Opioid Prescribing for Noncancer Patients—Issues of Drug Therapy Safety: Results from a German Study Based on Routine Data
Abstract
1. Introduction
2. Materials and Methods
- Anxiolytics/hypnotics/sedatives N05B and N05C without herbal ingredients;
- Laxatives (drugs treating constipation) A06A;
- Drugs to control the antiemetic effect of opioids: antiemetic drugs (A04AA, A04AB, A04AD); propulsives (A03FA) and antipsychotics: haloperidol, chlorpromazine and levopromazine (N05AD01, N05AA01/-02), dexamethasone (H02AB02/-BX02), olanzapine (N05AH03/-53), and lorazepam (N05BA06/-56).
3. Results
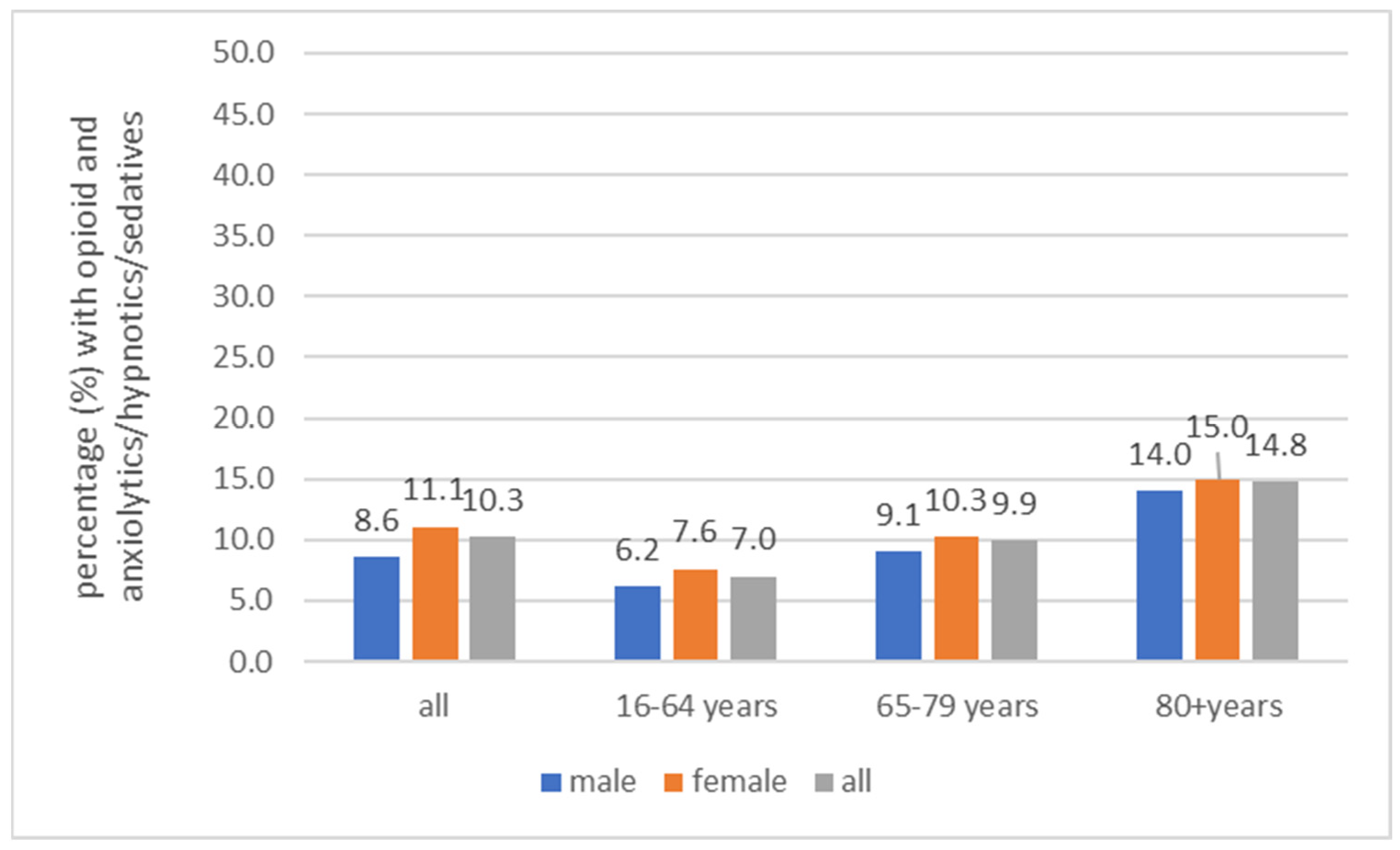
3.1. Anxiolytics/Hypnotics/Sedatives
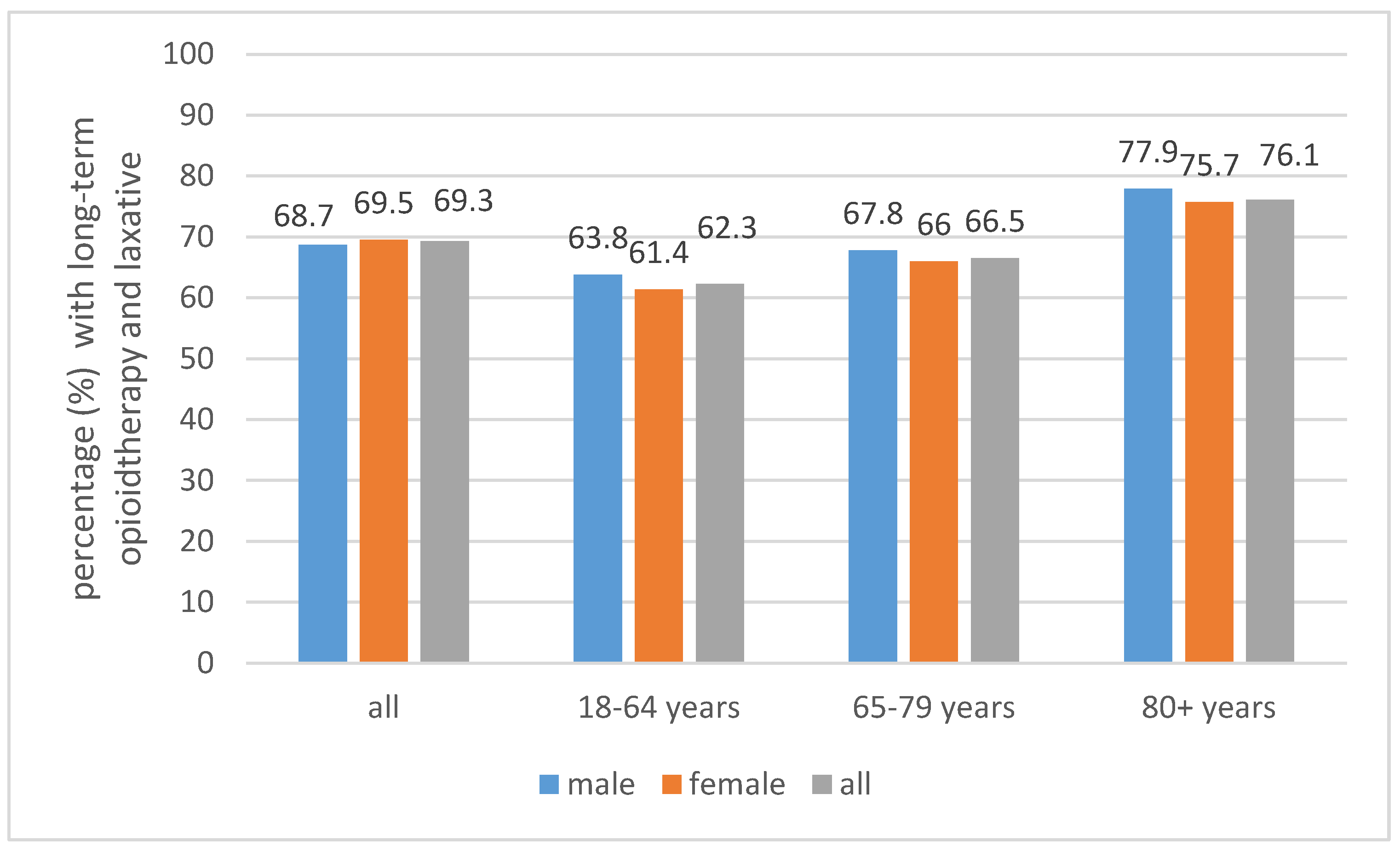
3.2. Laxatives
3.3. Antiemetic Drugs
3.4. Pretreatment with Non-Opioids
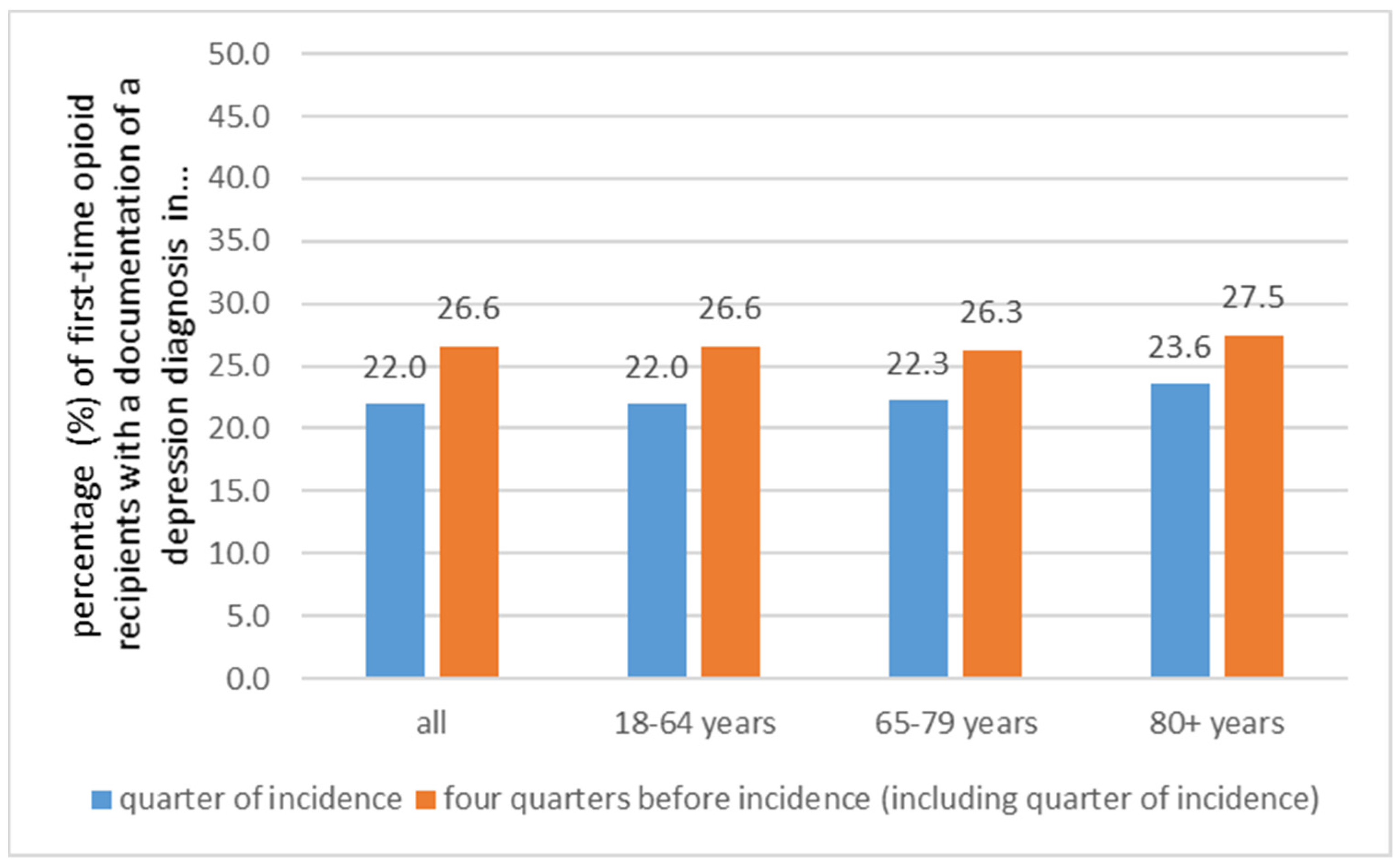
3.5. Depression
4. Discussion
4.1. Comparison with Other Studies
4.2. Strengths and Limitations
4.3. Implications for Policy, Practice, and Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gustafsson, M.; Matos, C.; Joaquim, J.; Scholl, J.; van Hunsel, F. Adverse Drug Reactions to Opioids: A Study in a National Pharmacovigilance Database. Drug Saf. 2023, 46, 1133–1148. [Google Scholar] [CrossRef]
- Bedson, J.; Chen, Y.; Ashworth, J.; Hayward, R.A.; Dunn, K.M.; Jordan, K.P. Risk of adverse events in patients prescribed long-term opioids: A cohort study in the UK Clinical Practice Research Datalink. Eur. J. Pain 2019, 23, 908–922. [Google Scholar] [CrossRef]
- Lalic, S.; Ilomäki, J.; Bell, J.S.; Korhonen, M.J.; Gisev, N. Prevalence and incidence of prescription opioid analgesic use in Australia. Br. J. Clin. Pharmacol. 2019, 85, 202–215. [Google Scholar] [CrossRef]
