Abstract
We described insulin glargine (originator) and insulin glargine-yfgn (biosimilar) treatment patterns, assessed effectiveness and safety outcomes, and identified reasons for switching back to the originator product from the biosimilar. This retrospective study included 328,463 Veterans 18 years of age and older who received one or more outpatient prescriptions for insulin glargine and/or insulin glargine-yfgn between 1 June 2021 and 31 December 2022. Patients were assigned to subgroups based on the initial prescription during the study period, prevalent versus incident use for originator insulin glargine, and prior versus no prior use of the originator before the biosimilar (i.e., prevalent originator non-switcher (n = 189,734), originator switch to biosimilar (n = 81,010), incident originator non-switcher (n = 49,401), and incident biosimilar (n = 8318)). There were no differences in the outcome of mean HbA1c (7.9% for all subgroups). There were also no differences in the unadjusted rates of hospitalization and/or emergency room visits for hyper- and hypoglycemia between the prevalent originator non-switcher and originator switched to biosimilar subgroups (p = 0.09 and 0.38, respectively) or the incident originator non-switcher and incident biosimilar subgroups (p = 0.054 and 0.61, respectively). Finally, none of the HbA1c or hyperglycemia outcomes adjusted for baseline characteristics were statistically different. Adjusted analyses for rates of hospitalization and/or emergency room visits for hypoglycemia could not be performed due to the low number of events. Overall, patients who received insulin glargine-yfgn had similar effectiveness and safety outcomes as patients who received the originator.
1. Introduction
The number of biosimilars approved by the US Food and Drug Administration (FDA), as well as the number of products participating in the FDA Biosimilar Development Program, continues to expand [1]. As the prevalence of biological products (biologics) for disease management grows, interest in biosimilars as cost-effective alternatives to the originator (i.e., initially approved or reference) product also increases. Biosimilars are analogous to generic medications in that both mimic the initially approved biologic or brand name drug, respectively. However, the biosimilar and originator biologics may have differences in structural variation due to their size and molecular complexity [2,3]. These differences are not clinically meaningful with regard to safety, purity, or potency, but they are sufficient to prevent the automatic substitution of the biosimilar for the originator. If biosimilars meet additional FDA requirements to demonstrate interchangeability for safety and efficacy, then they may be substituted for the originator without a new prescription [2].
In 2021, insulin glargine-yfgn was the first biosimilar to be granted interchangeability status by the FDA. Originator insulin glargine and its biosimilars are long-acting synthetic formulations of human insulin used for glycemic control by providing a basal insulin level for patients with type 1 or type 2 diabetes mellitus [4]. Results from two non-inferiority studies (INSTRIDE 1 and 2) [5,6] were used to support approval of the biosimilar, and one equivalence study (INSTRIDE 3) was used to support interchangeability between the originator and biosimilar products [7]. However, we were unable to find any published post-marketing studies with insulin glargine-yfgn; these data are important because they provide information on safety and effectiveness in diverse populations with more complex comorbidities. Real-world studies can help to inform more appropriate use and increase patients’ and providers’ confidence in biosimilars.
Therefore, the overall goal of this study was to provide additional information on the effectiveness and safety of the biosimilar product (insulin glargine-yfgn) as compared with the originator (insulin glargine) in the Veterans Health Administration (VHA). (There is another biosimilar, insulin glargine-aglr, but it has not been used in VHA to date.) The objectives were to (1) describe biosimilar and originator insulin glargine treatment patterns; (2) evaluate the effectiveness of the biosimilar versus the originator; (3) assess the rates of select adverse events among patients who receive the biosimilar versus the originator; and (4) identify the reasons for switching back to the originator from the biosimilar product, as applicable.
2. Results
2.1. Patient Cohort
Between 1 June 2021 and 31 December 2022, 339,536 Veterans received an outpatient prescription for the originator or biosimilar insulin glargine product (Figure 1). Throughout this study, “insulin glargine products” refer to originator insulin glargine and insulin glargine-yfgn, the biosimilar. Of these patients, 334,957 (98.7%) had a prescription for the U-100 formulation of insulin glargine or insulin glargine-yfgn. Patients with prescriptions for both the originator and biosimilar on the index date were excluded (i.e., date of first prescription for insulin glargine during the study period), leaving 334,947 Veterans. After restricting the cohort to Veterans with a history of using VHA services, 328,463 were included and divided into subgroups based on the index prescription, prevalent versus incident use for originator insulin glargine, and prior versus no prior use of the originator before incident use of the biosimilar. The prevalent originator non-switcher subgroup was the largest with 189,734 patients, followed by originator switch to incident biosimilar (n = 81,010), incident originator non-switcher (n = 49,401), and incident biosimilar (n = 8318) subgroups.
