Cost Estimations of Managing Adverse Drug Reactions in Hospitalized Patients: A Systematic Review of Study Methods and Their Influences
Abstract
1. Introduction
2. Results
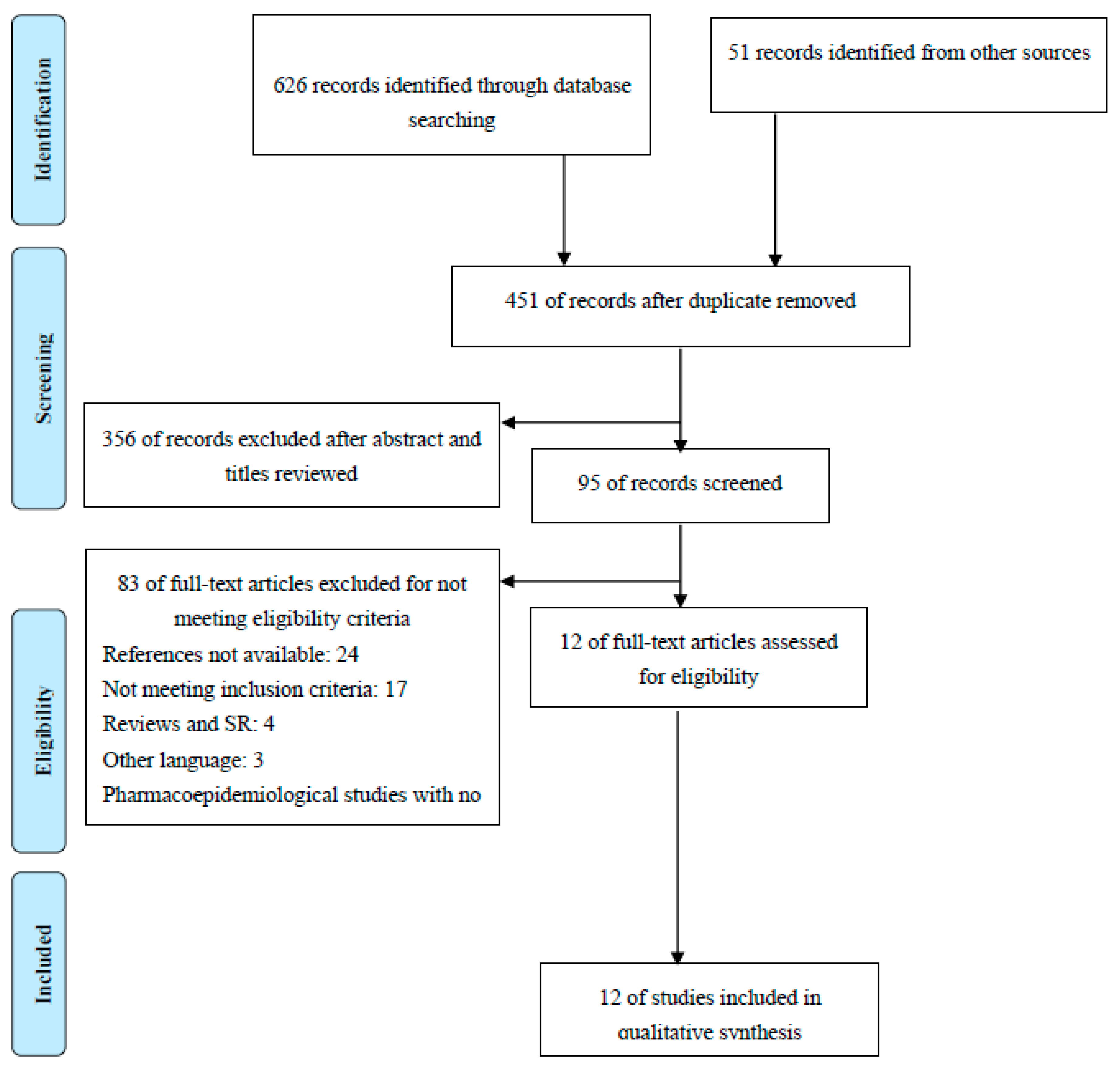
2.1. Characteristics of the Included Studies
2.2. ADR Identification Methods, Incidence, and Length of Stay (LOS)
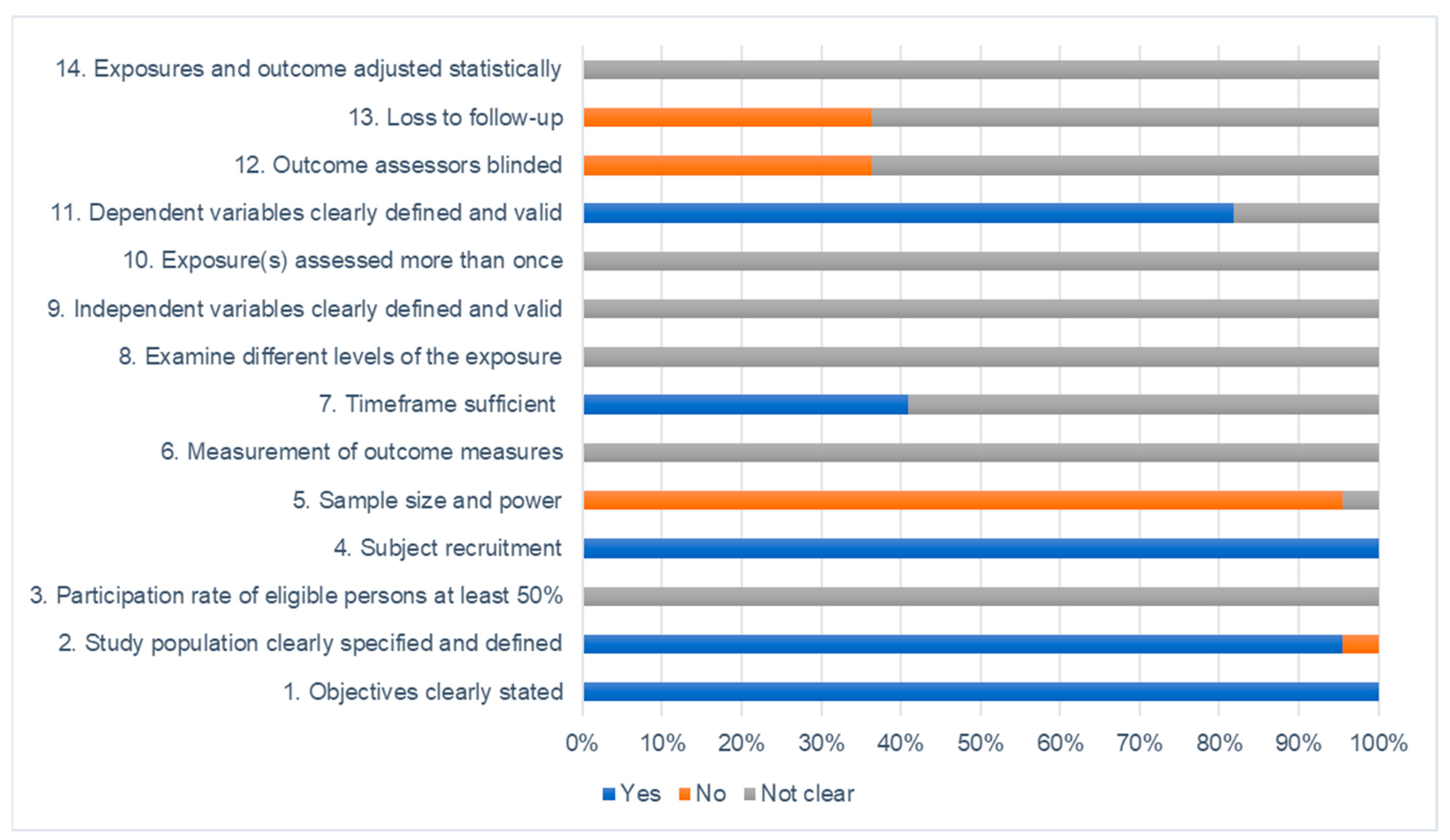
2.3. Study Quality and Risk of Bias
2.4. Method of Cost Calculation
2.5. Cost of ADR
3. Discussion
Streamlining ADR Costing Methods
4. Methods
4.1. Search Strategy
4.2. Study Selection Criteria
4.3. Data Extraction
4.4. Quality Assessments
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Safety of Medicines: A Guide to Detecting and Reporting Adverse Drug Reactions: Why Health Professionals Need to Take Action; WHO: Geneva, Switzerland, 2002.
- Dormann, H.; Muth-Selbach, U.; Krebs, S.; Criegee-Rieck, M.; Tegeder, I.; Schneider, H.T.; Hahn, E.G.; Levy, M.; Brune, K.; Geisslinger, G. Incidence and costs of adverse drug reactions during hospitalisation. Drug Saf. 2000, 22, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.; Raehl, C.L.; Franke, T. Clinical pharmacy services, pharmacy staffing, and the total cost of care in United States hospitals. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2000, 20, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Ernst, F.R.; Grizzle, A.J. Drug-related morbidity and mortality: Updating the cost-of-illness model. J. Am. Pharm. Assoc. 2001, 41, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J. Survey of the spontaneous adverse drug reaction reporting schemes in fifteen countries. Br. J. Clin. Pharmacol. 1986, 22, 83S. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, J.; Pomeranz, B.H.; Corey, P.N. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998, 279, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Alhawassi, T.; Krass, I.; Bajorek, B.; Pont, L. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin. Interv. Aging 2014, 9, 2079–2086. [Google Scholar] [CrossRef]
- Evans, R.S.; Pestotnik, S.L.; Classen, D.C.; Bass, S.; Burke, J. Prevention of adverse drug events through computerized surveillance. In Proceedings of the Annual Symposium on Computer Application in Medical Care, Baltimore, Maryland, 8–11 November 1992; p. 437. [Google Scholar]
- Beijer, H.; De Blaey, C. Hospitalisations caused by adverse drug reactions (ADR): A meta-analysis of observational studies. Pharm. World Sci. 2002, 24, 46–54. [Google Scholar] [CrossRef]
