Abstract
Background/Objectives: This study explores the evolution and morphology of the human mandible, focusing on recent changes and adaptations over the last 2000 years. It aims to examine how functional, genetic, and environmental factors influence mandibular size, shape, and sexual dimorphism by analyzing key anatomical landmarks—the horizontal ramus (HR), ascending ramus (AR), and mandibular angle (MA). Methods: A retrospective approach was employed using computed tomography (CT) scans of 39 mandibular samples from various historical periods, ranging from the Roman Imperial Age to the present day. Imaging was conducted using a 64-slice multislice computed tomography (MSCT) scanner, and the resulting data were processed to generate detailed 3D reconstructions for morphological assessment. Results: The analysis reveals that present-day samples exhibit significantly less variation in AR and MA compared to archaeological specimens, suggesting a trend of gracilization over time. Statistically significant differences were found in MA, likely influenced by environmental, dietary, and cultural factors. Correlation analysis showed moderate to weak relationships between AR, HR, and MA across sample groups, with significant sexual dimorphism in AR within the archaeological sample. Principal Component Analysis (PCA) further supported these findings, demonstrating a clear distinction between gracile modern mandibles and more robust ancient ones. Conclusions: These findings provide insights into the evolutionary trajectory of the human mandible, underscoring the influence of dietary and cultural shifts on mandibular structure over the past two millennia.
1. Introduction
The mandible, or lower jaw, represents one of the most functionally significant and evolutionarily informative structures in the human skeleton. As the largest and strongest bone in the face [], it plays a central role in mastication, respiration, speech, and overall craniofacial morphology []. Its morphology reflects a combination of genetic inheritance and phenotypic plasticity, responding dynamically to environmental factors like diet and mechanical loading [,,,].
The evolutionary trajectory of the mandible within the hominin lineages reveals a complex interplay of functional, ecological, and cultural factors []. Early hominins such as Australopithecus had robust mandibles adapted for coarse, plant-based diets, characterized by thick cortical bone and large post-canine teeth [].
With the emergence of Homo around 2.5 million years ago, mandibular morphology began to reflect changes in diet and tool use. Early Homo species, such as H. habilis and H. erectus, showed reduced jaw size and tooth dimensions, likely due to increased meat consumption, food processing, and possibly cooking []. These innovations reduced the selective pressure for strong, heavy jaws, leading to a trend toward gracilization [,].
By the time of Homo sapiens and Neanderthals, mandibular differences had become more specialized. Neanderthals retained robust jaws and large teeth, reflecting diets requiring intense mechanical processing. In contrast, anatomically modern humans had more gracile mandibles, thinner symphyses, and reduced prognathism, consistent with the adoption of cooking and softer diets [,,,].
Alongside functional adaptation, sexual dimorphism also played a role in mandibular evolution [,]. Throughout the hominin lineage, males consistently show more robust features—larger bicondylar widths, more prominent mental protuberances, and deeper mental fossae—linked to higher bite forces and larger masticatory muscles [,]. Females, by contrast, tend to have smaller, more rounded mandibles with thinner cortical bone []. These differences stem from both genetic and hormonal influences and reflect sex-specific growth patterns [,].
Mandibular development begins in embryogenesis with Meckel’s cartilage, which forms parts of the jaw through endochondral ossification [,].
Postnatally, mandibular growth is region-specific. The condylar cartilage drives vertical growth of the ramus through mechanical loading, while the body elongates through bone deposition and resorption [].
Sexual dimorphism emerges during adolescence, with androgenic hormones stimulating greater mandibular growth in males and estrogen influencing the more rounded contours seen in females [].
These morphological distinctions are also shaped by functional loading during mastication. Populations with diets requiring more chewing develop thicker cortical bone and more robust mandibles. Conversely, populations with soft, processed diets exhibit smaller mandibles and reduced ramus height []. This developmental plasticity reflects environmental and cultural variation, particularly in relation to diet.
In modern populations, the transition from hunter-gatherer diets to agricultural and industrial diets has further reduced masticatory demands [,]. This shift correlates with smaller mandibles, increased dental crowding, and a rise in malocclusion and TMJ dysfunction [,,].
These trends are consistent across global populations and underscore the influence of cultural change on craniofacial development [,,].
This manuscript aims to provide an explorative analysis dealing with mandibular morphology, focusing on the recent morphological changes—tracing the morphological adaptations of the mandible from people living in the last 2000 years—trying to support the current observation about the genetic and functional mechanisms underlying mandibular growth and shape.
This study aims to evaluate recent changes in mandibular morphology using a cost-effective and anatomically informative method, focusing on three key skeletal landmarks: the horizontal ramus (HR), ascending ramus (AR), and mandibular angle (MA).
These landmarks are readily identifiable on most well-preserved mandibles and can be measured using standard osteometric tools or basic imaging techniques, without the need for expensive, high-resolution 3D scanning or complex morphometric software.
We would explore the capability of these components, when analyzed collectively, in providing a comprehensive understanding of mandibular morphology.
This methodological choice allows for the systematic assessment of mandibular size, proportion, and shape across a wide range of archaeological and clinical samples, even in cases where preservation is partial or imaging resources are limited. The horizontal and ascending rami, when analyzed together, provide robust insights into mandibular proportions and biomechanical loading, while the mandibular angle serves as a reliable indicator of muscle attachment patterns and masticatory adaptation.
The integration of the three landmarks might be relevant for the inference of functional adaptation [], particularly considering the ascending ramus and mandibular angle, reflecting adaptations to masticatory forces and muscle attachments.
Similarly, the analysis of the changes in the dimensions and relationships of these components over time can reveal patterns for sexual dimorphism, assisting in individual identification and population-level comparisons. Importantly, this framework balances analytical power with accessibility, making it particularly suitable for studies in resource-limited settings or those dealing with large sample sizes. It facilitates comparative research across diverse populations and time periods, while still providing meaningful insight into both evolutionary and developmental processes. In summary, we propose the tri-landmark approach as a practical, scalable, and cost-efficient method for exploring mandibular morphology, capable of supporting broader anatomical, anthropological, clinical, and evolutionary interpretations without the need for highly specialized equipment or computational resources.
2. Materials and Methods
The study employed a retrospective approach to explore the anatomical characteristics of the mandible, utilizing computed tomography (CT) scans of samples from various recent historical periods. A total of 39 mandibular bones were evaluated (Table 1), representing adult individuals from a diachronic time transect spanning from the Roman Imperial Age to the present day. All necessary permissions for the archaeological series were granted by the Parco Archeologico di Ostia Antica, from where the samples were collected. The series was managed in accordance with the latest guidelines issued by the Italian Ministry of Culture [] and following international ethical recommendations []. The museum specimens, ranging between the 15th and 18th centuries CE, were obtained with authorization from the Director of MUSA—University of Campania “L. Vanvitelli”, while the present-day individuals provided informed consent before their imaging records were used. The primary criterion for sample recruitment was the overall preservation of the specimens, although the ancient series may include specimens with missing areas due to diagenesis. For both the archaeological series and museum samples, sex and age at death were determined using established osteological protocols. The demographic parameters obtained for the ancient specimens were compared with data extrapolated from previous archaeological and anthropological evaluations, which also provided contextual information.