- Hamina, A.; Muller, A.E.; Clausen, T.; Skurtveit, S.; Hesse, M.; Tjagvad, C.; Thylstrup, B.; Odsbu, I.; Zoega, H.; Jónsdóttir, H.L.; et al. Prescription opioids among older adults: Ten years of data across five countries. BMC Geriatr. 2022, 22, 429. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, N.; Funk, M.J.; Proescholdbell, S.; Hirsch, A.; Ribisl, K.M.; Marshall, S. Cohort Study of the Impact of High-Dose Opioid Analgesics on Overdose Mortality. Pain Med. 2016, 17, 85–98. [Google Scholar] [CrossRef] [PubMed]
- García-Sempere, A.; Hurtado, I.; Robles, C.; Llopis-Cardona, F.; Sánchez-Saez, F.; Rodriguez-Bernal, C.; Peiró-Moreno, S.; Sanfélix-Gimeno, G. Initial opioid prescription characteristics and risk of opioid misuse, poisoning and dependence: Retrospective cohort study. BMJ Qual. Saf. 2024, 33, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Keto, J.; Heiskanen, T.; Hamunen, K.; Kalliomäki, M.L.; Linna, M. Opioid trends in Finland: A register-based nationwide follow-up study. Sci. Rep. 2022, 12, 7261. [Google Scholar] [CrossRef]
- Muller, A.E.; Clausen, T.; Sjøgren, P.; Odsbu, I.; Skurtveit, S. Prescribed opioid analgesic use developments in three Nordic countries, 2006–2017. Scand. J. Pain 2019, 19, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B.; Neicun, J.; Yang, J.C.; Roman-Urrestarazu, A. Opioid prescription patterns in Germany and the global opioid epidemic: Systematic review of available evidence. PLoS ONE 2019, 14, e0221153. [Google Scholar] [CrossRef]