- Bordet, R.; Gautier, S.; Le Louet, H.; Dupuis, B.; Caron, J. Analysis of the direct cost of adverse drug reactions in hospitalised patients. Eur. J. Clin. Pharmacol. 2001, 56, 935–941. [Google Scholar] [CrossRef]
- Johnson, J.; Bootman, J. Drug-related morbidity and mortality: A cost of illness model. Arch. Intern. Med. 1995, 155, 1949–1956. [Google Scholar] [CrossRef]
- Waller, P.C.; Lee, E.H. Responding to drug safety issues. Pharmacoepidemiol. Drug Saf. 1999, 8, 535–552. [Google Scholar] [CrossRef]
- Gautier, S.; Bachelet, H.; Bordet, R.; Caron, J. The cost of adverse drug reactions. Expert Opin. Pharmacother. 2003, 4, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Coyle, D.; Lee, K.M. Evidence-based economic evaluation: How the use of different data sources can impact results. In Evidence-Based Health Economics: From Effectiveness to Efficiency in Systematic Review; Donaldson, C., Mugford, M., Vale, L., Eds.; BMJ Publishing Group: London, UK, 2002; pp. 55–66. [Google Scholar]
- Howard, R.; Avery, A.; Howard, P.; Partridge, M. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: Observational study. BMJ Qual. Saf. 2003, 12, 280–285. [Google Scholar] [CrossRef]
- Marques, F.B.; Penedones, A.; Mendes, D.; Alves, C. A systematic review of observational studies evaluating costs of adverse drug reactions. Clin. Outcomes Res. CEOR 2016, 8, 413. [Google Scholar]
- Al Hamid, A.; Ghaleb, M.; Aljadhey, H.; Aslanpour, Z. A systematic review of qualitative research on the contributory factors leading to medicine-related problems from the perspectives of adult patients with cardiovascular diseases and diabetes mellitus. BMJ Open 2014, 4, e005992. [Google Scholar] [CrossRef] [PubMed]
- Cano, F.G.; Rozenfeld, S. Adverse drug events in hospitals: A systematic review. Cad. Saúde Pública 2009, 25, S360–S372. [Google Scholar] [CrossRef]
- Dechanont, S.; Maphanta, S.; Butthum, B.; Kongkaew, C. Hospital admissions/visits associated with drug–drug interactions: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2014, 23, 489–497. [Google Scholar] [CrossRef]
- Impicciatore, P.; Choonara, I.; Clarkson, A.; Provasi, D.; Pandolfini, C.; Bonati, M. Incidence of adverse drug reactions in paediatric in/out-patients: A systematic review and meta-analysis of prospective studies. Br. J. Clin. Pharmacol. 2001, 52, 77–83. [Google Scholar] [CrossRef]
- Khan, L.M. Comparative epidemiology of hospital-acquired adverse drug reactions in adults and children and their impact on cost and hospital stay–a systematic review. Eur. J. Clin. Pharmacol. 2013, 69, 1985–1996. [Google Scholar] [CrossRef]