To assess recent changes in mandibular morphology, this study focuses on a cost-effective and anatomically informative approach based on three key skeletal landmarks: the horizontal ramus (HR), ascending ramus (AR), and mandibular angle (MA). HR is the main horizontal bony structure extending from the mandibular symphysis at the midline, where it fuses with its counterpart, to the angle of the mandible. This segment is responsible for supporting the alveolar part of the mandible, which contains the dental sockets, for the lower teeth. The external surface is convex and provides attachment points for facial muscles, while the internal surface is marked by the mylohyoid line, which serves as an attachment for the mylohyoid muscle. The ascending ramus (AR) is the vertically oriented section of the mandible stretching upwards from HR. The ramus is marked by two processes: the anterior, thin, triangular eminence called the Coronoid process that serves as an attachment for the temporalis muscle, and the Condylar Process, the posterior projection that articulates with the temporal bone to form the temporomandibular joint. The internal surface of the ramus features the mandibular foramen, leading into the mandibular canal, transmitting the inferior alveolar nerve and vessels. The angle of the mandible (MA) is the junction where the lower border of HR meets the posterior border of AR. This bony angle is significant for providing robust muscular attachment for the Masseter Muscle, which attaches externally, aiding in the elevation of the mandible, and the Medial Pterygoid Muscle, going internally, contributing to mastication movements (Figure 1).
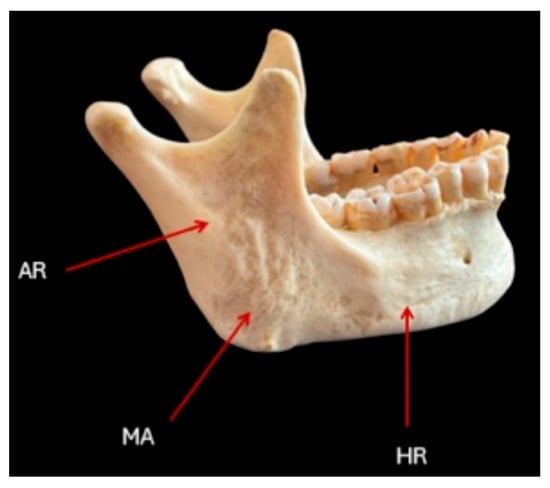
Figure 1.
An informative drawing of a skeletal specimen from which measurements were taken from the lateral projections. AR, MA, and HR refer to the ascending ramus, mandibular angle, and horizontal ramus, respectively.
The bone specimens were subjected to computed tomography (CT) analysis [,,,,]. Prior to imaging, each specimen was catalogued according to an internal system, receiving an individual identification number. All data were entered into the Radiological Information System (RIS) to ensure traceability during the subsequent analysis phases. Before CT scanning, the bone fragments were carefully cleaned, removing superficial soil and debris while preserving the integrity of the surfaces. Each specimen was then individually positioned in the CT gantry using radiolucent supports specifically designed to stabilize the bone and to maintain, as far as possible, its anatomical orientation. This arrangement was essential to facilitate correct acquisition planes (axial, sagittal, and coronal) and improve the quality of subsequent reconstructions. The imaging was performed using a 64-slice multislice computed tomography (MSCT) scanner (Revolution EVO, GE Healthcare, Little Chalfont, UK). The acquisition parameters were standardized across all specimens: Collimation: 64 × 0.6 mm, Gantry rotation time: 3.30 s, Pitch: variable between 0.2 and 0.5 depending on the specimen’s size and preservation, Tube current: 330 mA, Tube voltage: 100–120 kV, adjusted according to the density and dimensions of the fragment, Field of view (FOV): adapted to each specimen to optimize image resolution.
Each acquisition was followed by image reconstruction using a slice thickness of 0.6 mm. Both bone and soft tissue reconstruction algorithms were applied to enhance the visualization of cortical and trabecular structures. Multiplanar reconstructions (MPR) were generated in axial, coronal, and sagittal planes, and, where useful, 3D reconstructions (volume rendering and surface shaded display) were performed to support morphological interpretation.
In selected cases, dual-energy CT (DECT) scans were carried out to improve contrast resolution and to differentiate between bone tissue and possible infiltrations or foreign materials. For each mandibular fragment, the following features were systematically analyzed: Cortical bone thickness and regularity, Architecture and density of trabecular bone, Presence and typology of fractures (ante-mortem, peri-mortem, or post-mortem); Areas of bone necrosis; Signs of inflammatory or infectious processes (lytic or sclerotic lesions); Presence of foreign bodies or other alterations; and Taphonomic modifications, including weathering, erosion, and other post-depositional alterations.
The CT scans generated detailed three-dimensional images of the bones, allowing for an accurate assessment of mandibular morphology without altering or damaging the samples.
The .dcm files obtained by the CT scanning were processed with the open-source software Blue Sky Plan 4 (Blue Sky Bio Inc., Libertyville, IL, USA), which facilitated the implementation of a measurement protocol. Specifically, it was possible to build a latero-lateral radiograph for each of the mandibles. For each one, the following anatomical landmarks were identified: (a) Pogonion (the most anterior and prominent point of the horizontal ramus, Point P); (b) Gonion (Mandibular angle, the point of transition between the lower edge of the horizontal ramus and the vertical ramus), Point G; (c) Superior margin of the condyle (identified by the plane passing through the upper border of the condyle and parallel to the lower edge of the mandible), Point C. From the intersection between the plane passing through Point C and that passing through Point G and the posterior edge of the vertical ramus, Point C^1 is derived.
Once the landmarks were identified, the following segments were measured in millimeters: PG, from Pogonion to Gonion; PC^1, from Pogonion to Point C^1.
To measure the divergence between the vertical and horizontal branches of the mandible, the angle C^1-G-P was measured in degrees (Figure 2).
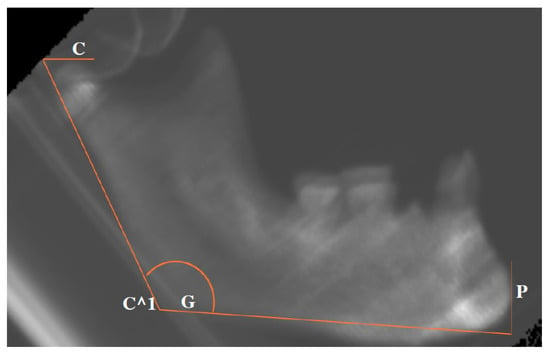
Figure 2.
Graphical representation of the measurements taken from the latero–lateral projections. The location of each C, C^1, G, and P is referred to in the main text.
The evaluation of the imaging data was conducted by multiple expert scholars, including specialists in Imaging and Odontostomatology, who independently assessed the cases to ensure inter-observer reliability. Each landmark was evaluated by at least two independent experts to minimize bias and enhance the accuracy of the measurements. Additionally, intra-observer reliability was verified by having the same examiner re-evaluate a subset of cases after a specified time interval (one week), ensuring consistency in the measurements taken. This dual-level approach, combining both inter-observer and intra-observer validation, was essential to ensure the reproducibility of the landmark measurements and to increase confidence in the reliability of the data derived from CT-based imaging.
