Guidelines for Establishing Safety in Ayahuasca and Ibogaine Administration in Clinical Settings
Abstract
:1. Introduction
1.1. Ayahuasca
1.2. Ibogaine
2. Materials and Methods
3. Results
3.1. Screening and Selection
3.2. Use of Medications
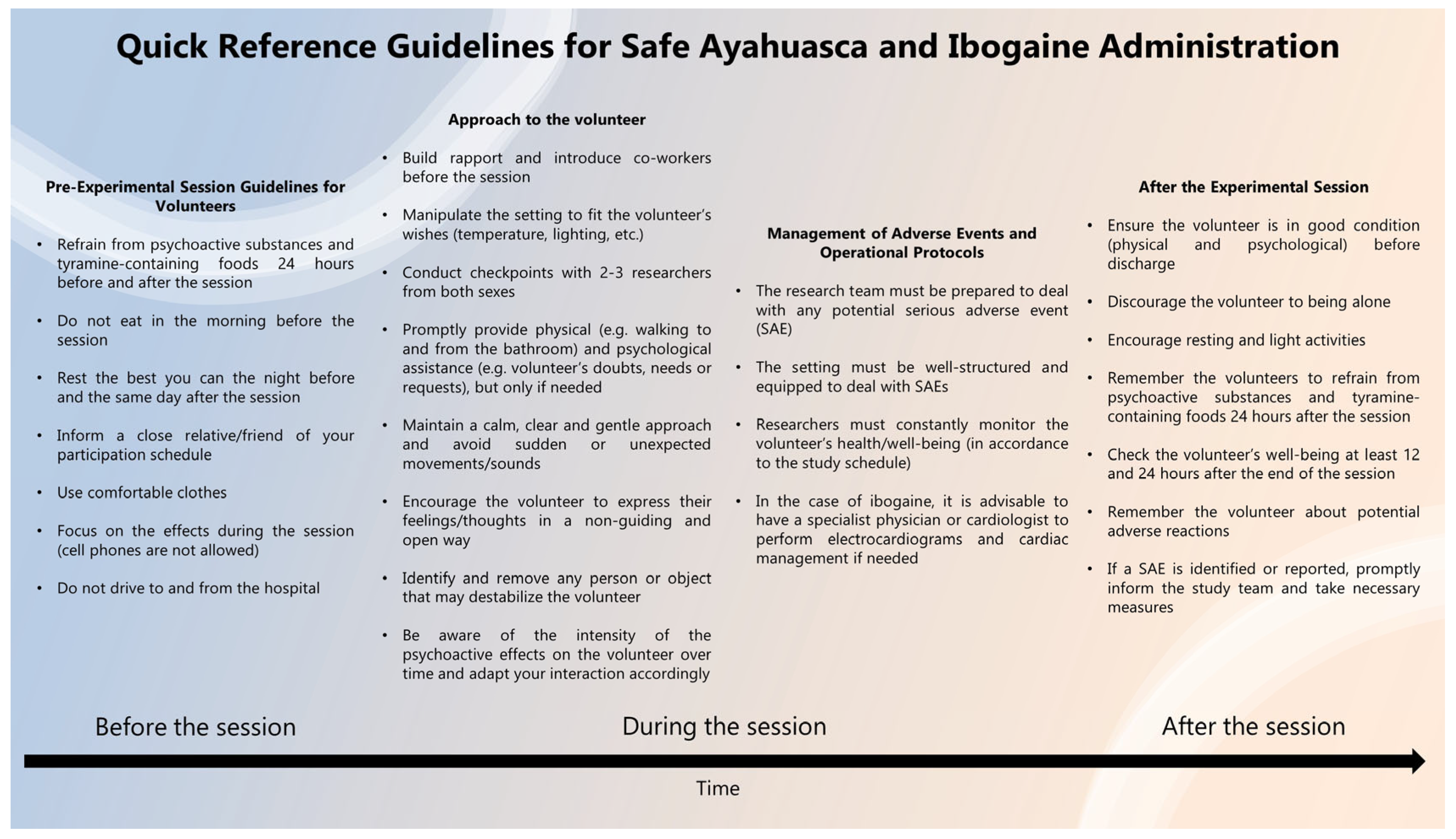
3.3. Experimental Session
3.3.1. Setting
3.3.2. Approach the Volunteer
- Introduce yourself and all team members at the site before the intervention, even those who are not expected to be in direct contact with the volunteer;
- Inquire about the participant’s preferences regarding the intensity of light, temperature, use of blankets, and other comfort items;
- Conduct assessments with 2–3 people;
- Prohibit the volunteers from using their cell phones, especially to solve problems and work-related tasks;
- Provide assistance with bathroom visits and walking, if needed, being attentive to any effects that may lead to potential accidents, such as impaired motor coordination and intense alterations in sensory perception;
- Maintain a calm and gentle approach by speaking softly and pausing and avoiding sudden or unexpected movements;
- Refrain from taking notes during the interaction—your attention should be on the volunteer;
- Facilitate communication—questions must be clear and straightforward;
- Encourage the volunteer to express their feelings in a welcoming and open way. Listen attentively in a non-judgmental or confrontational way and do not guide or interpret their experience;
- Emphasize that the subjective and/or physical effects of the substance are transitory;
- Respond promptly to the individual’s needs and requests;
- Identify and remove any individuals who may destabilize the participant;
- Avoid unnecessary physical contact with the volunteer and ask for their permission before any physical contact is made;
- After drug administration and before the start of the effects, the participant may be talkative. Be attentive to when psychoactivity starts and avoid maintaining unnecessary conversations/interactions.
3.3.3. Management of Adverse Events and Operational Protocols
- The research team must be prepared to deal with any potential serious adverse event, no matter how infrequent it may be;
- The setting must be well-structured and equipped with the necessary tools to deal with serious adverse effects;
- The clinical team should be aware, and the management protocol should be aligned among the team;
- Regular monitoring of volunteers’ health status must be performed according to the study schedule;
- Especially in the case of ibogaine, it is advisable to have a specialist physician or cardiologist perform electrocardiograms and cardiac management if needed.
3.4. After the Experimental Session
- Ensure, after clinical evaluation, that the participant is in good condition (both physical and psychological) to safely return home before discharging them;
- Ensure that the participant is in the company of someone they trust (such as a close friend or a relative). Discourage them to be alone after the experimental session;
- Encourage resting and light activities. Instruct the participant to avoid intense, stressful, or demanding activities;
