Abstract
Although statins have served as the cornerstone for pharmacological lowering of lipid levels in atherosclerotic cardiovascular disease (ASCVD) risk reduction, many patients are unable to achieve target doses of statin medication due to side effects or target levels of cholesterol reduction on statin monotherapy. The landscape of lipid-lowering strategies has expanded in recent years, with the emergence of therapies that make use of small interfering RNA (siRNA) and antisense oligonucleotides, in addition to traditional small-molecule agents. Non-statin therapies that have shown promising results in randomized controlled trials include adenosine triphosphate-citrate lyase inhibitors, proprotein convertase subtilisin/kexin 9 (PCSK9)-inhibiting antibodies and siRNA, omega-3 polyunsaturated fatty acids, and lipoprotein(a) gene-inhibiting siRNA and ASOs, in addition to older therapies such as ezetimibe. In contrast, cholesteryl ester transfer protein (CETP) inhibitors have shown less promising results in randomized trials. The purpose of this narrative review is to summarize the evidence for these medications, with a focus on phase III randomized trials.
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of morbidity and mortality worldwide [1], affecting over 500 million individuals globally and accounting for 19 million deaths annually [2]. The pathogenesis of ASCVD, also known as atherogenesis, depends on the presence of various lipoproteins to initiate a cascade that culminates in the deposition of plaques made of cholesterol, fibrin, and calcium in vessels [3]. Uncontrolled elevations in atherogenic lipoproteins, including low-density lipoprotein cholesterol (LDL-C), triglyceride-rich lipoproteins, and lipoprotein A (Lp(a)), along with inflammatory molecules, have been shown to be important predictors of ASCVD outcomes [4]. While statins have been the bedrock of lipid-lowering therapies (LLTs), the landscape of LLTs has expanded in recent years, with the emergence of numerous non-statin therapies targeting these atherogenic lipoproteins via novel molecular methods [5].
Given the challenge of deciding which non-statin therapy is most appropriate for a given patient, the present review seeks to provide a comprehensive evaluation of contemporary non-statin-based LLTs by appraising the pivotal phase III cardiovascular outcomes trials for each LLT, their current indications in the American Heart Association (AHA) and American College of Cardiology (ACC) guidelines, and their Food and Drug Administration (FDA) status. In contrast to previous articles that focused on specific classes of atherogenic lipoproteins, our review addresses contemporary and evolving therapies targeting LDL, triglyceride-rich lipoproteins, and Lp(a). We include clinical trial results that were not available in previous review articles, as well as future lipid-focused treatments targeting multiple classes of atherogenic lipoproteins. Additionally, our review considers the cost-effectiveness of each class of LLT, which may be of utility to clinicians and health systems when determining broad population-based approaches versus targeted high-risk approaches (including primary vs. secondary prevention populations) to the selection of LLTs.
2. Methods
Our team searched through the recent literature on both FDA-approved and emerging non-statin lipid-lowering medications. Using the PubMed, Google Scholar, and clinicaltrials.gov databases, we identified major phase III and IV randomized controlled trials (RCTs) that were either completed or ongoing. We identified studies in our review through keyword-search utilization and a review of meta-analyses between 2018 and 2023 [6,7]. Given that most studies regarding therapies targeting Lp(a) began in the past few years, we expanded our Lp(a) search to include phase II trials. After collecting information on study population, inclusion/exclusion criteria, primary/secondary outcomes, and data across LDL-C, triglyceride, Lp(a), and cardiovascular outcome reduction, RCTs were categorized by drug of interest, population of interest (primary prevention vs. secondary prevention vs. combined), and outcome (lipid reduction vs. cardiovascular event outcomes vs. plaque reduction). For each LLT, we summarized (i) the mechanism of action, (ii) the results of randomized trials as described above, (iii) the FDA-approved indications and dosing on package labels, (iv) the indications recommended in the current AHA/ACC guidelines, (v) the common side effects, and (vi) cost considerations.
3. The Statin-Based Approach to ASCVD
Building on evidence showing that lower LDL-C levels are more cardioprotective, especially in high-risk populations, national subspecialty societies have increasingly tightened the recommended target LDL-C levels for ASCVD risk reduction [8,9]. The core framework of ASCVD risk reduction prioritizes lifestyle interventions as the first-line approach in both primary and secondary prevention of ASCVD (2019 ACC/AHA guidelines [5]). Lifestyle interventions involve strategies to attenuate cardiovascular risk, such as weight loss, blood pressure control, blood sugar control, smoking cessation, dietary changes, and regular exercise. Both statin and non-statin based therapies can be used in conjunction with lifestyle modifications to provide additional LDL-C reduction via pharmacological mechanisms (2019 ACC/AHA guidelines [5]).
Statins serve as the cornerstone of all lipid-lowering strategies, with current guidelines indicating that all individuals with ASCVD scores ≥ 7.5% should be on some form of statin-based therapy for primary prevention of cardiovascular events, and for secondary prevention in patients with a known history of ASCVD (2022 ACC guidelines [9]). By competitively inhibiting the enzyme HMG-CoA reductase, these drugs decrease hepatic cholesterol synthesis [10]. This results in the upregulation of hepatic LDL receptors, thereby increasing LDL-C uptake and, ultimately, lowering serum LDL-C levels [6,10]. Statins have been shown to reduce LDL-C by 25 to 60% [11] and produce dose-dependent reductions in adverse cardiovascular events and total mortality in both primary and secondary prevention [12].
Limitations to Statin-Based Therapies
Despite the numerous established benefits of statins in cardiovascular risk reduction, in isolation, they may be insufficient for achieving target LDL-C levels in patients with dyslipidemia. In addition, intolerance and non-adherence to statins remain a major challenge. Previous large-scale studies have shown discontinuation rates of nearly 50% [13], with patients often reporting concerns over side effects and/or statin intolerance as their reason for discontinuation [14]. Overall, about 10–15% of patients report experiencing statin intolerance, which includes a wide range of symptoms: myalgias (most common), chronic headaches, nausea, sleep disorders, and erectile dysfunction [15]; interestingly, the recent literature shows that myalgia reports are similar between statin-controlled and placebo-controlled patients [16], thereby suggesting that myalgia reporting may be influenced by a nocebo effect in which patients anticipate and may be more hypervigilant for unrelated symptoms that are then attributed to the statin. Rare side effects of statin use include myopathy, rhabdomyolysis, and acute tubular necrosis [17].
Non-statin-based therapies can provide additional lowering of atherogenic lipoproteins such as LDL-C, triglycerides, or Lp(a), reduce rates of adverse cardiac events, and improve overall mortality. Additionally, non-statin therapies tend to result in fewer myalgias, and although there are overlapping as well as distinct side-effect profiles, they may be more tolerable to certain patients; additionally, alternative modes of delivery (e.g., subcutaneous injection) that allow for longer intervals between doses may improve adherence. In this review, we will provide an overview of non-statin-based therapies: their mechanisms of action, major randomized trials examining their effects, and which patient populations stand to benefit from them.
4. LDL-Lowering Therapies
Table 1 provides an overview of the current FDA-approved non-statin-based medications for lipid management: their mechanisms of action, indications, and side effects. Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9 provide an overview of major randomized controlled trials involving non-statin-based therapies that target LDL-C reduction. Table S1 provides a detailed overview of the major inclusion and exclusion criteria for phase III randomized controlled trials for LDL-lowering therapies and phase II trials for Lp(a)-lowering therapies. The majority of FDA-approved non-statin therapies target additional LDL-C reduction as an adjunct to maximally tolerated statin therapy; however, some medications, like bempedoic acid, have also demonstrated utility as standalone therapies in statin-intolerant patients. Figure 1 describes the various mechanisms of action for each of the non-statin-based therapies described here. The following drugs targeting additional reductions in LDL-C are mentioned below. Figure 2 provides a summary of phase III randomized clinical trials that focused on cardiovascular outcomes, along with their study populations.

Table 1.
Overview of FDA-approved medications for lipid lowering.
Table 1.
Overview of FDA-approved medications for lipid lowering.
| Drug, Mechanism | Indication on FDA Label | Dosage, Route, Side Effects (SE) |
|---|---|---|
| Ezetimibe Inhibits serol NPC1L1 | As an adjunct to statin and diet to ↓ LDL-C in adults with 1° HLD including HeFH | Dose: 10 mg daily. Route: Oral. SE: Diarrhea, arthralgia, upper respiratory infection, flu-like symptoms |
| Bempedoic acid Inhibits ATP citrate lyase | As an adjunct to diet and maximally tolerated statins for LDL-C reduction in (a) patients with ASCVD and (b) adults with HeFH | Dose: 180 mg daily. Route: Oral. SE: Hyperuricemia, myopathy, leukopenia, thrombocytopenia, ↑ liver enzymes |
| Ezetimibe + bempedoic acid | As an adjunct to diet and maximally tolerated statins for LDL-C reduction in (a) patients with ASCVD and (b) adults with HeFH | Dose: 180 mg bempedoic acid, 10 mg ezetimibe daily. Route: Oral. SE: Upper respiratory infection, muscle spasms, hyperuricemia, back/abdominal pain, bronchitis, anemia, ↑ liver enzymes, diarrhea |
| Fibrates Agonizes PPAR-α | As an adjunct to diet (a) in patients with 1° HLD or mixed HLD to ↓ LDL-C, TC, TG, apoB, and ↑ HDL-C, and (b) in patients with severe hypertriglyceridemia for TG ↓ | Dose: 200 mg TID (bezafibrate), 100–200 mg PO daily (ciprofibrate), 34–201 mg QD (fenofibrate), 60 mg PO BID (gemfibrozil). Route: Oral. SE: Abdominal pain, constipation, myopathy, ↑ liver enzymes, ↑ Cr |
| Alirocumab, evolocumab Antibody that inhibits PCSK9’s interaction with LDL-C receptors | (a) To ↓ risk of MI, UA, and stroke requiring hospitalization in adults with ASCVD; (b) as adjunct to diet, alone or with LDL-C-lowering therapies in adults with 1° HLD and HeFH; (c) as adjunct to LDL-C-lowering therapies in adults with HoFH | Dose for alirocumab: 75 mg/mL biweekly, 150 mg/mL biweekly, 300 mg monthly. Dose for evolocumab: (a) In adults with ASCVD or 1° HLD: 140 mg biweekly, 420 mg monthly; (b) in patients with HoFH: 420 mg monthly. Route: Subcutaneous. SE: Nasopharyngitis, injection site reactions, flu-like symptoms, myalgia, non-cardiac chest pain |
| Nicotinic acid Inhibits hormone-sensitive lipase | To ↓ TC, LDL-C, TG, and Apo B levels, and increase HDL-C, in patients with 1° HLD and mixed HLD | Dose: 500 mg–2000 mg daily. Route: Oral. SE: Flushing, diarrhea, nausea, vomiting, increased cough, pruritus |
| Inclisiran Binds to PCSK9 mRNA | Use in conjunction with statins and diet for LDL-C ↓ in patients with (a) 1° HLD or mixed HLD, or (b) HeFH | Dose: 284 mg/mL every 6 months (second dose is provided at 3 months, every 6 months afterwards). Route: Subcutaneous. SE: Injection site reactions, arthralgia, bronchitis |
| Mipomersen Inhibits ApoB synthesis | As an adjunct to diet for additional ↓ of LDL-C, apoB, TC, and non-HDL-C in patients with HoFH | Dose: 200 mg weekly. Route: Subcutaneous. SE: Injection site reactions, flu-like symptoms, ↑ liver enzymes |
| Lomitapide Blocks VLDL assembly | As an adjunct to diet for additional ↓ of LDL-C, apoB, TC, and non-HDL-C in patients with HoFH | Dose: 5–60 mg daily. Route: Oral. SE: Diarrhea, nausea, dyspepsia, abdominal pain |
| Icosapent ethyl Reduces hepatic VLDL-TG synthesis/secretion | (1) As an adjunct to maximally tolerated statins to ↓ incidence of CV events in adults with elevated TG > 150 mg/dL and (a) ASCVD or (b) diabetes mellitus and two or more additional risk factors for CVD. (2) As an adjunct to diet to decrease TG levels in adults with TG > 500 mg/dL | Dose: 0.5 mg daily, 1 mg BID. Route: Oral. SE: Atrial fibrillation/flutter, bleeding (especially with anticoagulant and antiplatelet use), allergic reactions in patients with fish allergy, GI dysfunction |

Table 2.
Major phase III randomized clinical trials involving ezetimibe.
Table 2.
Major phase III randomized clinical trials involving ezetimibe.
| Trial, Sample Size | Study Population | Intervention and Comparator | 1° Outcome, Follow-Up Period | 1° Outcome Result |
|---|---|---|---|---|
| Cannon (2015) IMPROVE-IT [18] n = 18,144 | 2° prevention—Patients ≥ 50 years old stabilized within 10 days of an ACS with LDL-C between 50 and 100 mg/dL if on statins, and between 50 and 125 mg/dL if not receiving statins | Intervention: Simvastatin 40 mg daily in combination with ezetimibe 10 mg daily. Comparator: Simvastatin 40 mg daily in combination with placebo. | A composite of CV death, MI, documented unstable angina requiring hospital admission, PCI/CABG ≥ 30 days after randomization, and non-fatal stroke. F/U: 6 years | Kaplan–Meier event rate for 1° outcome was 32.7% in the simvastatin–ezetimibe group, and 34.7% in the simvastatin monotherapy group (absolute risk difference, 2.0 percentage points; HR, 0.936; 95% CI, 0.89 to 0.99; p = 0.016) |
| Ouchi (2019) EWTOPIA 75 [19] n = 3796 | 1° and 2° prevention—Patients ≥ 75 y/o with elevated LDL-C ≥ 140 mg/dL and no history of CAD | Intervention: Oral ezetimibe 10 mg daily. Control: Usual care (no placebo). | A composite of sudden cardiac death, myocardial infarction, coronary revascularization, or stroke. F/U: 4.1 years | Ezetimibe significantly ↓ the incidence of the 1° outcome (HR, 0.66; 95% CI, 0.50–0.86; p = 0.002) |
| Tsujita (2015): PRECISE-IVUS [20] n = 202 | 2° prevention—Patients 30–85 years of age with CAD, with LDL-C ≥ 100 mg/dL, who underwent successful coronary angiography or percutaneous coronary intervention (PCI) to treat ACS or stable angina pectoris | Intervention: Atorvastatin + ezetimibe 10 mg/day. Comparator: Atorvastatin alone | Absolute change in percent atheroma volume (PAV). F/U: 9–12 months | A −1.538% difference in PAV between intervention and control (95% CI: −3.079% to 0.003%). Absolute change in PAV showed superiority for the dual lipid-lowering strategy (−1.4%; 95% CI: −3.4% to −0.1% vs. −0.3%; 95% CI: −1.9% to 0.9% with atorvastatin alone; p = 0.001). |

Table 3.
Major phase III randomized controlled trials involving bempedoic acid.
Table 3.
Major phase III randomized controlled trials involving bempedoic acid.
| Trial, Sample Size | Study Population | Intervention and Comparator | 1° Outcome, Follow-Up Period | 1° Outcome Result |
|---|---|---|---|---|
| Ballantyne (2018) CLEAR Tranquility [21] n = 269 | 1° and 2° prevention—Patients ≥ 18 y/o with statin intolerance or on no statin with LDL-C ≥ 100 mg/dL | Intervention: Oral bempedoic acid 180 mg daily + ezetimibe 10 mg daily. Comparator: Oral placebo daily + ezetimibe 10 mg daily. | Percent change in LDL-C level at week 12. F/U: 12 weeks | Bempedoic acid resulted in a placebo-corrected difference in mean change in LDL-C of −28.5% (95% CI: −34.4%, −22.5%; p < 0.001) |
| Ray (2019) CLEAR Harmony [22] n = 2230 | HeFH and 2° prevention—Patients ≥ 18 years old with ASCVD, HeFH, or both, with LDL-C ≥ 70 mg/dL, on a maximally tolerated statin | Intervention: Oral bempedoic acid 180 mg daily. Comparator: Oral placebo daily. | Overall safety (incidence of AEs and changes in laboratory safety values). F/U: 52 weeks | Bempedoic acid resulted in a ↓ in the mean LDL-C level by 19.2 mg/dL, a change of −16.5% from baseline, and a placebo-corrected difference of −18.1% (95% CI, −20.0 to −16.1; p < 0.001) |
| Goldberg (2019) CLEAR Wisdom [23] n = 779 | HeFH and 2° prevention—Patients ≥ 18 years old with ASCVD, HeFH, or both, with LDL-C ≥ 70 mg/dL, on maximally tolerated LLT | Intervention: oral bempedoic acid 180 mg daily. Comparator: oral placebo daily. | Percent change in LDL-C level at week 12. F/U: 52 weeks | Bempedoic acid resulted in a −15.1% change from baseline in LDL-C levels (placebo-corrected difference of −17.4% [95% CI, −21.0% to −13.9%]; p < 0.001) |
| Laufs (2019) CLEAR Serenity [24] n = 345 | 1° and 2° prevention—Patients ≥ 18 years old with statin intolerance with LDL-C ≥ 130 mg/dL (1° prevention) and ≥ 100 mg/dL (2° prevention) | Intervention: Oral bempedoic acid 180 mg daily. Comparator: Oral placebo daily. | Percent change in LDL-C level at week 12. F/U: 24 weeks | Bempedoic acid resulted in a placebo-corrected difference of −21.4% in LDL-C levels [95% CI, −25.1% to −17.7%]; p < 0.001 |
| Nissen (2023) CLEAR Outcomes [25] n = 13,970 | 1° and 2° prevention—Patients 18–85 years old with or at high risk of ASCVD, statin intolerance, and LDL-C ≥ 100 mg/dL | Intervention: Oral bempedoic acid 180 mg daily. Comparator: Oral placebo daily. | A composite of CV death, non-fatal MI, non-fatal stroke, or coronary revascularization. F/U: 40.6 months | The incidence of a 1° endpoint event was significantly lower with bempedoic acid than with the placebo (11.7% vs. 13.3%; HR, 0.87; 95% CI 0.79 to 0.96; p = 0.004) |

Table 4.
Major phase III randomized controlled trials involving evolocumab.
Table 4.
Major phase III randomized controlled trials involving evolocumab.
| Trial | Study Population | Intervention and Comparator | 1° Outcome, Follow-Up Period | 1° Outcome Data |
|---|---|---|---|---|
| Koren (2014) MENDEL-2 [26] n = 614 | 1° prevention—Patients aged 18 to 80 years with LDL-C ≥ 100 mg/dL and <190 mg/dL, TG ≤ 400 mg/dL, and 10-year Framingham coronary heart disease risk scores ≤ 10% | Interventions: Oral placebo and evolocumab 140 mg biweekly, or oral placebo and evolocumab 420 mg monthly. Comparators: Oral placebo and subcutaneous placebo biweekly; oral placebo and subcutaneous placebo monthly; ezetimibe and subcutaneous placebo every two weeks; ezetimibe and subcutaneous placebo monthly. | Percent change in LDL-C level averaged at weeks 10 and 12, and at week 12 | At 12 weeks: −57.1% (−61.1, −53.1) biweekly evolocumab vs. placebo; −39.3% (−43.3, −35.3) biweekly evolocumab vs. ezetimibe; −54.8% (−58.5, −51.1) monthly evolocumab vs. placebo; −37.6% (−41.2, −33.9) monthly evolocumab vs. ezetimibe |
| Robinson (2014) LAPLACE-2 [27] n = 1896 | 1° prevention—Patients aged 18 to 80 years with LDL-C ≥ 150 mg/dL if not on statins at screening, ≥100 mg/dL if on non-intensive statin, or ≥80 mg/dL if on intensive statins, and TG < 400 mg/dL | Intervention: Evolocumab (140 mg biweekly or 420 mg monthly). Comparator: Matching placebo or ezetimibe (10 mg or placebo daily; atorvastatin patients only) | Percent change from baseline in LDL-C level at the mean of weeks 10 and 12, and at week 12 | |
| Stroes (2014) GAUSS-2 [28] n = 307 | 1° prevention—Patients aged 18 to 80 years on no or low-dose statins, with LDL-C ≥ 70 mg/dL, and who had previous intolerance to ≥2 statins | Interventions: Oral placebo and subcutaneous evolocumab 140 mg biweekly, or oral placebo and evolocumab 420 mg monthly. Comparators: daily ezetimibe 10 mg and subcutaneous placebo every two weeks; daily ezetimibe 10 mg and subcutaneous placebo monthly. | Percent change in LDL-C at the mean of weeks 10 and 12, and at week 12 | Difference between evolocumab and ezetimibe at 12 weeks at the following doses: Evolocumab 140 mg biweekly + placebo daily: −36.9% (−42.3, −31.6). Evolocumab 420 mg monthly + placebo daily: −38.7% (−43.1, −34.3) |
| Blom (2014) DESCARTES [29] n = 901 | 1° and 2° prevention—Patients aged 18 to 75 years with LDL-C ≥ 75 mg/dL, and a TG < 400 mg/dL | Intervention: Evolocumab (420 mg) monthly. Comparator: placebo every 4 weeks. Background LLT included diet alone or diet plus atorvastatin | Percent change in LDL-C at week 52 | Treatment differences, baseline vs. placebo: Diet only: −63.8% (4.2); Diet + atorvastatin 10 mg: −64.4% (2.8); Diet + atorvastatin 80 mg: −57.9% (5.9); Diet + atorvastatin 80 mg + ezetimibe 10 mg: −49.1% (5.6); Overall: −59.3% (2.3) |
| Raal (2015) RUTHERFORD-2 [30] n = 331 | 1° prevention—Patients aged 18 to 80 years with HeFH and on a stable dose of a statin | Intervention: Evolocumab 140 mg biweekly or 420 mg monthly. Comparator: Matching placebo biweekly or monthly | Percent change in LDL-C at week 12, and at the mean of weeks 10 and 12 | Treatment differences (95% CI) from baseline: Evolocumab 140 mg every 2 weeks vs. placebo: −59.2% (−65.1 to −53.4); Evolocumab 420 mg monthly vs. placebo: −61·3% (−69.0 to −53.6) |
| Sabatine (2015) OSLER-2 [31] n = 3141 | Patients recruited from parent trials ranged from LDL-C ≥ 85 mg/dL to ≥100 mg/dL, including patients on statins and those with statin intolerance | Intervention: Evolocumab (140 mg biweekly or 420 mg monthly) plus standard therapy. Comparator: standard therapy alone | Incidence of adverse events. F/U: a median of 11.1 months | Adverse events occurred in 2060 of 2976 patients (69.2%) in the evolocumab group, and in 965 of 1489 patients (64.8%) in the standard therapy group |
| Nissen (2016) GAUSS-3 [32] n = 199 | 1° and 2° prevention—Patients aged 18 to 80 years with LDL-C ≥ 100 mg/dL if CAD, ≥130 mg/dL if ≥2 risk factors, ≥160 mg/dL with ≥1 risk factor, or ≥190 mg/dL with no risk factors, and statin intolerance | Intervention: Evolocumab injections (420 mg) monthly. Comparator: Oral ezetimibe (10 mg) daily, or matched placebo | Percent change in LDL-C at week 24 for evolocumab vs. ezetimibe, and percent change in LDL-C at weeks 22 and 24 for evolocumab vs. ezetimibe | At week 24 for evolocumab vs. ezetimibe: −52.8% vs. −16.7%, p < 0.001; At weeks 22 and 24 for evolocumab vs. ezetimibe: −54.5% vs. −16.7%, p < 0.001 |
| Sabatine (2017) FOURIER [33] n = 27,564 | 2° prevention—Patients aged 40 to 85 years with ASCVD, one major risk factor (T1DM, T2DM, age ≥ 65, or current smoking), or two minor risk factors with LDL-C ≥ 70 mg/dL or a non-HDL-C ≥ 100 mg/dL on stable LLT | Intervention: Subcutaneous injections of evolocumab (either 140 mg every 2 weeks or 420 mg monthly, according to patient preference). Comparator: Subcutaneous injections of matching placebo. | A composite of CV death, MI, stroke, hospitalization for unstable angina, or coronary revascularization. F/U: A median of 2.2 years | The 1° endpoint occurred in 1344 patients (9.8%) in the evolocumab group vs. 1563 patients (11.3%) in the placebo group. HR (95% CI) 0.85 (0.79 to 0.92) p < 0.001 |
| Rosenson (2019) BANTING [34] n = 421 | 1° and 2° prevention—Patients aged 18 years and older with T2DM on stable pharmacological therapy for T2DM and the maximum tolerated statin dose | Intervention: Evolocumab 420 mg once per month. Comparator: Placebo once per month | Percent change in LDL-C from baseline to week 12, and at the mean of weeks 10 and 12 | Week 12 results: −54.3% (1.4). Mean treatment difference: −53.1% (2.3). |
| O’Donoghue (2022) FOURIER-OLE [35] n = 6635 | 2° prevention—Patients with a history of established ASCVD and LDL-C ≥ 70 mg/dL or non–HDL-C ≥ 100 mg/dL on statin therapy | Original parent trial: Intervention was evolocumab, comparator was placebo. This open-label trial administered evolocumab at either 140 mg every 2 weeks or 420 mg every month, per patient preference | Subject incidence of treatment-emergent adverse events. F/U: a median of 5.0 years | Overall annualized incidence rates for safety events of interest for patients randomized to evolocumab did not exceed the annualized incidence rate for patients treated with the placebo |
| Nicholls (2021) HUYGENS [36] n = 164 | 2° prevention—Patients ≥ 18 years with at least one non-culprit epicardial coronary stenosis ≥ 20% on angiography during NSTEMI, with intervention of the culprit lesion and a target vessel suitable for imaging with ≤50% visual obstruction, on maximally tolerated statin and LDL-C at the time of NSTEMI ≥ 130 mg/dL if not taking a statin, ≥80 mg/dL if on a low- or moderate-intensity statin, or ≥60 mg/dL if on a high-intensity statin | Intervention: Evolocumab 420 mg once per month. Comparator: Placebo once per month | Nominal change in minimum fibrous cap thickness at any point throughout the matched arterial segment, defined by proximal and distal side branches, from baseline to week 50 | Greater increase in minimum fibrous cap thickness (+42.7 vs. +21.5 μm; p = 0.015) and decrease in maximum lipid arc (−57.5 vs. −31.4; p = 0.04) and macrophage index (−3.17 vs. −1.45 mm; p = 0.04) throughout the arterial segment, with similar findings in lipid-rich plaque regions, and greater regression of % atheroma volume (−2.29% ± 0.47% vs. −0.61% ± 0.46%; p = 0.009) in the evolocumab groups |
| TIMI (ongoing) VESALIUS-CV [37] n = 12,301 | 1° prevention—Patients aged ≥ 50 (men) or ≥55 (women) and ˂80 years of age with LDL-C ≥ 90 mg/dL or non-HDL-C ≥ 120 mg/dL, or apoB ≥ 80 mg/dL without prior MI or stroke, and with evidence of CAD, atherosclerotic cerebrovascular disease, PAD, DM, and at least one high-risk factor | Intervention: Evolocumab 140 mg biweekly. Comparator: Placebo biweekly | A composite of coronary death, MI, and ischemic stroke. F/U: a median of 4.5 years | Not yet published |
| (Ongoing) EVOLVE-MI (NCT05284747) n = 4000 | 2° prevention—Patients aged 18 years and older hospitalized for NSTEMI or STEMI due to presumed atherosclerotic disease | Intervention: Open-label evolocumab biweekly plus routine lipid management. Comparator: Routine lipid management | Total (first and subsequent) composite of MI, ischemic stroke, any arterial revascularization procedure, and all-cause death. F/U: a median of 3.5 years | Not yet published |
| (Not yet published) YELLOW III (NCT04710368) n = 137 | 1° prevention—Patients undergoing elective PCI with a non-obstructive lesion (30–50% stenosis identified by angiography in a non-culprit vessel with lipid-rich plaques) and an optimal background statin | Intervention: Subcutaneous evolocumab 140 mg biweekly. No comparator | Changes in the minimal fibrous cap thickness, by OCT, and max 4 mm lipid core burden index, by near-infrared spectroscopy at week 26 | Not yet published |

Table 5.