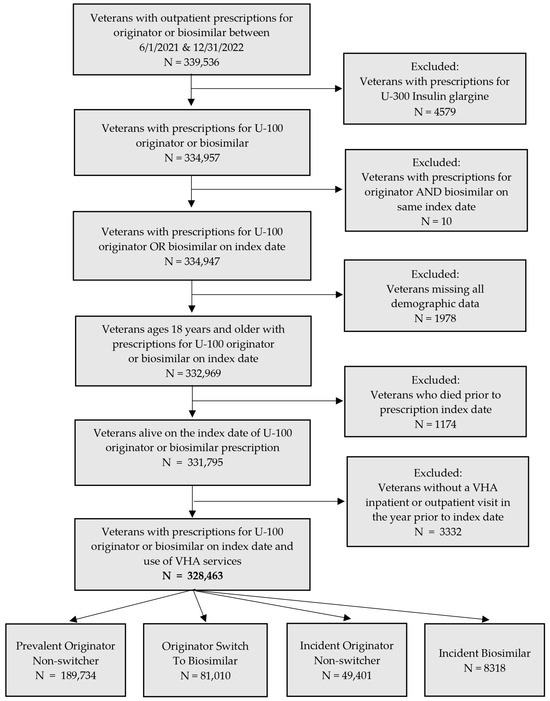
Figure 1.
Flowchart of included and excluded patients and subgroups.
2.2. Prescribing Patterns
Of the 239,135 patients with an index prescription for the originator, 79.3% were prevalent users, and 20.7% were incident users (Figure 2). None of these patients were switched to the biosimilar during the study period. Patients who were switched to the biosimilar from originator insulin glargine (24.7%) either remained on the biosimilar, switched back to the originator, or switched at least one additional time for a total of three or more changes in insulin glargine prescriptions. These patients who switched to the biosimilar were either prevalent or incident users of the originator. Patients who initiated therapy with the biosimilar during the study period (2.5%) either remained on the biosimilar, switched to the originator, switched back to the biosimilar, or switched at least one additional time for a total of three or more changes in insulin glargine prescriptions.
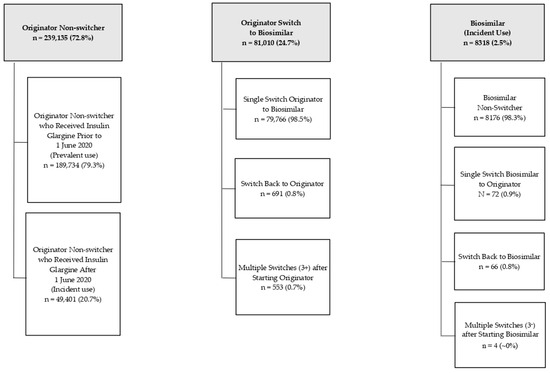
Figure 2.
Relationship between prescribing pattern of originator and/or biosimilar product and sub-cohort groups.
2.3. Baseline Patient Characteristics
Patients across the subgroups were predominantly non-Hispanic White males (Table 1). The mean (SD) age ranged from 66.2 (12.5) years for the incident originator subgroup to 69.4 (10.8) years for the prevalent originator subgroup. The proportion of patients with comorbidities of interest was lower in the incident originator and biosimilar subgroups. The mean number of concomitant antidiabetic medications at baseline was similar across subgroups, but the specific classes of medications varied. For example, a higher proportion of incident users of both originator and biosimilar insulin glargine received sulfonylureas as compared with prevalent users of originator insulin glargine and those who switched from the originator to biosimilar. Mean (SD) baseline glycated hemoglobin (i.e., HbA1c) was higher in the incident originator (9.4% [2.6]) and incident biosimilar (9.3% [2.5]) subgroups, and the proportion of patients with an HbA1c less than 7% was also lower in these two subgroups. Finally, the proportion of patients with VHA hospitalizations and/or emergency room (ER) visits for hyperglycemia at baseline was greater in the incident biosimilar and originator subgroups compared with prevalent users of the originator and those who switched from the originator to biosimilar.