- Tell the participants to avoid recreational drugs and keep the tyramine-free diet for another 24 h;
- Contact the volunteer at least 12 and 24 h after the end of the experimental session. Ask for their general condition, adverse effects, and the eventual use of recreational or prescription drugs, focusing on addressing the participant’s needs;
- Educate the volunteer about potential adverse reactions and provide contact numbers of the research team to be reached if needed;
- If some severe adverse effect is identified or reported, promptly inform the study coordinator and the psychiatrist in charge.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luna, L.E. Indigenous and Mestizo Use of Ayahuasca. An Overview. In The Ethnopharmacology of Ayahuasca; Transworld Research Network: Kerala, India, 2011; pp. 1–21. [Google Scholar]
- Gonçalves, J.; Luís, Â.; Gallardo, E.; Duarte, A.P. A Systematic Review on the Therapeutic Effects of Ayahuasca. Plants 2023, 12, 2573. [Google Scholar] [CrossRef] [PubMed]
- James, E.; Keppler, J.; LRobertshaw, T.; Sessa, B. N,N-dimethyltryptamine and Amazonian ayahuasca plant medicine. Hum. Psychopharmacol. 2022, 37, e2835. [Google Scholar] [CrossRef]
- McKenna, D.J.; Towers, G.; Abbott, F. Monoamine Oxidase Inhibitors in South American Hallucinogenic Plants: Tryptamine and Beta-Carboline Constituents of Ayahuasca. J. Ethnopharmacol. 1984, 10, 195–223. [Google Scholar] [CrossRef] [PubMed]
- Riba, J.; Valle, M.; Urbano, G.; Yritia, M.; Morte, A.; Barbanoj, M.J. Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion, and Pharmacokinetics. J. Pharmacol. Exp. Ther. 2003, 306, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Riba, J.; McIlhenny, E.H.; Valle, M.; Bouso, J.C.; Barker, S.A. Metabolism and Disposition of N,N-Dimethyltryptamine and Harmala Alkaloids after Oral Administration of Ayahuasca. Drug Test. Anal. 2012, 4, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Landeira-Fernandez, J.; Strassman, R.; Motta, V.; Cruz, A. Effects of Ayahuasca on Psychometric Measures of Anxiety, Panic-Like, and Hopelessness in Santo Daime Members. J. Ethnopharmacol. 2007, 112, 507–513. [Google Scholar] [CrossRef]
- Osório, F.d.L.; Sanches, R.F.; Macedo, L.R.; dos Santos, R.G.; Maia-De-Oliveira, J.P.; Wichert-Ana, L.; de Araujo, D.B.; Riba, J.; Crippa, J.A.; Hallak, J.E. Antidepressant Effects of a Single Dose of Ayahuasca in Patients with Recurrent Depression: A Preliminary Report. Braz. J. Psychiatry 2015, 37, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sanches, R.F.; De Lima Osório, F.; Santos, R.G.D.; Macedo, L.R.H.; Maia-De-Oliveira, J.P.; Wichert-Ana, L.; De Araujo, D.B.; Riba, J.; Crippa, J.A.S.; Hallak, J.E.C. Antidepressant Effects of a Single Dose of Ayahuasca in Patients With Recurrent Depression: A SPECT Study. J. Clin. Psychopharmacol. 2016, 36, 77–81. [Google Scholar] [CrossRef]