Major phase III randomized clinical trials involving alirocumab.
Table 5.
Major phase III randomized clinical trials involving alirocumab.
| Trial | Study Population | Intervention and Comparator | 1° Outcome, Follow-Up Period | 1° Outcome Data |
|---|---|---|---|---|
| Schwartz (2018) and Szarek (2019) ODYSSEY Outcomes [38] n = 18,924 | 2° Prevention—Patients ≥ 40 years old hospitalized for an ACS and with inadequate lipid control (LDL-C ≥ 70 mg/dL, non-HDL-C ≥ 100 mg/dL, or apoB ≥ 80 mg/dL) on a maximally tolerated dose | Intervention: Subcutaneous injections of alirocumab 75 mg every 2 weeks. Comparator: Subcutaneous injections of matched-dose placebo every 2 weeks. | A composite of coronary death, non-fatal MI, fatal or non-fatal ischemic stroke, and hospitalization for unstable angina. F/U: median of 2.8 years | The composite 1° endpoint event occurred in 903 patients (9.5%) in the alirocumab group and in 1052 patients (11.1%) in the placebo group. HR (95% CI) 0.85 (0.78–0.93), p < 0.001) |
| Robinson (2015) ODYSSEY LONG TERM [39] n = 2341 | 1° and 2° prevention—Patients aged 18 and older with LDL-C ≥ 70 mg/dL and with HeFH, ASCVD, or high-risk 1° prevention, including CKD or DM with additional risk factors, on maximally tolerated statins | Intervention: Subcutaneous injections of alirocumab 150 mg every two weeks. Control: Subcutaneous injections of matched placebo every two weeks. | Percent change in LDL-C level at week 24 | At week 24, the difference between the alirocumab and placebo groups was −62% (p < 0.001) |
| Moriarty (2015) ODYSSEY ALTERNATIVE [40] n = 314 | 1° prevention—Patients aged 18 years and older with statin intolerance, and at a moderate, high, or very high CV risk | Intervention: Subcutaneous injections of alirocumab 75 mg every 2 weeks. Comparators: Ezetimibe 10 mg daily or atorvastatin 20 mg daily | Percent change in LDL-C at week 24 | Percent change from baseline: −45.0% (2.2). Comparator-corrected difference: −30.4% (3.1), CI −36.6 to −24.2, p < 0.0001 |
| Kastelein (2015) ODYSSEY FH I [41] n = 486 | 1° and 2° prevention—Patients with HeFH without a history of CV events, or patients with a history of MI or ischemic stroke, and with LDL-C levels not on target according to current guidelines | Intervention: Alirocumab 75 mg every 2 weeks. Comparator: Placebo every 2 weeks. | Percent change in LDL-C at week 24 | Percent change from baseline: −48.8% (1.6). Placebo-corrected difference: −57.9% (2.7), CI −63.3% to −52.6%, p < 0.0001 |
| Kastelein (2015) ODYSSEY FH II [41] n = 249 | 1° and 2° prevention—Patients with HeFH without a history of CV events, or patients with a history of MI or ischemic stroke, and with LDL-C levels not on target according to current guidelines | Intervention: Alirocumab 75 mg every 2 weeks. Comparator: Placebo every 2 weeks | Percent change in LDL-C at week 24 | Percent change from baseline: −48.7% (1.9). Placebo-corrected difference: −51.4% (3.4), CI −58.1% to −44.8%, p < 0.0001 |
| Ginsberg (2016) ODYSSEY HIGH FH [42] n = 107 | 1° and 2° prevention—Patients with HeFH and LDL-C ≥ 160 mg/dL on a maximally tolerated statins | Intervention: Subcutaneous alirocumab 150 mg every 2 weeks. Comparator: Placebo every 2 weeks | Percent change in LDL-C at week 24 | Percent change from baseline: −45.7% (3.5). Placebo-corrected difference: −39.1% (6.0), CI −51.1% to −27.1%, p < 0.0001 |
| Bays (2015) ODYSSEY OPTIONS I [43] n = 355 | 1° and 2° prevention—Patients aged 18 years and older at very high risk of CVD (a history of CVD including CHD, or T2DM with target organ damage) and with LDL-C ≥ 70 mg/dL, or at high risk (no history of CVD or CHD but with other risk factors: 10-year risk of fatal CVD of 5% or greater, moderate CKD, or T2DM with no target organ damage) and with LDL-C ≥ 100 mg/dL | Intervention: Subcutaneous injections of alirocumab 75 mg. Comparators: Add-on therapy with ezetimibe 10 mg daily, doubling of atorvastatin dose to 80 mg daily, or a switch to rosuvastatin 40 mg daily | Percent change in LDL-C at week 24 | Atorvastatin 20 mg group: Alirocumab −44.1% vs. ezetimibe −20.5% (p < 0.001) and doubling of atorvastatin dose −5.0% (p < 0.001). Atorvastatin 40 mg group: Alirocumab −54.0% (p < 0.001), ezetimibe −22.6% (p < 0.001), doubling of atorvastatin dose 4.8% (p < 0.001), and switching atorvastatin 40 mg to rosuvastatin 40 mg −21.4% (p < 0.001) |
| Farnier (2016) ODYSSEY OPTIONS II [44] n = 305 | 1° and 2° prevention—Patients with hypercholesterolemia at very high or high risk of CV, receiving rosuvastatin 10 or 20 mg/day | Intervention: Add-on subcutaneous injection via a prefilled pen of alirocumab 75 mg every 2 weeks. Comparators: Add-on ezetimibe 10 mg daily, or double-dose rosuvastatin | Percent change in LDL-C at week 24 | Rosuvastatin 10 mg group: Alirocumab −50.6% vs. ezetimibe −14.4% (p < 0.0001) and double-dose rosuvastatin −16.3% (p < 0.0001). Rosuvastatin 20 mg group: Alirocumab −36.3% vs. ezetimibe −11.0% (p = 0.0136) and double-dose rosuvastatin −15.9% (p < 0.0453) [pre-specified threshold for significance (p < 0.0125)] |
| Kereiakes (2015) ODYSSEY COMBO I [45] n = 316 | 1° and 2° prevention—Patients aged 18 years and older with LDL-C ≥ 70 mg/dL and established CVD, or LDL-C ≥ 100 mg/dL with CHD risk equivalents (DM with other risk factors or CKD) | Intervention: Alirocumab (75 mg) every 2 weeks. Comparator: Placebo every 2 weeks | Percent change in LDL-C at week 24 | Percent change from baseline: −48.2% (95% CI −52.0 to −44.4). Placebo-corrected difference: −45.9 (95% CI −52.5 to −39.3), p < 0.0001 |
| Cannon (2015) ODYSSEY COMBO II [46] n = 720 | 1° and 2° prevention—Patients with HLD and established CHD or CHD risk equivalents (ischemic stroke, PAD, moderate CKD, or DM plus ≥2 additional risk factors), on maximally tolerated statins | Intervention: Subcutaneous injections of alirocumab 75 mg every 2 weeks plus oral placebo. Comparator: Oral ezetimibe 10 mg daily plus subcutaneous placebo | Percent change in LDL-C at week 24 | Percent change from baseline: −50.6% (1.4). Placebo-corrected difference: −29.8% (2.3; 95% CI −34.4 to −25.3), p < 0.0001 |
| Roth (2016) ODYSSEY CHOICE I [47] n = 803 | 1° and 2° prevention—Patients with T2DM and inadequately controlled hypercholesterolemia and who were at moderate CVD risk with no statin, moderate-to-very-high CVD risk with statin-associated muscle symptoms, or moderate-to-very-high CVD risk with maximally tolerated statins | Intervention: Alirocumab 300 mg monthly. Comparator: Matching placebo monthly | Percent change in LDL-C at week 24 and time-averaged LDL-C over weeks 21 to 24 | Percent change from baseline: −57.4% (3.3). Placebo-corrected difference: −61.6% (5.6), p < 0.0001 |
| Stroes (2016) ODYSSEY CHOICE II [48] n = 233 | 1° prevention—Patients aged 18 years and older with hypercholesterolemia receiving fenofibrate, ezetimibe, or diet alone | Intervention: Alirocumab 150 mg monthly or 75 mg biweekly, with dose adjustment to 150 mg biweekly at week 12 if predefined LDL-C target levels were not met. Comparator: Matching placebo | Percent change in LDL-C at week 24 | Alirocumab 75 mg biweekly: % change from baseline −53.5% (1.6). Placebo-corrected difference: −58.2% (2.8), p < 0.0001. Alirocumab 150 mg monthly: % change from baseline −51.7% (2.3). Placebo-corrected difference: −56.4% (3.3), p < 0.0001 |
| Teramoto (2016) ODYSSEY JAPAN [49] n = 216 | 1° and 2° prevention—Patients aged 18 and older with HeFH, with or without a history of documented CAD, or patients with non-FH at high CVD risk with a history of documented CAD, or classified as JAS category III (1° prevention), with inadequately controlled cholesterol levels | Intervention: Alirocumab 75 mg every 2 weeks, with increase to 150 mg if predefined LDL-C target levels were not met. Comparator: Matching placebo | Percent change in LDL-C at week 24 | Percent change from baseline: −62.5% (1.3). Placebo-corrected difference: −64.1% (2.2; 95% CI −68.5% to −59.8%), p < 0.0001 |
| Koh (2018) ODYSSEY KT [50] n = 199 | 1° prevention—Patients aged 18 years or older with high CV risk who had inadequately controlled hypercholesterolemia on maximally tolerated statins | Intervention: Alirocumab 75 mg every 2 weeks, with dose increase to 150 mg every 2 weeks at week 12 if predefined LDL-C target levels were not met. Comparator: Matching placebo | Percent change in LDL-C at week 24 | Percent change from baseline: −57.1% (3.0). Placebo-corrected difference: −63.4% (4.2; 95% CI −71.6 to −55.2), p < 0.0001 |
| Teramoto (2019) ODYSSEY NIPPON [51] n = 163 | 1° prevention—Patients with LDL-C ≥ 100 mg/dL (HeFH or non-FH with CHD) or LDL-C ≥ 120 mg/dL (non-FH, Japan Atherosclerosis Society category III) on atorvastatin 5 mg/day or non-statin LLT | Intervention: Subcutaneous alirocumab 150 mg monthly or alirocumab 150 mg biweekly. Comparator: Matching placebo | Percent change in LDL-C at week 12 | Alirocumab monthly: % change from baseline −43.8% (2.2). Placebo-corrected difference: −39.5% (3.1), p < 0.0001 Alirocumab biweekly: % change from baseline −70.1% (2.3). Placebo-corrected difference: −65.8% (3.1), p < 0.0001 |
| Han (2020) ODYSSEY EAST [52] n = 615 | 1° and 2° prevention—Patients with hypercholesterolemia and established CHD or CHD risk equivalents who were inadequately controlled with stable maximally tolerated statins | Intervention: Alirocumab 75 mg every 2 weeks, with dose increase to 150 mg biweekly at week 12 if predefined LDL-C target levels were not met. Comparator: Ezetimibe 10 mg daily | Percent change in LDL-C at week 24 | Percent change from baseline: −56.0% (1.5). Placebo-corrected difference: −35.6 (2.5; 95% CI −40.6 to −30.7), p < 0.0001 |
| Perez de Isla (2023) ARCHITECT [53] n = 104 | 1° prevention—Patients with FH, without clinical ASCVD, with LDL-C ≥ 100 mg/dL on maximal statins, a global coronary PB > 30% at baseline, and prescribed alirocumab by the treating physician | Intervention: Subcutaneous alirocumab (150 mg) every 14 days. Comparator: No comparator | Changes in coronary plaque burden. F/U: 78 weeks | Coronary plaque burden changed from 34.6% (32.5 to 36.8) to 30.4% (27.4 to 33.4) at follow-up, p < 0.001. There was an increase in the proportion of calcified (+0.3%; p < 0.001) and mainly fibrous (+6.2%; p < 0.001) plaque, and a decrease in the % fibro-fatty (−3.9%; p < 0.001) and necrotic plaque (−0.6%; p < 0.001). |
| Sugizaki (2020) ALTAIR [54] n = 24 | 1° prevention—Patients aged 20 years and older who underwent PCI for ACS or stable angina pectoris, with LDL-C > 70 mg/dL despite statin treatment, and OCT evaluation of TCFA characteristics in non-culprit, angiographically intermediate lesions causing 30–70% diameter stenosis | Intervention: Alirocumab 75 mg every 2 weeks and 10 mg rosuvastatin daily. Comparator: Standard-of-care 10 mg rosuvastatin daily | Absolute change in fibrous cap thickness. F/U: 36 weeks | The absolute increase in the fibrous cap thickness was 140 μm (78 to 163 μm) (%age change 273% [155% to 293%]) in the alirocumab group vs. 45 μm (10 to 78 μm) in the standard-of-care group (100% [20% to 155%]), p = 0.002 |
| Räber (2022) PACMAN-AMI [55] n = 300 | 2° prevention—Patients aged 18 years and older who underwent PCI for STEMI or NSTEMI, with LDL-C > 125 mg/dL, and who were suitable for intracoronary imaging | Intervention: Subcutaneous injections of alirocumab 150 mg biweekly. Comparator: Placebo biweekly | Change in intravascular ultrasound-derived % atheroma volume from baseline to week 52 | The mean change in % atheroma volume was −2.13% with alirocumab vs. −0.92% with placebo (difference, −1.21% [95% CI, −1.78% to −0.65%], p < 0.001) |
| Ako (2019) ODYSSEY J-IVUS [56] n = 206 | 2° prevention—Patients aged 20 years and older who had been hospitalized for ACS, had LDL-C ≥ 100 mg/dL at ACS diagnosis, had undergone IVUS imaging as part of usual clinical practice in Japan, and had an analyzable IVUS image of the culprit or non-culprit vessel with ≥50% angiographic stenosis of the culprit vessel within 1 week after ACS onset | Intervention: Alirocumab 75 mg every 2 weeks and up to 150 mg biweekly. Comparator: Standard of care (atorvastatin ≥ 10 mg daily or rosuvastatin ≥ 5 mg daily) | Percent change in normalized total atheroma volume from baseline to week 36 | At week 36, the mean % change in normalized total atheroma volume from baseline was −3.1% (1.0) with the standard of care vs. −4.8% (1.0) with alirocumab (between-group difference: −1.6 [1.4]; p = 0.23). The absolute change from baseline in % atheroma volume was −1.3% (0.4) with the standard of care and −1.4% (0.4) with alirocumab, p = 0.79) |
| Gao (2021) Impact of PCSK9 Inhibitors on Coronary Plaque Composition and Vulnerability Assessed by Optical Coherence Tomography [57] n = 61 | 2° prevention—Patients aged 18 to 80 years with stable CAD or ACS on admission, planned to have clinically indicated coronary angiography and identified as having at least one intermediate lesion (50–70% diameter stenosis) on de novo coronary arteries, identified with elevated LDL-C ≥ 70 mg/dL for patients with ACS or ≥ 100 mg/dL for non-ACS patients, despite maximally tolerated statins | Intervention: Alirocumab 75 mg biweekly plus statin (atorvastatin 20 mg daily or rosuvastatin 10 mg daily) therapy. Comparator: Standard-of-care statin | Optimal-coherence-tomography-derived absolute changes in minimum fibrous cap thickness between baseline and follow-up. F/U: 36 weeks | The increase in minimum fibrous cap thickness in the alirocumab group was 18.0 μm (10.8–29.2) vs. 13.2 μm (7.4–18.6) in the standard-of-care group, p = 0.029. The increase in minimum lumen area in the alirocumab group was 0.20 mm2 (0.10–0.33) vs. 0.13 mm2 (0.12–0.24) in the standard-of-care group, p = 0.006. The diminution in maximum lipid arc in the alirocumab group was 15.1 (7.8–24.5) vs. 8.4% (2.0–10.5) in the standard-of-care group p = 0.008. |

Table 6.
Major phase III randomized clinical trials involving bococizumab.
Table 6.
Major phase III randomized clinical trials involving bococizumab.
| Trial | Study Population | Intervention and Comparator | 1° Outcome, Follow-Up Period | 1° Outcome Data |
|---|---|---|---|---|
| Ridker (2017) SPIRE-HR [58] n = 711 | 1° and 2° prevention—Patients ≥ 18 and older with LDL-C ≥ 70 mg/dL on the highest statin dose and with a history of CAD, other ASCVD, T1DM, T2DM, or CKD, and with TG ≤ 400 mg/dL | Intervention: Subcutaneous injections of bococizumab 150 mg self-administered every 2 weeks. Comparator: Subcutaneous injections of matched placebo self-administered every 2 weeks. | Percent change in LDL-C at week 12 and persistence for 12 months. F/U: 52 weeks | Meta-analysis of LDL-C ↓ in SPIRE-HR, SPIRE-LDL, SPIRE-FH, SPIRE-LL, SPIRE-SI, and SPIRE-AI: −55.2% at 12 weeks; −42.5% at 52 weeks |
| Ridker (2017) SPIRE-LDL [58] n = 2139 | 1° and 2° prevention—Patients ≥ 18 and older with LDL-C ≥ 70 mg/dL on the highest statin dose if a history of CAD, other ASCVD, T1DM, T2DM, or CKD was present, or with LDL-C ≥ 100 mg/dL on the highest statin dose if equivalent risk factors were present | Intervention: Bococizumab 150 mg subcutaneously self-administered every 2 weeks. Comparator: Placebo. | Percent change in LDL-C at week 12 and long-term persistence of any effects on the LDL-C level for 12 months. F/U: 52 weeks | |
| Ridker (2017) SPIRE-FH [58] n = 370 | HeFH 1° and 2° prevention—Patients ≥ 18 and older with HeFH and LDL-C ≥ 70 mg/dL on highest statin dose if a history of CAD, other ASCVD, T1DM, T2DM, or CKD is present, or LDL-C ≥ 100 mg/dL on highest statin dose if 1° prevention, and with TG ≤ 400 mg/dL | Intervention: Subcutaneous injections of bococizumab 150 mg self-administered every 2 weeks. Comparator: Subcutaneous injections of matched placebo self-administered every 2 weeks. | Percent change in LDL-C at week 12 and long-term persistence of any effects on the LDL-C level for 12 months. F/U: 52 weeks | |
| Ridker (2017) SPIRE-LL [58] n = 746 | 1° and 2° prevention—Patients ≥ 18 and older with LDL-C ≥ 100 mg/dL with ASCVD, T1DM, T2DM, CDK, or equivalent risk factors | Intervention: Subcutaneous injections of bococizumab 150 mg self-administered every 2 weeks. Comparator: Subcutaneous injections of matched placebo self-administered every 2 weeks. | Percent change in LDL-C at week 12 and long-term persistence of any effects on the LDL-C level for 12 months. F/U: 52 weeks | |
| Ridker (2017) SPIRE-SI [58] n = 184 | 1° and 2° prevention—Patients ≥ 18 and older with LDL-C ≥ 70 mg/dL and with statin intolerance | Intervention: Subcutaneous injections of bococizumab 150 mg self-administered every 2 weeks. Comparators: Oral atorvastatin 40 mg or subcutaneous injections of matched placebo self-administered every 2 weeks | Percent change in LDL-C at week 12 and long-term persistence of any effects on the LDL-C level. F/U: 6 months | |
| Ridker (2017) SPIRE-AI [58] n = 299 | 1° and 2° prevention—Patients ≥ 18 and older with LDL-C ≥ 70 mg/dL on stable statins | Interventions: Subcutaneous injections of bococizumab 150 or 75 mg administered with an autoinjector device every 2 weeks. Comparators: Subcutaneous injections matching doses of placebo administered with an autoinjector device every 2 weeks. | Percent change in LDL-C and long-term persistence of any effects on the LDL-C level. F/U: 12 weeks | |
| Ridker (2017) SPIRE-1 [59] n = 16,817 | 1° and 2° prevention—Patients ≥ 18 and older if 2° prevention, ≥35 years (men) and ≥45 years (women) if elevated LDL-C, and ≥50 years (men) and ≥60 years (women) if 1° prevention, with LDL-C ≥ 70 mg/dL or non-HDL-C ≥ 100 mg/dL, and on stable statins | Intervention: Subcutaneous injections of bococizumab 150 mg every 2 weeks (dose was lowered if LDL-C < 10 mg/dL). Comparator: Subcutaneous injections of matching placebo every 2 weeks. | A composite of CV death, non-fatal MI, non-fatal stroke, and hospitalization for unstable angina needing urgent revascularization. F/U: Median of 7 months | HR (95% CI) 0.99 (0.80–1.22) |
| Ridker (2017) SPIRE-2 [59] n = 10,621 | 1° and 2° prevention—Patients ≥ 18 years if 2° prevention, ≥35 years (men) and ≥45 years (women) if elevated LDL-C, and ≥50 years (men) and ≥60 years (women) if 1° prevention with LDL-C ≥ 100 mg/dL or non-HDL-C ≥ 130 mg/dL, and on stable statins | Intervention: Subcutaneous injections of bococizumab 150 mg every 2 weeks (dose was lowered if LDL-C < 10 mg/dL). Comparator: Subcutaneous injections of matching placebo every 2 weeks. | A composite of CV death, non-fatal MI, non-fatal stroke, and hospitalization for unstable angina needing urgent revascularization. F/U: Median of 12 months | HR (95% CI) 0.79 (0.65–0.97) |

Table 7.