Table 1.
Baseline characteristics of patients receiving originator insulin glargine or insulin glargine-yfgn (i.e., biosimilar).
2.4. Effectiveness Outcomes
The means of the most recent HbA1c values were the same across the four subgroups (7.9%, Table 2). There were statistically significant differences in the proportion of patients with the most recent HbA1c < 7% in the prevalent originator versus originator to biosimilar subgroups (p = 0.01) and in the incident originator versus incident biosimilar subgroups (p = 0.02). However, the proportions were numerically similar. Also, the incident originator (31.2%) and biosimilar (28.7%) subgroups had slightly higher percentages of patients with the most recent HbA1c < 7%, compared with the prevalent originator (26.6%) or the originator to biosimilar (27.5%) subgroups. The arithmetic mean HbA1c that included all results after 3 months of insulin therapy was also similar across all subgroups (7.9% or 8%).

Table 2.
Unadjusted effectiveness outcomes.
2.5. Safety Outcomes
For the safety outcomes assessed (Table 3), there was no difference in the crude rates of hyperglycemia resulting in hospitalizations and/or ER visits between the prevalent originator non-switcher and originator switched to biosimilar subgroups (p = 0.09) or the incident originator non-switcher and incident biosimilar subgroups (p = 0.054). However, the rate (per 100 patient-years) of hospitalizations and ER visits for hyperglycemia was greater in the incident originator (4.8) and biosimilar subgroups (6.1) compared with the prevalent originator (2.2) and originator switched to biosimilar subgroups (2.5). The number of hypoglycemic events was small and too few to provide an accurate estimate in the biosimilar subgroups. However, there was no difference in the crude rates of hypoglycemia resulting in hospitalizations and/or ER visits between the prevalent originator non-switcher and originator switched to biosimilar subgroups (p = 0.38) or the incident originator non-switcher and incident biosimilar subgroups (p = 0.61).

Table 3.
Unadjusted safety outcomes.
2.6. Adjusted Regression Analyses
The adjusted regression analyses for the effectiveness and safety outcomes are presented in Table 4. Hypoglycemia was excluded from the analyses because the number of events was too small to estimate the risk difference between groups while adjusting for potential confounders. All analyses are adjusted for the characteristics in Table 1. None of the differences are statistically significant.

Table 4.
Adjusted regression analyses for effectiveness and safety outcomes 1.
2.7. Switching Back to Originator Insulin Glargine
Of the 691 patients who received originator insulin glargine within one year prior to the index prescription for the biosimilar, 139 (20%) were randomly sampled, and their electronic health records were reviewed to determine the reason patients were switched back to originator insulin glargine (Table 5). Most patients were switched back because the originator was the only insulin glargine the VA facility pharmacy had in stock (i.e., an administrative switch) (61.2%). Of the remaining patients sampled, most were inadvertently identified as switching back to the originator (30.2%). When their electronic health records were reviewed, originator insulin glargine was ordered on the prescription, but the medication dispensed was the biosimilar. Upon further investigation, this could have been due to the ordering profile set-up for biosimilars at certain facilities. Of the 42 patients, 20 were from the same site.

Table 5.
Reasons for switching back to originator insulin glargine (i.e., originator to biosimilar to originator) (n = 139).