- Martins, A.; Giordani, F.; Rozenfeld, S. Adverse drug events among adult inpatients: A meta-analysis of observational studies. J. Clin. Pharm. Ther. 2014, 39, 609–620. [Google Scholar] [CrossRef]
- Miguel, A.; Azevedo, L.F.; Araújo, M.; Pereira, A.C. Frequency of adverse drug reactions in hospitalized patients: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2012, 21, 1139–1154. [Google Scholar] [CrossRef]
- Siltharm, C.; Thavorncharoensap, M. Cost of adverse drug reactions (ADRs) induced hospitalization: A systematic review. Mahidol Univ. J. Pharm. Sci. 2013, 40, 40–49. [Google Scholar]
- Smyth, R.M.D.; Gargon, E.; Kirkham, J.; Cresswell, L.; Golder, S.; Smyth, R.; Williamson, P. Adverse drug reactions in children—A systematic review. PLoS ONE 2012, 7, e24061. [Google Scholar] [CrossRef] [PubMed]
- Vallano, A.F.; Agustí, A.E.; Pedrós, C.X.; de Bolós Arnau, J. Systematic review of studies assessing the cost of adverse drug reactions. Gac. Sanit. 2012, 26, 277–283. [Google Scholar]
- Wiffen, P.; Gill, M.; Edwards, J.; Moore, A. Adverse Drug Reactions in Hospital Patients. A Systematic Review of the Prospective and Retrospective Studies; Centre for Reviews and Dissemination: York, UK, 2002; pp. 1–16. [Google Scholar]
- Gyllensten, H.; Jönsson, A.K.; Rehnberg, C.; Carlsten, A. How are the Costs of Drug-Related Morbidity Measured? Drug Saf. 2012, 35, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Geer, M.; Koul, P.; Tanki, S.; Shah, M. Frequency, types, severity, preventability and costs of adverse drug reactions at a tertiary care hospital. J. Pharmacol. Toxicol. Methods 2016, 81, 323–334. [Google Scholar] [CrossRef]
- Patel, K.; Kedia, M.; Bajpai, D.; Mehta, S.; Kshirsagar, N.; Gogtay, N. Evaluation of the prevalence and economic burden of adverse drug reactions presenting to the medical emergency department of a tertiary referral centre: A prospective study. BMC Clin. Pharmacol. 2007, 7, 8. [Google Scholar] [CrossRef]
- Pattanaik, S.; Dhamija, P.; Malhotra, S.; Sharma, N.; Pandhi, P. Evaluation of cost of treatment of drug-related events in a tertiary care public sector hospital in Northern India: A prospective study. Br. J. Clin. Pharmacol. 2009, 67, 363–369. [Google Scholar] [CrossRef]
- Wasserfallen, J.-B.; Livio, F.; Buclin, T.; Tillet, L.; Yersin, B.; Biollaz, J. Rate, type, and cost of adverse drug reactions in emergency department admissions. Eur. J. Intern. Med. 2001, 12, 442–447. [Google Scholar] [CrossRef]
- Yee, J.L.; Hasson, N.K.; Schreiber, D.H. Drug-related emergency department visits in an elderly veteran population. Ann. Pharmacother. 2005, 39, 1990–1995. [Google Scholar] [CrossRef]