The data analyses were performed using R version 4.4.2 and the packages stats and tidyverse [,]. The power analyses for this study were conducted using Cohen’s d as the measure of effect size for pairwise comparisons between the groups. Cohen’s d is a widely used statistic that quantifies the magnitude of the difference between two groups [,], and it was calculated for each pairwise comparison: Archeo vs. Museum, Museum vs. Present, and Archeo vs. Present. To ensure that the significance of these comparisons was properly evaluated in the context of multiple testing, the alpha error probability (α = 0.05) was adjusted using Bonferroni correction [], dividing the original alpha value by the number of comparisons (3 comparisons), resulting in an α = 0.0167 for each individual test. These calculations were implemented in G*Power v.3.1 [,], a tool designed for conducting power analysis and sample size calculations. The adjusted alpha value was used in conjunction with the Cohen’s d values to estimate the required sample sizes and to assess whether the study had sufficient power (at least 80%) to detect the expected effect sizes. Specifically, the power analysis helped determine whether the study could reliably identify significant differences, particularly for larger effect sizes, while acknowledging the limitations posed by the relatively small sample sizes in some groups.
3. Results
The CT scans allow for the collection of reliable measurements (Table 1; Figure 2, Figure 3 and Figure 4).
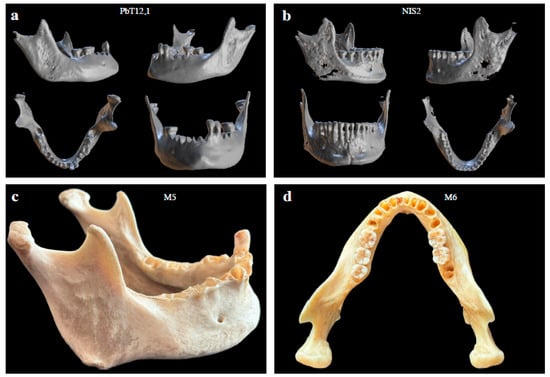
Figure 3.
Examples of mandibular specimens analyzed in this study. The upper panels (a,b) show 3D reconstructions of archaeological mandibles from different views (oblique, lateral, superior, and frontal). The lower panels (c,d) show photographic images of Museum mandibular specimens, illustrating their morphology and state of preservation.
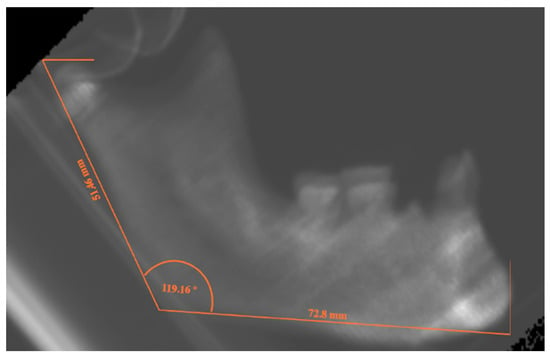
Figure 4.
Latero-lateral projection of PbT12,1 mandible from the Archeo group, with the lines evoking the measurements for AR, HR and MA.

Table 1.
Measurements for each specimen, with their meta-data relating to biological sex and time-transect. For sex determination: F: Female; M: Male; na: not conclusive. For SUB PERIOD: I: Imperial Age; TA: Late Antiquity; M: Middle Age; Mu: Specimens from Museum collection; PD: present-day. For measurements: AR and HR measurements are expressed in mm; MA results are in degrees; na: not available due to wearing and/or diagenetic fractures.
Table 1.
Measurements for each specimen, with their meta-data relating to biological sex and time-transect. For sex determination: F: Female; M: Male; na: not conclusive. For SUB PERIOD: I: Imperial Age; TA: Late Antiquity; M: Middle Age; Mu: Specimens from Museum collection; PD: present-day. For measurements: AR and HR measurements are expressed in mm; MA results are in degrees; na: not available due to wearing and/or diagenetic fractures.
| ID | Sex | DATE CLASS | MACRO | AR | HR | MA |
|---|---|---|---|---|---|---|
| NIS1 | F | I | Archeo | 51.54 | 71.69 | 113.66 |
| VRBT11 | F | TA | Archeo | 57.57 | 77.53 | 121.99 |
| Pb157 | F | M | Archeo | 59.24 | 71.4 | 127.77 |
| S.I TVII | M | TA | Archeo | 61.12 | 71.51 | 114.07 |
| S.I TXIV | M | TA | Archeo | 69.44 | 72.16 | 115.98 |
| NIS2 | M | I | Archeo | 70.26 | 74.14 | 114.63 |
| Pb89 T44, A | M | I | Archeo | 64.97 | 72.96 | 120.31 |
| Pb89 T44, C | na | I | Archeo | 62.48 | 74.38 | 139.23 |
| Pb89 T21, 1 | M | M | Archeo | 60.98 | 80.18 | 116.6 |
| PbT12,1 | F | M | Archeo | 51.46 | 72.8 | 119.16 |
| VRBT9 | M? | TA | Archeo | 56.79 | 71.79 | 123.29 |
| VRBT5 | F | TA | Archeo | 55.79 | 73.35 | 122.22 |
| S.I. TXV | M | TA | Archeo | 65.46 | 79.14 | 129.09 |
| Pb89T22,2 | M | TA | Archeo | 54.25 | 76.85 | 111.89 |
| Pb Ed.4,2 | na | I | Archeo | 61.59 | 73.99 | 108.18 |
| M1 | M | Mu | Museum | 70.28 | 73.58 | 104.73 |
| M2 | F | Mu | Museum | 59.44 | 78.45 | 102.88 |
| M3 | F | Mu | Museum | 60.21 | 71.36 | 113.4 |
| M4 | F | Mu | Museum | 62.17 | 70.38 | 121.11 |
| M5 | M | Mu | Museum | 64.6 | 69.62 | 111.82 |
| M6 | F | Mu | Museum | 63.29 | 76.16 | 112.44 |
| M7 | M | Mu | Museum | 61.63 | 61.49 | 120.28 |
| Patient1 | F | PD | Present | 51.9 | 73.6 | 124.25 |
| Patient2 | M | PD | Present | 61.08 | 73.18 | 124.62 |
| Patient3 | F | PD | Present | 61.95 | 63.17 | 123.94 |
| Patient4 | F | PD | Present | 58.04 | 66.34 | 121.88 |
| Patient5 | F | PD | Present | 57.56 | 65.01 | 124.75 |
| Patient6 | F | PD | Present | 62.94 | 75.17 | 124.94 |
| Patient7 | F | PD | Present | 59.01 | 66.52 | 127.02 |
| Patient8 | M | PD | Present | 57.98 | 70.87 | 125.81 |
Figure 5 illustrates the distribution of the three landmarks (AR: Ascending Ramus; HR: Horizontal Ramus; MA: Mandible Angle) within the defined clusters based on the grouping of archeological samples (Archeo), specimens collected in the Anatomical Museum (Museum), and present-day patients (Present). Among the 39 scanned mandibles, 6 specimens were excluded from the analysis due to missing measurements, while 3 archeological samples were excluded for the determination of the length of the ascending ramus due to diagenetic wear on the condyles.
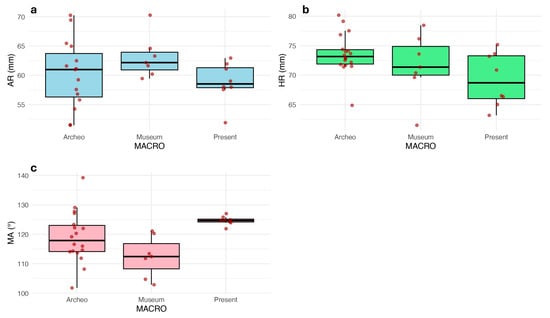
Figure 5.