Major phase III randomized clinical trials involving inclisiran.
Table 7.
Major phase III randomized clinical trials involving inclisiran.
| Trial, Sample Size | Study Population | Intervention and Comparator | 1° Outcome, Follow-Up Period | 1° Outcome Result |
|---|---|---|---|---|
| Raal (2020) ORION-9 [60] n = 482 | HeFH 1° and 2° prevention—Patients ≥ 18 years old with HeFH, LDL-C ≥ 100 mg/dL, on maximally tolerated statin dose, or with statin intolerance | Intervention: Inclisiran sodium 300 mg on days 1, 90, 270, and 450. Comparator: Subcutaneous placebo injection on days 1, 90, 270, and 450. | (1) Percent change in LDL-C level at day 510. (2) Time-adjusted % change in LDL-C level between day 90 and day 540. F/U: 540 days | At day 510, inclisiran resulted in a placebo-adjusted difference of −47.9% age points (95% CI, −53.5 to −42.3; p < 0.001) in LDL-C. The time-averaged % change in LDL-C was a placebo-adjusted difference of −44.3 percentage points (95% CI, −48.5 to −40.1; p < 0.001) |
| Ray (2020) ORION-10 [61] n = 1561 | 2° prevention—Patients ≥ 18 years old with ASCVD and LDL-C ≥ 70 mg/dL on maximally tolerated statins or with statin intolerance | Intervention: Inclisiran sodium 284 mg on days 1, 90, 270, and 450. Comparator: Subcutaneous placebo injection on days 1, 90, 270, and 450. | (1) Percent change in LDL-C level at day 510. (2) Time-adjusted % change in LDL-C level from baseline after day 90 and up to day 540. F/U: 540 days | At day 510, inclisiran resulted in a placebo-adjusted difference of −52.3% (95% CI, −55.7 to −48.8; p < 0.001) in LDL-C levels. Inclisiran resulted in a placebo-adjusted difference of −53.8% (95% CI, −56.2 to −51.3; p < 0.001) in time-adjusted changes in LDL-C between day 90 and day 540 |
| Ray (2020) ORION-11 [61] n = 1617 | 1° and 2° prevention—Patients with ASCVD and high-risk 1° prevention | Intervention: Inclisiran sodium 284 mg on days 1, 90, 270, and 450. Comparator: Subcutaneous placebo injection on days 1, 90, 270, and 450. | (1) Percent change in LDL-C level at day 510. (2) Time-adjusted % change in LDL-C level from baseline after day 90 and up to day 540. F/U: 540 days | At day 510, inclisiran resulted in a placebo-adjusted difference of −49.9% (95% CI, −53.1 to −46.6; p < 0.001) in LDL-C levels. Inclisiran resulted in a placebo-adjusted difference of −49.2% (95% CI, −51.6 to −46.8; p < 0.001) in time-adjusted changes in LDL-C between day 90 and day 540 |
| Ray (2023) ORION-3 [62] n = 382 | 1° and 2° prevention—Patients ≥ 18 years old with LDL-C ≥ 70 mg/dL with ASCVD (2° prevention), or with LDL-C ≥ 100 mg/dL at high risk of ASCVD (1° prevention), on statins or with statin intolerance | Intervention: Inclisiran sodium 300 mg twice yearly (after 200, 300, or 500 mg on day 1, or 100, 200, or 300 mg on days 1 and 90 in ORION-1). Comparator: Subcutaneous evolocumab 140 mg every 2 weeks for up to 1 year, followed by subcutaneous inclisiran sodium 300 mg with random allocation to staged switch (day 336, final dose evolocumab; day 360, first dose inclisiran) or concurrent switch (day 360, final dose evolocumab, first dose inclisiran), then 90 days later (day 450) and 6-monthly thereafter (after matched placebo in ORION-1). | Percent change in LDL-C at day 210 of ORION-3 (570 days from the start of ORION-1). F/U: 4 years | At day 210, inclisiran usage resulted in a −47.5% difference (95% CI −50.7 to −44.3; p < 0.0001) in baseline LDL-C levels |

Table 8.
Major phase III randomized controlled trials involving mipomersen.
Table 8.
Major phase III randomized controlled trials involving mipomersen.
| Trial, Sample Size | Study Population | Intervention and Comparator | 1° Outcome, Follow-Up Period | 1° Outcome Result |
|---|---|---|---|---|
| Stein (2012) RADICHOL II [63] n = 114 | HeFH 2° prevention—Patients ≥ 18 years old with HeFH and CAD with LDL-C ≥ 100 mg/dL on maximally tolerated statins | Intervention: Subcutaneous injections of mipomersen 200 mg self-administered weekly. Comparator: Subcutaneous injections of matched placebo self-administered weekly. | Percent change in LDL-C at week 28, or 2 weeks after the last dose for non-completers. F/U: 28 weeks | Mipomersen significantly ↓ LDL-C by −28% from baseline (95% CI: [−34.0% to −22.1%]), compared with a 5.2% (95% CI: [−0.5% to 10.9%]) increase with placebo (p < 0.001). |
| McGowan (2012) Randomized, Placebo-Controlled Trial of Mipomersen in Patients with Severe Hypercholesterolemia Receiving Maximally Tolerated Lipid-Lowering Therapy [64] n = 58 | 1° and 2° prevention—Patients ≥ 18 years old with severe hypercholesterolemia, with and without ASCVD, on maximally tolerated LLT | Intervention: Subcutaneous injections of mipomersen 200 mg self-administered weekly. Comparator: Subcutaneous injections of matched placebo self-administered weekly. | Percent change in LDL-C at week 28, or 2 weeks after the last dose for non-completers. F/U: 28 weeks | Mipomersen significantly ↓ LDL-C from baseline by −36% (95% CI, −51.3, −15.3; p < 0.001), compared to a 12.5% increase in the placebo group (95% CI, −10.8 to 35.8) |
| Thomas (2013) Safety and Efficacy of Mipomersen (ISIS 301012) As Add-on Therapy in High Risk Hypercholesterolemic Patients [65] n = 104 | 1° and 2° prevention—Patients on statins with LDL-C ≥ 100 mg/dL, with or at high risk of cardiovascular disease | Intervention: Subcutaneous injections of mipomersen 200 mg self-administered weekly. Comparator: Subcutaneous injections of matched placebo self-administered weekly. | Percent change in LDL-C at week 28, or 2 weeks after the last dose for non-completers. F/U: 24 weeks | Mipomersen significantly ↓ LDL-C from baseline by −36.9%, compared with a −4.5% decrease in the placebo group (p < 0.001) |

Table 9.
Major clinical trials involving CETP inhibitors.
Table 9.
Major clinical trials involving CETP inhibitors.
| Trial, Sample Size, Drug | Study Population | Intervention and Comparator | 1° Outcome, Follow-Up Period | 1° Outcome Result |
|---|---|---|---|---|
| Raal (2023) LIBerate-HeFH [66] n = 478 Lerodalcibep | HeFH 1° and 2° prevention—Patients ≥ 18 years old with HeFH and LDL-C ≥ 100 mg/dL (primary prevention) or ≥70 mg/dL (secondary prevention) | Intervention: Subcutaneous injections of lerodalcibep 300 mg monthly. Comparator: Subcutaneous injections of matched placebo monthly. | Percent change in LDL-C at week 24, and the mean of weeks 22 and 24. F/U: 24 weeks | Lerodalcibep reduced LDL-C by a placebo-adjusted −58.61 (3.25)% at week 24 (p < 0.0001). For the mean of weeks 22 and 24, lerodalcibep reduced LDL-C by 2.28 (0.10) mmol/L (95% CI −2.47 to −2.09) and −65.0 (2.87)% compared with the placebo (p < 0.0001 for both) |
| Schwartz (2012) dal-OUTCOMES [67] n = 15,871 Dalcetrapib | 2° prevention—Patients ≥ 45 years old hospitalized for acute ACS | Intervention: Oral dalcetrapib 600 mg daily. Comparator: Oral matched placebo daily. | A composite of death from CAD, non-fatal MI, ischemic stroke, unstable angina, or cardiac arrest with resuscitation. F/U: 31 months | Compared to the placebo, dalcetrapib did not alter the risk of the primary endpoint or total mortality (cumulative event rates: 8.0% and 8.3%, respectively; HR with dalcetrapib, 1.04; 95% CI [0.93–1.16]; p = 0.52) |
| HPS3/TIMI55–Reveal Collaborative Group (2017) REVEAL [68] n = 30,449 Anacetrapib | 2° prevention—Patients with established ASCVD | Intervention: Oral anacetrapib 100 mg daily. Comparator: Oral matched placebo daily. | A composite of death from CAD, MI, or coronary revascularization. F/U: 4.1 years | Compared to the placebo, anacetrapib resulted in a lower incidence of major coronary events (1640 of 15,225 patients [10.8%] vs. 1803 of 15,224 patients [11.8%]; rate ratio, 0.91; 95% CI, 0.85 to 0.97; p = 0.004) |
| Barter (2007) ILLUMINATE [69] n = 15,067 Torcetrapib | 1° and 2° prevention—Patients aged 45–75 years with established ASCVD (secondary prevention), and patients with type 2 diabetes without previous ASCVD (primary prevention) | Intervention: Atorvastatin and oral torcetrapib Comparator: Atorvastatin only. | A composite of death from coronary heart disease, non-fatal MI, stroke, and hospitalization for unstable angina. F/U: 4.5 years | Torcetrapib therapy increased the risk of cardiovascular events (HR, 1.25; 95% CI, 1.09 to 1.44; p = 0.001) and death from any cause (HR, 1.58; 95% CI, 1.14 to 2.19; p = 0.006) relative to the placebo. |
| Lincoff (2017) ACCELERATE [70] n = 12,092 Evacetrapib | 2° prevention—Patients ≥ 18 years old with ASCVD on statin therapy | Intervention: Oral dose of 130 mg of evacetrapib once daily, in addition to statin therapy. Comparator: Matching placebo and statin therapy. | A composite of death from cardiovascular causes, MI, stroke, coronary revascularization, and hospitalization from unstable angina. F/U: 26 months | At 26 months, the primary endpoint occurred in 12.9% of patients receiving evacetrapib and in 12.8% of patients receiving the placebo (HR, 1.01; 95% CI, 0.91 to 1.11; p = 0.91) |
| Nicholls (2022) [71] n = 120 Obicetrapib | 1° prevention—Patients 18–75 years old with LDL-C > 70 mg/dL, without significant ASCVD, on high-intensity statin therapy | Intervention: 10 mg oral dose of obicetrapib. Control: matched placebo | Percent change in LDL-C after 8 weeks of treatment. F/U: 8 weeks | Obicetrapib 5 mg and 10 mg significantly reduced LDL-C levels by 42.9% and 45.7%, respectively, compared to 0.0% for the placebo group (p < 0.0001) |
| (Ongoing) BROOKLYN (NCT05425745) n = 300 Obicetrapib | HeFH, 1° and 2° prevention—Patients ≥ 18 years old with HeFH and LDL-C ≥ 70 mg/dL on maximally tolerated statin therapy | Intervention: 10 mg oral dose of obicetrapib. Control: Matched placebo | Percent change in LDL-C after 12 weeks of treatment. F/U: 12 weeks | Results pending |
| (Ongoing) BROADWAY (NCT05142722) n = 2532 Obicetrapib | HeFH and 2° prevention—Patients > 60 years old with either HeFH or ASCVD and a history of MI in the past year, with LDL-C ≥ 100 mg/dL, on maximally tolerated LLT | Intervention: 10 mg oral dose of obicetrapib. Control: Matched placebo | Percent change in LDL-C after 12 weeks of treatment. F/U: 12 weeks | Results pending |
| (Ongoing) PREVAIL (NCT05202509) n = 9000 Obicetrapib | 2° prevention—Patients ≥ 18 years old with established ASCVD and LDL-C ≥ 70 mg/dL on maximally tolerated LLT | Intervention: 10 mg oral dose of obicetrapib. Control: Matched placebo | A composite of major adverse cardiac events. F/U: 32 months | Results pending |
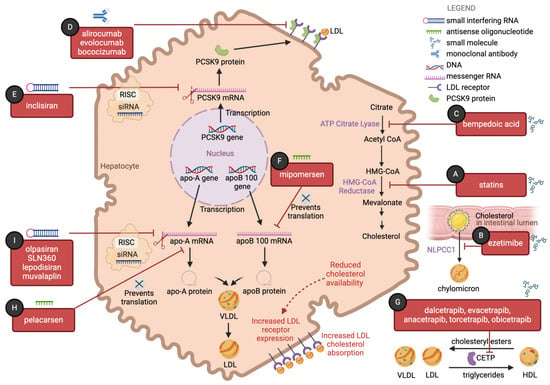
Figure 1.
The landscape of lipid-lowering strategies has expanded in recent years with the emergence of therapies that target multiple pathways in cholesterol metabolism: (A) Statins competitively inhibit the enzyme β-hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase, leading to reduced hepatic cholesterol synthesis. Reduced intracellular cholesterol availability will lead to upregulation of low-density lipoprotein (LDL) receptor expression and consequent increased LDL cholesterol absorption, a common downstream effect of many medications in this figure. (B) Ezetimibe acts at the small intestinal brush border to reduce cholesterol absorption. It binds to the transmembrane protein Niemann–Pick C1-like 1 (NPC1L1) and prevents uptake of cholesterol-rich luminal micelles into enterocytes. (C) Bempedoic acid inhibits adenosine triphosphate-citrate lyase (ACL), an essential enzyme in cholesterol biosynthesis upstream of the enzyme HMG-CoA reductase. (D) Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a serine protease that irreversibly binds the LDL–LDL receptor complex in hepatocytes, leading to the lysosomal degradation of the LDL receptor. Alirocumab, evolocumab, and bococizumab are monoclonal IgG antibodies that inhibit PCSK9, preventing premature degradation of the LDL receptor and promoting continued LDL absorption. (E) Inclisiran is a small interfering RNA (siRNA) that binds to PCSK9 messenger ribonucleic acid (mRNA) with the help of an RNA-induced silencing complex (RISC), thereby inhibiting translation of PCSK9 mRNA to protein. (F) Mipomersen is an antisense oligonucleotide (ASO) that targets the apoB 100 mRNA, thereby inhibiting translation of apoB 100 mRNA to apoB 100 protein. (G) Dalcetrapib, evacetrapib, anacetrapib, and torcetrapib are inhibitors of cholesteryl ester transfer protein (CETP), a protein that mediates the transfer of cholesteryl esters from high-density lipoprotein (HDL) to LDL and very-low-density lipoprotein (VLDL), as well as the transfer of triglycerides from LDL and VLDL to HDL, (H) Pelacarsen is an ASO that targets the LPA gene mRNA. (I) Olpasiran, SLN360, lepodisiran, and muvalaplin are siRNAs that bind to apolipoprotein A (apo-A) mRNA, with the help of an RISC, thereby inhibiting translation of apo-A mRNA to protein. Designed in BioRender.
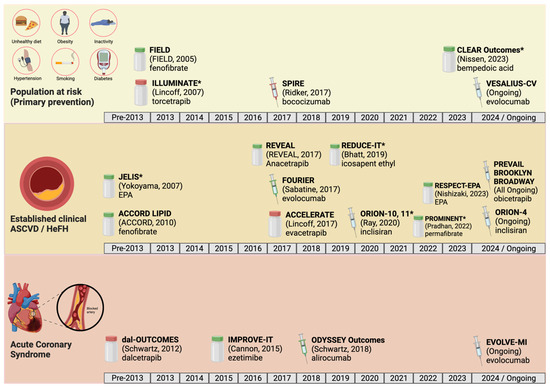
Figure 2.
The landscape of major phase III randomized controlled trials of agents that lower atherogenic lipoprotein has expanded in recent years in the realms of primary prevention (top row), secondary prevention in patients with established atherosclerotic cardiovascular disease (ASCVD) (middle row), and secondary prevention in patients with recent acute coronary syndrome (ACS) (bottom row). Syringe icons denote medications administered by injection, whereas pill bottle icons denote medications administered orally. Red icons denote trials whose primary outcome was negative, while green icons denote trials whose primary outcome was positive. Asterisks denote studies that had a combined primary and secondary prevention population. Designed in BioRender. Please refer to the Abbreviations section for full trial names, and to the corresponding tables for the results of each trial [18,25,33,37,38,58,61,67,68,69,70,72,73,74,75,76,77].
4.1. Ezetimibe
4.1.1. Mechanism of Action
While most non-statin therapies manipulate cholesterol and triglyceride production and recycling, ezetimibe displays a unique mechanism of action that results in decreased intestinal cholesterol absorption [78]. Ezetimibe operates at the small intestinal brush border, where it binds to the transmembrane protein NPC1L1 (Niemann–Pick C1-like 1) and prevents the uptake of cholesterol-rich luminal micelles into enterocytes [79].
4.1.2. Results of Randomized Trials
Ezetimibe’s initial approval from the FDA in 2002 was granted on the basis of LDL-lowering trials, without a demonstration of any corresponding reduction in cardiovascular events, leading to calls for evidence that ezetimibe improves hard outcomes [80]. Table 2 provides an overview of major phase III randomized controlled clinical trials related to ezetimibe usage, and it contains test statistics for all reported values. IMPROVE-IT (2015) was the largest trial to date examining the impact of ezetimibe on both LDL-C reduction and cardiac mortality, and it focused specifically on individuals with acute coronary syndrome (ACS) or myocardial infarction (MI) in the past 10 days and mildly elevated LDL-C levels (50–125 mg/dL) [18]. Starting from a mean baseline LDL-C of 93.8 mg/dL, ezetimibe plus simvastatin reduced LDL-C levels to a mean of 53.2 mg/dL, 24% lower than the mean LDL-C of 69.9 md/dL achieved in the simvastatin-only group (p < 0.001). The primary endpoint of cardiovascular mortality, major cardiovascular event, or non-fatal stroke occurred in 32.7% of the ezetimibe–simvastatin group and 34.7% of the simvastatin-only group, representing an absolute risk difference of 2% (hazard ratio [HR], 0.936; 95% confidence interval [CI], 0.89 to 0.99; p = 0.016). This corresponded to a necessary treatment group size of 50. This landmark study was the first large trial to demonstrate that the addition of ezetimibe to moderate-intensity statin therapy significantly reduces cardiovascular events. Because this trial was designed prior to the current guidelines, the participants were treated with a moderate-intensity statin instead of a high-intensity statin, raising the possibility that the effect size may have been less pronounced had a high-intensity statin been used.
Given that the volume of atherosclerotic plaques correlates with major adverse cardiovascular events (MACE) [81], PRECISE-IVUS (2015) aimed to assess the effect of adding ezetimibe to atorvastatin on atherosclerotic plaque volume, as assessed by intravascular ultrasound, as well as LDL-C levels in patients who had undergone coronary angiography or percutaneous coronary intervention for ACS or stable ischemic heart disease [20]. Starting from a mean LDL-C of 109.8 mg/dL and 108.3 mg/dL, respectively, ezetimibe–atorvastatin combination therapy reduced LDL-C levels to 63.2 mg/dL (−42.4%), compared to 73.3 mg/dL (−33.2%) in the atorvastatin-only group (p < 0.001). More interestingly, ezetimibe–atorvastatin combination therapy led to a change in percent atheroma volume of −1.4%, compared to −0.3% in the atorvastatin-only group (95% CI: −3.4% to −0.1% vs. −0.3%; 95% CI: −1.9% to 0.9% with atorvastatin alone; p = 0.001), corresponding to an absolute change in percent atheroma volume of −1.1% (p = 0.001). This trial was the first to suggest that ezetimibe combination therapy could reduce both LDL-C and atherosclerotic plaque volume in patients with clinical ASCVD.
Subsequently, EWTOPIA 75 (2019) investigated the effects of ezetimibe standalone therapy for primary prevention in older adults (≥75 years old) whose LDL-C level was ≥140 mg/dL [19]. Ezetimibe reduced the rate of the primary composite outcome of sudden cardiac death, myocardial infarction, coronary revascularization, or stroke, compared with usual care, with a HR of 0.66 (95% CI, 0.50–0.86; p = 0.002). Moreover, ezetimibe reduced the rate of coronary revascularization (HR 0.38, 95% CI, 0.18–0.79; p = 0.007), thereby supporting the use of ezetimibe in aging populations. Notably, this study focused exclusively on patients who were beyond the standard age range for statin-based therapies for primary prevention. EWTOPIA was the first trial to show the benefit of a non-statin agent as monotherapy for primary prevention in patients with elevated LDL-C. However, EWTOPIA is limited by its open-label nature, as participants in the usual care arm did not receive a placebo pill. In summary, both IMPROVE-IT and EWTOPIA 75 reported improved cardiovascular event rates and formed the basis of the ezetimibe recommendations in the 2019 and 2022 AHA/ACC guidelines [5,9].
4.1.3. Current Indications
Ezetimibe is currently approved for usage in combination with statin therapy or alone as an adjunct to diet to reduce LDL-C in adults with primary hyperlipidemia, in heterozygous familial hypercholesterolemia (HeFH) patients ≥10 years old, in patients with mixed hyperlipidemia (as an adjunct to fibrates and diet), and to reduce sitosterol and campesterol in patients ≥9 years old with homozygous familial sitosterolemia [82].
