Do Propylene Glycol, Benzyl Alcohol, and Ethanol in Concomitant Drugs Influence Clinical Outcomes Following Intravenous Acetaminophen in Critically Ill Neonates?
Abstract
:1. Introduction
2. Results
2.1. Demographic Details
2.2. Concomitant Drugs Containing BA, PG, or Ethanol
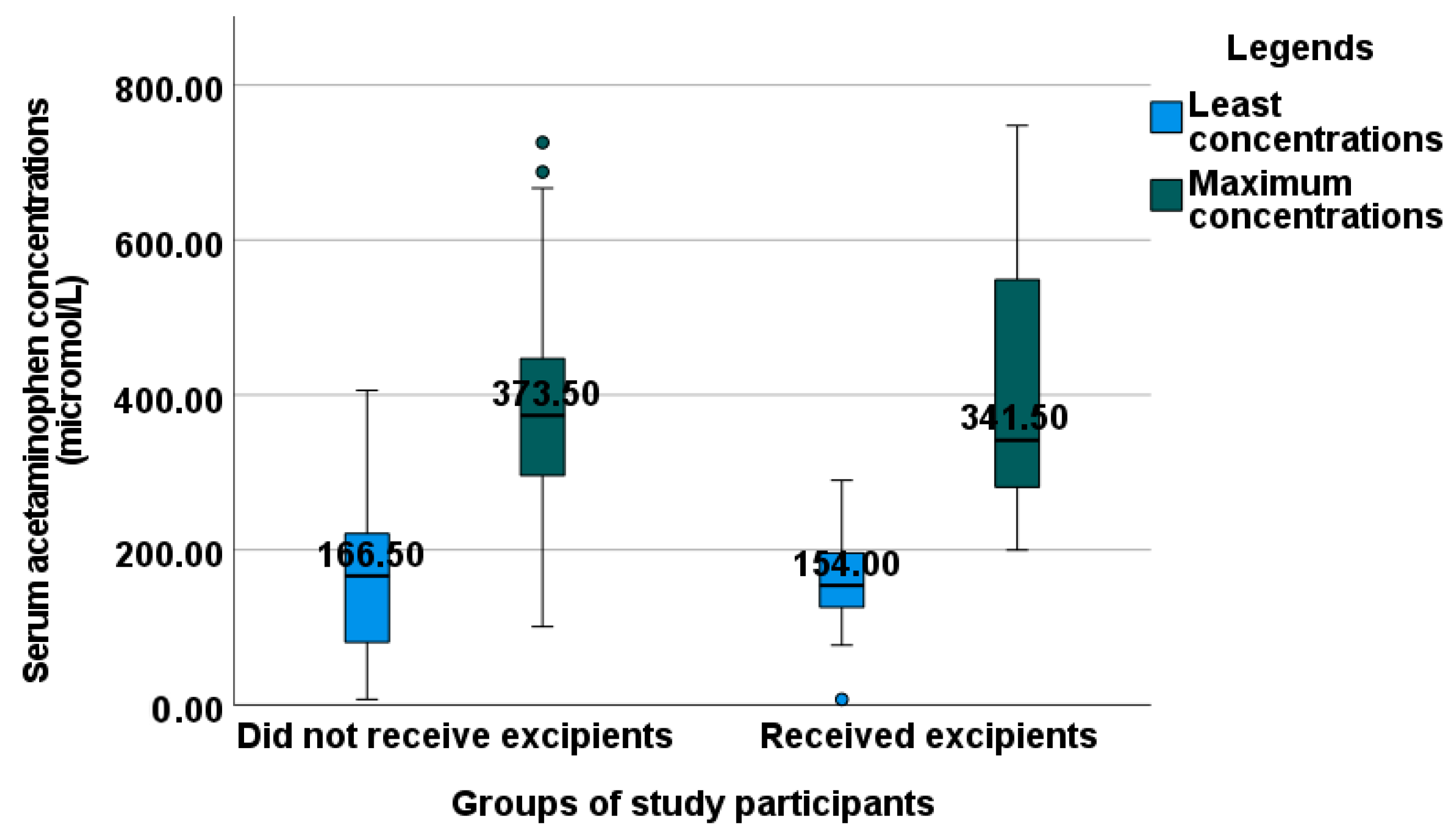
2.3. Evaluation of Serum Acetaminophen Concentrations
2.4. Presence of Excipients and PDA Outcomes
2.5. Liver and Renal Function Tests
3. Discussion
4. Materials and Methods
4.1. Study Ethics and Design
4.2. Study Procedure
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bucaretchi, F.; Fernandes, C.B.; Branco, M.M.; De Capitani, E.M.; Hyslop, S.; Caldas, J.P.; Moreno, C.A.; Porta, G. Acute liver failure in a term neonate after repeated paracetamol administration. Rev. Paul. Pediatr. 2014, 32, 144–148. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Huang, H.; Whelan, S.; Liu, L.; Kautza, B.; Luciano, J.; Wang, G.; Chen, G.; Stratimirovic, S.; Tsung, A.; et al. Benzyl alcohol attenuates acetaminophen-induced acute liver injury in a Toll-like receptor-4-dependent pattern in mice. Hepatology 2014, 60, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; McGill, M.R.; Xie, Y.; Jaeschke, H. Benzyl alcohol protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes but causes mitochondrial dysfunction and cell death at higher doses. Food Chem. Toxicol. 2015, 86, 253–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, M.S.; Loft, S.; Roberts, D.W.; Poulsen, H.E. Cytochrome P4502E1 inhibition by propylene glycol prevents acetaminophen (paracetamol) hepatotoxicity in mice without cytochrome P4501A2 inhibition. Pharmacol. Toxicol. 1995, 76, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Caparrotta, T.M.; Antoine, D.J.; Dear, J.W. Are some people at increased risk of paracetamol-induced liver injury? A critical review of the literature. Eur. J. Clin. Pharmacol. 2018, 74, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, A.; Ferrari, A.; Ottani, A.; Guerzoni, S.; Tacchi, R.; Leone, S. Paracetamol: New vistas of an old drug. CNS Drug Rev. 2006, 12, 250–275. [Google Scholar] [CrossRef]
- Nellis, G.; Metsvaht, T.; Varendi, H.; Toompere, K.; Lass, J.; Mesek, I.; Nunn, A.J.; Turner, M.A.; Lutsar, I.; ESNEE consortium. Potentially harmful excipients in neonatal medicines: A pan-European observational study. Arch. Dis. Child. 2015, 100, 694–699. [Google Scholar] [CrossRef]