Distribution of the measurements for (a) AR; (b) HR; (c) MA. The analysis of variance (ANOVA) was conducted to assess the impact of heterogeneous series, following a normality check using the Shapiro–Wilk test. Notably, the distribution of HR in the Archeo group was the only one showing a significant deviation from normality (Table 2 and Table 3).

Table 2.
Table showing the results of the Shapiro–Wilk test for normality, indicating the distributional characteristics of the data across groups.
Table 2.
Table showing the results of the Shapiro–Wilk test for normality, indicating the distributional characteristics of the data across groups.
| AR | HR | MA | |
|---|---|---|---|
| Archeo | W = 0.96, P = 0.78 | W(15) = 0.86, P = 0.03 | W(15) = 0.94, P = 0.42 |
| Museum | W = 0.88, P = 0.25 | W(7) = 0.94, P = 0.76 | W(7) = 0.92, P = 0.53 |
| Present | W = 0.91, P = 0.37 | W(8) = 0.91, P = 0.40 | W(8) = 0.95, P = 0.83 |
Accordingly, the variation trend highlighted for HR was analyzed by Kruskal–Wallis test, which returned a trend of significant difference (H = 5.60; P = 0.06). Conversely, ANOVA for AR did not show significant variation across the diachronic grouping (F = 1.52; P = 0.24), while MA distribution returns a significant variation across the three clusters (F = 6.54; P = 0.005), pointing out a difference that is not due by chance. Consequently, the Tuckey post hoc test was able to identify the diverging pair as the Museum vs. Present-day (Padj = 0.004).

Table 3.
Table showing the descriptive statistics for the three landmarks across groups. * Median and IQR are reported as indicators of central tendency and dispersion for HR in Archeo.
Table 3.
Table showing the descriptive statistics for the three landmarks across groups. * Median and IQR are reported as indicators of central tendency and dispersion for HR in Archeo.
| Sample | Indicator | AR (mm) | HR (mm) | MA (Degree) |
|---|---|---|---|---|
| Archeo | Average | 60.2 | 73.35 * | 119.9 |
| N = 15 | SD | 5.8 | 5.1 * | 7.9 |
| Museum | Average | 63.1 | 71.6 | 112.4 |
| N = 7 | SD | 3.6 | 5.5 | 6.9 |
| Present | Average | 58.8 | 69.2 | 124.7 |
| N = 8 | SD | 3.4 | 4.5 | 1.5 |
In order to explore the complex interaction among the forces driving the shaping of the human mandible, we tested the correlation among the three markers. In a general view, the three landmarks appear not significantly correlated (AR vs. HR Pearson’s R = 0.06; P = 0.74; AR vs. MA Pearson’s R = −0.17; P = 0.37; and HR vs. MA Pearson’s R = −0.15; P = 0.40).
However, the dissection of the data groupings uncovers noteworthy correlations and trends across different sample sets. Specifically, the relationship between the AR (Ascending Ramus) and HR (Horizontal Ramus) demonstrates interesting variations. For instance, the Archeo group shows a slight positive correlation between these two variables, whereas the more recent groups (Museum and Present) show negligible or even negative correlations. This contrast suggests a potential shift in mandibular development patterns over time, which is further explored in the relationships between other mandibular measurements.
In the exploration of the relationship between AR and MA (Mandible Angle) in the Museum group, a moderate negative correlation (R = −0.29) is observed. The negative correlation becomes even stronger when looking at the HR vs. MA (R = −0.75). This finding suggests that, in more recent groups, an increase in one variable tends to coincide with a decrease in the other, indicating a possible compensatory relationship between different aspects of mandibular morphology.
Conversely, the Archeo and Present groups reveal moderate positive correlations between the variables, with R = 0.19 and R = 0.15, respectively (Figure 6a–c). These findings are intriguing, as they suggest different developmental patterns between past and present populations, though no consistent trend emerges across all periods. This could indicate the influence of environmental or social changes that differently impacted the populations from different time periods, even in historical times, while that was already suggested for more remote ones [].
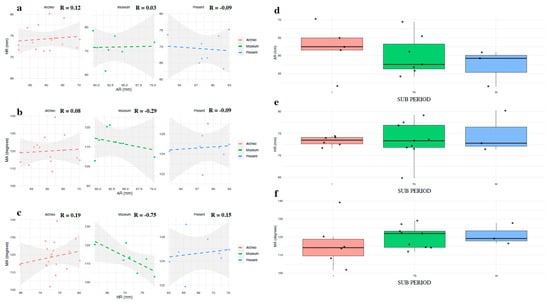
Figure 6.
Scatterplots showing correlation (a) AR vs. HR; (b) AR vs. MA; (c) HR vs. MA across the three Periods (Archeo, Museum, and Present Day). (d) Distribution of AR in Archeo samples, according to their SUB PERIOD. (e) Distribution of HR in Archeo samples, according to their SUB PERIOD. (f) Distribution of MA in Archeo samples, according to their SUB PERIOD.
Further exploration of the Archeo group, specifically when split by period (Imperial Roman, I; Late Antiquity, TA; and Medieval, M), did not reveal significant associations between the AR, HR, or MA variables and specific time periods. F-values and p-values all exceeded the 0.05 threshold, indicating that the trends observed may not be statistically significant due to a small sample size. While some trends are visible, they do not confidently support the notion of significant differences across these time periods.
An interesting tendency can be noted in the AR variable, especially when the Archeo samples are binned by period (Figure 6d–f). The Imperial Roman samples (I) appear to have larger ascending rami compared to the Medieval samples (M).
As it is known that morphological variation in the mandible can be linked to secondary dimorphic traits, we aimed to explore whether the three landmarks (AR, HR, and MA) vary according to individual gender and to trace their trajectory over time. Since the demographic data for the Archeo samples were obtained from well-contextualized research and the sex identification was double-checked with that obtained by the evaluation of the whole skeletal characteristics, we rely on those samples to investigate the variation in AR, HR, and MA and to compare these findings with the Present samples (Figure 7) [,].
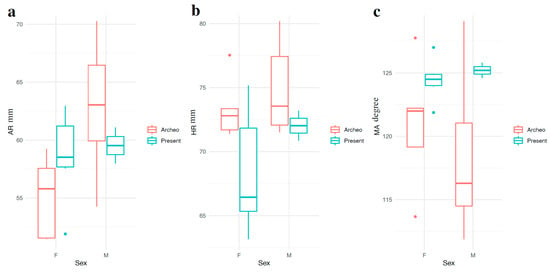
Figure 7.
Distribution of (a) AR, (b) HR, and (c) MA by sex in Archeo and Present Day samples.
Differences between males and females were assessed using the Mann–Whitney U (MW) test (Table 4), a non-parametric test that compares the distributions of two independent groups, since the data did not meet the assumptions of normality.

Table 4.
Mann–Whitney test for comparison between Archeo and Present Day samples.
The MW test revealed no significant differences in the landmarks for either diachronic group, except for AR in the Archeo group, which showed a significant difference between males and females (U = 5; p = 0.03) (Table 4, Figure 7). Specifically, AR was significantly larger in Archeo males compared to females. In contrast, AR values in the Present samples were similar between genders; however, the small sample size warrants cautious interpretation of this finding.
The joint evaluation of the three landmarks appears effective in identifying putative trends within our samples, providing meaningful insights into the recent evolution of mandible shape. To explore this, we performed a Principal Component Analysis (PCA) to combine the three markers and reduce the dataset’s dimensionality while preserving as much of the original variation as possible. The original data points were projected onto the new principal component axes, creating a lower-dimensional representation of the data.