The 2022 AHA/ACC guidelines provide three populations in which the use of ezetimibe is indicated [9]. Among individuals with clinical ASCVD who are at very high risk, ezetimibe is indicated in those who are unable to achieve a ≥50% reduction in LDL-C, or whose LDL-C remains >55 mg/dL (or whose non-HDL remains ≥85 mg/dL) on maximally tolerated statin therapy. In individuals with clinical ASCVD who are not at very high risk, ezetimibe is indicated in those who are unable to achieve a ≥50% reduction in LDL-C, or whose LDL-C remains ≥70 mg/dL (or whose non–HDL remains ≥100 mg/dL) on maximally tolerated statin therapy. In individuals with clinical ASCVD with elevated baseline LDL-C at ≥190 mg/dL that is not due to familial hyperlipidemia or other secondary etiologies, ezetimibe is indicated in those who are unable to achieve a ≥50% reduction in LDL-C or whose LDL-C remains ≥70 mg/dL (or whose non–HDL remains ≥100 mg/dL) on maximally tolerated statin therapy. In all of the above scenarios, the guidelines allow for the selection of ezetimibe based on its low cost (given the availability of the generic formulation), its ease of use as an oral agent, and patient preference. Ezetimibe is prescribed as a fixed dose of 10 mg daily.
4.1.4. Safety Profile
Ezetimibe is considered to be a safe drug without major side effects. Some adverse effects reported include headache, sore throat, and runny nose, and less commonly, body aches, back pain, diarrhea, joint pain, fatigue, and hepatotoxicity. There have been reports of rhabdomyolysis in combination with statin therapy. Ezetimibe is contraindicated in patients with active liver disease. The use of ezetimibe during pregnancy and lactation has not been studied [83].
4.1.5. Cost-Effectiveness
Ezetimibe is one of the more cost-effective non-statin medications available to reduce cholesterol. A meta-analysis examining the incremental net benefit of ezetimibe compared to other lipid-lowering agents showed that ezetimibe was significantly cost-effective [84], with a pooled incremental net benefit (INB) of USD 4274. Subgroup analyses demonstrated that it is cost-effective in high-income countries and for primary prevention; however, fewer data exist on the cost-effectiveness of ezetimibe in lower–middle-income countries (LMICs) [84]. When used as an adjunct to statin therapy, ezetimibe has been found to modestly increase life expectancy by an additional 0.05 to 0.07 quality-adjusted life years (QALYs), with a net cost between USD 43,600 and USD 91,500 per QALY in patients with chronic kidney disease [85]. In another study, the incremental cost-effectiveness ratio (ICER) of ezetimibe as an adjunct to statin therapy, compared to standalone statin therapy, was CNY 47,102.99 (USD 6971.24) per QALY [86].
4.2. Bempedoic Acid
4.2.1. Mechanism of Action
Bempedoic acid functions as a prodrug that undergoes intracellular activation in order to inhibit cholesterol production. Specifically, it acts by inhibiting adenosine triphosphate-citrate lyase (ACL), an essential enzyme in cholesterol biosynthesis upstream of the enzyme HMG-CoA reductase, which is inhibited by statin therapies. It thus lowers LDL-C by the same mechanism as statins, reducing cellular LDL-C production, thereby upregulating LDL receptors and increasing LDL-C clearance [87]. Its unique formulation as a hepatocyte-only-activated prodrug allows for action in hepatocytes but not in myocytes, thereby decreasing the risk of myopathy compared to statin therapy [87].
4.2.2. Results of Randomized Trials
Table 3 provides an overview of the major phase III randomized-controlled clinical trials related to bempedoic acid usage, and it contains test statistics for all reported values. The efficacy of bempedoic acid was studied in patients with high-risk ASCVD on maximally tolerated statin therapy (CLEAR Harmony [22] and Wisdom [23]), and in statin-intolerant patients (CLEAR Serenity [24], Tranquility [21], and Outcomes [25]). CLEAR Harmony studied the use of bempedoic acid in individuals who had a history of ASCVD and/or HeFH, whereas CLEAR Wisdom studied individuals with high cardiovascular risk and concomitant hypercholesterolemia despite the use of maximally tolerated statin therapy [22,23]. Both included secondary prevention patients with a baseline LDL-C ≥ 70 mg/dL. In CLEAR Harmony, starting with a mean LDL-C of 103.29 mg/dL, bempedoic acid significantly reduced LDL-C levels, with a placebo-corrected difference of −18.1% (95% CI, −20.0 to −16.1; p < 0.001) [22]. In CLEAR Wisdom, starting from a baseline LDL-C of 120.4 mg/dL, bempedoic acid significantly reduced LDL-C levels, with a placebo-corrected difference of −17.4% (95% CI, −21.0% to −13.9%; p < 0.001) [23].
CLEAR Tranquility (2018) [21] and CLEAR Serenity (2019) [24] both investigated the use of bempedoic acid for patients with statin intolerance, compared to placebo. CLEAR Tranquility studied individuals who had a history of intolerance of two or more statins due to muscle-related side effects, whereas CLEAR Serenity more broadly included individuals who had any history of intolerance of at least one statin. Both included primary and secondary prevention patients with a baseline LDL-C of ≥100 mg/dL, although CLEAR Serenity required the baseline LDL-C to be ≥130 mg/dL in its primary prevention patients. In CLEAR Tranquility, starting from a mean LDL-C of 127.6 mg/dL, bempedoic acid significantly reduced LDL-C, with a placebo-corrected difference of −28.5% (95% CI: −34.4%; p < 0.001) [21]. In CLEAR Serenity, starting from a baseline LDL-C of 157.6 mg/dL, bempedoic acid significantly reduced LDL-C, with a placebo-corrected difference of −21.4% (95% CI, −25.1% to −17.7%; p < 0.001) [24].
The first trial to examine cardiovascular events beyond LDL-C reduction from bempedoic acid was CLEAR Outcomes (2023) [25]. CLEAR Outcomes studied individuals who had a history of intolerance of two or more statins, with a baseline LDL-C of ≥100 mg/dL, and like its predecessor trials it included both primary and secondary prevention patients. Patients were also required to provide written documentation of statin intolerance and inability or unwillingness to take statin therapy despite knowing that they stand to derive cardiovascular benefits from statin therapy. The incidence of the primary endpoint, which was four-component MACE (non-fatal MI, non-fatal stroke, coronary revascularization, or cardiovascular death), was lower in the bempedoic acid group (11.7% vs. 13.3% in placebo), with an associated HR of 0.87 (95% CI 0.79 to 0.96; p = 0.004).
In light of the results of the CLEAR trials, some have questioned whether bempedoic acid would still demonstrate these effects when layered on top of statin therapy. A phase III trial by Ballantyne et al. randomized individuals with multiple cardiovascular risk factors or HeFH, whose baseline LDL-C was ≥100 mg/dL, to bempedoic acid, ezetimibe, bempedoic acid plus ezetimibe, or placebo groups, on top of maximally tolerated statin therapy [88]. Combination bempedoic acid–ezetimibe therapy resulted in a placebo-corrected LDL-C reduction of −38.0% (p < 0.001), which was more effective than ezetimibe alone (−23.2%) or bempedoic acid alone (−17.2%). These studies provide supportive evidence for the use of bempedoic acid as both monotherapy and combination therapy to lower LDL-C, and as monotherapy to reduce cardiovascular events. Bempedoic acid may serve as a useful medication for cholesterol reduction in statin-intolerant patients, as well as in patients on maximally tolerated statin therapy.
4.2.3. Current Indications
Bempedoic acid received FDA approval for usage in 2020 as an adjunct to diet and maximally tolerated statin therapy for individuals with HeFH or established ASCVD who require additional lowering of LDL-C [89]. Furthermore, the FDA expanded the indications for bempedoic acid and bempedoic acid plus ezetimibe for primary hyperlipidemia and removed the prerequisite for patients to be on maximally tolerated statin therapy in December 2023 [89].
The 2022 AHA/ACC guidelines provide four populations in which the use of bempedoic acid is indicated [9]: In individuals with clinical ASCVD who are at very high risk, bempedoic acid can be considered in those who are unable to achieve a >50% reduction in LDL-C or whose LDL-C remains ≥55 mg/dL (or whose non–HDL-C remains ≥85 mg/dL) despite maximally tolerated statin therapy, ezetimibe, and a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor. In individuals with clinical ASCVD who are not at very high risk, bempedoic acid can be considered in those who are unable to achieve a >50% reduction in LDL-C or whose LDL-C remains ≥0 mg/dL (or whose non–HDL-C remains ≥100 mg/dL) despite maximally tolerated statin therapy, ezetimibe, and/or a PCSK9 inhibitor. In both scenarios, the guidelines note that bempedoic acid should be considered in the setting of documented statin intolerance. Third, the guidelines allow for the use of bempedoic acid if other evidence-based agents are contraindicated or not tolerated, and for ease of use for patients who prefer to avoid injectable medications. Because the 2022 AHA/ACC guidelines were issued prior to the results of CLEAR Outcomes, the guidelines advised a preference for ezetimibe and PCSK9 inhibitors as first- and second-line non-statin agents. It remains to be seen whether bempedoic acid is elevated to a second-line non-statin agent in future guidelines. Fourth, in individuals with clinical ASCVD with a baseline LDL-C elevated to ≥190 mg/dL that is not due to familial hyperlipidemia or other secondary etiologies, bempedoic acid is indicated in those who are unable to achieve a ≥50% reduction in LDL-C or whose LDL-C remains ≥70 mg/dL (or whose non–HDL remains ≥100 mg/dL) after the addition of ezetimibe and/or a PCSK9 inhibitor.
Bempedoic acid is prescribed as a fixed dose of 180 mg orally once daily, or as a combination therapy with ezetimibe 10 mg daily [89].
4.2.4. Safety Profile
Bempedoic acid has not been associated with a significant side-effect profile in clinical trials, but real-world clinical experience is still limited. Rare adverse effects observed with bempedoic acid compared to placebo included increased blood uric acid levels (2.1% vs. 0.5%), gout (1.4% vs. 0.4%), decreased glomerular filtration rate (0.7% vs. <0.1%), and increased levels of hepatic enzymes (2.8% vs. 1.3%) [90]. It is also associated with cholelithiasis (RR: 1.87; CI: 1.43–2.44) and is relatively contraindicated in patients with comorbid gout.
4.2.5. Cost-Effectiveness
Bempedoic acid’s long-term cost-effectiveness in the United States remains to be further evaluated. Bempedoic acid/ezetimibe in combination with statin therapy produced a net cost of USD 186,000 per QALY gained, which does not meet commonly cited thresholds of cost-effectiveness, which lie between USD 100,000 and USD 150,000 per QALY [91]. In 2021, the Institute for Clinical and Economic Review published an evidence report evaluating the clinical effectiveness and value of cholesterol-lowering medications; it recommended a health-benefit price benchmark of USD 1600 to 2600 per year for bempedoic acid/ezetimibe, which would require a 30–60% discount off the treatment’s current wholesale acquisition cost [92].
4.3. Monoclonal Antibodies Targeting Proprotein Convertase Subtilisin/Kexin Type 9
4.3.1. Mechanism of Action
Epidemiological observations have shown that loss-of-function mutations in PCSK9—a serine protease that irreversibly binds the LDL–LDL receptor complex in hepatocytes, leading to the lysosomal degradation of the LDL receptor—are associated with a lower risk of cardiovascular disease [93], pointing to PCSK9 as a therapeutic target. Three monoclonal antibodies (mAbs) targeting PCSK9 and inhibiting its interaction with the LDL receptor have been studied in phase III clinical trials. PCSK9 mAbs include two fully human mAbs, the immunoglobulin G1 mAb alirocumab, the immunoglobulin G2 mAb evolocumab, and bococizumab, a humanized IgG-λ mAb with a remnant murine sequence [94].
4.3.2. Results of Randomized Trials of Evolocumab
Table 4 provides an overview of the major clinical trials related to evolocumab. Over the last decade, clinical trials under the Program to Reduce LDL-C and Cardiovascular Outcomes Following Inhibition of PCSK In Different Populations (PROFICO) have evaluated the efficacy of evolocumab in reducing LDL-C levels, adverse cardiovascular events, and atherosclerotic burden in several target patient populations. Significant reductions in LDL-C levels were shown in patients with hypercholesterolemia, no evidence of ASCVD, and not treated with statins who were given evolocumab either biweekly (140 mg) or monthly (420 mg) in the MENDEL-2 trial [26], in patients treated with various statins in the LAPLACE-2 trial [27], in statin-intolerant patients in the GAUSS-2 [28], GAUSS-3 [32], and GAUSS-4 trials [95], in patients with hypercholesterolemia on a lipid-lowering diet or taking statins or ezetimibe in the DESCARTES trial [29], in patients with HeFH in the RUTHERFORD-2 trial [30], and in patients with type 2 diabetes mellitus in the BANTING trial [34], when compared to placebo, ezetimibe, or both. Similar findings were shown in two extension studies, OSLER-1 and OSLER-2 [31]. In the YUKAWA-2 trial, Japanese patients with hyperlipidemia or mixed dyslipidemia and a high cardiovascular risk taking atorvastatin experienced marked reductions in LDL-C levels when randomized to evolocumab compared to placebo [96]. Self-administration of evolocumab with an autoinjector or an automated mini-doser was shown to successfully lower LDL-C levels in the THOMAS-1 and the THOMAS-2 trials [97].
The FOURIER trial, the most practice-changing evolocumab trial, enrolled 27,564 patients between 40 and 85 years of age, with LDL-C levels greater than or equal to 70 mg/dL or non-HDL-C levels greater than 100 mg/dL, with triglycerides less than or equal to 400 mg/dL, and treated with moderate- or high-intensity statin therapy, and it excluded patients with New York Heart Association (NYHA) class III or IV heart failure or a left ventricular ejection fraction of less than 30%, uncontrolled hypertension, a recurrent or an uncontrolled ventricular tachycardia, hypothyroidism or hyperthyroidism, homozygous FH, and patients undergoing apheresis of plasma or LDL [33]. Participants were randomized to subcutaneous evolocumab or placebo, administered biweekly or once per month, and followed for a median of 26 months. The investigators reported an absolute reduction of 1.5% in the evolocumab group (9.8% vs. 11.3% in the placebo group), with an HR of 0.85 (95% CI, 0.79–0.92) in the primary endpoint, a composite of time to cardiovascular mortality, myocardial infarction, stroke, hospitalization for unstable angina, and coronary revascularization; and a 1.5% absolute risk reduction in the evolocumab group (5.9% vs. 7.4% in the placebo group), with an HR of 0.80 (95% CI, 0.73–0.88) in the secondary endpoint, a composite of cardiovascular mortality, myocardial infarction, and stroke. Overall, these results were similar across subgroups, stratified by demographics and background LDL-C-lowering therapy. When analyzed separately, however, the primary endpoint in patients with polyvascular disease, with baseline LDL-C between 92 and 109 mg/dL or greater than 109 mg/dL, or treated with ezetimibe did not reach statistical significance, which may have been a result of the small sample size in these groups. The reduction in MACE was driven by a significant reduction in myocardial infarction, stroke, and coronary revascularization. Evolocumab did not reduce cardiovascular or all-cause mortality. A reanalysis of the mortality data of the FOURIER trial pointed to possible discrepancies between the reported results and information in the clinical study reports [98].
In two extension studies of the FOURIER trial (FOURIER-OLE and FOURIER-OLE in Selected European Countries, which was terminated by the sponsor), patients received evolocumab irrespective of their randomization group in the parent study [35]. After a median follow-up of 5 years, patients previously randomized to evolocumab (and therefore exposed to it for a longer duration) experienced a 0.6% absolute reduction in the primary endpoint compared to patients originally randomized to placebo. In addition, the investigators reported an absolute reduction of 0.22% in cardiovascular mortality in patients previously randomized to evolocumab.
The GLAGOV trial explored the reduction in coronary plaque burden via serial intravascular ultrasonography in adult patients randomized to either evolocumab or placebo. Patients were included if they demonstrated evidence of coronary heart disease and a fasting LDL-C greater than or equal to 80 mg/dL if no additional risk factors were present, or greater than or equal to 60 mg/dL if other risk factors were present. The study’s results demonstrated a 1% absolute reduction in coronary atheroma volume (95% CI, −1.8% to −0.64%; p < 0.001), which may partially explain the drug’s ability to reduce major adverse cardiovascular events [99].
4.3.3. Results of Randomized Trials of Alirocumab
Table 5 provides an overview of the major clinical trials related to alirocumab. Alirocumab led to a greater reduction in LDL-C levels compared to ezetimibe when administered without additional lipid-lowering therapies in the ODYSSEY-MONO trial [100], when administered with statins in the ODYSSEY-COMBO I [45] and ODYSSEY-COMBO 2 [46] trials compared to placebo and ezetimibe, in patients with HeFH when added to statin therapy in the ODYSSEY-FH I, ODYSSEY-FH II [41], and ODYSSEY HIGH FH [42] trials compared to placebo, in patients with a high risk of ASCVD events compared to ezetimibe and statin therapy in the ODYSSEY-OPTIONS I [43] and ODYSSEY-OPTIONS II trials [44], and in patients with moderate-to-high risk of ASCVD events either taking or not taking statins in the ODYSSEY CHOICE I [47] and ODYSSEY CHOICE II trials [48]. The ODYSSEY LONG TERM trial further showed a significant 62% reduction (p < 0.001) in LDL-C in high-risk patients, including patients with HeFH, established CAD, or equivalent risk factors [39]. While not designed to investigate cardiovascular outcomes, the ODYSSEY LONG TERM trial suggested that alirocumab may be beneficial in reducing adverse cardiovascular events [39].
The ODYSSEY OUTCOMES trial enrolled 18,924 patients of 40 years of age and older who were hospitalized for ACS at least 1 month and no more than 12 months before randomization, and with an LDL-C level greater than 70 mg/dL, a non-HDL-C level greater than 100 mg/dL, or an apoB level greater than 80 mg/dL receiving high-intensity statin therapy, and with a triglycerides level of less than 400 mg/dL [38]. Patients with NYHA class III or IV heart failure and those treated with fibrates were excluded. The trial investigators randomized participants to receive alirocumab (75 mg) subcutaneously or placebo biweekly and followed them for a median of 2.8 years. Patients randomized to alirocumab whose LDL-C levels remained above 50 mg/dL underwent titration to a higher alirocumab dose (150 mg). A total of 22.2% of the total alirocumab treatment time was at the 150 mg dose. The trial investigators reported an absolute risk reduction of 1.6% in the alirocumab group (9.5% vs. 11.1% in the placebo group), with an HR of 0.85 (95% CI, 0.78–0.93) in the primary endpoint, a composite of coronary death, non-fatal MI, ischemic stroke (fatal and non-fatal), and hospitalization for unstable angina. They also reported a 1.6% absolute risk reduction in the alirocumab group (12.7% vs. 13.3% in the placebo group), with an HR of 0.88 (95% CI, 0.81–0.95) in a major secondary endpoint (a composite of coronary death, non-fatal MI, hospitalization for unstable angina, and coronary revascularization). Alirocumab did not reduce coronary mortality. An analysis of cardiovascular events that included the first non-fatal event and all subsequent events showed a reduction in non-fatal events (including MI, stroke, hospitalization for unstable angina, hospitalization for heart failure, and ischemic-driven coronary revascularization), with an HR of 0.87 (95% CI, 0.82–0.93), and in all-cause mortality, with an HR of 0.83 (95% CI, 0.71–0.97) in the alirocumab group compared to placebo [101].
4.3.4. Results of Randomized Trials of Bococizumab
Table 6 provides an overview of the major clinical trials related to bococizumab. Bococizumab reduced LDL-C, TC, non-HDL-C, apoB, and Lp(a) after 3 months in a series of phase III trials including patients with varying levels of risk for ASCVD events (SPIRE-HR, SPIRE-LDL, and SPIRE-LL), including patients with HeFH (SPIRE-FH) and patients who were statin-intolerant (SPIRE-SI), and including a trial in which bococizumab was administered via an autoinjector (SPIRE-AI) [58]. Close to one-half of enrolled patients had detectable antidrug antibodies after 1 year, the levels of which were associated with a decline in the observed lipid profile biomarkers’ response. Following these results, bococizumab was discontinued by the sponsor, as two phase III trials were underway assessing the drug’s effects on MACE. The SPIRE-1 and SPIRE-2 outcome trials were halted after a median follow-up of 7 and 12 months, respectively, and showed a reduction in MACE in higher- (LDL-C ≥ 100 mg/dL) but not lower-risk (LDL-C ≥ 70 mg/dL) patients randomized to bococizumab [59].
4.3.5. Current Indications
Alirocumab and evolocumab received FDA approval in 2015 for use in LDL-C reduction as adjuncts to diet and statin therapy, as well as for standalone therapy in patients with clinical ASCVD or HeFH [102,103]. Both alirocumab and evolocumab have received FDA approval for secondary prevention in patients with established ASCVD. Bococizumab is not FDA-approved, due to concerns about variability in clinical responses and immunogenicity [58].
The 2022 AHA/ACC guidelines provide three populations in which the use of PCSK9 inhibitors is indicated [9]: In individuals with clinical ASCVD who are at very high risk, PCSK9 inhibitor can be considered in those who are unable to achieve a >50% reduction in LDL-C or whose LDL-C remains ≥55 mg/dL (or whose non–HDL-C remains ≥85 mg/dL) despite maximally tolerated statin therapy, or who are unable to tolerate even low-intensity statin therapy or alternative statin therapy dosing regimens (every other day, twice weekly, or weekly). PCSK9 inhibitors can be considered as first-line treatments over ezetimibe in patients who require >25% additional lowering of LDL-C, or based on clinician–patient decision-making. In individuals with clinical ASCVD who are not at very high risk, PCSK9 inhibitors can be considered in those who are unable to achieve a >50% reduction in LDL-C or whose LDL-C remains ≥70 mg/dL (or whose non–HDL-C remains ≥100 mg/dL) despite maximally tolerated statin–ezetimibe combination therapy or non-statin combination therapy in the setting of documented statin intolerance, if the patient prefers PCSK9 inhibition after patient–clinician discussion. Third, in individuals with clinical ASCVD with a baseline LDL-C elevated to ≥190 mg/dL that is not due to familial hyperlipidemia or other secondary etiologies, PCSK9 inhibitors are indicated in those who are unable to achieve a ≥50% reduction in LDL-C or whose LDL-C remains ≥70 mg/dL (or whose non–HDL remains ≥100 mg/dL) despite maximally tolerated statin therapy, and who require >25% additional lowering of LDL-C or who have very high additional risk factors.
The FDA-recommended dosing for alirocumab and evolocumab is shown in Table 1. The majority of patients taking alirocumab self-administer 75 mg once every 2 weeks via a subcutaneous injection, but patients who prefer a less frequent dosing regimen can be prescribed a 300 mg injection once every 4 weeks [103]. Patients taking evolocumab with established ASCVD, primary hyperlipidemia, and/or HeFH can self-inject either 140 mg every 2 weeks or 420 mg monthly via subcutaneous injection. Patients with HoFH are administered a dose of 420 mg once monthly [102].
4.3.6. Safety Profile
PCSK9 inhibitors originally faced criticism based on concerns surrounding their unique side-effect profiles, as established by various outcomes studies. For example, the ODYSSEY LONG TERM trial demonstrated that patients taking alirocumab experienced more overall adverse events, myalgia, injection site reactions, and ophthalmologic and neurocognitive events compared to the placebo group [33]. Additionally, the OSLER-1 and OSLER-2 studies demonstrated increased incidence of adverse neurocognitive events in patients receiving evolocumab compared to placebo [31]. Moreover, PCSK9 genetic variants associated with decreased LDL-C levels were found to be correlated with an increased risk of new-onset diabetes [104]. These findings raised skepticism around PCSK9 usage; however, recent meta-analyses have demonstrated that PCSK9 inhibitors are well tolerated, with no significant differences in serious adverse effects (RR 0.937, 95% CI: 0.896–0.980), diabetes-related adverse events (RR: 0.967, 95% CI: 0.845–0.987), neurological adverse effects (RR 1.031, 95% CI: 0.913–1.163), liver enzyme elevation (RR 0.94, 95% CI: 0.84–1.06), rhabdomyolysis (RR 0.90, 95% CI: 0.62–1.31), or allergic reactions (RR 1.04, 95% CI: 0.97–1.12) compared to placebo [105,106]. The most common adverse effects associated with PCSK9 inhibitors include injection site reactions, influenza-like illnesses, and myalgias [107].