3. Discussion
In our study of the effectiveness and safety of originator insulin glargine versus biosimilar insulin glargine-yfgn in a large Veteran population, we did not find any clinically relevant differences in outcomes between the subgroups of incident users of the originator and biosimilar and the subgroups of the prevalent originator and originator switchers to the biosimilar. Some unadjusted HbA1c, or effectiveness, outcomes were statistically different due to the large numbers of patients in the subgroups (i.e., we had enough power to detect very small differences). The rates of hospitalizations and ER visits for hyperglycemia were comparable among the incident user subgroups and among the prevalent originator and originator switchers to the biosimilar subgroups; however, the incident user subgroups both had higher rates of hyperglycemia. This is not unexpected because providers are more frequently adjusting insulin doses in those starting therapy. During this time, providers are more likely to proceed with conservative adjustments in doses to avoid hypoglycemia in older adults who do not tolerate low blood glucose values as well as a younger population [8]. These incident users also had higher HbA1cs, and lower proportions with an HbA1c < 7% at baseline, which is anticipated because they had no history of insulin glargine use within one year prior to the index date. The rates of hospitalization and ER visits for hypoglycemia were low in all subgroups and too small to provide an accurate estimate in the biosimilar subgroups, but the differences between the groups were not statistically significant in the unadjusted analyses. Our results are not surprising, but they are reassuring as we expected the outcomes to be similar among those receiving the originator versus the biosimilar based on previously published studies.
At this time, we were unable to find any real-world comparative effectiveness and safety studies with originator insulin glargine and insulin glargine-yfgn. Phase 3 trials evaluated the efficacy and safety of insulin glargine-yfgn with the originator in patients with type 1 diabetes (INSTRIDE 1) [5] and type 2 diabetes (INSTRIDE 2) [6] and found the biosimilar to be non-inferior to the originator based on the primary endpoint of HbA1c change. INSTRIDE 3 compared the efficacy and safety of switching from the biosimilar to the originator and back to the biosimilar with a group of patients with type 1 diabetes who remained on the originator throughout the trial. The outcomes were similar in both groups, and these data were used to support the approval of insulin glargine-yfgn as interchangeable with the originator (INSTRIDE 3) [7].
In addition, there are post-marketing studies comparing originator insulin glargine with the follow-on biologic version. Follow-on biologics are also similar to the originator, but they are FDA-approved under the Food, Drug, and Cosmetic Act 505(b)(2) rather than the newer Public Health Service Act, Section 501(k) pathway that has been in place for biologics since 23 March 2020 [9]. Follow-on biologics cannot be substituted for the originator without a new prescription. Similar to our results, the real-world studies comparing the follow-on biologic with the originator found no significant differences in effectiveness and safety [10,11,12,13].
Given the lack of clinically relevant differences among the subgroups regarding effectiveness and safety outcomes, our results support switching patients from insulin glargine to insulin glargine-yfgn with no resulting clinically pertinent changes, on average, in HbA1c scores and rates of hospitalization and ER visits for hyper- and hypoglycemia. Although the results for hypoglycemia could not be adjusted for potential confounders due to the occurrence of few events, it is unlikely that non-significant findings would become significant in adjusted analyses. The VA Center for Medication Safety, which is part of VA Pharmacy Benefits Management Services, has been evaluating the safety and effectiveness of other biosimilars (e.g., rituximab, infliximab), and they have not identified problems when switching from the originator to biosimilar product.
Transition to insulin glargine-yfgn from the originator occurred asynchronously across VA facilities due to changes in the amount of biosimilar available from the manufacturer, with many sites beginning the transition in the last two months of the study period. However, some sites quickly switched most of their patients to the biosimilar shortly after it was available due to the lower cost. Therefore, if the study period was longer, more patients would have likely switched to the biosimilar from the originator or initiated therapy with the biosimilar. This could potentially lead to additional cases of hypoglycemia in the biosimilar subgroups and permit a more robust comparison with the originator product. However, given our results and those in the literature, there is unlikely to be a difference.
Although our study evaluated clinically relevant outcomes in a large, national Veteran population, there are limitations. First, the sample size was too small to assess effectiveness and safety in patients who underwent multiple switches between products (i.e., 3+). This is an important area for future study given the lack of information in these patients and concern about the formation of antibodies. Second, there was another biosimilar for the originator insulin glargine (insulin glargine-aglr) that was not evaluated because it was not used in VHA during the study timeframe [14]. Comparing effectiveness and safety among biosimilars will be useful as more receive FDA approval. Third, we did not evaluate insulin doses because the directions on the prescription may not be updated every time the patient’s dose is changed, especially in new starts. However, INSTRIDE 1 and 2 included this outcome and found no difference between the biosimilar and originator [5,6]. Fourth, there is the potential for residual confounding due to the lack of data on variables such as BMI, adherence, diabetes duration, socioeconomic status, educational level, urban/rural continuum, and physical activity. However, the inclusion of other possible confounders is unlikely to change the conclusions given that there was no difference in outcomes between the groups. Finally, we did not include events that occurred outside of VHA, although we would not expect differential use of non-VHA hospitals/ERs among the subgroups. However, this likely contributed to the low number of hypoglycemic events.