- Carrasco-Garrido, P.; de Andrés, L.A.; Barrera, V.H.; de Miguel, G.Á.; Jiménez-García, R. Trends of adverse drug reactions related-hospitalizations in Spain (2001–2006). BMC Health Serv. Res. 2010, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Rottenkolber, D.; Schmiedl, S.; Rottenkolber, M.; Farker, K.; Saljé, K.; Mueller, S.; Hippius, M.; Thuermann, P.A.; Hasford, J.; Centers, N.o.R.P. Adverse drug reactions in Germany: Direct costs of internal medicine hospitalizations. Pharmacoepidemiol. Drug Saf. 2011, 20, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.; Pantaleo, N. Evaluation of outpatient adverse drug reactions leading to hospitalization. Am. J. Health-Syst. Pharm. 2003, 60, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.-C.; Woodall, B.S.; Shin, S.-K.; Santis, E.R.H.-D. Clinical and economic impact of adverse drug reactions in hospitalized patients. Ann. Pharmacother. 2000, 34, 1373–1379. [Google Scholar] [CrossRef]
- Chan, A.L.; Lee, H.Y.; Ho, C.-H.; Cham, T.-M.; Lin, S.J. Cost evaluation of adverse drug reactions in hospitalized patients in Taiwan: A prospective, descriptive, observational study. Curr. Ther. Res. 2008, 69, 118–129. [Google Scholar] [CrossRef]
- Rajakannan, T.; Mallayasamy, S.; Guddattu, V.; Kamath, A.; Vilakkthala, R.; Rao, P.G.; Bairy, L.K. Cost of adverse drug reactions in a South Indian tertiary care teaching hospital. J. Clin. Pharmacol. 2012, 52, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000, 356, 1255–1259. [Google Scholar] [CrossRef]
- Rawlins, M.D.; Thompson, J.W. Mechanisms of adverse drug reaction. In Textbook of Adverse Drug Reactions; Davies, D.M., Ed.; Oxford University Press: Oxford, UK, 1991; pp. 18–45. [Google Scholar]
- IMF. International Monetary Fund: World Economic Outlook Database; IMF: Bretton Woods, NH, USA, 2016. [Google Scholar]
- Hartwig, S.C.; Siegel, J.; Schneider, P.J. Preventability and severity assessment in reporting adverse drug reactions. Am. J. Health-Syst. Pharm. 1992, 49, 2229–2232. [Google Scholar] [CrossRef]
- Bowling, A. Mode of questionnaire administration can have serious effects on data quality. J. Public Health 2005, 27, 281–291. [Google Scholar] [CrossRef]
- Ridyard, C.H.; Hughes, D.A. Methods for the collection of resource use data within clinical trials: A systematic review of studies funded by the UK Health Technology Assessment program. Value Health 2010, 13, 867–872. [Google Scholar] [CrossRef]
- World Health Organization. Public Spending on Health: A Closer Look at Global Trends; World Health Organization: Geneva, Switzerland, 2018.