- Fischer, B.; Robinson, T. The marked oscillatory pattern in prescription opioid utilization in Canada since 2000: Selected observations and questions for outcomes and policy. Pharmacoepidemiol. Drug Saf. 2024, 33, e5748. [Google Scholar] [CrossRef]
- Spithoff, S.; Leece, P.; Sullivan, F.; Persaud, N.; Belesiotis, P.; Steiner, L. Drivers of the opioid crisis: An appraisal of financial conflicts of interest in clinical practice guideline panels at the peak of opioid prescribing. PLoS ONE 2020, 15, e0227045. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Petzke, F.; Radbruch, L.; Tölle, T.R. The opioid epidemic and the long-term opioid therapy for chronic noncancer pain revisited: A transatlantic perspective. Pain Manag. 2016, 6, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Böger, R. Symptomatische Behandlung von Schmerz, Fieber und Entzündung. In Arzneiverordnungs-Report; Ludwig, W.D., Mühlbauer, B., Seifert, R., Eds.; Springer: Berlin, Germany, 2022; pp. 387–412. [Google Scholar]
- Häuser, W.; Bock, F.; Hüppe, M.; Nothacker, M.; Norda, H.; Radbruch, L.; Schiltenwolf, M.; Schuler, M.; Tölle, T.; Viniol, A.; et al. Empfehlungen der zweiten Aktualisierung der Leitlinie LONTS. Der Schmerz 2020, 34, 204–244. [Google Scholar] [CrossRef] [PubMed]
- Busse, J.W.; Craigie, S.; Juurlink, D.N.; Buckley, D.N.; Wang, L.; Couban, R.J.; Agoritsas, T.; Akl, E.A.; Carrasco-Labra, A.; Cooper, L.; et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ 2017, 189, E659–E666. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, C.; Fisher, E.; Thomas, K.H.; Hearn, L.; Derry, S.; Stannard, C.; Knaggs, R.; Moore, R.A. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst. Rev. 2017, 11, Cd010323. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.D.; Reardon, R.; Weppler, C. Opioids for chronic noncancer pain: A new Canadian practice guideline. CMAJ 2010, 182, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Kahan, M.; Mailis-Gagnon, A.; Wilson, L.; Srivastava, A. Canadian guideline for safe and effective use of opioids for chronic noncancer pain: Clinical summary for family physicians. Part 1: General population. Can. Fam. Physician Med. Fam. Can. 2011, 57, 1257–1266, e1407-1218. [Google Scholar]
- Kim, E.D.; Lee, J.Y.; Son, J.S.; Byeon, G.J.; Yeo, J.S.; Kim, D.W.; Yoo, S.H.; Hong, J.H.; Park, H.J. Guidelines for prescribing opioids for chronic non-cancer pain in Korea. Korean J. Pain 2017, 30, 18–33. [Google Scholar] [CrossRef]
- Manchikanti, L.; Kaye, A.M.; Knezevic, N.N.; McAnally, H.; Slavin, K.; Trescot, A.M.; Blank, S.; Pampati, V.; Abdi, S.; Grider, J.S.; et al. Responsible, Safe, and Effective Prescription of Opioids for Chronic Non-Cancer Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician 2017, 20, S3–S92. [Google Scholar] [CrossRef]
- Espnes, K.A.; Nøst, T.H.; Handal, M.; Skurtveit, S.O.; Langaas, H.C. Can academic detailing reduce opioid prescriptions in chronic non-cancer pain? BMC Prim. Care 2023, 24, 84. [Google Scholar] [CrossRef]
- Grandt, D.; Lappe, V.; Schubert, I. Arzneimittelreport 2023: Medikamentöse Schmerztherapie Nicht-Onkologischer Ambulanter Patientinnen und Patienten; BARMER: Berlin, Germany, 2023. [Google Scholar]
- Manchikanti, L.; Abdi, S.; Atluri, S.; Balog, C.C.; Benyamin, R.M.; Boswell, M.V.; Brown, K.R.; Bruel, B.M.; Bryce, D.A.; Burks, P.A.; et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2—Guidance. Pain Physician 2012, 15, S67–S116. [Google Scholar]