4.3.7. Cost-Effectiveness
Several studies have examined the cost-effectiveness of PCSK-9 inhibitors based on their trends in pricing since introduction to the market. A 2019 study of evolocumab (added to standard background therapy vs. standard background therapy alone) in the FOURIER trials following its 60% price reduction in 2018 revealed that evolocumab produces a 0.39 to 0.44 increase in QALYs and a range of ICERs from USD 56,655 to USD 7677 per QALY gained, with variation largely determined by the baseline cardiovascular event rate. These results demonstrate that evolocumab meets current cost-effectiveness thresholds [108]. A 2022 study demonstrated that alirocumab has an ICER of 54,211 pounds (US dollars 67,221.64) per QALY, which also meets current cost-effectiveness thresholds [109].
4.4. Inclisiran
4.4.1. Mechanism of Action
While PCSK9 inhibitors utilize antibodies to block PCSK9 activity post-production, small interfering RNAs (siRNAs) are able to directly inhibit PCSK9 formation. Inclisiran is a novel siRNA that inhibits PCSK9 production by binding to mRNA precursors, thereby inhibiting translation [110]. Its downstream effects are similar to those of PCSK9 inhibitors, causing increased hepatocyte recycling of LDL receptors and thereby decreasing levels of LDL-C.
4.4.2. Results of Randomized Trials
The clinical efficacy and safety of inclisiran has been established through the ORION trial series. ORION-9, 10, and 11 were the major phase III trials that examined LDL-lowering from inclisiran in large populations. Table 7 provides an overview of the major clinical trials related to inclisiran. The ORION-9 trial examined the efficacy of inclisiran usage in patients with HeFH. Starting from a mean baseline LDL-C of 104.7, inclisiran therapy significantly reduced LDL-C levels, with a placebo-corrected difference of −47.9% (95% CI, −53.5 to −42.3, p < 0.001). This study also demonstrated a significant reduction in PCSK9 levels, with a placebo-corrected difference of −78.4%, and a placebo-corrected difference in ApoB levels of −2.1% (p < 0.001 for both) [60]. The ORION-10 trial examined the efficacy of inclisiran therapy in patients with clinical ASCVD. Starting from a mean baseline of 104.7 mg/dL, inclisiran therapy significantly reduced LDL-C, with a placebo-corrected difference of −2.3% (95% CI, −55.7 to −48.8, p < 0.001). This study also demonstrated a placebo-corrected difference in PCSK9 levels of −3.3% (p < 0.001) [61]. The ORION-11 trial built upon its predecessor by also examining patients with ASCVD risk equivalents. Starting from a mean baseline LDL-C of 105.5 mg/dL, inclisiran therapy significantly reduced LDL-C levels, with a placebo-corrected difference of −9.9% (95% CI, −53.1 to −46.6; p < 0.001) and a −79.3% difference in PCSK9 levels (p < 0.001) [61].
At the time of writing, there are no completed trials of inclisiran that have examined cardiovascular events as a primary outcome. This will change with ORION-4 (NCT03705234) and VICTORIAN-2 PREVENT (NCT05030428), which are anticipated for completion in 2026 and 2027, respectively. Both ORION-4 and VICTORIAN-2 PREVENT are randomized, placebo-controlled trials of inclisiran in patients with known ASCVD. The primary outcome will be the time to first occurrence of MACE, defined slightly differently in each trial.
4.4.3. Current Indications
Inclisiran was approved by the FDA in 2021 for use as an adjunct to maximal statin therapy for patients with clinical ASCVD and those with HeFH in need of additional LDL-C reduction [111]. More recently, inclisiran has also been approved for primary prevention in patients with hyperlipidemia [112]. The drug is a provider-administered subcutaneous injection on day 1 and is followed by a subsequent injection on day 90. Afterwards, the drug is administered every 6 months.
The 2022 AHA/ACC guidelines provide two populations in which the use of inclisiran is indicated [9]: In individuals with clinical ASCVD who are at very high risk, inclisiran can be considered in those who are unable to achieve a >50% reduction in LDL-C or whose LDL-C remains ≥55 mg/dL (or whose non–HDL-C remains ≥85 mg/dL) despite maximally tolerated statin therapy, instead of a PCSK9 inhibitor, in individuals with poor adherence to PCSK9 inhibitor injections or who have experienced adverse effects from PCSK9 inhibition. In individuals with clinical ASCVD who are not at very high risk, inclisiran can be considered in those who are unable to achieve a >50% reduction in LDL-C or whose LDL-C remains ≥70 mg/dL (or whose non–HDL-C remains ≥100 mg/dL) despite maximally tolerated statin therapy, instead of a PCSK9 inhibitor, in individuals with poor adherence to PCSK9 inhibitor injections or who have experienced adverse effects from PCSK9 inhibition.
4.4.4. Safety Profile
Inclisiran has been found to be well tolerated across a variety of patient populations, with little evidence of serious side effects. Meta-analyses of the data indicate that the most common side effects of administration include injection site reactions (OR 5.86, 95% CI: 3.44–9.98) and bronchitis (OR 1.58, 95% CI: 1.10–2.26) [113].
4.4.5. Cost-Effectiveness
Recent cost-effectiveness analyses of inclisiran in patients with atherosclerotic cardiovascular report an annual price below USD 9000, an ICER of USD 51,686 per QALY, and a 100% probability of being cost-effective [114].
4.5. Mipomersen
4.5.1. Mechanism of Action
Mipomersen is an antisense oligonucleotide targeted against apolipoprotein B (apoB)-100, the primary apolipoprotein in LDL, very-low-density lipoprotein (VLDL), and Lp(a). By binding to mRNA, it can interfere with subsequent translational products and thereby decrease the synthesis of apoB-100, leading to decreased cholesterol production [115]. The drug comes in a long-acting subcutaneous injection that is usually self-administered weekly.
4.5.2. Results of Randomized Trials
Table 8 provides an overview of the major clinical trials related to mipomersen. The majority of studies have examined patients with HoFH [116], but several trials have examined its use outside of HoFH: in patients with severe hypercholesterolemia [64], as an adjunct to statin-based therapy [65], and in patients with HeFH (the RADICHOL II trial) [63]. In these studies, mipomersen was shown to decrease LDL-C by 32.4–48.5% relative to placebo (p < 0.001 for all measurements). Additionally, these studies indicate that mipomersen therapy leads to significant reductions in Lp(a), apoB, and total non-HDL cholesterol [64,65]. While these findings are promising, mipomersen usage has been discouraged in non-HoFH patients due to risks of hepatotoxicity and a lack of evidence supporting reductions in adverse cardiovascular outcomes [7].
4.5.3. Current Indications
Mipomersen is currently FDA-approved for use in patients with HoFH only. Further research is needed in this area to determine whether mipomersen can be FDA-approved as an adjunct to statin therapy in patients without HoFH. Mipomersen does not appear in the recent AHA/ACC guidelines.
4.5.4. Safety Profile
Data from meta-analyses reveal that treatment with mipomersen is associated with injection site reactions (OR 11.41, p < 0.001), liver enzyme elevation (OR 3.61, p < 0.001), hepatic steatosis (OR 4.96, p = 0.001), and development of flu-like symptoms (OR 2.02, p < 0.001) [117]. These symptoms all contribute to an increased rate of treatment discontinuation amongst patients taking mipomersen (OR 2.02, p < 0.001) [117].
4.5.5. Cost-Effectiveness
As a newer drug, mipomersen is more expensive than other cholesterol-lowering medications. The average wholesale price for a 30-day supply of mipomersen is around USD 23,038.60 [118]. The cost-effectiveness of mipomersen has not been fully evaluated.
4.6. CETP Inhibitors
4.6.1. Mechanism of Action
Since the discovery that individuals in Japan with cholesteryl ester transfer protein (CETP) gene mutations had not only depleted CETP but elevated HDL-C and decreased LDL-C [119], there has been considerable interest in CETP as a pharmacological target for LDL reduction. When bound to HDL, CETP allows for the exchange of both cholesteryl ester and triglycerides between HDL and lipoproteins that contain apolipoprotein B100, such as LDL and VLDL. Ultimately, CETP functions to transfer cholesterol esters from HDL to LDL and VLDL in exchange for triglycerides [120]. By inhibiting CETP, these drugs decrease the amount of cholesterol esters incorporated into LDL-C, increase HDL, and increase hepatic LDL-C recycling [121]. CETP inhibitors include dalcetrapib, evacetrapib, anacetrapib, torcetrapib, and obicetrapib.
4.6.2. Results of Randomized Trials
Despite their promising impact on HDL-C production and LDL-C reduction in animal models, phase III trials involving CETP inhibitors have yielded disappointing results due to unknown mechanisms. Table 9 provides an overview of the clinical trials related to CETP inhibitors. ILLUMINATE (2007) was the first large CETP trial, investigating torcetrapib plus atorvastatin versus atorvastatin alone in patients with known cardiovascular disease. Despite a 72.1% increase in HDL (p < 0.001) and a 24.9% decrease in LDL-C compared to baseline (p < 0.001), participants in the torcetrapib group experienced increased adverse cardiovascular events (HR 1.25, 95% CI, 1.09 to 1.44; p = 0.001) and all-cause mortality (HR 1.58, 95% CI, 1.14 to 2.19; p = 0.006), leading to a premature termination of the study [69].
The dal-OUTCOMES trial (2009) investigated dalcetrapib versus placebo on top of background statin therapy in patients in the post-acute phase (within 3 months) after acute coronary syndrome (ACS) [67]. From a mean HDL-C of 42 mg/dL and LDL-C of 76 mg/dL, dalcetrapib increased HDL-C by 31–40% in the dalcetrapib group and by 4–11% in the placebo group (p < 0.001 for all measurements), but it had no effect on the risk of the primary composite endpoint of death from coronary heart disease, non-fatal MI, ischemic stroke, unstable angina, or cardiac arrest with resuscitation. The dal-OUTCOMES trial was terminated early due to futility.
Subsequently, ACCELERATE (2017) investigated evacetrapib, which was thought to have fewer off-target effects compared to torcetrapib, versus placebo on top of background statin therapy in populations with known high-risk vascular disease [70]. From a mean HDL-C of 45.3 mg/dL and LDL-C of 81.3 mg/dL, evacetrapib increased HDL-C by 133.2% (compared to 1.6% from placebo) and decreased LDL-C by 31.1% (compared to 6.0% from placebo) (p < 0.001 for all measurements). However, there was no difference in the composite endpoint of death from cardiovascular causes, MI, stroke, coronary revascularization, or hospitalization for unstable angina. ACCELERATE was terminated early due to futility. In summary, both dal-OUTCOMES and ACCELERATE were stopped for futility, with both showing nearly identical rates of adverse cardiac events despite significant increases in HDL and reductions in LDL-C, while ILLUMINATE was stopped due to harm.
REVEAL (2017) was the first large study to show positive outcomes from CETP inhibitor use [68]. In this study, patients with known ASCVD were randomized to anacetrapib plus high-intensity atorvastatin versus atorvastatin alone. The results showed significantly decreased incidence of adverse cardiac events (composite of coronary death, MI, or coronary revascularization) in the anacetrapib group (rate ratio 0.91, p = 0.004). In addition, there was a relative difference of +104% in HDL in the anacetrapib group compared to the placebo group, as well as a relative difference of −18% in total non-HDL cholesterol, −17% in LDL-C, and −25% in Lp(a).
Several theories have been proposed to explain the discrepant results of the above phase III trials. First, CETP-inhibitor-related elevations in blood pressure and plasma aldosterone may have offset any potentially beneficial effects of lipid reduction [120]. Second, during CETP inhibitor use, LDL-C levels are underestimated both by the Friedewald equation and by direct LDL-C assays compared with beta quantification [122]. Third, dal-OUTCOMES studied patients who were within three months of a recent ACS event, a phase of disease in which the risk of recurrent ASCVD events is likely too high to be meaningfully modified by lipid modification [120]. Furthermore, REVEAL was carried on for 4 years, gathering 40% more patients than the other three phase III trials combined, and showed that cardiovascular benefit only emerged in years 3 and 4. Whether ACCELERATE would have also demonstrated a similar cardiovascular benefit at years 3 and 4 remains unknown.
In a recent phase II trial, the CETP inhibitor obicetrapib showed robust effects in reducing LDL-C by up to 51% and apolipoprotein B by 30% (p < 0.0001 for both measurements), with an acceptable safety profile [71]. Obicetrapib is currently being evaluated in three phase III trials: BROADWAY (NCT05142722), BROOKLYN (NCT05425745), and PREVAIL (NCT05202509). PREVAIL is examining patients with ASCVD, BROOKLYN is examining patients with HeFH, and BROADWAY is examining patients with either ASCVD or HeFH. The results of BROADWAY and BROOKLYN are expected in 2024, while the results of PREVAIL are expected in 2026 [110].
4.6.3. Current Indications
As a result of mixed studies on the safety and efficacy of CETP inhibitors, none of the drugs in this class have received FDA approval, and none of the CETP inhibitors are recommended in the AHA/ACC guidelines at this time.
4.6.4. Cost-Effectiveness
Given that CETP inhibitors have mixed safety and efficacy, resulting in a lack of FDA approval, there is no literature on the cost-effectiveness of CETP inhibitors to report.
5. Triglyceride-Lowering Therapies
5.1. Overview
Triglyceride-rich lipoproteins (TRLs) represent a class of circulating lipoproteins that includes chylomicrons, VLDL, intermediate-density lipoprotein (IDL), and remnant particles. These particles are chiefly responsible for the transport of dietary fats (in the form of chylomicrons) that are absorbed in the intestines, as well as transporting triglycerides and cholesterol esters (in the form of VLDL) from the liver to the periphery. Similar to LDL, TRLs contain apolipoprotein B (apoB) and are considered to be atherogenic. Observational studies indicate a positive association between higher plasma triglyceride levels and increased risk of atherosclerotic cardiovascular disease (ASCVD). The causality of this association is further supported by Mendelian randomization studies, particularly focusing on variants in lipoprotein lipase (LPL), and in randomized controlled trials of lipid-lowering therapies.
Elevated triglyceride levels (≥150 mg/dL in the fasting state, and ≥175 mg/dL in the non-fasting state) are often multifactorial. In addition to being due to rare monogenic or polygenic disorders (which will not be addressed in the current review), common causes of hypertriglyceridemia that must be considered in the initial evaluation include poorly controlled diabetes, thyroid disorders, hepatic or renal dysfunction, high body mass index, and poor dietary habits, including excessive alcohol consumption. In the 2019 ACC/AHA Primary Prevention guidelines, persistently elevated triglycerides (≥175 mg/dL) were considered to be a risk enhancer for individuals who may have a “borderline” or “intermediate” 10-year ASCVD risk to consider initiation of statin therapy [5]. Similarly, persistently elevated triglycerides, despite treatment with statin therapy, is an adverse prognostic factor for individuals with established ASCVD.
Herein, we provide a review of contemporary triglyceride-lowering therapies that are adjunctive to statin therapy and have been tested in large-scale cardiovascular outcome trials. Bile acid sequestrants are not addressed in this review because they are neither novel nor well tolerated.
5.2. Omega-3 Fatty Acids (Including Eicosapentaenoic Acids)
5.2.1. Mechanism of Action
Interest in omega-3 fatty acids for cardiovascular protection dates back to the Greenland Eskimos, who historically had very low rates of myocardial infarction (MI), which were, in turn, attributed to the high consumption of omega-3 fatty acids in their diet. The underlying mechanisms for this purported benefit were wide-ranging and included effects on platelet activation/aggregation, endothelial function, blood pressure, heart rate and variability, modulating inflammation, reducing circulating atherogenic particles (particularly TRLs), and prevention of ischemia-induced ventricular arrhythmias [123]. Epidemiological studies of fish intake and circulating biomarkers of omega-3 fatty acids have consistently shown benefit across multiple cardiovascular outcomes, particularly in patients with fatal coronary artery disease (CAD) [124,125].
5.2.2. Results of Randomized Trials
With respect to randomized trials of omega-3 treatments, the evidence remains relatively mixed. Initial studies with low-dose omega-3 fatty acid mixtures showed benefit with respect to reducing CVD outcomes. The GISSI-P (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico-Prevenzione; 1999) study showed that in patients with recent (within ≤3 months) MI randomized to n-3 polyunsaturated fatty acids, vitamin E, both, or none, treatment with n-3 PUFAs, but not vitamin E, lowered the risk of the primary endpoint (composite of death, non-fatal MI, and stroke) [126]. It should be noted that this study was conducted in a population with relatively low utilization of statin therapy. Subsequent trials that were conducted with more contemporary background medical therapy in secondary prevention populations have generally shown no benefit with omega-3 fatty acid treatment [127].
Following these studies, two large-scale trials, A Study of Cardiovascular Events in Diabetes (ASCEND; 2018) [128] and the Vitamin D and Omega-3 Trial (VITAL; 2019) [129], were conducted to assess the efficacy of 1 g/d of omega-3 fatty acids vs. matching olive oil placebo in the primary prevention setting. While both trials failed to meet their primary endpoint (composite of cardiovascular death, non-fatal MI, and non-fatal stroke), potential efficacy of omega-3 fatty acids was seen for certain secondary outcomes and/or subgroups. In ASCEND, a nominally significant reduction in vascular death was observed, with an HR of 0.81 (95% CI, 0.67, 0.99) [128]. In VITAL, there was a nominally significant reduction in total MI, with an HR of 0.72 (95% CI, 0.59, 0.90) [129]. Additionally, in subgroup analyses, there was a signal for greater benefit on the primary outcome with respect to individuals who consumed less than 1.5 servings of fish per week, as well as in non-Hispanic black individuals. Nevertheless, since the benefits in ASCEND and VITAL were predominantly seen for secondary outcomes (not adjusted for multiple testing) or in subgroups after demonstrating no significant efficacy for the primary endpoint, these findings should be interpreted in the context of other low-dose EPA + DHA trials and, overall, they do not provide strong evidence of the clinical efficacy of omega-3 fatty acids for the primary or secondary prevention of ASCVD.
In more contemporary trials, evidence for the benefits of omega-3 fatty acids has been most consistent for high-dose eicosapentaenoic acid (EPA). Table 10 provides an overview of EPA-related clinical trials. This was initially shown in the JELIS trial, in which 18,645 participants (mixed primary/secondary prevention population) from Japan were randomized to 1.8 g of EPA (in the form of ethyl esters) or control, on a background of statin therapy [72]. EPA led to a lower incidence of the primary outcome, with an HR of 0.81 (95% CI, 0.69, 0.95). In particular, individuals with elevated triglycerides and low HDL at baseline experienced a 53% reduction in incident CVD events, with an HR of 0.47 (95% CI, 0.23, 0.98). Subsequently, the landmark REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial) trial randomized 8179 patients with established ASCVD or diabetes with additional cardiovascular risk factors to treatment with icosapent ethyl (IPE; highly purified EPA) at 4 g per day vs. a mineral oil placebo [73]. IPE led to a 25% reduction in the primary endpoint, with an HR of 0.75 (95% CI, 0.68, 0.83) with a number needed to treat of 21 (95% CI, 15–33). Interestingly, the findings were generally similar irrespective of baseline or achieved triglyceride levels, suggesting possible pleiotropic effects on modifying cardiovascular risk. Shortly after the publication of REDUCE-IT, concerns were raised about the neutrality of the mineral oil placebo, as patients randomized to this treatment experienced increases in LDL-C, as well as in several inflammatory biomarkers [130]. Part of the controversy over the efficacy of IPE and the potential deleterious effects of the mineral oil placebo was further fueled by the publication of the STRENGTH (Long-Term Outcomes Study to Assess Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia) trial [131]. STRENGTH randomized 13,078 with either established ASCVD or high risk of ASCVD to 4 g/d of omega-3 carboxylic acid (free fatty acid formulation that included both EPA and DHA) or corn oil. The trial was terminated early for clinical futility, with an HR of 0.99 (95% CI, 0.90, 1.09) comparing the omega-3 intervention to corn oil. Moreover, an increased risk of atrial fibrillation and gastrointestinal disorders was reported for the omega-3 intervention group. Debate remains as to the precise reason(s) for the discrepancy between the two trials, including differences in drug formulation (EPA alone vs. EPA + DHA; ethyl ester preparation vs. free fatty acid), trial population (higher proportion of secondary prevention participants in REDUCE-IT, requiring low HDL-C as an inclusion criterion in STRENGTH), and comparator group (mineral oil vs. corn oil, the former of which was seen to be associated with increases in LDL-C and CRP).

Table 10.
Major phase III randomized controlled trials involving eicosapentaenoic acid.
More recently, a randomized trial conducted in Japan provided further support for the potential cardiovascular benefits of EPA. The Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy–Statin and Eicosapentaenoic Acid (RESPECT-EPA) [74] was a multicenter, open-label trial that randomized 2506 Japanese patients with a history of chronic coronary artery disease to either 1.8 g/d of EPA or control. It should be noted that individuals for this trial were not identified by elevated triglycerides but, rather, had to have a low EPA/AA (arachidonic acid) ratio (defined as <0.4) to be randomized. The trial showed a non-significant reduction with EPA for the primary endpoint (composite of cardiovascular death, non-fatal MI, non-fatal stroke, unstable angina, and coronary revascularizations), with 10.9% in the EPA arm vs. 14.9% in the control arm (HR 0.785; 95% CI 0.616, 1.001) [132]. On the other hand, the secondary efficacy outcome (composite of MI, sudden cardiac death, unstable angina, and coronary revascularizations) was significantly lower in the EPA arm (8.0%) compared to the control arm (11.3%), with an HR of 0.734 (95% CI, 0.554, 0.973). In summary, while RESPECT-EPA narrowly missed its primary outcome, taken together with JELIS and REDUCE-IT, it provides additional support for potential cardiovascular benefits with respect to high-dose EPA.
5.2.3. Current Indications
IPE received FDA approval in 2012 for triglyceride reduction, and since the publication of REDUCE-IT it has received an expanded indication, i.e., it is to be used as an adjunct to maximally tolerated statin therapy among individuals with fasting triglyceride levels ≥ 150 mg/dL and either established ASCVD or diabetes mellitus with two or more additional cardiovascular risk factors [133]. Both IPE and omega-3-acid ethyl ester capsules are indicated in conjunction with diet to reduce triglyceride levels in adults with severe hypertriglyceridemia (TG ≥ 500 mg/dL) [134].
In the 2021 ACC Expert Consensus Decision Pathway on the Management of ASCVD Risk Reduction in Patients with Persistent Hypertriglyceridemia, several populations were enumerated for consideration of IPE use [135]. In adults with ASCVD and fasting triglycerides 150–499 mg/dL (or non-fasting triglycerides 175–499 mg/dL), IPE can be considered in those with LDL-C ≥ 70 mg/dL. In those with LDL-C < 70 mg/dL, IPE can be considered, but with caution, as it was associated with a 1% increase in hospitalization for atrial fibrillation or flutter in REDUCE-IT. In adults without ASCVD and with fasting triglycerides 150–499 mg/dL (or non-fasting triglycerides 175–499 mg/dL), IPE can be considered for those aged ≥ 50 years with diabetes mellitus and one other ASCVD risk factor. In adults aged ≥ 20 years with triglycerides ≥ 500 mg/dL, IPE can be considered to reduce the risk of pancreatitis.