4. Conclusions
Slightly less than three-quarters of Veterans received only originator insulin glargine during the study period, and approximately one-quarter were switched from the originator to the biosimilar. Of these patients, less than one percent were switched back to the originator, mainly because it was the only insulin glargine the pharmacy had in stock. Veterans who were incident users of insulin glargine-yfgn had similar HbA1c results and rates of hospitalization and/or emergency room visits for hyper- and hypoglycemia to those who were incident users of the originator. The same was true when comparing Veterans who were switched from the originator to insulin glargine-yfgn with those who were prevalent users of the originator. These real-world findings should help patients and providers feel more comfortable with insulin glargine biosimilars. Studies in patients who switch multiple times among available biosimilars and the originator are crucial.
5. Materials and Methods
5.1. Study Design and Participants
This national retrospective cohort study included Veterans aged ≥ 18 years who had at least one outpatient prescription for originator insulin glargine and/or biosimilar insulin glargine-yfgn filled within VHA between 1 June 2021 and 31 December 2022 (i.e., the study period). Patients who did not use the VHA at baseline (i.e., no inpatient or outpatient visit within one year prior to the index prescription date) were excluded because it is liable for there to be limited data available within VHA records for these patients. Veterans receiving combination products (e.g., insulin glargine + lixisenatide) or concentrated insulin glargine at 300 units/mL were also excluded as these patients were less likely to be candidates to switch to the biosimilar. In addition, patients with prescriptions for both the originator and biosimilar on the same index date were excluded because we did not know which biologic they used.
The index date was defined as the release date of the first prescription for the originator or biosimilar product during the study period. For outcomes other than prescribing patterns, patients were followed until they switched products (e.g., biosimilar to originator), had prescriptions for both the originator and biosimilar released on the same date, reached the index date +364 days or at the end of the study period, died, or discontinued the insulin glargine product, which was defined as no new prescription within 1.5 times the days’ supply of the last insulin glargine prescription for patients who did not have another prescription for the biosimilar or originator during the remainder of the study period. Throughout this study, “insulin glargine products” refer to originator insulin glargine and insulin glargine-yfgn, the biosimilar. This study was approved by the IRBs at the Edward Hines, Jr. VA Medical Center and the VA Pittsburgh Healthcare System.
5.2. Data Sources and Data Collection
The Pharmacy Benefits Management (PBM) outpatient prescription database v3.0 was used to identify patients who received insulin glargine and insulin glargine-yfgn during the study period. This database includes the drug name, strength, dosing instructions, days’ supply, prescriber, station number, and release dates for outpatient prescriptions. Other validated PBM databases were used to collect demographic information, comorbidities, pertinent lab values, and hospitalizations and ER visits within VHA. The Vital Status File was used to determine the date of death during the study timeframe, as applicable.
Baseline data were collected during the 12 months prior to the index date and included age, sex, race/ethnicity, marital status, smoking status, comorbidities, HbA1c, concomitant antidiabetic medications, use of a glucose monitoring sensor, and the number of VHA hospitalizations and ER visits with an ICD-10-CM code for hypoglycemia and hyperglycemia in the primary position (Table S1) [15,16,17]. Data on body mass index (BMI) were also available, but the information was excluded due to a high proportion of missing results for the cohort (>40%). Comorbidities included those within the Charlson Comorbidity Index, as well as potential complications of diabetes (i.e., neuropathy, ischemic stroke, hypertension, retinopathy, and nephropathy) [18]. Outpatient visits and hospitalizations with ICD-10-CM codes for these comorbidities were used to obtain the data. The PBM Outpatient Prescription database was used to pull information on concomitant use of biguanides, fast-acting insulin, sulfonylureas, glucagon-like peptide-1 (GLP-1) receptor agonists, thiazolidinediones, meglitinides, sodium-glucose cotransporter 2 (SGLT2) inhibitors, dipeptidyl peptidase 4 (DPP-4) inhibitors, alpha-glucosidase inhibitors, amylinomimetics, and glucose monitoring sensors. Concomitant use of antidiabetic medications was defined as possessing a days’ supply of the medication that overlapped with the index prescription release date for originator insulin glargine or the biosimilar.