- Chevat, C.; Peña, B.M.; Al, M.J.; Rutten, F.F. Healthcare resource utilisation and costs of treating NSAID-associated gastrointestinal toxicity. Pharmacoeconomics 2001, 19, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Natanaelsson, J.; Hakkarainen, K.M.; Hägg, S.; Sundell, K.A.; Petzold, M.; Rehnberg, C.; Jönsson, A.K.; Gyllensten, H. Direct and indirect costs for adverse drug events identified in medical records across care levels, and their distribution among payers. Res. Soc. Adm. Pharm. 2017, 13, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.; Wang, P.; Barber, B.; Long, S.; Bagalman, J.; Wagner, V.; Song, X.; Zhao, Z. Clinical and economic impact of infusion reactions in patients with colorectal cancer treated with cetuximab. Ann. Oncol. 2010, 21, 1455–1461. [Google Scholar] [CrossRef]
- Lundkvist, J.; Jönsson, B. Pharmacoeconomics of adverse drug reactions. Fundam. Clin. Pharmacol. 2004, 18, 275–280. [Google Scholar] [CrossRef]
- Thürmann, P.A. Methods and systems to detect adverse drug reactions in hospitals. Drug Saf. 2001, 24, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.F.; Marques, F.B.; Ribeiro, C.F. Can decisional algorithms replace global introspection in the individual causality assessment of spontaneously reported ADRs? Drug Saf. 2006, 29, 697–702. [Google Scholar] [CrossRef]
- Briggs, A.H.; Levy, A.R. Pharmacoeconomics and pharmacoepidemiology. Pharmacoeconomics 2006, 24, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Rozich, J.; Haraden, C.; Resar, R. Adverse drug event trigger tool: A practical methodology for measuring medication related harm. BMJ Qual. Saf. 2003, 12, 194–200. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guide to Identifying the Economic Consequences of Disease and Injury; World Health Organization: Geneva, Switzerland, 2009.
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Atiqi, R.; Cleophas, T.; Van, E.B.; Zwinderman, A. Meta-analysis of recent studies on patients admitted to hospital due to adverse drug effects. Int. J. Clin. Pharmacol. Ther. 2009, 47, 549–555. [Google Scholar] [CrossRef]
- National Institutes of Health. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2014. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 1 July 2018).
| Author (Publication Date) | Suh et al. (2000) [38] | Bordet et al. (2001) [10] | Wasserfallen et al. (2001) [33] | Wu and Pantaleo (2003) [37] | Yee et al. (2005) [34] | Patel et al. (2007) [31] | Chan et al. (2008) [39] | Pattanaik et al. (2009) [32] | Carrasco-Garrido et al. (2010) [35] | Rottenkolber et al. (2011) [36] | Rajakannan et al. (2012) [40] | Geer et al. (2016) [30] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | USA | France | Switzerland | USA | USA | India | Taiwan | India | Spain | Germany | India | India |
| Type of study design | Prospective | Prospective | Prospective | Retro-spective | Retro-spective | Prospective | Prospective | Prospective | Retro-spective | Retro-spective | Prospective | Prospective |
| Single/Multi centre | Single centre | Single centre | Single centre | Single centre | Single centre | Single centre | Single centre | Single centre | Multicentre | Multicentre | Single centre | Single centre |
| Study duration (months) | 5 | 18 | 5 | 24 | 3 | 1.5 | 36 | 4 | 72 | 24 | 6 | 9 |
| No. Patients included | 9311 | 16,916 | 3195 | 191 | 2225 | 2046 | 142,295 | 1833 | 20,712,399 | 57,000 | 1438 | 5483 |
| Age range | Mean 56.6 (SD 20.3) | Mean 66.0 (SD 2.0) | Mean 61.4 (Range 16–93) | Mean/median age (NR) | Mean 60.2 (SD 14.2) | Mean 40.0 (NR) | Mean 66.0 (SD 2.0) | Mean or median age (NR) | Mean or median age (NR) | Mean 71.0 (14.7) Median 74 (17–103) | Mean 45.9 (SD 15.8) | Mean 62.0 (SD 2.3) |
| Gender | Male: 50.4% Female: 49.6% | Male: 55.3% Female: 44.7% | Male: 47.0% Female: 53.0% | Male: 44.0% Female: 56.0% | Male: 92.3% Female: 7.3% | NR | Male: 54.0% Female: 46.0% | Male: 57.7% Female: 42.3% | Male: 50.5% Female: 49.5% | Male: 41.8% Female: 58.2% | Male: 42.3% Female: 57.7% | Male: 38.6% Female: 61.4% |
| Setting (medical specialty) | General medical | Medical, surgical, paediatrics, and ICU | Emergency | Emergency | Emergency | Emergency | General medical | Medical emergency | General hospital admission | Internal medicine | Medical wards | Internal medicine and emergency |
| Author (Publication Date) | Suh et al. (2000) [38] | Bordet et al. (2001) [10] | Wasserfallen et al. (2001) [33] | Wu and Pantaleo (2003) [37] |