5.2.4. Safety Profile
In terms of adverse effects, low-dose omega-3 fatty acids are generally well tolerated, with minimal side effects. For high-dose omega-3 fatty acid treatments, an increased risk of gastrointestinal disorders, atrial fibrillation, and minor bleeding has been reported [136]. With respect to atrial fibrillation, this appeared to be dose-dependent and was predominantly seen with high-dose omega-3 treatments, whereas low-dose treatment (~1 g/d) was associated with minimal risk of atrial fibrillation, an observation that was confirmed when assessing biomarkers of omega-3 fatty acid intake [137]. Individuals with true documented allergies to fish or fish products are not recommended to take these products, though whether these patients are also unable to take IPE, which is believed to be highly purified and theoretically free of allergens from fish, remains less certain.
5.2.5. Cost-Effectiveness
Omega-3 fatty acids have been shown to be cost-effective in many scenarios, including for the prevention of secondary cardiovascular events in men [138] and for primary and secondary prevention [139]. In terms of icosapent ethyl, there are conflicting data on its cost-effectiveness. A 2019 study determined that icosapent ethyl was not cost-effective and revealed an ICER of 59,036 (USD 41,053 USD) per QALY [140]. However, a recent study found an ICER range of USD 31,823–70,427 per QALY gained, with a lifetime horizon ICER of USD 32,925 per QALY, suggesting that icosapent ethyl is a cost-effective strategy for the secondary prevention for ischemic cardiovascular events [141].
5.3. Fibric Acid Derivatives
5.3.1. Mechanism of Action
The fibric acid derivative or fibrate class of medications activates the nuclear transcription factor peroxisome proliferator-activated receptor (PPAR)-alpha (PPARα) [142]. This, in turn, regulates levels of Apo-CIII, ApoA-I, and lipoprotein lipase (LPL), leading to lowering of triglyceride levels and increases in HDL-C.
5.3.2. Results of Randomized Trials
Table 11 provides an overview of fibrate-related clinical trials. The clinical efficacy of the fibrate class of medications was initially demonstrated in the Veterans Affairs HDL Intervention Trial (VA-HIT; 1999), in which 2531 patients with established coronary artery disease and low HDL-C were randomized to gemfibrozil 1200 mg/d or placebo [143]. Gemfibrozil led to a 22% lower risk of the primary outcome of non-fatal myocardial infarction or death from coronary causes, as well as a 24% risk reduction in the secondary outcome, which additionally included stroke. These findings were corroborated by the results from the Helsinki Heart Study [144,145]. However, due to the impact of gemfibrozil on statin metabolism, potentiating the risk of rhabdomyolysis, the utility of gemfibrozil in contemporary practice is limited, as current guidelines continue to recommend statin therapy for the primary pharmacological management of moderate-to-severe triglyceridemia.

Table 11.
Major phase III randomized clinical trials involving fibrates.
Among statin-treated patients, two trials with fenofibrate have been conducted. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD; 2005) [77] trial randomized 9795 patients with type 2 diabetes, with and without established ASCVD, to micronized fenofibrate 200 mg daily or placebo. It should be noted that none of the patients were on statin therapy at the beginning of the trial, although a subset of patients initiated statin therapy during the course of the trial (higher percentage in the placebo arm). The primary outcome of coronary heart disease mortality and non-fatal MI was not significantly reduced with fenofibrate, with an HR of 0.89 (95% CI, 0.75, 1.05). Nevertheless, certain secondary outcomes, including non-fatal MI, total cardiovascular events, and revascularizations, were significantly reduced. The Action to Control Cardiovascular Risk in Diabetes (ACCORD; 2010) [76] lipid trial randomized 5518 patients with type 2 diabetes treated with open-label simvastatin to fenofibrate or placebo. Treatment with fenofibrate did not lead to a lower incidence of the primary outcome (defined as a composite of non-fatal MI, non-fatal stroke, and cardiovascular death), with an HR of 0.92 (95% CI, 0.79, 1.08). All secondary outcomes were similarly neutral with respect to randomization to fenofibrate or placebo. In both FIELD and ACCORD, there were hints of clinical benefit for the subgroup of patients with high triglycerides or low HDL-C who received fenofibrate compared to placebo.
Partially spurred by the encouraging findings of possible benefit with respect to fibrate therapy in FIELD and ACCORD among patients with more severe underlying dyslipidemia, the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) trial [75] was launched to investigate the clinical efficacy of the selective PPARα modulator pemafibrate. PROMINENT enrolled 10,497 patients with type 2 diabetes, high triglycerides, and low HDL-C (approximately 67% secondary prevention) to either 0.2 mg twice daily or a matching placebo. Ultimately, despite significantly reducing triglycerides and VLDL cholesterol by approximately 25%, pemafibrate did not reduce clinical ASCVD events, with an HR of 1.03 (95% CI, 0.91, 1.15) for the primary composite outcome. Similarly neutral results were observed for secondary CV outcomes, as well as in all examined subgroups. Interestingly, while triglycerides and TRLs were decreased, LDL-C and total apoB-containing particles were slightly increased, suggesting conversion of TRLs to other apoB-containing particles rather than elimination, which may explain the potential lack of clinical benefit with this approach.
5.3.3. Current Indications
The FDA reports that fibrates are indicated as an adjunct to diet control to reduce LDL-C, total cholesterol, triglycerides, and ApoB, and to increase HDL-C, in patients with primary hypercholesterolemia or mixed dyslipidemia. Additionally, fibrates can be used for triglyceride reduction in patients with severe hypertriglyceridemia [146]. The AHA also recommends fibrate usage for triglyceride reduction in patients with severe hypertriglyceridemia.
5.3.4. Safety Profile
Fibrates are generally well tolerated. Side effects include GI upset, cutaneous manifestations, acute kidney injury, and hepatotoxicity (typically reversible with cessation of the drug).
5.3.5. Cost-Effectiveness
Studies have demonstrated that fibrates are cost-effective in the primary prevention of coronary heart disease, meeting a societal cost-effectiveness threshold of USD 50,000 per QALY [147]. One study in Germany demonstrated that fibrates were viable low-cost medications to lower triglycerides [139]. A systematic review demonstrated that fenofibrate was cost-effective as compared with statin monotherapy [148].
6. Lipoprotein-A-Lowering Therapies
6.1. Overview
Lipoprotein A (Lp(a)) is a newer target for lipid-lowering medications. It has long been known to be a risk factor for the development of ASCVD, but pharmacological treatment options have been challenging to develop due to its low affinity for clearance. It consists of an apolipoprotein B particle covalently bonded to an apolipoprotein(a) unit. It is synthesized in the liver, specifically in the hepatocyte cell membranes. Elevated Lp(a) levels are defined as levels above 50 mg/dL and have been found to be associated with an increased risk of cardiovascular disease [149]. Unlike other atherogenic lipoproteins, Lp(a) levels are estimated to be around 70 to >90% genetically determined and is minimally affected by lifestyle or existing lipid-lowering therapies [150]. Table 12 provides an overview of the major clinical trials related to Lp(a) reduction therapies.

Table 12.
Major clinical trials involving Lp(a)-targeted therapies.
6.1.1. Current Indications
As of now, only lipoprotein apheresis is approved by the Food and Drug Administration (FDA) for the treatment of elevated Lp(a) levels. Clinical indications consist of patients with progressive cardiovascular disease and Lp(a) > 60 mg/dL in Germany; it is not currently included among class I or II recommendations in the AHA/ACC guidelines [153]. However, there are novel therapies targeting Lp(a) reduction that are in various phases of clinical trials.
6.1.2. Mechanism of Action
Lp(a) contributes to cardiovascular risk through oxidation of these lipoproteins to the coronary arterial wall, resulting in inflammation, atherosclerosis, and thrombosis, although the specific pathophysiology remains under investigation [150]. Lp(a)-lowering therapies currently under investigation include pelacarsen, olpasiran, SLN360, LY3473329, and LY3819469. Pelacarsen is an antisense oligonucleotide agent, while the remaining therapies are small interfering RNA agents. All of these agents contain N-acetylgalactosamine (GalNAc), which allows for rapid, specific uptake via the asialoglycoprotein receptors on hepatocytes. Due to the relative nascency of trials in this area, we will focus on the results of phase II trials of Lp(a)-lowering therapies, while phase III trials are ongoing.
6.2. Pelacarsen
Pelacarsen, also known as ISIS 681257, IONIS APO(a)-LRx, AKCEA-APO(a)-LRx, and TQJ230, is a hepatocyte-directed antisense oligonucleotide that targets the LPA gene mRNA, thus inhibiting Lp(a) synthesis in the liver [154].
In the phase II dose-ranging trial AKCEA-APO(a)-LRx (2020) [154], adults with established cardiovascular disease (CVD) and Lp(a) > 150 nmol/L were randomized to pelacarsen (20, 40, or 60 mg every 4 weeks; 20 mg every 2 weeks; or 20 mg every week) or subcutaneous saline placebo for 6 to 12 months. Starting from a median baseline Lp(a) level of 204.5 to 246.6 nmol/L for the six dose groups, pelacarsen injections led to significant decreases in Lp(a), ranging from 35% (for the 20 mg every 4 weeks dose) to 80% (for the 20 mg every week dose) compared to the placebo (p-values ranged from 0.003 to <0.001). Importantly, there were no differences in liver and kidney measures or platelet counts between the pelacarsen and placebo arms. The most common adverse events were injection site reactions.
AKCEA-APO(a)-LRx demonstrated that pelacarsen could reduce Lp(a) in a dose-dependent manner, without significant adverse effects, and paved the way for two ongoing phase III trials, Lp(a)HORIZON and TQJ230. In the secondary prevention trial Lp(a)HORIZON, adults with elevated LDL-C levels ≥ 70 mg/dL and established CVD will be randomized to pelacarsen (80 mg monthly) versus placebo. The primary outcome will be the time to occurrence of a major adverse cardiac event, including cardiovascular death, non-fatal MI, non-fatal stroke, and urgent coronary revascularization requiring hospitalization (NCT04023552). These results may shed light on whether a pelacarsen-mediated reduction in Lp(a) translates into improved cardiovascular outcomes.
Meanwhile, the TQJ230 trial is enrolling adults with Lp(a) > 60 mg/dL and prior CVD undergoing lipoprotein apheresis in Germany. TQJ230 will randomize participants to pelacarsen versus placebo, with the primary outcome being the rate of lipoprotein apheresis sessions performed over 52 weeks normalized to the weekly lipoprotein apheresis schedule (NCT05900141). The investigators hope that pelacarsen will demonstrate a greater reduction in the rate of lipoprotein apheresis sessions compared to placebo.
Safety Profile
Efficacy data from phase II trials indicate that pelacarsen has a favorable safety profile, with no significant differences in liver and renal function, platelet counts, or flu-like symptoms compared to placebo. The most common adverse events associated with pelacarsen usage are injection site reactions [152].
6.3. Olpasiran
Olpasiran is a small interfering RNA (siRNA) agent directed towards the LPA gene that prevents assembly of the Lp(a) particle in hepatocytes by preventing translation of the apolipoprotein(a) protein.
The phase II dose-ranging trial OCEAN(a) (2022) evaluated olpasiran in adults with fasting lipoprotein A > 150 nmol/L and a history of ASCVD [151]. Starting from a median Lp(a) level of 260.3 nmol/L, lipoprotein A levels decreased in a dose-dependent manner with the administration of olpasiran: −70.5% with the 10 mg dose, −97.4% with the 75 mg dose, −101.1% with the 225 mg dose administered every 12 weeks, and −100.5% with the 225 mg dose administered every 24 weeks (p < 0.001 for all comparisons with the baseline, placebo-adjusted). Significant adverse events were infrequent and similar across the olpasiran and placebo arms.
Given the success of OCEAN(a), the phase III trial OCEAN(a) Outcomes is now enrolling 6000 participants with a history of ASCVD and Lp(a) > 200 nmol/L to evaluate cardiovascular outcomes—specifically, the time to CHD death, MI, or urgent coronary revascularization (NCT05581303).
Safety Profile
Efficacy data from phase II trials indicate that olpasiran has a favorable side-effect profile, with no differences in overall adverse events, myalgias, renal or liver function, or neuropathy compared to placebo. Local hypersensitivity and injection site reactions are the most commonly reported adverse events associated with olpasiran usage [151].
6.4. SLN360
SLN360 is a siRNA targeting the LPA gene. It is involved in the ongoing randomized phase II trial SLN360, evaluating varying doses (30, 100, 300, and 600 mg) of SLN360 and its effect on LpA levels in adults at high risk of ASCVD events and with Lp(a) > 150 nmol/L (NCT05537571). Preliminary results have demonstrated large reductions in Lp(a), with the 300 mg dose causing a 96% reduction in Lp(a) and the 600 mg dose causing a 98% reduction in Lp(a), as noted at follow-up on day 150 (IQR 89.98% and 97.98%, respectively) [155]. The study is expected to finish in June 2024.
6.5. LY3473329 (Muvalaplin)
The drug LY3473329, or muvalaplin, is a small-molecule inhibitor of Lp(a) formation and siRNA. It is currently the only orally administered Lp(a)-lowering therapy under investigation. The dose-ranging phase II trial KRAKEN is evaluating adults with LpA > 175 nmol/L at high risk of cardiovascular events, with the goal of determining percent change from baseline in Lp(a) (NCT05563246). It has excluded adults with a history of ASCVD, including MI, stroke, or coronary/carotid/peripheral arterial vascularization, focusing only on adults at high risk of these events. This study is expected to be completed in January 2024.
6.6. LY3819469 (Lepodisiran)
LY3819469, or lepodisiran, is another siRNA targeting the Lp(a) gene. It was previously evaluated in a phase I trial, in which it showed reductions in Lp(a) and was well tolerated [156]. It is now in an ongoing dose-ranging randomized phase II trial that will also evaluate adults with LpA > 175 nmol/L at high risk of cardiovascular events, with the aim of determining the percent change in Lp(a) levels by the end of the intervention (between days 60 and 180) (NCT05565742). This study is expected to be completed in October 2024.
7. Conclusions and Future Directions
In this review, we have summarized the current landscape of non-statin therapies and their respective major clinical trials, with the aim of assisting clinicians in understanding recent advances in non-statin cholesterol-lowering therapies for reducing the risk of ASCVD. Significant strides have been made in the development of non-statin LLTs for cardiovascular risk reduction, offering a range of options for patients who have statin intolerance or who need further lipid reduction despite maximally tolerated statin therapy. Several areas warrant further exploration in the coming years.
With regard to triglyceride reduction, conflicting evidence on the cardiovascular benefits in the aforementioned triglyceride-lowering therapies have cast uncertainty on whether triglyceride-lowering, per se, is sufficient for reducing the risk of CVD. In the case of IPE, the benefit appeared to be driven by the achieved EPA levels rather than reduced triglyceride levels. Fibrates have generally not proven to have incremental benefits when added to statin therapy, and this may be related to their effect of converting TRLs to LDL particles, leading to minimal overall changes in ApoB. Ongoing clinical investigations of agents that inhibit Apo-CIII and ANGPTL-3, approaches that were discovered through leveraging human genetic observations, appear to show marked reductions in TRLs and, in the case of ANGPTL-3, also reduce ApoB. Large-scale cardiovascular outcome trials will be needed to determine the clinical efficacy of these approaches.
While the repertoire of LDL-lowering agents has significantly expanded, and the burgeoning repertoire of Lp(a)-lowering agents has shown promising early results, several areas need further investigation. First, clinical guidelines regarding non-statin therapies will depend on the results of ongoing cardiovascular outcomes trials such as OCEAN(a), Lp(a) HORIZON, and ORION-4. Second, long-term safety and outcomes data will need to be tracked. Third, the cost-effectiveness and accessibility of these medications will need to be addressed so that patients who could stand to benefit from these agents will be able to receive them. Fourth, the effects of combination therapies should be explored as the repertoire of non-statin options increasingly expands. Fifth, the interplay between LDL and Lp(a) lowering and that of other risk factors, such as systemic inflammation, requires further investigation. Finally, personalized medicine approaches involving genetic, metabolic, and lifestyle factors, as well as considerations in the cardio–kidney–metabolic space, can be used to optimize therapy selection.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharma3010009/s1, Table S1: Overview of study inclusion and exclusion criteria for non-statin phase III randomized control trials.
Author Contributions
Conceptualization: J.-R.H., E.S.S. and A.G.; Methodology: T.K., B.A., R.S.C. and F.Q.; Software: T.K., J.-R.H., M.S. and B.A.; Validation: T.K. and B.A.; Formal Analysis: T.K., B.A., R.S.C. and F.Q.; Investigation: T.K., B.A., R.S.C., F.Q. and J.-R.H.; Resources: T.K., B.A., R.S.C., M.W.R. and F.Q.; Data Curation: T.K., B.A., R.S.C. and F.Q.; Writing—Original Draft Preparation: T.K., B.A., R.S.C. and F.Q. Writing—Review and Editing: J.-R.H., T.K., E.S.S., A.G., M.S. and F.Q.; Visualization: J.-R.H., M.S. and T.K.; Supervision: J.-R.H., E.S.S. and A.G.; Project Administration: J.-R.H.; Funding Acquisition: none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable (this study did not involve humans or animals).
Informed Consent Statement
Not applicable because this study did not involve humans.
Data Availability Statement
No publicly archived datasets were used and no new data were created during this study.
Acknowledgments
Further interactive educational materials on this topic can be found at www.ASCVD.org, URL accessed on 10 February 2024.
Conflicts of Interest
The authors do not declare any conflicts of interest.