- Palhano-Fontes, F.; Barreto, D.; Onias, H.; Andrade, K.C.; Novaes, M.M.; Pessoa, J.A.; Mota-Rolim, S.A.; Osório, F.L.; Sanches, R.; dos Santos, R.G.; et al. Rapid Antidepressant Effects of the Psychedelic Ayahuasca in Treatment-Resistant Depression: A Randomized Placebo-Controlled Trial. Psychol. Med. 2019, 49, 655–663. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Rocha, J.M.; Rossi, G.N.; Osório, F.L.; Ona, G.; Bouso, J.C.; de Oliveira Silveira, G.; Yonamine, M.; Marchioni, C.; Crevelin, E.J.; et al. Effects of ayahuasca on the endocannabinoid system of healthy volunteers and in volunteers with social anxiety disorder: Results from two pilot, proof-of-concept, randomized, placebo-controlled trials. Hum. Psychopharmacol. Clin. Exp. 2022, 37, e2834. [Google Scholar] [CrossRef]
- Cesar, P.; Barbosa, R.; Mizumoto, S.; Bogenschutz, P.; Strassman, R.J. Health status of ayahuasca users. Drug Test Anal. 2012, 4, 601–609. [Google Scholar] [CrossRef]
- Barbosa, P.C.R.; Tófoli, L.F.; Bogenschutz, M.P.; Hoy, R.; Berro, L.F.; Marinho, E.A.V.; Areco, K.N.; Winkelman, M.J. Assessment of Alcohol and Tobacco Use Disorders Among Religious Users of Ayahuasca. Front. Psychiatry 2018, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.O.; Daldegan-Bueno, D.; Wießner, I.; Araujo, D.B.; Tófoli, L.F. Ayahuasca’s therapeutic potential: What we know–and what not. Eur. Neuropsychopharmacol. 2023, 66, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Schlag, A.K.; Aday, J.; Salam, I.; Neill, J.C.; Nutt, D.J. Adverse effects of psychedelics: From anecdotes and misinformation to systematic science. J. Psychopharmacol. 2022, 36, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.M.M.; Rossi, G.N.M.; Osório, F.d.L.; Bouso, J.C.; Silveira, G.M.d.O.; Yonamine, M.; Campos, A.C.; Bertozi, G.B.; Hallak, J.E.C.; dos Santos, R.G. Effects of Ayahuasca on the Recognition of Facial Expressions of Emotions in Naive Healthy Volunteers: A Pilot, Proof-of-Concept, Randomized Controlled Trial. J. Clin. Psychopharmacol. 2021, 41, 267–274. [Google Scholar] [CrossRef]
- Rossi, G.N.M.; Rocha, J.M.M.; Osório, F.L.; Bouso, J.C.; Ona, G.M.; Silveira, G.d.O.; Yonamine, M.; Bertozi, G.B.; Crevelin, E.J.; Queiroz, M.E.; et al. Interactive Effects of Ayahuasca and Cannabidiol in Social Cognition in Healthy Volunteers: A Pilot, Proof-of-Concept, Feasibility, Randomized-Controlled Trial. J. Clin. Psychopharmacol. 2023, 43, 339–349. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.G.; Bouso, J.C.; Hallak, J.E.C. Ayahuasca, dimethyltryptamine, and psychosis: A systematic review of human studies. Ther. Adv. Psychopharmacol. 2017, 7, 141–157. [Google Scholar] [CrossRef]
- Gómez-Sousa, M.M.; Jiménez-Garrido, D.F.M.; Ona, G.M.; dos Santos, R.G.; Hallak, J.E.C.; Alcázar-Córcoles, M.; Bouso, J.C. Acute psychological adverse reactions in first-time ritual ayahuasca users: A prospective case series. J. Clin. Psychopharmacol. 2021, 41, 163–171. [Google Scholar] [CrossRef]
- Bouso, J.C.; Andión, Ó.; Sarris, J.J.; Scheidegger, M.; Tófoli, L.F.; Opaleye, E.S.; Schubert, V.; Perkins, D. Adverse effects of ayahuasca: Results from the Global Ayahuasca Survey. PLoS Glob. Public Health 2022, 2, e0000438. [Google Scholar] [CrossRef]