| ADR definition and type classification | WHO | WHO | WHO | WHO |
| Method of ADR detection (ADR defined by WHO) | Assessed by healthcare providers (pharmacists, nurses) from ADR reporting system and medical records. | Assessed by healthcare professionals (physicians and nurses) upon admission at all units and reviewing medical records. | Assessed by healthcare providers (research investigators) from hospital admission book. | Assessed by healthcare providers from ADR-related hospital admissions and patient’s medication profiles. |
| Method of ADR severity | No reference Mild: 30.0% Moderate: 53.0% Severe: 17.0% | WHO Mild: 53% Moderate: 34% Severe: 10% | No reference Evaluated using 5-point scale | NR |
| Causality assessment of ADR | Naranjo probability scale Definite: 8.0% Probable: 69.0% Possible: 21.0% Doubtful: 2.0% | French method Very likely: 1.0% Likely: 21.0% Possible: 25.0% Doubtful: 53.0% | WHO algorithm for imputability Certain: 18.0% Likely: 26.0% Possible: 56.0% | NR |
| Admission due to ADR (%) | 2.1 | 2.2 | 7.1 | NR |
| Preventable ADR (%) | NR | NR | 20.0 (10-item Qs, Livio 1998) | NR |
| Length of hospitalization due to ADR (days) | 1–3 ADR: 10.3 (10.7) [mean] >4 ADR: 12.8 (6.8) [mean] | 11 (NR) [mean] | 9.0 (0.6) [mean] | 8.0 (3.0) [mean] 5.0 (NR) [median] (Range 0 to 99 days) |
| Top five causative agents of ADR (therapeutic group) | Anti-infectives (17.1%) CVS drugs (16.5%) Antineoplastic (14.6%) NSAIDs (14.6%) Psychotropics (5.5%) | CVS Agents (36.0%) Contrast media (20.0%) Anti-infectives (14.0%) Anticoagulant (13.0%) Diuretics (6.0%) | Antineoplastic (22.7%) Anticoagulant (8.4%) NSAIDs (8.1%) Analgesics (8.1%) Antihypertensive (7.3%) | Antidiabetics (27.8%) CVS drugs (26.2%) Anticoagulant (15.2%) Psychotropics (11.5%) Analgesics (10.0%) |
| Top five ADRs | Gastrointestinal (24.4%) Dermatology (18.6%) Immunology (14.5%) CNS (13.2%) Hematological (9.9%) | Cutaneous (24.0%) CVS condition (21.0%) Metabolic cond. (12.0%) Coagulation (10.0%) CNS (10.0%) | GI bleeding (22.2%) Febrile neutropenia (14.4%) Hypotension (7.9%) Enterocolitis (5.7%) Hypoglycemia (4.6%) | Endocrine (28.3%) CVS condition (24.1%) Hematological (15.2%) Neurologic (14.1%) Renal (14.1%) |
| Author (Publication Date) | Yee et al. (2005) [34] | Patel et al. (2007) [31] | Chan et al. (2008) [39] | Pattanaik et al. (2009) [32] |
| ADR definition and type classification | WHO | WHO | WHO | WHO |
| Method of ADR detection (ADR defined by WHO) | Assessed by healthcare professionals upon admission at ED and reviewing electronic medical record system. | Assessed by healthcare professionals (senior lecturers and authors) upon admission at ED. | Assessed by healthcare providers from hospital admission cases. | Assessed by healthcare professionals (doctors, pharmacists, nurses) upon admission at medical ED. |
| Method of ADR severity | NR | Modified Hartwig and Siegel Scale. Mild: 18.5% Moderate: 74.7% Severe: 6.8% | WHO Mild: 34.4% Moderate: 58.5% Severe: 7.1% | NR |
| Causality assessment of ADR | Naranjo probability scale Definite:1.0% Probable: 31.0% Possible: 68.0% | Naranjo probability scale Definite:3.8% Probable: 85.9% Possible: 10.3% | Naranjo probability scale Definite: 4.1% Probable: 73.2% Possible: 22.3% Doubtful: 0.4% | Naranjo probability scale Definite: 86.0% Probable: 6.0% Possible: 8.0% |
| Admission due to ADR (%) | 1.1 | 6.9 | 0.03 | 1.4 |
| Preventability of ADR (%) | NR | 59.6 (Hallas 1990) | NR | NR |
| Length of hospitalization due to ADR (days) | 6.3 (NR) [mean] | 5 (NR) [median] (95% CI 5.37 to 7.11) | NR | 3 (NR) [median] |
| Top five drugs causing ADR (therapeutic group) | Anticoagulant (22.1%) Anti-infectives (13.2%) Antineoplastic (13.2%) Antidiabetics (10.3%) Diuretics (8.8%) | Anti-TB agents (19.6%) Antiepileptic (13.6% Antimalarials (11.3%) Anticoagulants (9.4%) OHA (6.0%) | Anti-infectives (38.8%) Analgesics (11.0%) CVS drugs (9.9%) NSAIDs (5.7%) Antiepileptic (5.1%) | NR |
| Top five nature of ADR occurred | Dermatologic allergic reaction (75.6%) | Hepatitis (10.6%) GI bleeding (9.1%) Gastritis (8.3%) | Cutaneous (52.5%) Hematological (10.8%) CVS condition (9.6%) Hepatic (5.9%) GI effects (5.0%) | NR |
| Author (Publication Date) | Carrasco-Garrido et al. (2010) [35] | Rottenkolber et al. (2011) [36] | Rajakannan et al. (2012) [40] | Geer et al. (2016) [30] |