5.3. Creation of Subgroups
Patient transition to the insulin glargine-yfgn was asynchronous across VHA. Contributing factors included a temporary supply shortage until the manufacturer was able to meet demand, local guidance, and prescriber preferences. Consequently, patients may have been switched to the available insulin glargine product one or more times during the study period. A switch between products was defined as a new prescription for insulin glargine that was different from the prior insulin glargine product.
Patients who received originator insulin glargine and/or insulin glargine-yfgn during the study period were separated into four subgroups based on the index prescription, prevalent versus incident use for originator insulin glargine, and prior versus no prior use of the originator before incident use of the biosimilar (i.e., prevalent originator non-switcher, originator switch to biosimilar, incident originator non-switcher, and incident biosimilar). Patients in the category of prevalent originator non-switcher received insulin glargine within one year prior to the index date of the originator prescription in the study period and continued throughout the study with no switches to the biosimilar. Patients in the originator switch to biosimilar subgroup received originator insulin glargine within one year prior to the index date of the biosimilar prescription in the study period. This included patients who had a single switch from the originator to the biosimilar, switched back to the originator, or had multiple switches starting with an index prescription for the originator. The incident originator non-switcher subgroup included patients who initiated originator insulin glargine during the study period, had no history of receiving the originator, and remained on the originator throughout. The final subgroup, incident biosimilar, included Veterans who started insulin glargine-yfgn during the study period and had no history of receiving originator insulin glargine. This group included patients who never switched after starting the biosimilar, switched once to the originator, switched back to the biosimilar, or had multiple switches. Incident and prevalent users of insulin glargine were separated due to potential differences in dosing trends, monitoring, and outcomes. Patients who received insulin glargine-yfgn were separated into subgroups based on prior use of the originator for the same reasons. For example, patients receiving a stable dose of originator insulin glargine may not require as many dose adjustments when switched to the biosimilar compared to an incident biosimilar patient.
5.4. Outcomes
5.4.1. Prescribing Patterns
To determine the prescribing patterns of insulin glargine products for each Veteran, outpatient prescription data between 1 June 2021 and 31 December 2022 were examined. Data for each patient were reviewed from the index prescription to the last prescription released for insulin glargine or insulin glargine-yfgn during the study period. A patient was described as a non-switcher, single switcher, switch back (two switches), or multiple switcher (three or more switches between insulin glargine products).
5.4.2. Effectiveness and Safety
Effectiveness outcomes included the mean of the most recent HbA1c values prior to censoring, proportion of patients with the most recent HbA1c less than 7%, and overall arithmetic mean HbA1c. To be included in the effectiveness analysis, patients must have received at least three months of insulin therapy as HbA1c averages three months of blood glucose levels [19]. Baseline characteristics of Veterans included in the effectiveness outcomes analyses are provided in Table S2.
Safety outcomes were hospitalizations and/or ER visits for hyperglycemia and hypoglycemia based on ICD-10-CM codes in the primary position (Table S1) [15,16,17]. Both unadjusted and adjusted results were assessed. Although evaluating outcomes among prevalent users can be more prone to bias, we included the results so providers could compare prevalent originator non-switchers with those who switched from the originator to the biosimilar as this is an area of interest.
5.4.3. Reasons for Switching Back to Originator from Biosimilar
To ascertain reasons for patients being switched back to originator insulin glargine from the biosimilar (i.e., originator to biosimilar to originator), reviews of the electronic medical record were performed by three pharmacovigilance pharmacist co-investigators (S.W., A.A., C.S.) on a 20% random sample of these patients. This was conducted to identify potential issues with switching from originator to insulin glargine-yfgn such as adverse drug events and decreased effectiveness.