We used the three landmarks for each sample to generate a two-dimensional space, which accounted for 74.9% of the cumulative variance (Figure 8). A noteworthy trend emerged: PC1, which explains the largest portion of variability (43.6%), separates the samples based on both period and biological sex. Negative values on PC1 are primarily associated with gracile mandibles, typically found in female individuals, and Present specimens, typically characterized by the gracilization due to the dietary-related loss of strain. Conversely, positive values on PC1 are more frequently linked to robust mandibles, predominantly observed in male and Archeo individuals, with only a few exceptions. Certainly, the inter-individual morphological heterogeneity can be detrimental for a clear clustering [,], and it is not surprising to note the exceptions. Indeed, two Archeo samples pertaining to the Roman Imperial Age, lacking sex determination for the non-conclusive assessment by multiple osteological methods [,,,], can be tentatively addressed by their specific location in the two groups derived from the PC1 eigenvalues.
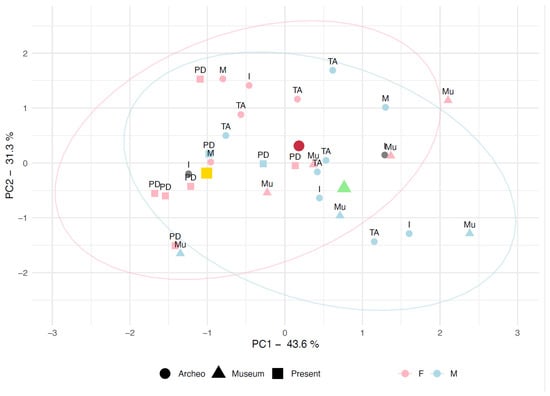
Figure 8.
PCA built using the whole set of available data for AR, HR, and MA. The shapes refer to the Periods, the colors refer to Sex. Yellow, Dark Red and Green markers are the centroids for each Period distribution.
The Museum specimens, though well-preserved and providing reliable measurements, are distributed across PC1. Indeed, the distribution of museum specimens appears somewhat misleading, as most individuals are positioned on the positive side of PC1. However, the centroids for the samples grouped by period indicate a consistent pattern of gracilization in present-day individuals (with the centroid on the negative side of PC1) compared to the ancient samples (Archeo and Museum), which are scattered through the positive side of the plot. Even when considering only biological sex, the PC1 data reveals a notable trend: female individuals are generally more dispersed across the left side of the plot, while male individuals predominantly occupy the right side, confirming the general trend towards an overall gracilization in the last 2000 years (Figure 9).
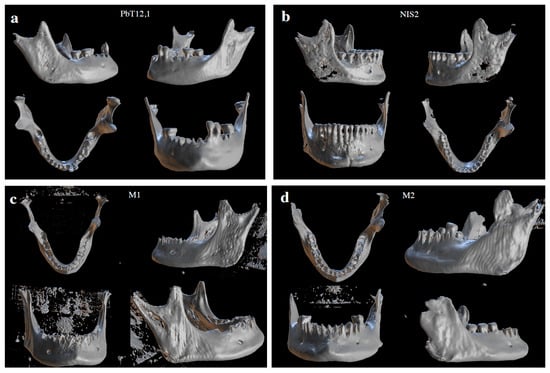
Figure 9.
Three-dimensional rendering of mandibles included in the study. (a) PbT12,1, the mandible pertaining to a female individual in Archeo, and the one with the lowest AR; (b) NIS2, the mandible pertaining to a male individual, characterized by the largest AR in the Archeo group. (c) M1, the mandible pertaining to a male individual in the Museum’s collection, characterized by the largest AR and an intermediate HR. (d) M2, the mandible pertaining to a female individual from the Museum’s series, characterized by the shortest AR, the longest HR and the least wide MA.
4. Discussion
The results of this study offer new perspectives on the recent evolutionary trajectory of the human mandible, revealing notable morphological shifts over the past two millennia. Based on the analysis of 39 mandibular samples from the Roman Imperial period to the present day, the findings underscore how cultural practices, dietary changes, and biomechanical demands have influenced mandibular form and function over time.
The distribution of the AR variable shows a wide range for the archaeological samples, exhibiting significant variation. In contrast, for the more recent classes, a trend emerges, with the Present-day individuals representing the class with the least variability. This trend is further reinforced for MA, where the present-day patients exhibit the narrowest distribution, which overall shows a wider MA than that of the historical specimens. Notably, HR follows an opposite trend, indicating a reduction in the length of the body of the mandible over time, consistent with the overall gracilization process [].
Even though the sample size of this exploratory evaluation is restricted, we tried to quantify the differences across the clusters. Unfortunately, we do not have precise dating for the Museum specimens; however, they should pertain to an intermediate time transect between Archeo and Present, as most of the MUSA anatomical museum collections are. Accordingly, we can explore the relatively rapid morphological variation in the mandibular angle that could have happened in the last 2000 years. This observation led to speculation about the factors that likely influenced this variation, accounting for a combination of evolutionary, cultural, and environmental factors specific to that period. Indeed, by 2000 years ago, food processing technologies, such as grinding grains or cooking food, became widespread. These technologies further reduced the need for chewing tough, raw food, contributing to a reduction in the size and robusticity of the mandible, which may have impacted the angle of the mandible, as verified in mice models []. Over time, the development and widespread use of tools to prepare and organize food (e.g., knives, grinding devices) meant that people could process food without needing to exert significant force with their jaws. This likely reduced the selective pressure on maintaining a large and robust jaw, leading to gradual changes in the shape and angle of the mandible—but also the reduction in its body—as reported for HR—over time [].
Similarly, the ability to cook and prepare softer foods has led to a reduced need for strong jaw muscles and a more robust jawbone structure. As food preparation became easier, requiring less mechanical processing, the strength of the jaw muscles (such as the masseter and temporalis) decreased, which in turn could have caused a reduction in the overall robustness of the jawbone, as seen in models []. However, it should be noted that a diet rich in softer food and reducing the need for strong chewing forces will lead the masseter and temporalis, responsible for chewing, to be less developed because they would not be used as much. Accordingly, over time, the bones associated with these muscles may become less robust. The mandible angle is influenced by the size and strength of the jaw muscles, and a reduction in muscle strength would likely result in a decrease in the angle, making it smaller and less pronounced.
Specifically, the reduction in MA has significant biomechanical implications, particularly by reducing the attachment site area for key masticatory muscles, which in turn decreases the leverage for force generation. This is especially concerning for the attachment and function of critical masticatory muscles, such as the Masseter and Temporalis. The Masseter is the primary muscle responsible for closing the jaw and generating vertical force during mastication, while the Temporalis aids in elevating the mandible and controlling jaw movements []. The MA region serves as a pivotal point for the insertion of these muscles, and any changes in the shape and robustness of this area directly impact the force and leverage these muscles can generate. The MA reduction also has significant biomechanical implications for the Pterygoid muscles, which assist in elevating the mandible and enabling lateral jaw movements. A smaller MA suggests that the mechanical load on these muscles has diminished. Consequently, the Pterygoid muscles are likely generating less force during chewing, which aligns with the evolutionary shift towards softer, processed diets that require less masticatory effort [].
Additionally, a reduction in tooth size or shifts in facial posture and breathing patterns may have also contributed to adjustments in the mandible’s angle across generations [,].