- Sridharan, K.; Hasan, H.M.; Al Jufairi, M.; Al Daylami, A.; Al Ansari, E.; Qader, A.M.; Pasha, S.A.A. Possible effects of excipients used in the parenteral drugs administered in critically ill adults, children, and neonates. Expert Opin. Drug Saf. 2020, 19, 1625–1640. [Google Scholar] [CrossRef]
- Marek, E.; Kraft, W.K. Ethanol pharmacokinetics in neonates and infants. Curr. Ther. Res. Clin. Exp. 2014, 76, 90–97. [Google Scholar] [CrossRef] [Green Version]
- De Cock, R.F.; Knibbe, C.A.; Kulo, A.; de Hoon, J.; Verbesselt, R.; Danhof, M.; Allegaert, K. Developmental pharmacokinetics of propylene glycol in preterm and term neonates. Br. J. Clin. Pharmacol. 2013, 75, 162–171. [Google Scholar] [CrossRef] [Green Version]
- FDA notifications. Kaletra revisions to packaging approved. AIDS Alert 2010, 25, 34. [Google Scholar]
- Osterberg, R.E.; See, N.A. Toxicity of excipients-a Food and Drug Administration perspective. Int. J. Toxicol. 2003, 22, 377–380. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.G.; Fletcher, A.B.; Johnson, E.L.; Boeckx, R.L.; Getson, P.R.; Miller, M.K. The potential toxicity to neonates of multivitamin preparations used in parenteral nutrition. JPEN J. Parenter. Enter. Nutr. 1987, 11, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Valeur, K.S.; Holst, H.; Allegaert, K. Excipients in Neonatal Medicinal Products: Never Prescribed, Commonly Administered. Pharm. Med. 2018, 32, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Ganetsky, M.; Berg, A.H.; Solano, J.J.; Salhanick, S. Inhibition of CYP2E1 With Propylene Glycol Does Not Protect against Hepatocellular Injury in Human Acetaminophen Daily-Dosing Model. J. Clin. Pharmacol. 2019, 59, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Snider, N.T.; Portney, D.A.; Willcockson, H.H.; Maitra, D.; Martin, H.C.; Greenson, J.K.; Omary, M.B. Ethanol and Acetaminophen Synergistically Induce Hepatic Aggregation and TCH346-Insensitive Nuclear Translocation of GAPDH. PLoS ONE 2016, 11, e0160982. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.P.; Liao, J.T.; Cheng, Y.W.; Wu, T.L.; Lee, S.L.; Liu, J.K.; Yin, S.J. Inhibition of human alcohol and aldehyde dehydrogenases by acetaminophen: Assessment of the effects on first-pass metabolism of ethanol. Alcohol 2013, 47, 559–565. [Google Scholar] [CrossRef]