| ADR definition and type classification | WHO | WHO Edwards and Aronson (2000) classification | WHO | WHO Rawlins and Thomson classification |
| Method of ADR detection (ADR defined by WHO) | Assessed by healthcare professionals (physicians) from hospital admission database. | Identified by evaluators from pharmacovigilance database and reviewing medical history. | Identified by investigator based on indicator list developed during manual screening of patients. | Assessed by healthcare professionals (multidisciplinary medical team) through hospital admission cases. |
| Method of ADR severity | NR | Hartwig severity scale Hosp: 89.3% Intensive: 9.1% Harm: 0.7% Fatal: 0.9% | Hartwig severity scale Mild: 36.6% Moderate: 61.5% Severe: 1.9% | Modified Hartwig and Siegel Scale. Mild: 41.5% Moderate: 48.8% Severe: 9.7% |
| Causality assessment of ADR | NR | Begaud et al. (1985) algorithm Predictable: 91.1% Unpredictable: 7.8% | Naranjo scale Definite: 1.0% Probable: 61.2% Possible: 37.8% | Naranjo scale Definite: 5.3% Probable: 78.6% Possible: 16.1% |
| Admission due to ADR (%) | 1.69 (1.65, 1.73) | 3.25 | 17.11 | 1.24 |
| Preventability of ADR (%) | NR | 20.1 (Schumock and Thornton 1992) | NR | 81.6 (Hallas 1990) |
| Length of hospitalization due to ADR (days) | 8.0 (10) [median] | 9.3 (7.1) [mean] | 5.0 (Range 5–28) [median] | 7 (NR) [median] |
| Top five drugs causing ADR (therapeutic group) | Antineoplastic (21.5%) Steroids (13.5%) Anticoagulant (7.5%) CVS drugs (7.0%) Anti-infectives (6.3%) | Anticoagulant (18.3%) Antidiabetics (15.9%) Diuretics (10.0%) | Anti-infectives (27.6%) | Anti-infectives (40.9%) Anti-TB agents (13.2%) Steroids (14.0%) Anticoagulant (8.8%) NSAIDs (7.9%) |
| Top five nature of ADR occurred | Neutropenia (5.0%) Chronic bronchitis (4.9%) CVS condition (3.1%) Neoplastic (3.0%) Pneumonia (2.9%) | GI bleeding (16.5%) Hypoglycemic (13.3%) Bradycardia (5.5%) Colitis (3.9%) Gastric ulcer (3.8%) | GI effects (19.5%) CNS condition (18.6%) Dermatology (15.4%) Metabolic cond. (15.1%) Hepatic (12.0%) | GI effects (23.7%) Dermatology (12.6%) CNS condition (11.7%) Hematological (10.0%) Metabolic cond. (9.6%) |
| Study | Author | Year Published | Quality Assessment (Risk of Bias) 1 | Baseline Clinical Data 2 | Resource Use 2 | Costs 2 |
|---|---|---|---|---|---|---|
| 1 | Suh et al. | 2000 | High | 1++ | 1++ | 1++ |
| 2 | Bordet et al. | 2001 | High | 1++ | 1++ | 1++ |
| 3 | Wasserfallen et al. | 2001 | High | 1++ | 1+ | 1+ |
| 4 | Wu and Pantaleo | 2003 | Moderate | 1+ | 1++ | 1++ |
| 5 | Yee et al. | 2005 | Moderate | 1+ | 1++ | 1++ |
| 6 | Patel et al. | 2007 | Moderate | 1++ | 1++ | 1++ |
| 7 | Chan et al. | 2008 | High | 1++ | 1++ | 1++ |
| 8 | Pattanaik et al. | 2009 | Moderate | 1++ | 1++ | 1++ |
| 9 | Carrasco-Garrido et al. | 2010 | High | 1+ | 1++ | 1++ |
| 10 | Rottenkolber et al. | 2011 | High | 1+ | 1+ | 1+ |
| 11 | Rajakannan et al. | 2012 | High | 1++ | 1++ | 1++ |
| 12 | Geer et al. | 2016 | Moderate | 1++ | 1++ | 1++ |
| Authors (Date Published) | Type of Study Design | No. of Pat. (Cost Analysis) | Data Extraction (Database Used) | Cost Analysis Perspective | Cost Calculation | LOS Definition | Sensitivity Analysis | Total Cost ADR (Duration) | Cost/Case (As Reported, Mean (SD)) | Cost/Case, USD (Adjusted 2016) |
|---|---|---|---|---|---|---|---|---|---|---|
| Micro-costing or unit cost (component of resource use estimated and unit cost derived) | ||||||||||
| Wasserfallen et al. (2001) [33] | Prospective | 229 | Admission database and medical records | Provider (health sector) | Hospital marginal costs divided by no. of patients in ward and computed according to no. of stays | Number of days spent in different wards | Imputability and avoidability figures (10–100%) | NR | CHF 3586.00 (342.00) | 2908.77 |
| Yee et al. (2005) [34] | Retrospective | 274 | Admission database (ED) and medical records (electronic) and VAD system | Provider (health sector) | Costs from activity-based costing system implemented | NA | NR | USD 333,433.00 (12 wk) | USD 3704.00 (NR) | 4463.59 |
| Patel et al. (2007) [31] | Prospective | 141 | Hospital admissions reports and patients’ profiles | Provider (health sector) | Products of total admission days for all patients admitted with ADR | Number of days admitted to hospital due to ADR | NR | INR 1.12 million (6 wk) | INR 6197.00 (NR) | 581.74 |