Abbreviations
| ↑ | Increase |
| ↓ | Decrease |
| 1° | Primary |
| 2° | Secondary |
| ACC | American College of Cardiology |
| ACCELERATE | Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition with Evacetrapib in Patients at a High Risk for Vascular Outcomes |
| ACCORD | Action to Control Cardiovascular Risk in Diabetes |
| ACL | Adenosine triphosphate-citrate lyase |
| ACS | Acute coronary syndrome |
| ApoB | Apolipoprotein B |
| ASCVD | Atherosclerotic cardiovascular disease |
| AHA | American Heart Association |
| ALTAIR | The Efficacy of Alirocumab for Thin-cap fIbroatheroma in Patients With Coronary Artery Disease Estimated by Optical Coherence Tomography |
| ARCHITECT | Alirocumab and Plaque Burden In Familial Hypercholesterolaemia |
| BANTING | Evaluation of Evolocumab Efficacy in Diabetic Adults With Hypercholesterolemia/Mixed Dyslipidemia |
| BID | Twice daily |
| BROADWAY | Randomized Study to Evaluate the Effect of Obicetrapib on Top of Maximum Tolerated Lipid-Modifying Therapies |
| BROOKLYN | Evaluate the Effect of Obicetrapib in Patients With HeFH on Top of Maximum Tolerated Lipid-Modifying Therapies |
| CAD | Coronary artery disease |
| CABG | Coronary artery bypass grafting |
| CETP | Cholesteryl ester transfer protein |
| CI | Confidence interval |
| CLEAR | Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen |
| CV | Cardiovascular |
| dal-OUTCOMES | Effects of Dalcetrapib in Patients with a Recent Acute Coronary Syndrome |
| DESCARTES | Durable Effect of PCSK9 Antibody Compared with placebo Study |
| EPA | Eicosapentaenoic acid |
| EVOLVE-MI | Evolocumab Very Early After Myocardial Infarction |
| EWTOPIA 75 | Ezetimibe Lipid-Lowering Trial on Prevention of Atherosclerotic Cardiovascular Disease in 75 or Older |
| F/U | Follow-up |
| FDA | Food and Drug Administration |
| FIELD | Fenofibrate Intervention and Event Lowering in Diabetes |
| FOURIER | Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk |
| FOURIER-OLE | Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk Open-label Extension |
| GAUSS-2 | Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects 2 |
| GAUSS-3 | Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects 3 |
| HDL | High-density lipoprotein |
| HeFH | Heterozygous familial hypercholesterolemia |
| HLD | hyperlipidemia |
| HMG-CoA | 3-Hydroxy-3-methylglutaryl coenzyme A |
| HoFH | Homozygous familial hypercholesterolemia |
| HR | Hazard ratio |
| HUYGENS | High-Resolution Assessment of Coronary Plaques in a Global Evolocumab Randomized Study |
| IDL | Intermediate-density lipoprotein |
| iLLUMINATE | Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia |
| IMPROVE-IT | IMProved Reduction of Outcomes: Vytorin Efficacy International Trial |
| IPE | Icosapent ethyl |
| JELIS | Japan EPA Lipid Study |
| KRAKEN | A Study of LY3473329 in Adult Participants With Elevated Lipoprotein(a) at High Risk for Cardiovascular Events |
| LAPLACE-2 | LDL-C Assessment With PCSK9 Monoclonal Antibody Inhibition Combined With Statin Therapy-2 |
| LDL | Low-density lipoprotein cholesterol |
| LIBerate-HeFH | Long-term efficacy and safety of lerodalcibep in heterozygous familial hypercholesterolaemia |
| LLT | Lipid-lowering therapy |
| Lp(a) | Lipoprotein A |
| Lp(a) HORIZON | A Randomized Double-blind, Placebo-controlled, Multicenter Trial Assessing the Impact of Lipoprotein (a) Lowering With TQJ230 on Major Cardiovascular Events in Patients With Established Cardiovascular Disease |
| MACE | Major adverse cardiovascular event |
| MENDEL-2 | Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL-C in Subjects Currently Not Receiving Drug Therapy for Easing Lipid Levels-2 |
| mg/dL | Milligrams per deciliter |
| MI | Myocardial infarction |
| mRNA | Messenger ribonucleic acid |
| NPC1L1 | Niemann–Pick C1-like 1 |
| NYHA | New York Heart Association |
| OCT | Optical coherence tomography |
| OCEAN (a) | Olpasiran Trials of Cardiovascular Events and Lipoprotein(a) Reduction |
| ODDYSEY FH I | Efficacy and Safety of Alirocumab (SAR236553/REGN727) Versus Placebo on Top of Lipid-Modifying Therapy in Patients With Heterozygous Familial Hypercholesterolemia Not Adequately Controlled With Their Lipid-Modifying Therapy) |
| ODDYSEY FH II | Study of Alirocumab in Patients With heFH Who Are Not Adequately Controlled With Their Lipid-Modifying Therapy |
| ODYSSEY ALTERNATIVE | Study of Alirocumab in Patients With Primary Hypercholesterolemia and Moderate, High, or Very High Cardiovascular Risk, Who Are Intolerant to Statins |
| ODYSSEY CHOICE I | Study to Evaluate the Efficacy and Safety of an Every Four Weeks Treatment Regimen of Alirocumab (REGN727/SAR236553) in Patients With Primary Hypercholesterolemia |
| ODYSSEY CHOICE II | Phase III Study To Evaluate Alirocumab in Patients With Hypercholesterolemia Not Treated With a Statin |
| ODYSSEY COMBO I | Efficacy and Safety of Alirocumab (SAR236553/REGN727) Versus Placebo on Top of Lipid-Modifying Therapy in Patients With High Cardiovascular Risk and Hypercholesterolemia |
| ODYSSEY COMBO II | Efficacy and Safety of Alirocumab (SAR236553/REGN727) Versus Ezetimibe on Top of Statin in High Cardiovascular Risk Patients With Hypercholesterolemia |
| ODYSSEY EAST | Evaluation of Alirocumab Versus Ezetimibe on Top of Statin in Asia in High Cardiovascular Risk Patients With Hypercholesterolemia |
| ODYSSEY HIGH FH | Efficacy and Safety of Alirocumab Versus Placebo on Top of Lipid-Modifying Therapy in Patients With Heterozygous Familial Hypercholesterolemia |
| ODYSSEY JAPAN | Efficacy and Safety Evaluation of Alirocumab in Patients With Heterozygous Familial Hypercholesterolemia or High Cardiovascular Risk Patients With Hypercholesterolemia on Lipid Modifying Therapy |
| ODYSSEY LONG TERM | RCT of alirocumab and reduction of lipids and cardiovascular events |
| ODYSSEY MONO | Efficacy and Safety of Alirocumab Versus Ezetimibe in Patients With Hypercholesterolemia |
| ODYSSEY NIPPON | Efficacy and Safety of Alirocumab in Patients With Hypercholesterolemia Not Adequately Controlled With Non-statin Lipid Modifying Therapy or the Lowest Strength of Statin |
| ODYSSEY OPTIONS I | Study of the Efficacy and Safety of Alirocumab (REGN727/SAR236553) in Combination With Other Lipid-modifying Treatment) |
| ODYSSEY OPTIONS II | Study of the Efficacy and Safety of Alirocumab (REGN727/SAR236553) in Combination With Other Lipid-modifying Treatment |
| ODYSSEY OUTCOMES | Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) |
| OR | Odds ratio |
| ORION-10 | Inclisiran for Participants With Atherosclerotic Cardiovascular Disease and Elevated Low-density Lipoprotein Cholesterol |
| ORION-11 | Inclisiran for Subjects With ASCVD or ASCVD-Risk Equivalents and Elevated Low-density Lipoprotein Cholesterol |
| ORION-3 | Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol |
| ORION-9 | Trial to Evaluate the Effect of Inclisiran Treatment on Low Density Lipoprotein Cholesterol (LDL-C) in Subjects With Heterozygous Familial Hypercholesterolemia (HeFH) |
| OSLER-2 | Open-Label Study of Long-Term Evaluation against LDL Cholesterol 2 |
| OYDSSEY KD | Evaluation of Alirocumab in Addition to Lipid-Modifying Therapy in Patients With High Cardiovascular Risk and Hypercholesterolemia in South Korea and Taiwan |
| PACMAN-AMI | Vascular Effects of Alirocumab in Acute MI-Patients |
| PAV | Percent atheroma volume |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| PCI | Percutaneous coronary intervention |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PRECISE-IVUS | Plaque Regression With Cholesterol Absorption Inhibitor or Synthesis Inhibitor Evaluated by Intravascular Ultrasound |
| PREVAIL | Placebo Controlled, Double Blind, Randomized Cardiovascular Outcome Study to Evaluate the Effect of 10 mg Obicetrapib in Participants With ASCVD Not Adequately Controlled Despite Maximally Tolerated Lipid Modifying Therapies |
| PROFICO | Program to Reduce LDL-C and Cardiovascular Outcomes Following Inhibition of PCSK In Different Populations |
| PROMINENT | Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes |
| RADIOCHOL II | Efficacy and Safety Study of ISIS 301012 as Add-on in Familial Hypercholesterolemic Patients With Coronary Artery Disease |
| RCT | Randomized controlled trial |
| REDUCE-IT | Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial |
| RESPECT-EPA | Randomized trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy-Statin and Eicosapentaenoic Acid |
| REVEAL | Randomized Evaluation of the Effects of Anacetrapib through Lipid-modification |
| RUTHERFORD-2 | Reduction of LDL-C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder Study-2 |
| SPIRE-1, SPIRE-2 | Studies of PCSK9 Inhibition and the Reduction of Vascular Events |
| SPIRE-AI | Efficacy, Safety, Tolerability And Actual Use Study Of Bococizumab And An Autoinjector (Pre-Filled Pen) In Subjects With Hyperlipidemia Or Dyslipidemia |
| SPIRE-FH | A 52 Week Study To Assess The Use Of Bococizumab In Subjects With Heterozygous Familial Hypercholesterolemia |
| SPIRE-HR, SPIRE-LDL | Randomized Clinical Trial Of Bococizumab In Subjects With Hyperlipidemia Or Mixed Dyslipidemia At Risk Of Cardiovascular Events |
| SPIRE-LL | Randomized Clinical Trial of Bococizumab (PF-04950615; RN316) in Subjects With Primary Hyperlipidemia or Mixed Dyslipidemia At Risk Of Cardiovascular Events |
| SPIRE-SI | Randomized Clinical Trial of Bococizumab in Subjects Who Are Intolerant to Statins |
| TC | Total cholesterol |
| TG | Triglycerides |
| TRL | Triglyceride-rich lipoprotein |
| UA | Unstable angina |
| VESALIUS-CV | Effect of Evolocumab in Patients at High Cardiovascular Risk Without Prior Myocardial Infarction or Stroke |
| VLDL | Very-low-density lipoprotein |
| YELLOW III | Effect of Evolocumab on Coronary Plaque Characteristics |
| Shaded rows represent names of trials. | |
References
- GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1603–1658. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Libby, P. Changing concepts of atherogenesis. J. Intern. Med. 2000, 247, 349–358. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis—No longer a theory. Clin. Chem. 2021, 67, 131–142. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e563–e595. [Google Scholar] [CrossRef]
- Abdul-Rahman, T.; Bukhari, S.M.A.; Herrera, E.C.; Awuah, W.A.; Lawrence, J.; de Andrade, H.; Patel, N.; Shah, R.; Shaikh, R.; Capriles, C.A.A.; et al. Lipid Lowering Therapy: An Era Beyond Statins. Curr. Probl. Cardiol. 2022, 47, 101342. [Google Scholar] [CrossRef]
- Beshir, S.A.; Hussain, N.; Elnor, A.A.; Said, A.S.A. Umbrella Review on Non-Statin Lipid-Lowering Therapy. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 437–452. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Writing, C.; Lloyd-Jones, D.M.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Covington, A.M.; DePalma, S.M.; Minissian, M.B.; Orringer, C.E.; Smith, S.C., Jr.; et al. 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 80, 1366–1418. [Google Scholar] [CrossRef]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, J.W.; Weiss, S.R.; Davidson, M.H.; Sprecher, D.L.; Schwartz, S.L.; Lupien, P.J.; Jones, P.H.; Haber, H.E.; Black, D.M. Reduction of LDL cholesterol by 25% to 60% in patients with primary hypercholesterolemia by atorvastatin, a new HMG-CoA reductase inhibitor. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 678–682. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists' (CTT) Collaboration; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Coupland, C.; Brindle, P.; Hippisley-Cox, J. Discontinuation and restarting in patients on statin treatment: Prospective open cohort study using a primary care database. BMJ 2016, 353, i3305. [Google Scholar] [CrossRef]
- Bradley, C.K.; Wang, T.Y.; Li, S.; Robinson, J.G.; Roger, V.L.; Goldberg, A.C.; Virani, S.S.; Louie, M.J.; Lee, L.V.; Peterson, E.D.; et al. Patient-Reported Reasons for Declining or Discontinuing Statin Therapy: Insights From the PALM Registry. J. Am. Heart. Assoc. 2019, 8, e011765. [Google Scholar] [CrossRef]
- Banach, M.; Rizzo, M.; Toth, P.P.; Farnier, M.; Davidson, M.H.; Al-Rasadi, K.; Aronow, W.S.; Athyros, V.; Djuric, D.M.; Ezhov, M.V.; et al. Statin intolerance—An attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch. Med. Sci. 2015, 11, 1–23. [Google Scholar] [CrossRef]
- Wood, F.A.; Howard, J.P.; Finegold, J.A.; Nowbar, A.N.; Thompson, D.M.; Arnold, A.D.; Rajkumar, C.A.; Connolly, S.; Cegla, J.; Stride, C.; et al. N-of-1 Trial of a Statin, Placebo, or No Treatment to Assess Side Effects. N. Engl. J. Med. 2020, 383, 2182–2184. [Google Scholar] [CrossRef]
- Arvanitis, M.; Lowenstein, C.J. Dyslipidemia. Ann. Intern. Med. 2023, 176, ITC81–ITC96. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Ouchi, Y.; Sasaki, J.; Arai, H.; Yokote, K.; Harada, K.; Katayama, Y.; Urabe, T.; Uchida, Y.; Hayashi, M.; Yokota, N.; et al. Ezetimibe Lipid-Lowering Trial on Prevention of Atherosclerotic Cardiovascular Disease in 75 or Older (EWTOPIA 75): A Randomized, Controlled Trial. Circulation 2019, 140, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, K.; Sugiyama, S.; Sumida, H.; Shimomura, H.; Yamashita, T.; Yamanaga, K.; Komura, N.; Sakamoto, K.; Oka, H.; Nakao, K.; et al. Impact of Dual Lipid-Lowering Strategy With Ezetimibe and Atorvastatin on Coronary Plaque Regression in Patients With Percutaneous Coronary Intervention: The Multicenter Randomized Controlled PRECISE-IVUS Trial. J. Am. Coll. Cardiol. 2015, 66, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Banach, M.; Mancini, G.B.J.; Lepor, N.E.; Hanselman, J.C.; Zhao, X.; Leiter, L.A. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: A randomized, placebo-controlled study. Atherosclerosis 2018, 277, 195–203. [Google Scholar] [CrossRef]
- Ray, K.K.; Bays, H.E.; Catapano, A.L.; Lalwani, N.D.; Bloedon, L.T.; Sterling, L.R.; Robinson, P.L.; Ballantyne, C.M.; Trial, C.H. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. N. Engl. J. Med. 2019, 380, 1022–1032. [Google Scholar] [CrossRef]
- Goldberg, A.C.; Leiter, L.A.; Stroes, E.S.G.; Baum, S.J.; Hanselman, J.C.; Bloedon, L.T.; Lalwani, N.D.; Patel, P.M.; Zhao, X.; Duell, P.B. Effect of Bempedoic Acid vs. Placebo Added to Maximally Tolerated Statins on Low-Density Lipoprotein Cholesterol in Patients at High Risk for Cardiovascular Disease: The CLEAR Wisdom Randomized Clinical Trial. JAMA 2019, 322, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Laufs, U.; Banach, M.; Mancini, G.B.J.; Gaudet, D.; Bloedon, L.T.; Sterling, L.R.; Kelly, S.; Stroes, E.S.G. Efficacy and Safety of Bempedoic Acid in Patients With Hypercholesterolemia and Statin Intolerance. J. Am. Heart Assoc. 2019, 8, e011662. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Lincoff, A.M.; Brennan, D.; Ray, K.K.; Mason, D.; Kastelein, J.J.P.; Thompson, P.D.; Libby, P.; Cho, L.; Plutzky, J.; et al. Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N. Engl. J. Med. 2023, 388, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Koren, M.J.; Lundqvist, P.; Bolognese, M.; Neutel, J.M.; Monsalvo, M.L.; Yang, J.; Kim, J.B.; Scott, R.; Wasserman, S.M.; Bays, H.; et al. Anti-PCSK9 monotherapy for hypercholesterolemia: The MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J. Am. Coll. Cardiol. 2014, 63, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Nedergaard, B.S.; Rogers, W.J.; Fialkow, J.; Neutel, J.M.; Ramstad, D.; Somaratne, R.; Legg, J.C.; Nelson, P.; Scott, R.; et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: The LAPLACE-2 randomized clinical trial. JAMA 2014, 311, 1870–1882. [Google Scholar] [CrossRef]
- Stroes, E.; Colquhoun, D.; Sullivan, D.; Civeira, F.; Rosenson, R.S.; Watts, G.F.; Bruckert, E.; Cho, L.; Dent, R.; Knusel, B.; et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: The GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J. Am. Coll. Cardiol. 2014, 63, 2541–2548. [Google Scholar] [CrossRef]
- Blom, D.J.; Hala, T.; Bolognese, M.; Lillestol, M.J.; Toth, P.D.; Burgess, L.; Ceska, R.; Roth, E.; Koren, M.J.; Ballantyne, C.M.; et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N. Engl. J. Med. 2014, 370, 1809–1819. [Google Scholar] [CrossRef]
- Raal, F.J.; Stein, E.A.; Dufour, R.; Turner, T.; Civeira, F.; Burgess, L.; Langslet, G.; Scott, R.; Olsson, A.G.; Sullivan, D.; et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): A randomised, double-blind, placebo-controlled trial. Lancet 2015, 385, 331–340. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Wiviott, S.D.; Raal, F.J.; Blom, D.J.; Robinson, J.; Ballantyne, C.M.; Somaratne, R.; Legg, J.; Wasserman, S.M.; et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1500–1509. [Google Scholar] [CrossRef]
- Nissen, S.E.; Stroes, E.; Dent-Acosta, R.E.; Rosenson, R.S.; Lehman, S.J.; Sattar, N.; Preiss, D.; Bruckert, E.; Ceska, R.; Lepor, N.; et al. Efficacy and Tolerability of Evolocumab vs. Ezetimibe in Patients With Muscle-Related Statin Intolerance: The GAUSS-3 Randomized Clinical Trial. JAMA 2016, 315, 1580–1590. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Daviglus, M.L.; Handelsman, Y.; Pozzilli, P.; Bays, H.; Monsalvo, M.L.; Elliott-Davey, M.; Somaratne, R.; Reaven, P. Efficacy and safety of evolocumab in individuals with type 2 diabetes mellitus: Primary results of the randomised controlled BANTING study. Diabetologia 2019, 62, 948–958. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Giugliano, R.P.; Wiviott, S.D.; Atar, D.; Keech, A.; Kuder, J.F.; Im, K.; Murphy, S.A.; Flores-Arredondo, J.H.; Lopez, J.A.G.; et al. Long-Term Evolocumab in Patients With Established Atherosclerotic Cardiovascular Disease. Circulation 2022, 146, 1109–1119. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Nissen, S.E.; Prati, F.; Windecker, S.; Kataoka, Y.; Puri, R.; Hucko, T.; Kassahun, H.; Liao, J.; Somaratne, R.; et al. Assessing the impact of PCSK9 inhibition on coronary plaque phenotype with optical coherence tomography: Rationale and design of the randomized, placebo-controlled HUYGENS study. Cardiovasc. Diagn. Ther. 2021, 11, 120–129. [Google Scholar] [CrossRef]
- Bohula, E.A.; Marston, N.A.; Ruzza, A.; Murphy, S.A.; De Ferrari, G.M.; Diaz, R.; Leiter, L.A.; Elliott-Davey, M.; Wang, H.; Bhatia, A.K.; et al. Rationale and design of the effect of evolocumab in patients at high cardiovascular risk without prior myocardial infarction or stroke (VESALIUS-CV) trial. Am. Heart. J. 2023, 269, 179–190. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, P.M.; Thompson, P.D.; Cannon, C.P.; Guyton, J.R.; Bergeron, J.; Zieve, F.J.; Bruckert, E.; Jacobson, T.A.; Kopecky, S.L.; Baccara-Dinet, M.T.; et al. Efficacy and safety of alirocumab vs. ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J. Clin. Lipidol. 2015, 9, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, J.J.; Ginsberg, H.N.; Langslet, G.; Hovingh, G.K.; Ceska, R.; Dufour, R.; Blom, D.; Civeira, F.; Krempf, M.; Lorenzato, C.; et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur. Heart J. 2015, 36, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Rader, D.J.; Raal, F.J.; Guyton, J.R.; Baccara-Dinet, M.T.; Lorenzato, C.; Pordy, R.; Stroes, E. Efficacy and Safety of Alirocumab in Patients with Heterozygous Familial Hypercholesterolemia and LDL-C of 160 mg/dL or Higher. Cardiovasc. Drugs. Ther. 2016, 30, 473–483. [Google Scholar] [CrossRef]
- Bays, H.; Gaudet, D.; Weiss, R.; Ruiz, J.L.; Watts, G.F.; Gouni-Berthold, I.; Robinson, J.; Zhao, J.; Hanotin, C.; Donahue, S. Alirocumab as Add-On to Atorvastatin Versus Other Lipid Treatment Strategies: ODYSSEY OPTIONS I Randomized Trial. J. Clin. Endocrinol. Metab. 2015, 100, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Farnier, M.; Jones, P.; Severance, R.; Averna, M.; Steinhagen-Thiessen, E.; Colhoun, H.M.; Du, Y.; Hanotin, C.; Donahue, S. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: The ODYSSEY OPTIONS II randomized trial. Atherosclerosis 2016, 244, 138–146. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Robinson, J.G.; Cannon, C.P.; Lorenzato, C.; Pordy, R.; Chaudhari, U.; Colhoun, H.M. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am. Heart. J. 2015, 169, 906–915. [Google Scholar] [CrossRef]
- Cannon, C.P.; Cariou, B.; Blom, D.; McKenney, J.M.; Lorenzato, C.; Pordy, R.; Chaudhari, U.; Colhoun, H.M.; Investigators, O.C.I. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: The ODYSSEY COMBO II randomized controlled trial. Eur. Heart J. 2015, 36, 1186–1194. [Google Scholar] [CrossRef]
- Roth, E.M.; Moriarty, P.M.; Bergeron, J.; Langslet, G.; Manvelian, G.; Zhao, J.; Baccara-Dinet, M.T.; Rader, D.J.; ODYSSEY CHOICE I investigators. A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add-on to statin: ODYSSEY CHOICE I. Atherosclerosis 2016, 254, 254–262. [Google Scholar] [CrossRef]
- Stroes, E.; Guyton, J.R.; Lepor, N.; Civeira, F.; Gaudet, D.; Watts, G.F.; Baccara-Dinet, M.T.; Lecorps, G.; Manvelian, G.; Farnier, M.; et al. Efficacy and Safety of Alirocumab 150 mg Every 4 Weeks in Patients with Hypercholesterolemia Not on Statin Therapy: The ODYSSEY CHOICE II Study. J. Am. Heart. Assoc. 2016, 5, e003421. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, T.; Kobayashi, M.; Tasaki, H.; Yagyu, H.; Higashikata, T.; Takagi, Y.; Uno, K.; Baccara-Dinet, M.T.; Nohara, A. Efficacy and Safety of Alirocumab in Japanese Patients With Heterozygous Familial Hypercholesterolemia or at High Cardiovascular Risk With Hypercholesterolemia Not Adequately Controlled With Statins—ODYSSEY JAPAN Randomized Controlled Trial. Circ. J. 2016, 80, 1980–1987. [Google Scholar] [CrossRef]
- Koh, K.K.; Nam, C.W.; Chao, T.H.; Liu, M.E.; Wu, C.J.; Kim, D.S.; Kim, C.J.; Li, I.; Li, J.; Baccara-Dinet, M.T.; et al. A randomized trial evaluating the efficacy and safety of alirocumab in South Korea and Taiwan (ODYSSEY KT). J. Clin. Lipidol. 2018, 12, 162–172.e166. [Google Scholar] [CrossRef]
- Teramoto, T.; Kiyosue, A.; Ishigaki, Y.; Harada-Shiba, M.; Kawabata, Y.; Ozaki, A.; Baccara-Dinet, M.T.; Sata, M. Efficacy and safety of alirocumab 150mg every 4 weeks in hypercholesterolemic patients on non-statin lipid-lowering therapy or lowest strength dose of statin: ODYSSEY NIPPON. J. Cardiol 2019, 73, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, J.; Chopra, V.K.; Zhang, S.; Su, G.; Ma, C.; Huang, Z.; Ma, Y.; Yao, Z.; Yuan, Z.; et al. ODYSSEY EAST: Alirocumab efficacy and safety vs. ezetimibe in high cardiovascular risk patients with hypercholesterolemia and on maximally tolerated statin in China, India, and Thailand. J. Clin. Lipidol. 2020, 14, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Perez de Isla, L.; Diaz-Diaz, J.L.; Romero, M.J.; Muniz-Grijalvo, O.; Mediavilla, J.D.; Argueso, R.; Sanchez Munoz-Torrero, J.F.; Rubio, P.; Alvarez-Banos, P.; Ponte, P.; et al. Alirocumab and Coronary Atherosclerosis in Asymptomatic Patients with Familial Hypercholesterolemia: The ARCHITECT Study. Circulation 2023, 147, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Sugizaki, Y.; Otake, H.; Kawamori, H.; Toba, T.; Nagano, Y.; Tsukiyama, Y.; Yanaka, K.I.; Yamamoto, H.; Nagasawa, A.; Onishi, H.; et al. Adding Alirocumab to Rosuvastatin Helps Reduce the Vulnerability of Thin-Cap Fibroatheroma: An ALTAIR Trial Report. JACC Cardiovasc. Imaging 2020, 13, 1452–1454. [Google Scholar] [CrossRef]
- Raber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Haner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.J.; Ondracek, A.S.; et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781. [Google Scholar] [CrossRef]
- Ako, J.; Hibi, K.; Tsujita, K.; Hiro, T.; Morino, Y.; Kozuma, K.; Shinke, T.; Otake, H.; Uno, K.; Louie, M.J.; et al. Effect of Alirocumab on Coronary Atheroma Volume in Japanese Patients With Acute Coronary Syndrome—The ODYSSEY J-IVUS Trial. Circ. J. 2019, 83, 2025–2033. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Z.J.; Ma, X.T.; Shen, H.; Yang, L.X.; Zhou, Y.J. Effect of alirocumab on coronary plaque in patients with coronary artery disease assessed by optical coherence tomography. Lipids Health Dis. 2021, 20, 106. [Google Scholar] [CrossRef]
- Ridker, P.M.; Tardif, J.C.; Amarenco, P.; Duggan, W.; Glynn, R.J.; Jukema, J.W.; Kastelein, J.J.P.; Kim, A.M.; Koenig, W.; Nissen, S.; et al. Lipid-Reduction Variability and Antidrug-Antibody Formation with Bococizumab. N. Engl. J. Med. 2017, 376, 1517–1526. [Google Scholar] [CrossRef]
- Ridker, P.M.; Revkin, J.; Amarenco, P.; Brunell, R.; Curto, M.; Civeira, F.; Flather, M.; Glynn, R.J.; Gregoire, J.; Jukema, J.W.; et al. Cardiovascular Efficacy and Safety of Bococizumab in High-Risk Patients. N. Engl. J. Med. 2017, 376, 1527–1539. [Google Scholar] [CrossRef]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.J.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Troquay, R.P.T.; Visseren, F.L.J.; Leiter, L.A.; Scott Wright, R.; Vikarunnessa, S.; Talloczy, Z.; Zang, X.; Maheux, P.; Lesogor, A.; et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): Results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. 2023, 11, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.A.; Dufour, R.; Gagne, C.; Gaudet, D.; East, C.; Donovan, J.M.; Chin, W.; Tribble, D.L.; McGowan, M. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: Results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation 2012, 126, 2283–2292. [Google Scholar] [CrossRef]
- McGowan, M.P.; Tardif, J.C.; Ceska, R.; Burgess, L.J.; Soran, H.; Gouni-Berthold, I.; Wagener, G.; Chasan-Taber, S. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS ONE 2012, 7, e49006. [Google Scholar] [CrossRef]
- Thomas, G.S.; Cromwell, W.C.; Ali, S.; Chin, W.; Flaim, J.D.; Davidson, M. Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: A randomized, double-blind, placebo-controlled trial. J. Am. Coll. Cardiol. 2013, 62, 2178–2184. [Google Scholar] [CrossRef]
- Raal, F.; Fourie, N.; Scott, R.; Blom, D.; De Vries Basson, M.; Kayikcioglu, M.; Caldwell, K.; Kallend, D.; Stein, E.; Investigators, L.I.-H. Long-term efficacy and safety of lerodalcibep in heterozygous familial hypercholesterolaemia: The LIBerate-HeFH trial. Eur. Heart J. 2023, 44, 4272–4280. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Olsson, A.G.; Abt, M.; Ballantyne, C.M.; Barter, P.J.; Brumm, J.; Chaitman, B.R.; Holme, I.M.; Kallend, D.; Leiter, L.A.; et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012, 367, 2089–2099. [Google Scholar] [CrossRef]
- Group, H.T.R.C.; Bowman, L.; Hopewell, J.C.; Chen, F.; Wallendszus, K.; Stevens, W.; Collins, R.; Wiviott, S.D.; Cannon, C.P.; Braunwald, E.; et al. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N. Engl. J. Med. 2017, 377, 1217–1227. [Google Scholar] [CrossRef]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.C.; Waters, D.D.; et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Nicholls, S.J.; Riesmeyer, J.S.; Barter, P.J.; Brewer, H.B.; Fox, K.A.A.; Gibson, C.M.; Granger, C.; Menon, V.; Montalescot, G.; et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N. Engl. J. Med. 2017, 376, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Ditmarsch, M.; Kastelein, J.J.; Rigby, S.P.; Kling, D.; Curcio, D.L.; Alp, N.J.; Davidson, M.H. Lipid lowering effects of the CETP inhibitor obicetrapib in combination with high-intensity statins: A randomized phase 2 trial. Nat. Med. 2022, 28, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Nishizaki, Y.; Miyauchi, K.; Iwata, H.; Inoue, T.; Hirayama, A.; Kimura, K.; Ozaki, Y.; Murohara, T.; Ueshima, K.; Kuwabara, Y.; et al. Study protocol and baseline characteristics of Randomized trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy-Statin and Eicosapentaenoic Acid: RESPECT-EPA, the combination of a randomized control trial and an observational biomarker study. Am. Heart. J. 2023, 257, 1–8. [Google Scholar] [CrossRef]
- Das Pradhan, A.; Glynn, R.J.; Fruchart, J.C.; MacFadyen, J.G.; Zaharris, E.S.; Everett, B.M.; Campbell, S.E.; Oshima, R.; Amarenco, P.; Blom, D.J.; et al. Triglyceride Lowering with Pemafibrate to Reduce Cardiovascular Risk. N. Engl. J. Med. 2022, 387, 1923–1934. [Google Scholar] [CrossRef]
- Group, A.S.; Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R., 3rd; Leiter, L.A.; Linz, P.; Friedewald, W.T.; Buse, J.B.; Gerstein, H.C.; et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar] [CrossRef]
- Keech, A.; Simes, R.J.; Barter, P.; Best, J.; Scott, R.; Taskinen, M.R.; Forder, P.; Pillai, A.; Davis, T.; Glasziou, P.; et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005, 366, 1849–1861. [Google Scholar] [CrossRef]
- Patel, J.; Sheehan, V.; Gurk-Turner, C. Ezetimibe (Zetia): A new type of lipid-lowering agent. In Baylor University Medical Center Proceedings; Taylor & Francis: Abingdon, UK, 2003; Volume 16, pp. 354–358. [Google Scholar] [CrossRef]
- Hammersley, D.; Signy, M. Ezetimibe: An update on its clinical usefulness in specific patient groups. Ther. Adv. Chronic. Dis. 2017, 8, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Mitka, M. Ezetimibe prescribing fails to keep up with evidence. JAMA 2014, 311, 1279–1280. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Hsu, A.; Wolski, K.; Hu, B.; Bayturan, O.; Lavoie, A.; Uno, K.; Tuzcu, E.M.; Nissen, S.E. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J. Am. Coll. Cardiol. 2010, 55, 2399–2407. [Google Scholar] [CrossRef]
- Organon. Zetia (Ezetimibe)–U.S. Food and Drug Website. Available online: https://www.organon.com/product/usa/pi_circulars/z/zetia/zetia_pi.pdf (accessed on 30 December 2023).