- Crockett, S.; Greer, K.B.; Sultan, S. Opioid-Induced Constipation (OIC) Guideline. Gastroenterology 2019, 156, 228. [Google Scholar] [CrossRef]
- Busse, R.; Blümel, M.; Knieps, F.; Bärnighausen, T. Statutory health insurance in Germany: A health system shaped by 135 years of solidarity, self-governance, and competition. Lancet 2017, 390, 882–897. [Google Scholar] [CrossRef]
- Sun, E.C.; Dixit, A.; Humphreys, K.; Darnall, B.D.; Baker, L.C.; Mackey, S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: Retrospective analysis. BMJ 2017, 356, j760. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Schubert, T.; Scherbaum, N.; Tölle, T. Long-term opioid therapy of non-cancer pain: Prevalence and predictors of hospitalization in the event of possible misuse. Schmerz 2018, 32, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Mellbye, A.; Svendsen, K.; Borchgrevink, P.C.; Skurtveit, S.; Fredheim, O.M. Concomitant medication among persistent opioid users with chronic non-malignant pain. Acta Anaesthesiol. Scand. 2012, 56, 1267–1276. [Google Scholar] [CrossRef]
- Jani, M.; Girard, N.; Bates, D.W.; Buckeridge, D.L.; Sheppard, T.; Li, J.; Iqbal, U.; Vik, S.; Weaver, C.; Seidel, J.; et al. Opioid prescribing among new users for non-cancer pain in the USA, Canada, UK, and Taiwan: A population-based cohort study. PLoS Med. 2021, 18, e1003829. [Google Scholar] [CrossRef] [PubMed]
- Sizar, O.; Genova, R.; Gupta, M. Opioid-Induced Constipation; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493184/?report=reader (accessed on 16 January 2024).