- Riba, J.; Rodríguez-Fornells, A.; Urbano, G.; Morte, A.; Antonijoan, R.; Montero, M.; Callaway, J.C.; Barbanoj, M.J. Subjective effects and tolerability of the South American psychoactive beverage Ayahuasca in healthy volunteers. Psychopharmacology 2001, 154, 85–95. [Google Scholar] [CrossRef]
- Rocha, J.M.M.; Rossi, G.N.M.; Osório, F.L.; Hallak, J.E.C.; dos Santos, R.G. Adverse Effects After Ayahuasca Administration in the Clinical Setting. J. Clin. Psychopharmacol. 2022, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.S. Executive Summary: Ayahuasca Global Consumption & Reported Deaths; International Center for Ethnobotanical Education, Research and Service (ICEERS): Catalonia, Spain, 2023. [Google Scholar]
- Alper, K.R. Chapter 1—Ibogaine: A Review in The Alkaloids: Chemistry and Biology; Academic Press: Cambridge, MA, USA, 2001; Volume 56, pp. 1–38. [Google Scholar] [CrossRef]
- Brown, T.K. Ibogaine in the treatment of substance dependence. Curr. Drug Abus. Rev. 2013, 6, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Mash, D.C.; Kovera, C.A.; Buck, B.E.; Norenberg, M.D.; Shapshak, P.; Hearn, W.L.; Sanchez-Ramos, J. Medication development of ibogaine as a pharmacotherapy for drug dependence. Ann. N. Y. Acad. Sci. 1998, 844, 274–292. [Google Scholar] [CrossRef] [PubMed]
- Alper, K.R.; Lotsof, H.S.; Kaplan, C.D. The ibogaine medical subculture. J. Ethnopharmacol. 2008, 115, 9–24. [Google Scholar] [CrossRef]
- Alper, K.R.; Stajić, M.; Gill, J.R. Fatalities temporally associated with the ingestion of ibogaine. J. Forensic Sci. 2012, 57, 398–412. [Google Scholar] [CrossRef]
- Ona, G.; Rocha, J.M.; Bouso, J.C.; Hallak, J.E.C.; Borràs, T.; Colomina, M.T.; dos Santos, R.G. The adverse events of ibogaine in humans: An updated systematic review of the literature (2015–2020). Psychopharmacology 2021, 239, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Koenig, X.; Hilber, K. The anti-addiction drug ibogaine and the heart: A delicate relation. Molecules 2015, 20, 2208–2228. [Google Scholar] [CrossRef]
- Litjens, R.P.W.; Brunt, T.M. How toxic is ibogaine? Clin. Toxicol. 2016, 54, 297–302. [Google Scholar] [CrossRef]
- Meisner, J.A.; Wilcox, S.R.; Richards, J.B. Ibogaine-Associated Cardiac Arrest and Death: Case Report and Review of the Literature. Ther. Adv. Psychopharmacol. 2016, 6, 95–98. [Google Scholar] [CrossRef]
- Schep, L.; Slaughter, R.; Galea, S.; Newcombe, D. Ibogaine for treating drug dependence. What is a safe dose? Drug Alcohol Depend. 2016, 166, 1–5. [Google Scholar] [CrossRef]
- Rocha, J.M.; Reis, J.A.S.; Bouso, J.C.; Hallak, J.E.C.; Dos Santos, R.G. Identifying setting factors associated with improved ibogaine safety: A systematic review of clinical studies. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 1527–1542. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Richards, W.; Griffiths, R. Human hallucinogen research: Guidelines for safety. J. Psychopharmacol. 2008, 22, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research (CDER). Psychedelic Drugs: Considerations for Clinical Investigations Guidance for Industry; U.S. Department of Health and Human Services/Food and Drug Administration: Montgomery, MD, USA, 2023.