- Lewek, J.; Banach, M. Dyslipidemia Management in Pregnancy: Why Is It not Covered in the Guidelines? Curr. Atheroscler. Rep. 2022, 24, 547–556. [Google Scholar] [CrossRef]
- Sasidharan, A.; Bagepally, B.S.; Kumar, S.S.; Jagadeesh, K.V.; Natarajan, M. Cost-effectiveness of Ezetimibe plus statin lipid-lowering therapy: A systematic review and meta-analysis of cost-utility studies. PLoS ONE 2022, 17, e0264563. [Google Scholar] [CrossRef]
- Schlackow, I.; Kent, S.; Herrington, W.; Emberson, J.; Haynes, R.; Reith, C.; Collins, R.; Landray, M.J.; Gray, A.; Baigent, C.; et al. Cost-effectiveness of lipid lowering with statins and ezetimibe in chronic kidney disease. Kidney Int. 2019, 96, 170–179. [Google Scholar] [CrossRef]
- Yang, H.; Li, N.; Zhou, Y.; Xiao, Z.; Tian, H.; Hu, M.; Li, S. Cost-Effectiveness Analysis of Ezetimibe as the Add-on Treatment to Moderate-Dose Rosuvastatin versus High-Dose Rosuvastatin in the Secondary Prevention of Cardiovascular Diseases in China: A Markov Model Analysis. Drug Des. Dev. Ther. 2020, 14, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Masana Marin, L.; Plana Gil, N. Bempedoic acid. Mechanism of action and pharmacokinetic and pharmacodynamic properties. Clin. Investig. Arterioscler. 2021, 33 (Suppl. 1), 53–57. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Laufs, U.; Ray, K.K.; Leiter, L.A.; Bays, H.E.; Goldberg, A.C.; Stroes, E.S.; MacDougall, D.; Zhao, X.; Catapano, A.L. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur. J. Prev. Cardiol. 2020, 27, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Nexlizethcp. Nexletol (Bempedoic Acid)–U.S. Food and Drug Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211616s000lbl.pdf (accessed on 30 December 2023).
- Banach, M.; Duell, P.B.; Gotto, A.M., Jr.; Laufs, U.; Leiter, L.A.; Mancini, G.B.J.; Ray, K.K.; Flaim, J.; Ye, Z.; Catapano, A.L. Association of Bempedoic Acid Administration With Atherogenic Lipid Levels in Phase 3 Randomized Clinical Trials of Patients With Hypercholesterolemia. JAMA Cardiol. 2020, 5, 1124–1135. [Google Scholar] [CrossRef]
- Agboola, F.; Lin, G.A.; Kazi, D.S.; McKenna, A.; Pearson, S.D. The effectiveness and value of bempedoic acid and inclisiran for heterozygous familial hypercholesterolemia and secondary prevention of ASCVD. J. Manag. Care Spec. Pharm. 2021, 27, 961–966. [Google Scholar] [CrossRef]
- ICER. Icer Publishes Evidence Report on Therapies for High Cholesterol. Available online: https://icer.org/news-insights/press-releases/icer-publishes-evidence-report-on-therapies-for-high-cholesterol/ (accessed on 11 February 2024).
- Benn, M.; Nordestgaard, B.G.; Grande, P.; Schnohr, P.; Tybjaerg-Hansen, A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J. Am. Coll. Cardiol. 2010, 55, 2833–2842. [Google Scholar] [CrossRef]
- Catapano, A.L.; Papadopoulos, N. The safety of therapeutic monoclonal antibodies: Implications for cardiovascular disease and targeting the PCSK9 pathway. Atherosclerosis 2013, 228, 18–28. [Google Scholar] [CrossRef]
- Koba, S.; Inoue, I.; Cyrille, M.; Lu, C.; Inomata, H.; Shimauchi, J.; Kajinami, K. Evolocumab vs. Ezetimibe in Statin-Intolerant Hyperlipidemic Japanese Patients: Phase 3 GAUSS-4 Trial. J. Atheroscler. Thromb. 2020, 27, 471–484. [Google Scholar] [CrossRef]
- Hirayama, A.; Honarpour, N.; Yoshida, M.; Yamashita, S.; Huang, F.; Wasserman, S.M.; Teramoto, T. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin-treated Japanese patients at high cardiovascular risk--primary results from the phase 2 YUKAWA study. Circ. J. 2014, 78, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Joshi, R.; Stephen Djedjos, C.; Legg, J.; Elliott, M.; Geller, M.; Meyer, D.; Somaratne, R.; Recknor, C.; Weiss, R. Evolocumab lowers LDL-C safely and effectively when self-administered in the at-home setting. Springerplus 2016, 5, 300. [Google Scholar] [CrossRef] [PubMed]
- Erviti, J.; Wright, J.; Bassett, K.; Ben-Eltriki, M.; Jauca, C.; Saiz, L.C.; Leache, L.; Gutierrez-Valencia, M.; Perry, T.L. Restoring mortality data in the FOURIER cardiovascular outcomes trial of evolocumab in patients with cardiovascular disease: A reanalysis based on regulatory data. BMJ Open 2022, 12, e060172. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016, 316, 2373–2384. [Google Scholar] [CrossRef]
- Roth, E.M.; McKenney, J.M. ODYSSEY MONO: Effect of alirocumab 75 mg subcutaneously every 2 weeks as monotherapy versus ezetimibe over 24 weeks. Future Cardiol. 2015, 11, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Szarek, M.; White, H.D.; Schwartz, G.G.; Alings, M.; Bhatt, D.L.; Bittner, V.A.; Chiang, C.E.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; et al. Alirocumab Reduces Total Nonfatal Cardiovascular and Fatal Events: The ODYSSEY OUTCOMES Trial. J. Am. Coll. Cardiol. 2019, 73, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Amgen. Repatha (Evolocumab)–U.S. Food and Drug Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125522orig2s000lbl.pdf (accessed on 30 December 2023).
- Sanofi, R.A. Praluent (Alirocumab)–U.S. Food and Drug Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125559s029s030lbl.pdf (accessed on 30 December 2023).
- Ference, B.A.; Robinson, J.G.; Brook, R.D.; Catapano, A.L.; Chapman, M.J.; Neff, D.R.; Voros, S.; Giugliano, R.P.; Davey Smith, G.; Fazio, S.; et al. Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. N. Engl. J. Med. 2016, 375, 2144–2153. [Google Scholar] [CrossRef]
- Guedeney, P.; Giustino, G.; Sorrentino, S.; Claessen, B.E.; Camaj, A.; Kalkman, D.N.; Vogel, B.; Sartori, S.; De Rosa, S.; Baber, U.; et al. Efficacy and safety of alirocumab and evolocumab: A systematic review and meta-analysis of randomized controlled trials. Eur. Heart J. 2022, 43, e17–e25. [Google Scholar] [CrossRef]
- Choi, H.D.; Kim, J.H. An Updated Meta-Analysis for Safety Evaluation of Alirocumab and Evolocumab as PCSK9 Inhibitors. Cardiovasc. Ther. 2023, 2023, 7362551. [Google Scholar] [CrossRef]
- Gurgoze, M.T.; Muller-Hansma, A.H.G.; Schreuder, M.M.; Galema-Boers, A.M.H.; Boersma, E.; Roeters van Lennep, J.E. Adverse Events Associated With PCSK9 Inhibitors: A Real-World Experience. Clin. Pharmacol. Ther. 2019, 105, 496–504. [Google Scholar] [CrossRef]
- Fonarow, G.C.; van Hout, B.; Villa, G.; Arellano, J.; Lindgren, P. Updated Cost-effectiveness Analysis of Evolocumab in Patients With Very High-risk Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2019, 4, 691–695. [Google Scholar] [CrossRef]
- Michaeli, D.T.; Michaeli, J.C.; Boch, T.; Michaeli, T. Cost-Effectiveness of Icosapent Ethyl, Evolocumab, Alirocumab, Ezetimibe, or Fenofibrate in Combination with Statins Compared to Statin Monotherapy. Clin. Drug. Investig. 2022, 42, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Migliorati, J.M.; Jin, J.; Zhong, X.B. siRNA drug Leqvio (inclisiran) to lower cholesterol. Trends Pharmacol. Sci. 2022, 43, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Albosta, M.S.; Grant, J.K.; Taub, P.; Blumenthal, R.S.; Martin, S.S.; Michos, E.D. Inclisiran: A New Strategy for LDL-C Lowering and Prevention of Atherosclerotic Cardiovascular Disease. Vasc. Health Risk Manag. 2023, 19, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Novartis. Leqvio (Inclisiran)–U.S. Food and Drug Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/214012s009lbl.pdf (accessed on 30 December 2023).
- Cicero, A.F.; Fogacci, F.; Zambon, A.; Toth, P.P.; Borghi, C. Efficacy and safety of inclisiran a newly approved FDA drug: A systematic review and pooled analysis of available clinical studies. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 13, 100127. [Google Scholar] [CrossRef]
- Desai, N.R.; Campbell, C.; Electricwala, B.; Petrou, M.; Trueman, D.; Woodcock, F.; Cristino, J. Cost Effectiveness of Inclisiran in Atherosclerotic Cardiovascular Patients with Elevated Low-Density Lipoprotein Cholesterol Despite Statin Use: A Threshold Analysis. Am. J. Cardiovasc. Drugs 2022, 22, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, A.; Jones, P.; Nambi, V. The role of antisense oligonucleotide therapy in patients with familial hypercholesterolemia: Risks, benefits, and management recommendations. Curr. Atheroscler. Rep. 2015, 17, 467. [Google Scholar] [CrossRef] [PubMed]
- Parham, J.S.; Goldberg, A.C. Mipomersen and its use in familial hypercholesterolemia. Expert Opin Pharmacother. 2019, 20, 127–131. [Google Scholar] [CrossRef]
- Fogacci, F.; Ferri, N.; Toth, P.P.; Ruscica, M.; Corsini, A.; Cicero, A.F.G. Efficacy and Safety of Mipomersen: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Drugs 2019, 79, 751–766. [Google Scholar] [CrossRef]
- Wong, E.; Goldberg, T. Mipomersen (kynamro): A novel antisense oligonucleotide inhibitor for the management of homozygous familial hypercholesterolemia. Pharm. Ther. 2014, 39, 119–122. [Google Scholar]
- Inazu, A.; Brown, M.L.; Hesler, C.B.; Agellon, L.B.; Koizumi, J.; Takata, K.; Maruhama, Y.; Mabuchi, H.; Tall, A.R. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N. Engl. J. Med. 1990, 323, 1234–1238. [Google Scholar] [CrossRef]
- Armitage, J.; Holmes, M.V.; Preiss, D. Cholesteryl Ester Transfer Protein Inhibition for Preventing Cardiovascular Events: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 477–487. [Google Scholar] [CrossRef]
- Tall, A.R.; Rader, D.J. Trials and Tribulations of CETP Inhibitors. Circ. Res. 2018, 122, 106–112. [Google Scholar] [CrossRef]
- Davidson, M.; Liu, S.X.; Barter, P.; Brinton, E.A.; Cannon, C.P.; Gotto, A.M., Jr.; Leary, E.T.; Shah, S.; Stepanavage, M.; Mitchel, Y.; et al. Measurement of LDL-C after treatment with the CETP inhibitor anacetrapib. J. Lipid Res. 2013, 54, 467–472. [Google Scholar] [CrossRef]
- Khoueiry, G.; Abi Rafeh, N.; Sullivan, E.; Saiful, F.; Jaffery, Z.; Kenigsberg, D.N.; Krishnan, S.C.; Khanal, S.; Bekheit, S.; Kowalski, M. Do omega-3 polyunsaturated fatty acids reduce risk of sudden cardiac death and ventricular arrhythmias? A meta-analysis of randomized trials. Heart Lung 2013, 42, 251–256. [Google Scholar] [CrossRef]
- Harris, W.S.; Tintle, N.L.; Imamura, F.; Qian, F.; Korat, A.V.A.; Marklund, M.; Djousse, L.; Bassett, J.K.; Carmichael, P.H.; Chen, Y.Y.; et al. Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat. Commun. 2021, 12, 2329. [Google Scholar] [CrossRef]
- Shen, S.; Gong, C.; Jin, K.; Zhou, L.; Xiao, Y.; Ma, L. Omega-3 Fatty Acid Supplementation and Coronary Heart Disease Risks: A Meta-Analysis of Randomized Controlled Clinical Trials. Front. Nutr. 2022, 9, 809311. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, J.; Badimon, L. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial–Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 1999, 354, 447–455. [Google Scholar]
- Aung, T.; Halsey, J.; Kromhout, D.; Gerstein, H.C.; Marchioli, R.; Tavazzi, L.; Geleijnse, J.M.; Rauch, B.; Ness, A.; Galan, P.; et al. Associations of Omega-3 Fatty Acid Supplement Use With Cardiovascular Disease Risks: Meta-analysis of 10 Trials Involving 77 917 Individuals. JAMA Cardiol. 2018, 3, 225–234. [Google Scholar] [CrossRef]
- Bowman, L.; Mafham, M.; Stevens, W.; Haynes, R.; Aung, T.; Chen, F.; Buck, G.; Collins, R.; Armitage, J.; Group, A.S.C. ASCEND: A Study of Cardiovascular Events iN Diabetes: Characteristics of a randomized trial of aspirin and of omega-3 fatty acid supplementation in 15,480 people with diabetes. Am. Heart J. 2018, 198, 135–144. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; MacFadyen, J.; Glynn, R.J.; Jiao, L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Tardif, J.C.; et al. Effects of Randomized Treatment With Icosapent Ethyl and a Mineral Oil Comparator on Interleukin-1beta, Interleukin-6, C-Reactive Protein, Oxidized Low-Density Lipoprotein Cholesterol, Homocysteine, Lipoprotein(a), and Lipoprotein-Associated Phospholipase A2: A REDUCE-IT Biomarker Substudy. Circulation 2022, 146, 372–379. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs. Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Gupta, K.; Hirsch, J.R.; Kalsi, J.; Patel, V.; Gad, M.M.; Virani, S.S. Highlights of Cardiovascular Disease Prevention Studies Presented at the 2022 American Heart Association Scientific Sessions. Curr. Atheroscler. Rep. 2023, 25, 31–41. [Google Scholar] [CrossRef]
- Amarin. Vascepa (Icosapent Ethyl)—U.S. Food and Drug Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202057s035lbl.pdf (accessed on 30 December 2023).
- Pharma, W. Lovaza (Omega-3-Acid Ethyl Esters)—U.S. Food and Drug Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021654s043lbl.pdf (accessed on 30 December 2023).
- Virani, S.S.; Morris, P.B.; Agarwala, A.; Ballantyne, C.M.; Birtcher, K.K.; Kris-Etherton, P.M.; Ladden-Stirling, A.B.; Miller, M.; Orringer, C.E.; Stone, N.J. 2021 ACC Expert Consensus Decision Pathway on the Management of ASCVD Risk Reduction in Patients With Persistent Hypertriglyceridemia: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 78, 960–993. [Google Scholar] [CrossRef]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef]
- Qian, F.; Tintle, N.; Jensen, P.N.; Lemaitre, R.N.; Imamura, F.; Feldreich, T.R.; Nomura, S.O.; Guan, W.; Laguzzi, F.; Kim, E.; et al. Omega-3 Fatty Acid Biomarkers and Incident Atrial Fibrillation. J. Am. Coll. Cardiol. 2023, 82, 336–349. [Google Scholar] [CrossRef]
- Schmier, J.K.; Rachman, N.J.; Halpern, M.T. The cost-effectiveness of omega-3 supplements for prevention of secondary coronary events. Manag. Care 2006, 15, 43–50. [Google Scholar]
- Michaeli, D.T.; Michaeli, J.C.; Boch, T.; Michaeli, T. Cost-Effectiveness of Lipid-Lowering Therapies for Cardiovascular Prevention in Germany. Cardiovasc. Drugs Ther. 2023, 37, 683–694. [Google Scholar] [CrossRef]
- Gao, L.; Moodie, M.; Li, S.C. The cost-effectiveness of omega-3 polyunsaturated fatty acids — The Australian healthcare perspective. Eur. J. Intern. Med. 2019, 67, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lachaine, J.; Charron, J.N.; Gregoire, J.C.; Hegele, R.A.; Leiter, L.A. Cost-Effectiveness of Icosapent Ethyl (IPE) for the Reduction of the Risk of Ischemic Cardiovascular Events in Canada. Clinicoecon Outcomes Res. 2023, 15, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Rubins, H.B.; Robins, S.J.; Collins, D.; Fye, C.L.; Anderson, J.W.; Elam, M.B.; Faas, F.H.; Linares, E.; Schaefer, E.J.; Schectman, G.; et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N. Engl. J. Med. 1999, 341, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Tenkanen, L.; Manttari, M.; Kovanen, P.T.; Virkkunen, H.; Manninen, V. Gemfibrozil in the treatment of dyslipidemia: An 18-year mortality follow-up of the Helsinki Heart Study. Arch. Intern. Med. 2006, 166, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Frick, M.H.; Elo, O.; Haapa, K.; Heinonen, O.P.; Heinsalmi, P.; Helo, P.; Huttunen, J.K.; Kaitaniemi, P.; Koskinen, P.; Manninen, V.; et al. Helsinki Heart Study: Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N. Engl. J. Med. 1987, 317, 1237–1245. [Google Scholar] [CrossRef]
- Tricor. Tricor (Fenofibrate)—U.S. Food and Drug Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021656s029lbl.pdf (accessed on 30 December 2023).
- Hay, J.W.; Sterling, K.L. Cost effectiveness of treating low HDL-cholesterol in the primary prevention of coronary heart disease. Pharmacoeconomics 2005, 23, 133–141. [Google Scholar] [CrossRef]
- Abushanab, D.; Al-Badriyeh, D.; Marquina, C.; Bailey, C.; Jaam, M.; Liew, D.; Ademi, Z. A Systematic Review of Cost-Effectiveness of Non-Statin Lipid-Lowering Drugs for Primary and Secondary Prevention of Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus. Curr. Probl. Cardiol. 2023, 48, 101211. [Google Scholar] [CrossRef]
- Maranhao, R.C.; Carvalho, P.O.; Strunz, C.C.; Pileggi, F. Lipoprotein (a): Structure, pathophysiology and clinical implications. Arq. Bras. Cardiol. 2014, 103, 76–84. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L.; et al. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e48–e60. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Rosenson, R.S.; Gencer, B.; Lopez, J.A.G.; Lepor, N.E.; Baum, S.J.; Stout, E.; Gaudet, D.; Knusel, B.; Kuder, J.F.; et al. Small Interfering RNA to Reduce Lipoprotein(a) in Cardiovascular Disease. N. Engl. J. Med. 2022, 387, 1855–1864. [Google Scholar] [CrossRef]
- Tsimikas, S.; Karwatowska-Prokopczuk, E.; Gouni-Berthold, I.; Tardif, J.C.; Baum, S.J.; Steinhagen-Thiessen, E.; Shapiro, M.D.; Stroes, E.S.; Moriarty, P.M.; Nordestgaard, B.G.; et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N. Engl. J. Med. 2020, 382, 244–255. [Google Scholar] [CrossRef]
- Thompson, G.; Parhofer, K.G. Current Role of Lipoprotein Apheresis. Curr. Atheroscler. Rep. 2019, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Prado, R.; Perez-Gomez, M.V.; Ortiz, A. Pelacarsen for lowering lipoprotein(a): Implications for patients with chronic kidney disease. Clin. Kidney J. 2020, 13, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Rambaran, C.; Wilson, R.J.; Swerdlow, D.I.; Campion, G.V.; Scrimgeour, A.C.; Nissen, S.E. Time Averaged Lipoprotein (a) Reduction With Sln360, a Novel Sirna Targeting Lp (a,) in Healthy Adults With Elevated Lp(a). Circulation 2022, 146, A10469. [Google Scholar] [CrossRef]
- Nissen, S.E.; Linnebjerg, H.; Shen, X.; Wolski, K.; Ma, X.; Lim, S.; Michael, L.F.; Ruotolo, G.; Gribble, G.; Navar, A.M.; et al. Lepodisiran, an Extended-Duration Short Interfering RNA Targeting Lipoprotein(a): A Randomized Dose-Ascending Clinical Trial. JAMA 2023, 330, 2075–2083. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).