ± 182 ‡ | 679 ± 157 ‡ | 0.610 |
| MS within 1 mm (dB) | 14.2 ± 5.72 ‡ | 13.3 ± 5.68 ‡ | 15.4 ± 5.88 ‡ | 0.427 |
| MS within 3 mm (dB) MS within 6 mm (dB) | 17.3 ± 5.15 ‡ 18.7 ± 4.91 ‡ | 17.1 ± 4.88 ‡ 18.6 ± 4.33 ‡ | 17.6 ± 5.75 ‡ 18.7 ± 5.80 ‡ | 0.847 0.959 |
| Final | ||||
| BCVA (logMAR) | 0.07 ± 0.19, 80.7 ± 12.3 ‡ | 0.03 ± 0.18, 83.8 ± 9.3 ‡ | 0.11 ± 0.21, 77.0 ± 14.9 ‡ | 0.341 |
| CMT (μm) | 264 ± 78 ‡ | 252 ± 71 ‡ | 278 ± 87 ‡ | 0.465 |
| MS within 1 mm (dB) | 22.3 ± 6.32 ‡ | 23.4 ± 3.75 ‡ | 20.9 ± 8.57 ‡ | 0.399 |
| MS within 3 mm (dB) MS within 6 mm (dB) | 23.5 ± 5.64 ‡ 23.3 ± 5.18 ‡ | 24.9 ± 3.01 ‡ 24.8 ± 2.74 ‡ | 21.6 ± 7.57 ‡ 21.5 ± 6.91 ‡ | 0.193 0.168 |
| Number of intravitreal injections | 4.2 ± 2.2 ‡ | 3.3 ± 2.3 ‡ | 5.2 ± 1.7 ‡ | 0.049 |
| Eyes that did not require PRN injections | 6 | 4 | 2 | 0.492 |
| Univariate | Multivariate Stepwise | |||
|---|---|---|---|---|
| Variable | Correlation Coefficient | p Value | Correlation Coefficient | p Value |
| BCVA at month 12 | ||||
| Age (yrs) | −0.05 | 0.834 | ||
| Duration of macular edema (days) | 0.18 | 0.463 | ||
| Baseline BCVA (log MAR) | −0.18 | 0.459 | ||
| Baseline CMT (μm) | −0.25 | 0.285 | ||
| Number of injections | 0.45 | 0.043 | 0.45 | 0.043 |
| Treatment regimen (1 + PRN vs. 3 + PRN) | 0.23 | 0.346 | ||
| Macular sensitivity within 1 mm at month 12 | ||||
| Age (yrs) | −0.41 | 0.073 | ||
| Duration of macular edema (days) | −0.57 | 0.008 | −0.57 | 0.008 |
| Baseline BCVA (log MAR) | −0.04 | 0.861 | ||
| Baseline CMT (μm) | −0.41 | 0.073 | ||
| Number of injections | −0.36 | 0.117 | ||
| Treatment regimen (1 + PRN vs. 3 + PRN) | −0.20 | 0.404 | ||
| Macular sensitivity within 3 mm at month 12 | ||||
| Age (yrs) | −0.45 | 0.043 | −0.45 | 0.043 |
| Duration of macular edema (days) | −0.43 | 0.058 | ||
| Baseline BCVA (log MAR) | −0.10 | 0.680 | ||
| Baseline CMT (μm) | −0.37 | 0.115 | ||
| Number of injections | −0.37 | 0.114 | ||
| Treatment regimen (1 + PRN vs. 3 + PRN) | −0.30 | 0.197 | ||
| Macular sensitivity within 6 mm at month 12 | ||||
| Age (yrs) | −0.47 | 0.034 | −0.47 | 0.034 |
| Duration of macular edema (days) | −0.33 | 0.164 | ||
| Baseline BCVA (log MAR) | −0.10 | 0.684 | ||
| Baseline CMT (μm) | −0.31 | 0.181 | ||
| Number of injections | −0.33 | 0.156 | ||
| Treatment regimen (1 + PRN vs. 3 + PRN) | −0.32 | 0.170 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niidome, E.; Noma, H.; Yasuda, K.; Yanagida, K.; Narimatsu, A.; Asakage, M.; Watarai, S.; Goto, H.; Shimura, M. Macular Sensitivity after Intravitreal Ranibizumab Injection for Macular Edema in Central Retinal Vein Occlusion: One versus Three Initial Monthly Injections. J. Vasc. Dis. 2022, 1, 43-52. https://doi.org/10.3390/jvd1010006
Niidome E, Noma H, Yasuda K, Yanagida K, Narimatsu A, Asakage M, Watarai S, Goto H, Shimura M. Macular Sensitivity after Intravitreal Ranibizumab Injection for Macular Edema in Central Retinal Vein Occlusion: One versus Three Initial Monthly Injections. Journal of Vascular Diseases. 2022; 1(1):43-52. https://doi.org/10.3390/jvd1010006
Chicago/Turabian StyleNiidome, Erina, Hidetaka Noma, Kanako Yasuda, Kosei Yanagida, Akitomo Narimatsu, Masaki Asakage, Sho Watarai, Hiroshi Goto, and Masahiko Shimura. 2022. "Macular Sensitivity after Intravitreal Ranibizumab Injection for Macular Edema in Central Retinal Vein Occlusion: One versus Three Initial Monthly Injections" Journal of Vascular Diseases 1, no. 1: 43-52. https://doi.org/10.3390/jvd1010006
APA StyleNiidome, E., Noma, H., Yasuda, K., Yanagida, K., Narimatsu, A., Asakage, M., Watarai, S., Goto, H., & Shimura, M. (2022). Macular Sensitivity after Intravitreal Ranibizumab Injection for Macular Edema in Central Retinal Vein Occlusion: One versus Three Initial Monthly Injections. Journal of Vascular Diseases, 1(1), 43-52. https://doi.org/10.3390/jvd1010006