Genetic factors might play a role as well. For example, specific loci inherited from archaic humans through interbreeding events in our recent evolutionary history—especially in Europe—are associated with reductions in tooth dimensions [], which in turn can be associated with the change in MA. In archeological contexts—referring to the Archeo samples—where populations are heterogeneous, with European and African ancestry or other mixed groups [], these genetic trends might be more difficult to detect. Conversely, more homogenous samples, particularly those related to specific ancestry lines such as the Present patients, could offer clearer insights into these evolutionary trends.
In more recent times, orthodontics and dental care (such as braces, tooth extraction, etc.) have become more common. These practices can ultimately influence the shape of the mandible and may contribute to subtle changes in its robustness over time [,]. Braces can modify the mandible angle, primarily by correcting malocclusions, improving the alignment of the upper and lower teeth, and encouraging proper jaw positioning []. Braces, by aligning the teeth and improving the bite, can help in cases where there is a class III malocclusion-where the lower jaw is too far forward compared to the upper jaw []. In these cases, braces can help reposition the teeth and jaw, which may lead to a slight widening of the mandible angle.
The difference in the AR variable, especially when the Archeo samples are binned by period (Figure 6d–f), could potentially be influenced by lifestyle changes, dietary habits, or physical activity patterns that varied over time. Such changes could reflect broader social and environmental pressures from each era, such as the impact of urbanization or the shift from agricultural to more sedentary lifestyles.
In line with previous research, certain populations, particularly those of Asian descent, tend to exhibit less development of the ascending ramus of the mandible []. However, these patterns can vary greatly depending on the specific ethnic group and individual. The Medieval samples from Ostia Antica might have been influenced by the arrival of the Barbarian armies in the 5th century, particularly the Visigoths, who sacked Rome in 410 AD [,]. This event likely had profound impacts on daily life in the region, which could have also influenced dietary patterns and social structures, thereby affecting anatomical traits such as the mandible.
The Visigoths, originating from regions in Eastern and Northern Europe (such as Scandinavia and the Russian steppes), migrated westward in search of land and resources. Their migration contributed to the broader decline of the Western Roman Empire, leading to significant cultural and demographic changes []. The impact of these invasions was likely felt not just in terms of culture but also in terms of the physical development of populations in areas like Ostia, which was closely connected to Rome and faced disruption during these periods [].
Contrastingly, the HR in Imperial Age specimens was notably shorter and more homogeneous. This can be partially explained by dietary habits, as populations with more robust dietary practices, such as those consuming tougher, fibrous foods, tend to have longer and more developed horizontal rami. Conversely, populations consuming softer diets may exhibit shorter horizontal rami.
Ancient Romans are known to have consumed a predominantly plant-based diet, supplemented by small amounts of meat, with foods such as pulses being staples [,,]. This softer diet could be linked to the homogeneously shorter horizontal rami observed in the Imperial samples. The reduction in the need for heavy chewing likely resulted in less development of the jaw muscles and the mandible’s bones. However, with the onset of the Barbarian invasions, there was a shift in dietary habits that had significant implications for the morphology of the mandible.
Isotopic studies [] have shown that during Late Antiquity and Medieval times, there was an increase in protein consumption due to greater reliance on animal-based foods, which are tougher and require more intensive chewing than the plant-based diet typical in the Imperial era. The introduction of more fibrous, tougher foods during and after the Barbarian invasions likely put additional strain on the jaw muscles, contributing to the development of more robust mandible bones. This could explain the differences observed in the horizontal ramus length between the Imperial and Medieval groups, as the Medieval population would have required stronger and robust jaw structures to process tougher foods.
Moreover, this dietary shift could also explain the observed widening of the mandible angle during Medieval times compared to the Imperial period. As previously noted, a diet rich in tougher, more resistant foodstuffs would likely lead to increased muscular activity in the jaw, promoting the development and widening of the mandible. This change in mandibular morphology is consistent with the increase in protein consumption and the transition from softer foods in the Imperial period to more robust foods during the Barbarian invasions, further reinforcing the relationship between diet and skeletal adaptation [,].
The results of the Mann–Whitney U (MW) test, applied to compare differences between males and females, showed no significant differences in the landmarks for either diachronic group, with one exception: the ascending ramus (AR) in the Archeo group. The AR was significantly larger in Archeo males compared to females (U = 5; p = 0.03), suggesting a potential gender-related morphological variation in this group. However, in the Present samples, AR values between males and females did not differ significantly, indicating a shift or potential stabilization of this trait over time. AR serves as the primary attachment site for the powerful Massete and Temporalis muscles, which are essential for the forceful chewing and grinding of food. Given that males generally exhibit larger muscle mass and greater chewing force [], it is plausible that the ascending ramus would show sexual dimorphism, with males having a larger and more robust ramus to accommodate stronger muscle attachments. This could suggest that, in ancient populations, males had more pronounced masticatory forces, possibly due to dietary habits or social behaviors that required more intensive chewing []. However, it is important to note that the small sample size in the Present group limits the strength of this conclusion and warrants caution in the interpretation of these results. The lack of significant differences in AR for the Present group may reflect modern population trends or other factors, but further research with larger sample sizes is needed to confirm these findings.
Notably, when using the three-landmark approach, a partial sex estimation based solely on mandibular characteristics allows for a provisional sex classification for each specimen. However, this classification shows only a slight alignment with the overall distribution (Figure 8).
Overall, this study provides valuable preliminary insights into recent diachronic variation in mandibular morphology, with a particular focus on the ascending ramus (AR), horizontal ramus (HR), and mandibular angle (MA). Unlike most studies that prioritize long-term evolutionary changes [,,,,], this research highlights the often-overlooked recent time transect, shedding light on more contemporary shifts in mandibular morphology. While the findings suggest moderate variation in AR and HR over time, the most significant shift is observed in MA, which appears to reflect broader evolutionary, dietary, and cultural influences. The study aligns with established trends in human evolution, including a reduction in jaw size and robustness, likely linked to changes in diet, food processing, and the decline in masticatory demands.
To assess the statistical power of this study and the relevance of the observed effect, we conducted Cohen’s d effect size calculations for pairwise comparisons across the three groups for each anatomical landmark and used them to perform a power evaluation [] (Table 5). Notably, if it is true that the study revealed moderate to large differences in AR morphology across the groups, with the most pronounced divergence observed between the Museum and Present-day groups. However, the power analysis indicated that the study may lack the statistical power to identify smaller, more subtle differences, especially in groups with fewer individuals.

Table 5.
Table summarizing the Cohen’s d effect sizes and variance values for pairwise comparisons across groups. Effect sizes are categorized as Large and Moderate based on Cohen’s d, with corresponding sample variances for each group comparison. * Note that for HR, the Archeo distribution does not meet the assumption of normality, even though it is only moderately skewed (skewness coefficient = 1). As such, while variance is reported for this distribution, its calculation may not fully capture the spread of the data. Additionally, the Kruskal–Wallis U test was used for this comparison, and the rank-biserial correlation was calculated for effect size, reported as an absolute value.
Furthermore, to assess the magnitude of the difference between the Archeo and Museum groups for Horizontal Ramus (HR), we needed to calculate the effect size using the rank-biserial correlation from the Mann–Whitney U test, which indicated moderate to large effect sizes, supporting the presence of a meaningful difference across the groups. Notably, the power analysis suggested that our evaluation had adequate power to detect the larger effect sizes, particularly for the Museum vs. Present-day comparison.