- Neuman, M.G. Synergetic signaling for apoptosis in vitro by ethanol and acetaminophen. Alcohol 2002, 27, 89–98. [Google Scholar] [CrossRef]
- Hedgpeth, B.; Missall, R.; Bambaci, A.; Smolen, M.; Yavuz, S.; Cottrell, J.; Chu, T.; Chang, S.L. A Review of Bioinformatics Tools to Understand Acetaminophen-Alcohol Interaction. Medicines 2019, 6, 79. [Google Scholar] [CrossRef] [Green Version]
- Johnsrud, E.K.; Koukouritaki, S.B.; Divakaran, K.; Brunengraber, L.L.; Hines, R.N.; McCarver, D.G. Human hepatic CYP2E1 expression during development. J. Pharmacol. Exp. Ther. 2003, 307, 402–407. [Google Scholar] [CrossRef]
- Prescott, L.F. Paracetamol, alcohol and the liver. Br. J. Clin. Pharmacol. 2000, 49, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Squires RHJr Shneider, B.L.; Bucuvalas, J.; Alonso, E.; Sokol, R.J.; Narkewicz, M.R.; Dhawan, A.; Rosenthal, P.; Rodriguez-Baez, N.; Murray, K.F.; Horslen, S.; et al. Acute liver failure in children: The first 348 patients in the pediatric acute liver failure study group. J. Pediatr. 2006, 148, 652–658. [Google Scholar] [CrossRef] [Green Version]
- Patman, G. Liver: Benzyl alcohol limits acute liver injury. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 396. [Google Scholar] [CrossRef]
- Palmer, G.M.; Atkins, M.; Anderson, B.J.; Smith, K.R.; Culnane, T.J.; McNally, C.M.; Perkins, E.J.; Chalkiadis, G.A.; Hunt, R.W.I.V. acetaminophen pharmacokinetics in neonates after multiple doses. Br. J. Anaesth. 2008, 101, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Zappitelli, M.; Ambalavanan, N.; Askenazi, D.J.; Moxey-Mims, M.M.; Kimmel, P.L.; Star, R.A.; Abitbol, C.L.; Brophy, P.D.; Hidalgo, G.; Hanna, M.; et al. Developing a neonatal acute kidney injury research definition: A report from the NIDDK neonatal AKI workshop. Pediatr. Res. 2017, 82, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, J.A.; Munoz, F.M.; Gonik, B.; Frau, L.; Cutland, C.; Mallett-Moore, T.; Kissou, A.; Wittke, F.; Das, M.; Nunes, T.; et al. Preterm birth: Case definition & guidelines for datacollection, analysis, and presentation of immunisation safety data. Vaccine 2016, 34, 6047–6056. [Google Scholar] [PubMed] [Green Version]