5.5. Analysis
For baseline characteristics, and effectiveness and safety outcomes, we compared the incident originator and biosimilar subgroups and the prevalent and switch from originator to biosimilar subgroups because new users of basal insulin are typically monitored more frequently and are at increased risk for adverse reactions. Given the large sample size of the study, baseline characteristics were compared using standardized differences, calculated as the difference in means or proportions divided by a pooled estimate of the standard deviation for each characteristic. A standardized difference < 0.1 is considered to be negligible [20]. A standardized difference provides a direct estimate of the magnitude of the difference independent of the sample size. For the effectiveness and safety outcomes, the unadjusted analyses were conducted using a t-test for the continuous outcome, chi-square test for the binary outcome, and negative binomial model for the count data. In the adjusted analyses, missing covariates were imputed using chained equations (no variables had >5% missingness) [21]. Multivariable regression analyses with robust variance were used to assess a difference in outcomes between originator and biosimilar insulin glargine subgroups while adjusting for baseline characteristics (e.g., demographics, comorbidities, concomitant antidiabetic medications, HbA1c). The linear regression for the continuous outcomes, logistic regression for the binary outcome, and negative binomial model for the count data were performed with the point estimate and 95% confidence intervals on mean difference, risk difference, and incidence rate difference presented as appropriate using Stata’s margins option after the models [21]. A two-sided p value < 0.05 was considered to be statistically significant. We conducted the descriptive analyses using SAS 9.4 (SAS Institute, Cary, NC, USA), and imputation and models using StataSE 17 (StataCorp LLC, College Station, TX, USA).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharma3010008/s1, Table S1: ICD-10-CM Codes for Hyperglycemia and Hypoglycemia and Table S2: Baseline Characteristics of Subsample of Patients Receiving Originator Insulin Glargine or Insulin Glargine-yfgn who were Followed for 3+ Months and had HbA1C Measured.
Author Contributions
Conceptualization, F.E.C., D.D. and S.L.A.; methodology, S.W., F.E.C., X.Z., D.D., P.A.G., D.R.M., D.K., A.A., C.S., K.B., Q.H. and S.L.A.; formal analysis, X.Z.; investigation, S.W., X.Z., D.D., A.A., C.S. and K.B.; data curation, S.W., X.Z. and S.L.A.; writing—original draft preparation, S.W. and S.L.A.; writing—review and editing, F.E.C., X.Z., D.D., P.A.G., D.R.M., D.K., A.A., C.S., K.B. and Q.H.; supervision, F.E.C. and S.L.A. All authors have read and agreed to the published version of the manuscript. The views expressed in this paper are those of the authors, and no official endorsement by the Department of Veteran Affairs or the United States Government is intended or should be inferred.
Funding
This research received no external funding. In-kind support was provided by the VA Center for Medication Safety, Hines, IL, and VA Center for Health Equity Research and Promotion, VA Pittsburgh Healthcare System, Pittsburgh, PA, USA.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Boards of Edward Hines, Jr. VA Medical Center (protocol #1701056, 11/22/22) and the VA Pittsburgh Healthcare System (protocol #1704409, 2/1/23).
Informed Consent Statement
The protocol is IRB exempt under Common Rule 2018, Category 4—Secondary research for which consent is not required.
Data Availability Statement
Data are unavailable due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Amgen 2022 Biosimilar Trends Report. Available online: https://www.amgenbiosimilars.com/commitment/-/media/Themes/Amgen/amgenbiosimilars-com/Amgenbiosimilars-com/pdf/USACBU81422-2022-Amgen-Biosimilars-Trend-Report-Oct-2022.pdf?#page=4 (accessed on 18 May 2023).
- US Food & Drug Administration. Biosimilar and Interchangeable Biologics: More Treatment Choices. Available online: https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices (accessed on 18 May 2023).
- Triplitt, C.; Hinnen, D.; Valentine, V. How similar are biosimilars? What do clinicians need to know about biosimilar and follow-on insulins? Clin. Diabetes 2017, 35, 209–216. [Google Scholar] [CrossRef]
- Cunningham, A.M.; Freeman, A.M. Glargine Insulin; StatPearls: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557756/ (accessed on 18 May 2023).