Similarly, Cohen’s d values indicated a medium to large difference in MA across the groups, and the power analysis suggested that the study had adequate power to detect the larger effect sizes.
Overall, we underline that this study highlights meaningful differences in mandibular morphology across the three groups, revealing patterns of variation in the considered landmarks.
However, several key limitations must be considered, particularly concerning the extremely restricted sample size, which is a fundamental constraint in this pilot study.
While the observed effect sizes, indicating moderate to large morphological variations between the groups, partially support the highlighted differences—suggesting significant changes in mandibular morphology across recent time, likely shaped by evolutionary and cultural factors—the study’s statistical power, although sufficient to detect large effect sizes, is limited by the relatively small sample sizes. This limitation may hinder the identification of more subtle, yet potentially important, variations. As a pilot study, this research tests the feasibility of the methodology and explores potential trends; however, the small sample size restricts the generalizability of the findings. Despite this, the study lays a solid foundation for understanding recent evolutionary and cultural influences on mandibular morphology, emphasizing the potential contributions of genetic, environmental, and cultural factors to craniofacial development.
Additionally, the relatively broad temporal categories used in this study—comparing the Archeo, Museum, and Present groups—may not fully capture the more subtle evolutionary transitions within these periods. A more refined temporal scale, obtained by combining precise dating technologies with an expanded sample size, would provide a clearer understanding of how mandibular morphology has evolved over recent time and help identify specific evolutionary events or cultural shifts that have influenced these changes.
Moreover, while the study’s focus on just three anatomical landmarks is both anatomically clear and methodologically efficient, it might limit the scope of insights into mandibular evolution. By concentrating on a limited set of key landmarks, the study may overlook other potentially significant anatomical features that could offer a more holistic understanding of the complex factors influencing mandibular morphology over time. Therefore, future research should consider incorporating additional anatomical landmarks, such as the condylar or coronoid processes, to provide a more comprehensive analysis of mandibular variation and its underlying evolutionary and cultural influences.
Despite these limitations, the pilot nature of this study offers valuable insights into the potential of investigating recent mandibular evolution using a cost-effective and accessible approach. The use of easily identifiable anatomical landmarks provides a promising method for exploring even recent evolutionary changes in human mandibles, especially in archaeological and fragmented skeletal samples. By addressing these limitations, future studies will be better equipped to offer a more comprehensive understanding of the adaptive strategies that have shaped human mandibular morphology throughout evolutionary history.
5. Conclusions
This study investigates recent diachronic changes in mandibular morphology, focusing on the ascending ramus (AR), horizontal ramus (HR), and mandibular angle (MA). The results show moderate variation in AR and HR over time, while MA experiences a significant shift, reflecting the impact of evolutionary, dietary, and cultural factors. The reduction in mandibular size and robustness, particularly in AR and HR, aligns with the process of gracilization, a trend linked to dietary changes, such as the increased consumption of softer, processed foods, which reduced the need for strong jaw muscles. The significant reduction in MA, especially between the Museum and Present-day groups, suggests a recent evolutionary adaptation.
The study also allows for identifying sexual dimorphism in the remote group, revealing distinct differences between male and female mandibles. These findings suggest that both biological factors, such as genetic inheritance and hormonal influences, and cultural pressures, such as social roles or behaviors tied to diet and lifestyle, have played a role in shaping mandibular morphology in recent times. Conversely, in more recent groups, as HR became more gracile, there was a corresponding decrease in the size of the MA, suggesting a shift toward more delicate craniofacial structures. This compensatory pattern highlights the complex ways in which evolutionary and cultural changes have influenced the development of cranial features over time.
As a pilot study focused on a recent time transect, the findings align with existing research on the complex interactions between genetic, environmental, and cultural influences on mandibular morphology. The study underscores the potential of using a cost-effective, anatomical approach to investigate mandibular evolution, particularly when resources or skeletal material are limited. Future research, incorporating larger and more diverse samples alongside refined chronological frameworks, is crucial for better understanding the relative contributions of evolutionary and cultural factors to the current shape of the human mandible.
Author Contributions
Conceptualization, F.D.A., A.R. (Anna Russo), A.N., V.G., P.F.R., A.D.L. and A.R. (Alfonso Reginelli); methodology, F.D.A., A.R. (Anna Russo) and A.N.; software, F.D.A., A.R. (Anna Russo) and A.R. (Alfonso Reginelli); validation, F.D.A., A.R. (Anna Russo), A.N., G.C., M.A.; formal analysis, F.D.A., A.R. (Anna Russo), A.N., M.A., S.I. and G.C.; resources, P.F.R., A.R. (Alfonso Reginelli), D.M. and A.D.L.; data curation, F.D.A., S.I. and P.F.R.; writing—original draft preparation, F.D.A. and G.C.; writing—review and editing, F.D.A., A.R. (Anna Russo), M.A. and G.C.; visualization, F.D.A., A.R. (Anna Russo), M.A. and A.N.; supervision, V.G., A.D.L., D.M. and A.R. (Alfonso Reginelli); Funding, V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This article is part of PRIN 2022 ‘Early-life adversities: writing a biological history of childhood through a transdisciplinary approach’ (202255L4YW—PI V. Gazzaniga) Financed by European Union—Next Generation EU, Mission 4 Component 1 CUP B53D23007790006.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the use of anonymized archaeological human remains curated in institutional collections. These specimens are historical and non-identifiable, and all analyses were conducted in accordance with ethical guidelines for research on human skeletal material. For modern samples, all data were obtained from existing anonymized CT scan databases. No personal identifiers were accessed by the researchers at any stage. Informed consent was obtained at the time of original data collection by the institutions or hospitals involved, and the use of these anonymized data for secondary research complies with institutional and legal ethical standards.
Informed Consent Statement
Informed consent was obtained from all living individuals involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Due to ethical restrictions and privacy concerns regarding human skeletal remains, access to some datasets may be limited. Processed data used for statistical analyses and 3D reconstructions are available upon request for academic and non-commercial purposes.