- Pacifici, G.M.; Allegaert, K. Clinical pharmacology of paracetamol in neonates: A review. Curr. Ther. Res. Clin. Exp. 2014, 77, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Sridharan, K.; Al Jufairi, M.; Al Ansari, E.; Al Marzooq, R.; Hubail, Z.; Hasan, S.J.R.; Al Madhoob, A. Intravenous acetaminophen (at 15 mg/kg/dose every 6 hours) in critically ill preterm neonates with patent ductus arteriosus: A prospective study. J. Clin. Pharm. Ther. 2021, 46, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
| Variables | Overall (n = 57) | No Exposure to Excipients (n = 42) | Exposure to Excipients (n = 15) | |
|---|---|---|---|---|
| Gestational age (weeks) $ | 28.7 (4.04) | 29.1 (4.1) | 26.8 (2.2) | |
| Category of gestational age (n) | Term | 4 | 4 | 0 |
| Late pre-term | 4 | 3 | 1 | |
| Very pre-term | 18 | 16 | 2 | |
| Extreme pre-term | 31 | 19 | 12 | |
| Birthweight (kg) $ | 1.2 (0.8) | 1.3 (0.8) | 0.9 (0.4) | |
| Category of birthweights (n) | Normal | 4 | 4 | 0 |
| Low | 6 | 5 | 1 | |
| Very low | 18 | 16 | 2 | |
| Extremely low | 29 | 17 | 12 | |
| Length (cm) $ | 35.2 (4.9) | 36.1 (5) | 33 (4.1) | |
| APGAR # | 1 min | 6 (1–9) | 7 (2–9) | 6 (1–9) |
| 5 min | 9 (5–10) | 9 (6–10) | 9 (5–10) | |
| 10 min | 10 (7–10) | 10 (7–10) | 10 (7–10) | |
| Male:Female (n) | 27:30 | 22:20 | 5:10 | |
| Duration of ICU stay (days) # | 40 (3–121) | 46 (8–121) | 21.5 (3–91) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sridharan, K.; Jufairi, M.A. Do Propylene Glycol, Benzyl Alcohol, and Ethanol in Concomitant Drugs Influence Clinical Outcomes Following Intravenous Acetaminophen in Critically Ill Neonates? Pharmacoepidemiology 2023, 2, 114-119. https://doi.org/10.3390/pharma2020011
Sridharan K, Jufairi MA. Do Propylene Glycol, Benzyl Alcohol, and Ethanol in Concomitant Drugs Influence Clinical Outcomes Following Intravenous Acetaminophen in Critically Ill Neonates? Pharmacoepidemiology. 2023; 2(2):114-119. https://doi.org/10.3390/pharma2020011
Chicago/Turabian StyleSridharan, Kannan, and Muna Al Jufairi. 2023. "Do Propylene Glycol, Benzyl Alcohol, and Ethanol in Concomitant Drugs Influence Clinical Outcomes Following Intravenous Acetaminophen in Critically Ill Neonates?" Pharmacoepidemiology 2, no. 2: 114-119. https://doi.org/10.3390/pharma2020011
APA StyleSridharan, K., & Jufairi, M. A. (2023). Do Propylene Glycol, Benzyl Alcohol, and Ethanol in Concomitant Drugs Influence Clinical Outcomes Following Intravenous Acetaminophen in Critically Ill Neonates? Pharmacoepidemiology, 2(2), 114-119. https://doi.org/10.3390/pharma2020011