| Chan et al. (2008 [39]) | Prospective | 564 | ADR reporting system and patients’ profiles | Provider (health sector) | Hospital cost for services related to ADR treatment based on hospital’s claim data system | NA | NR | USD 150,027.14 (36 mo) | USD 3489.00 (NR) | 3896.97 |
| Pattanaik et al. (2009) [32] | Prospective | 92 | Admissions reports (ED) and patients’ profiles | Provider and patients (health and societal) | Cost for healthcare (direct) and non-healthcare (indirect) in ADR treatment | NA | For variability in components of the indirect cost | EUR 5556.00 (4 mo) | EUR 214.00 (NR) | 346.25 |
| Rajakannan et al. (2012) [40] | Prospective | 246 | Patients’ medical records in wards (patient notes) | Payer (health sector) | Hospital cost for services related to ADR treatment based on hosp patient admin system | Number of days in ward due to ADR | NR | USD 36,451.00 (6 mo) | USD 115.00 (NR) | 327.38 |
| Geer et al. (2016) [30] | Prospective | 342 | Hospital admissions reports and patients’ profiles | Provider (health sector) | Products of total admission days for all patients admitted with ADR | Measured by excess days: difference duration of hosp stays of ADR patient and mean duration of hosp stays for non-ADR patients | NR | USD 22,469.00 (9 mo) | USD 65.00 (NR) | 65 |
| Case-mix group costing (gives cost by case or patient category, e.g., DRG) | ||||||||||
| Suh et al. (2000) [38] | Prospective | 131 | Institutional database | Provider and payer (health sector) | DRG-based estimates (using hospital-specific cost/charge ratio) | NA | NR | USD 22,775 (SD 21,088.00) (5 mo) | USD 20,745.00 (20,040.00) (1–3 ADR) USD 34,445.00 (24,025.00) (>4 ADR) (Values were based on ADR cost per patient) | 28,082.65 (1–3 ADR) 46,628.44 (>4 ADR) (Values were based on ADR cost per patient) |
| Carrasco-Garrido et al. (2010) [35] | Retrospective | 350,835 | Admission database (minimum basic data set, MBDS) and patients’ profiles | Payer (health sector) | DRG-based estimates (reimbursement) | NA | NR | EUR 272 million (12 mo) | EUR 4382.00 (NR) | 6668.84 |
| Rottenkolber et al. (2011) [36] | Retrospective | 1834 | Regional regulatory database and admissions reports | Payer (health sector) | DRG-based estimates (reimbursement) | NA | NR | EUR 434 million (12 mo) | EUR 2250.00 (1321.00) | 3102.71 |
| Average per diem (or daily cost) | ||||||||||
| Bordet et al. (2001) [10] | Prospective | 371 | Admission database and medical records | Provider (health sector) | Hospital charges converted using hospital-specific cost/charge ratio | NA | NR | EUR 1815 million (18 mo) | EUR 4150.00 (NR) | 6222.52 |
| Wu and Pantaleo (2003) [37] | Retrospective | 191 | Pharmacy depart. reports and medication profiles | Provider (health sector) | Hospital charges converted using hospital-specific cost/charge ratio | NA | NR | NR | USD 9491.00 (12,843.00) | 12,129.90 |
| Concept | Examples of Similar Phrases |
|---|---|
| Population | “adult”, “children”, or “pediatric” |
| ADR | “adverse drug reactions”, “drug toxicity”, “hospitalized adverse drug reactions”, “hospitalized side effect”, “hospitalized adverse effect”, “hospital acquired ADRs (MeSH)”, “hospital induced ADRs (MeSH)”, “ADRs occurred during hospitalization (MeSH)” |
| Surveillance | “drug monitoring (MeSH)”, “drug surveillance program”, “pharmacovigilance” |
| Cost | “ADR economic burden”, “direct cost”, “cost of illness”, “cost”, and “economic” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu, S.F.; Shafie, A.A.; Chandriah, H. Cost Estimations of Managing Adverse Drug Reactions in Hospitalized Patients: A Systematic Review of Study Methods and Their Influences. Pharmacoepidemiology 2023, 2, 120-139. https://doi.org/10.3390/pharma2020012
Abu SF, Shafie AA, Chandriah H. Cost Estimations of Managing Adverse Drug Reactions in Hospitalized Patients: A Systematic Review of Study Methods and Their Influences. Pharmacoepidemiology. 2023; 2(2):120-139. https://doi.org/10.3390/pharma2020012
Chicago/Turabian StyleAbu, Siti Fauziah, Asrul Akmal Shafie, and Haarathi Chandriah. 2023. "Cost Estimations of Managing Adverse Drug Reactions in Hospitalized Patients: A Systematic Review of Study Methods and Their Influences" Pharmacoepidemiology 2, no. 2: 120-139. https://doi.org/10.3390/pharma2020012
APA StyleAbu, S. F., Shafie, A. A., & Chandriah, H. (2023). Cost Estimations of Managing Adverse Drug Reactions in Hospitalized Patients: A Systematic Review of Study Methods and Their Influences. Pharmacoepidemiology, 2(2), 120-139. https://doi.org/10.3390/pharma2020012