- Blevins, T.C.; Barve, A.; Sun, B.; Ankersen, M. Efficacy and safety of MYL-1501D vs insulin glargine in patients with type 1 diabetes after 52 weeks: Results of the INSTRIDE 1 phase III study. Diabetes Obes. Metab. 2018, 20, 1944–1950. [Google Scholar] [CrossRef]
- Blevins, T.C.; Barve, A.; Sun, B.; Raiter, Y.; Aubonnet, P.; Muniz, R.; Athalye, S.; Ankersen, M. Efficacy and safety of MYL-1501D versus insulin glargine in patients with type 2 diabetes after 24 weeks: Results of the phase III INSTRIDE 2 study. Diabetes Obes. Metab. 2019, 21, 129–135. [Google Scholar] [CrossRef]
- Blevins, T.C.; Barve, A.; Raiter, Y.; Aubonnet, P.; Athalye, S.; Sun, B.; Muniz, R. Efficacy and safety of MYL-1501D versus insulin glargine in people with type 1 diabetes mellitus: Results of the INSTRIDE 3 phase 3 switch study. Diabetes Obes. Metab. 2020, 22, 365–372. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 13. Older Adults: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S244–S257. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, J.; Wang, Y.C.; Tran, D.; Seo, S.K. Leveraging Clinical Pharmacology Data to Assess Biosimilarity and Interchangeability of Insulin Products. Clin. Pharmacol. Ther. 2023, 113, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.; Kim, J.D.; Dormuth, C. The impact of mandatory nonmedical switching from originator to biosimilar insulin glargine. Clin. Ther. 2022, 4, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Pitlick, J.M.; Bryant, G.A.; Daly, M.W.; Koenigsfeld, C.F.; Lehman, N.; Brueggen, K.; McCormick, A.; Wellington, K. Real-World Evaluation of Dosing in Patients Converted from Insulin Glargine (Lantus) to Insulin Glargine (Basaglar). Ann. Pharmacother. 2020, 54, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Taki, K.; Koyanagi, M.; Nagaoka, S.; Shingaki, T. Treatment satisfaction, safety, and effectiveness of biosimilar insulin glargine is comparable in patients with type 2 diabetes mellitus after switching from insulin glargine or insulin degludec: A post-marketing safety study. Curr. Med. Res. Opin. 2020, 36, 1975–1983. [Google Scholar] [CrossRef]
- Shingaki, T.; Taki, K.; Koyanagi, M.; Nagaoka, S.; Yoshizawa, K.; Oki, N.; Yoshikawa, A.; Imaoka, T. Long-term safety and effectiveness of biosimilar insulin glargine in Japanese patients with diabetes mellitus in routine clinical practice: Results of a post-marketing safety study. Curr. Med. Res. Opin. 2020, 36, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Eli Lilly and Company. Rezvoglar (Insulin Glargine-Aglr) Injection [Package Insert]. Available online: https://pi.lilly.com/us/rezvoglar-uspi.pdf (accessed on 20 December 2023).
- Dugan, J.; Shubrook, J. International Classification of Diseases, 10th Revision, Coding for Diabetes. Clin. Diabetes 2017, 35, 232–238. [Google Scholar] [CrossRef]
- Lipscombe, L.L.; Austin, P.C.; Alessi-Severini, S.; Blackburn, D.F.; Blais, L.; Bresee, L.; Filion, K.B.; Kawasumi, Y.; Kurdyak, P.; Platt, R.W.; et al. Atypical antipsychotics and hyperglycemic emergencies: Multicentre, retrospective cohort study of administrative data. Schizophr. Res. 2014, 154, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.H.; Ziemba, R.; Shehab, N.; Geller, A.I.; Talreja, K.; Campbell, K.N.; Budnitz, D.S. Assessment of International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) code assignment validity for case finding of medication-related hypoglycemia acute care visits among Medicare beneficiaries. Med. Care. 2022, 60, 219–226. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Eyth, E.; Naik, R. Hemoglobin A1C; StatPearls: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549816/ (accessed on 18 May 2023).
- Mamdani, M.; Sykora, K.; Li, P.; Normand, S.L.; Streiner, D.L.; Austin, P.C.; Rochon, P.A.; Anderson, G.M. Reader’s guide to critical appraisal of cohort studies, 2: Assessing potential for confounding. BMJ 2005, 330, 960–962. [Google Scholar] [CrossRef]
- StataCorp. Multiple Imputation Reference Manual Release 13. Available online: https://www.stata.com/manuals13/mi.pdf (accessed on 20 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