- Dickinson, J.; McAlpin, J.; Wilkins, C.; Fitzsimmons, C.; Guion, P.; Paterson, T.; Greene, D.; Chaves, B.R. Clinical Guidelines for Ibogaine-Assisted Detoxification; The Global Ibogaine Therapy Alliance: Montreal, QC, Canada, 2015. [Google Scholar]
- Nichols, D.E. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. [Google Scholar] [CrossRef] [PubMed]
- Rush, B.; Marcus, O.; Shore, R.; Cunningham, L.; Thomson, N.; Rideout, K. Psychedelic Medicine: A Rapid Review of Therapeutic Applications and Implications for Future Research; Homewood Research Institute: Guelph, ON, Canada, 2022. [Google Scholar]
- Bender, D.; Hellerstein, D.J. Assessing the risk–benefit profile of classical psychedelics: A clinical review of second-wave psychedelic research. Psychopharmacology 2022, 239, 1907–1932. [Google Scholar] [CrossRef] [PubMed]
- La Torre, J.T.; Mahammadli, M.; Faber, S.C.; Greenway, K.T.; Williams, M.T. Expert Opinion on Psychedelic-Assisted Psychotherapy for People with Psychopathological Psychotic Experiences and Psychotic Disorders. Int. J. Ment. Health Addict. 2023, 1–25. [Google Scholar] [CrossRef]
- Köck, P.; Froelich, K.; Walter, M.; Lang, U.; Dürsteler, K.M. A systematic literature review of clinical trials and therapeutic applications of ibogaine. J. Subst. Abus. Treat. 2021, 138, 108717. [Google Scholar] [CrossRef]
- Del-Ben, C.M.; Sponholz-Junior, A.; Mantovani, C.; de Morais Faleiros, M.C.; de Oliveira, G.E.C.; Guapo, V.G.; de Azevedo Marques, J.M. Emergências psiquiátricas: Manejo de agitação psicomotora e avaliação de risco suicida. Rev. Med. Ribeirão Preto. 2017, 50, 98–112. [Google Scholar]
- de Paula Andrade, R.L.; Pedrão, L.J. Algumas considerações sobre a utilização de modalidades terapêuticas não tradicionais pelo enfermeiro na assistência de enfermagem psiquiátrica. Rev. Lat. Am. De Enferm. 2005, 13, 737–742. [Google Scholar] [CrossRef]
- Conselho Regional De Enfermagem De São Paulo (COREN-SP). Parecer COREN-SP 019/2012- CT, de 2012. Contenção de Pacientes Mediante a Prescrição por “Telemedicina” em APH e em Outras Situações. Available online: http://portal.coren-sp.gov.br/sites/default/files/parecer_coren_sp_2012_19.pdf (accessed on 5 April 2017).