- Sonohata, M.; Wada, S.; Koretaka, Y.; Morioka, Y.; Mishima, H.; Mawatari, M. A Survey of the Incidence of Constipation in Patients with Chronic Non-cancer Pain Using Opioid Analgesics in Japan. Pain Ther. 2022, 11, 845–859. [Google Scholar] [CrossRef]
- Andresen, V.; Banerji, V.; Hall, G.; Lass, A.; Emmanuel, A.V. The patient burden of opioid-induced constipation: New insights from a large, multinational survey in five European countries. United Eur. Gastroenterol. J. 2018, 6, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Varrassi, G.; Banerji, V.; Gianni, W.; Marinangeli, F.; Pinto, C. Impact and Consequences of Opioid-Induced Constipation: A Survey of Patients. Pain Ther. 2021, 10, 1139–1153. [Google Scholar] [CrossRef]
- Smith, H.S.; Laufer, A. Opioid induced nausea and vomiting. Eur. J. Pharmacol. 2014, 722, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, J.F.; Salas, J.; Miller-Matero, L.R.; Sullivan, M.D.; Ballantyne, J.C.; Debar, L.; Grucza, R.A.; Lustman, P.J.; Ahmedani, B. Long-term prescription opioid users’ risk for new-onset depression increases with frequency of use. Pain 2022, 163, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Grandt, D.; Lappe, V.; Schubert, I. Arzneimittelreport 2020: Sektorenübergreifende Arzneimitteltherapie; BARMER: Berlin, Germany, 2020. [Google Scholar]
- Phinn, K.; Liu, S.; Patanwala, A.E.; Penm, J. Effectiveness of organizational interventions on appropriate opioid prescribing for noncancer pain upon hospital discharge: A systematic review. Br. J. Clin. Pharmacol. 2023, 89, 982–1002. [Google Scholar] [CrossRef] [PubMed]
| Sex | Percentage of First-Time Opioid Recipients with Antiemetic Drugs | |||
|---|---|---|---|---|
| Percent | ||||
| Total | 18–64 years | 65–79 years | 80+ years | |
| Male | 4.2 | 4.0 | 4.2 | 5.4 |
| Female | 5.2 | 4.6 | 5.0 | 6.4 |
| Total | 4.8 | 4.4 | 4.8 | 6.2 |
| First-Time Opioid Recipients with a Non-Opioid Prescription Three Months before the Initiation of Opioids | |||
|---|---|---|---|
| Age | Sex | Percent | Mean DDD * |
| 18–64 | Male | 40.4 | 14 |
| Female | 44.5 | 16 | |
| All | 42.8 | 15 | |
| 65–79 | Male | 47.6 | 18 |
| Female | 53.2 | 20 | |
| All | 51.5 | 19 | |
| 80+ | Male | 50.7 | 17 |
| Female | 58.3 | 20 | |
| All | 56.9 | 19 | |
| Total | Male | 42.9 | 15 |
| Female | 49.6 | 18 | |
| All | 47.3 | 17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lappe, V.; Grandt, D.; Marschall, U.; Schubert, I. Opioid Prescribing for Noncancer Patients—Issues of Drug Therapy Safety: Results from a German Study Based on Routine Data. Pharmacoepidemiology 2024, 3, 94-102. https://doi.org/10.3390/pharma3010007
Lappe V, Grandt D, Marschall U, Schubert I. Opioid Prescribing for Noncancer Patients—Issues of Drug Therapy Safety: Results from a German Study Based on Routine Data. Pharmacoepidemiology. 2024; 3(1):94-102. https://doi.org/10.3390/pharma3010007
Chicago/Turabian StyleLappe, Veronika, Daniel Grandt, Ursula Marschall, and Ingrid Schubert. 2024. "Opioid Prescribing for Noncancer Patients—Issues of Drug Therapy Safety: Results from a German Study Based on Routine Data" Pharmacoepidemiology 3, no. 1: 94-102. https://doi.org/10.3390/pharma3010007
APA StyleLappe, V., Grandt, D., Marschall, U., & Schubert, I. (2024). Opioid Prescribing for Noncancer Patients—Issues of Drug Therapy Safety: Results from a German Study Based on Routine Data. Pharmacoepidemiology, 3(1), 94-102. https://doi.org/10.3390/pharma3010007