- Mantovani, G.; Migon, M.N.; Alheira, F.V.; Del-Ben, C.M. Manejo de paciente agitado ou agressivo. Rev. Bras. Psiquiatr. 2010, 32, S96–S103. [Google Scholar] [CrossRef]
- Novitayani, S.; Aiyub; Maulina; Martina. Restraint in Psychiatric Patients: A Literature Review. Sci. Technol. Publ. 2018, 1, 234–241. [Google Scholar]
- Garriga, M.; Pacchiarotti, I.; Kasper, S.; Zeller, S.L.; Allen, M.H.; Vázquez, G.; Baldaçara, L.; San, L.; McAllister-Williams, R.H.; Fountoulakis, K.N.; et al. Assessment and management of agitation in psychiatry: Expert consensus. World J. Biol. Psychiatry 2016, 7, 86–128. [Google Scholar] [CrossRef]
- Protocolo de contenção física e mecânica. Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto- FMRP/USP. 2022. Available online: https://www.ribeiraopreto.sp.gov.br/files/ssaude/pdf/protocolo-003.pdf (accessed on 1 September 2023).
- Ribeirão Preto. Prefeitura Municipal. Secretaria Municipal da Saúde. Departamento de Atenção à Saúde das Pessoas. Protocolo de contenção física e mecânica. Ribeirão Preto São Paulo 2018.
- de SB dos Santos, M.E.; dos A do Amor, J.; Del-Ben, C.M.; Zuardi, A.W. Serviço de emergências psiquiátricas em hospital geral universitário: Estudo prospectivo. Rev. Saúde Pública 2000, 34, 468–474. [Google Scholar] [CrossRef] [PubMed]
- da Silva Souza, L.M.; Santana, R.F.; da Silva Gabriel Capeletto, C.; Menezes, A.K.; Delvalle, R. Factors associated with mechanical restraint in the hospital environment: A cross-sectional study. Rev. Esc. Enferm. USP 2019, 53, e03473. [Google Scholar] [CrossRef] [PubMed]
- Zeferino, M.T.; do Horto Fontoura Cartana, M.; Fialho, M.B.; Huber, M.Z.; Bertoncello, K.C.G. Percepção dos trabalhadores da saúde sobre o cuidado às crises na Rede de Atenção Psicossocial. Esc. Anna Nery 2016, 20, e20160059. [Google Scholar]
- Baldaçara, L.; Rocha, G.A.; Leite, V.d.S.; Porto, D.M.; Grudtner, R.R.; Diaz, A.P.; Meleiro, A.; Correa, H.; Tung, T.C.; Quevedo, J.; et al. Brazilian Psychiatric Association guidelines for the management of suicidal behavior. Part 1. Risk factors, protective factors, and assessment. Braz. J. Psychiatry. 2021, 43, 525–537. [Google Scholar] [CrossRef]
- Martí, M.T. Protocolo Para la Detección y Manejo Inicial de la Ideación Suicida. Guía Desarrollada por el Centro de Psicología Aplicada (CPA) Universidad Autónoma de Madrid (UAM). 2013. Available online: https://psicologosemergenciasbaleares.files.wordpress.com/2018/01/protocolo_ideacion_suicida.pdf (accessed on 10 August 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, J.M.; Reis, J.A.S.; Rossi, G.N.; Bouso, J.C.; Hallak, J.E.C.; dos Santos, R.G. Guidelines for Establishing Safety in Ayahuasca and Ibogaine Administration in Clinical Settings. Psychoactives 2023, 2, 373-386. https://doi.org/10.3390/psychoactives2040024
Rocha JM, Reis JAS, Rossi GN, Bouso JC, Hallak JEC, dos Santos RG. Guidelines for Establishing Safety in Ayahuasca and Ibogaine Administration in Clinical Settings. Psychoactives. 2023; 2(4):373-386. https://doi.org/10.3390/psychoactives2040024
Chicago/Turabian StyleRocha, Juliana M., José Augusto S. Reis, Giordano N. Rossi, José Carlos Bouso, Jaime E. C. Hallak, and Rafael G. dos Santos. 2023. "Guidelines for Establishing Safety in Ayahuasca and Ibogaine Administration in Clinical Settings" Psychoactives 2, no. 4: 373-386. https://doi.org/10.3390/psychoactives2040024
APA StyleRocha, J. M., Reis, J. A. S., Rossi, G. N., Bouso, J. C., Hallak, J. E. C., & dos Santos, R. G. (2023). Guidelines for Establishing Safety in Ayahuasca and Ibogaine Administration in Clinical Settings. Psychoactives, 2(4), 373-386. https://doi.org/10.3390/psychoactives2040024