Acknowledgments
F.D.A. was supported by the project: MNESYS—a multiscale integrated approach to the study of the nervous system in health and disease (PNRR).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANOVA | Analysis of Variance |
| AR | Ascending Ramus |
| CT | Computed Tomography |
| DECT | Dual-Energy CT |
| HR | Horizontal Ramus |
| I | Imperial Roman |
| IQR | Inter-Quartile Range |
| M | Medieval |
| MA | Mandibular Angle |
| MPR | Multiplanar Reconstructions |
| MSCT | Multislice Computed Tomography |
| MW | Mann–Whitney |
| PC | Principal Component |
| PCA | Principal Component Analysis |
| TA | Late Antiquity |
| TMJ | Temporo-Mandibular Joint |
References
- Breeland, G.; Aktar, A.; Patel, B.C. Anatomy, Head and Neck, Mandible. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sella-Tunis, T.; Pokhojaev, A.; Sarig, R.; O’Higgins, P.; May, H. Human Mandibular Shape Is Associated with Masticatory Muscle Force. Sci. Rep. 2018, 8, 6042. [Google Scholar] [CrossRef]
- Bergmann, I.; Hublin, J.-J.; Gunz, P.; Freidline, S.E. How Did Modern Morphology Evolve in the Human Mandible? The Relationship between Static Adult Allometry and Mandibular Variability in Homo sapiens. J. Hum. Evol. 2021, 157, 103026. [Google Scholar] [CrossRef]
- Hersberger-Zurfluh, M.A.; Motro, M.; Kantarci, A.; Will, L.A.; Eliades, T.; Papageorgiou, S.N. Genetic and Environmental Impact on Mandibular Growth in Mono- and Dizygotic Twins during Adolescence: A Retrospective Cohort Study. Int. Orthod. 2024, 22, 100842. [Google Scholar] [CrossRef]
- Pokhojaev, A.; Avni, H.; Sella-Tunis, T.; Sarig, R.; May, H. Changes in Human Mandibular Shape during the Terminal Pleistocene-Holocene Levant. Sci. Rep. 2019, 9, 8799. [Google Scholar] [CrossRef]
- Raia, P.; Boggioni, M.; Carotenuto, F.; Castiglione, S.; Di Febbraro, M.; Di Vincenzo, F.; Melchionna, M.; Mondanaro, A.; Papini, A.; Profico, A.; et al. Unexpectedly Rapid Evolution of Mandibular Shape in Hominins. Sci. Rep. 2018, 8, 7340. [Google Scholar] [CrossRef] [PubMed]
- Strait, D.S.; Weber, G.W.; Neubauer, S.; Chalk, J.; Richmond, B.G.; Lucas, P.W.; Spencer, M.A.; Schrein, C.; Dechow, P.C.; Ross, C.F.; et al. The Feeding Biomechanics and Dietary Ecology of Australopithecus africanus. Proc. Natl. Acad. Sci. USA 2009, 106, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- Bastir, M.; Rosas, A. Cranial Base Topology and Basic Trends in the Facial Evolution of Homo. J. Hum. Evol. 2016, 91, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Decaup, P.-H.; Couture, C.; Garot, E. Is the Distribution of Cortical Bone in the Mandibular Corpus and Symphysis Linked to Loading Environment in Modern Humans? A Systematic Review. Arch. Oral Biol. 2023, 152, 105718. [Google Scholar] [CrossRef]
- Hernaiz-García, M.; Oxilia, G.; Benazzi, S.; Sarig, R.; Fu, J.; Kullmer, O.; Fiorenza, L. Diet of Neanderthals and Early Homo sapiens from Macrowear Analysis of Mandibular Molars. J. Archaeol. Sci. 2024, 164, 105950. [Google Scholar] [CrossRef]
- Rosas, A.; Martínez-Maza, C.; Bastir, M.; García-Tabernero, A.; Lalueza-Fox, C.; Huguet, R.; Ortiz, J.E.; Julià, R.; Soler, V.; de Torres, T.; et al. Paleobiology and Comparative Morphology of a Late Neandertal Sample from El Sidrón, Asturias, Spain. Proc. Natl. Acad. Sci. USA 2006, 103, 19266–19271. [Google Scholar] [CrossRef]
- Weaver, T.D. The Meaning of Neandertal Skeletal Morphology. Proc. Natl. Acad. Sci. USA 2009, 106, 16028–16033. [Google Scholar] [CrossRef] [PubMed]
- Chalazoniti, A.; Lattanzi, W.; Halazonetis, D.J. Shape Variation and Sex Differences of the Adult Human Mandible Evaluated by Geometric Morphometrics. Sci. Rep. 2024, 14, 8546. [Google Scholar] [CrossRef] [PubMed]
- Toneva, D.H.; Nikolova, S.Y.; Fileva, N.F.; Zlatareva, D.K. Size and Shape of Human Mandible: Sex Differences and Influence of Age on Sex Estimation Accuracy. Leg. Med. 2023, 65, 102322. [Google Scholar] [CrossRef] [PubMed]
- Balolia, K.L.; Wood, B. Comparative Context of Hard-Tissue Sexual Dimorphism in Early Hominins: Implications for Alpha Taxonomy. Evol. Anthropol. 2025, 34, e22052. [Google Scholar] [CrossRef]
- Reno, P.L.; Meindl, R.S.; McCollum, M.A.; Lovejoy, C.O. Sexual Dimorphism in Australopithecus Afarensis Was Similar to That of Modern Humans. Proc. Natl. Acad. Sci. USA 2003, 100, 9404–9409. [Google Scholar] [CrossRef]
- Shree, B.; Soni, S.; Sharma, S.K.; Handge, K.; Kumar, A.; Das, S.S.; Puri, N. Analytical Study of Mandible: Prerequisite for Sex Determination. J. Pharm. Bioallied Sci. 2023, 15, S1215–S1217. [Google Scholar] [CrossRef]
- Alfaro, J.M.; Manrique, R.; Santamaría, A.; Álvarez, E.; Manes, C.; Jiménez, M. Effects of Endocrine Disorders on Maxillary and Mandibular Growth in Colombian Children and Adolescents: A Cross-Sectional Study. Eur. Arch. Paediatr. Dent. 2024, 25, 17–25. [Google Scholar] [CrossRef]
- Kubo, N.; Awada, T.; Hirose, N.; Yanoshita, M.; Takano, M.; Nishiyama, S.; Tsuboi, E.; Kita, D.; Ito, S.; Nakatani, A.; et al. Longitudinal Effects of Estrogen on Mandibular Growth and Changes in Cartilage during the Growth Period in Rats. Dev. Biol. 2022, 492, 126–132. [Google Scholar] [CrossRef]
- Pitirri, M.K.; Durham, E.L.; Romano, N.A.; Santos, J.I.; Coupe, A.P.; Zheng, H.; Chen, D.Z.; Kawasaki, K.; Jabs, E.W.; Richtsmeier, J.T.; et al. Meckel’s Cartilage in Mandibular Development and Dysmorphogenesis. Front. Genet. 2022, 13, 871927. [Google Scholar] [CrossRef]
- Yuan, Y.; Chai, Y. Regulatory Mechanisms of Jaw Bone and Tooth Development. Curr. Top. Dev. Biol. 2019, 133, 91–118. [Google Scholar] [CrossRef]
- Martinez-Maza, C.; Rosas, A.; Nieto-Díaz, M. Postnatal Changes in the Growth Dynamics of the Human Face Revealed from Bone Modelling Patterns. J. Anat. 2013, 223, 228–241. [Google Scholar] [CrossRef]
- Suzuki, N.; Miyazaki, A.; Igarashi, T.; Dehari, H.; Kobayashi, J.-I.; Miki, Y.; Ogi, K.; Nagai, I.; Sonoda, T.; Yotsuyanagi, T.; et al. Relationship Between Mandibular Ramus Height and Masticatory Muscle Function in Patients with Unilateral Hemifacial Microsomia. Cleft Palate Craniofacial J. 2017, 54, 43–52. [Google Scholar] [CrossRef]
- Laird, M.F.; Ross, C.F.; O’Higgins, P. Jaw Kinematics and Mandibular Morphology in Humans. J. Hum. Evol. 2020, 139, 102639. [Google Scholar] [CrossRef]
- Simione, M.; Loret, C.; Le Révérend, B.; Richburg, B.; Del Valle, M.; Adler, M.; Moser, M.; Green, J.R. Differing Structural Properties of Foods Affect the Development of Mandibular Control and Muscle Coordination in Infants and Young Children. Physiol. Behav. 2018, 186, 62–72. [Google Scholar] [CrossRef]
- D’Amato, G.; Tofangchiha, M.; Sheikhdavoodi, N.; Mohammadi, Z.; Ranjbaran, M.; Jabbarian, R.; Patini, R. Relationship between Skeletal Malocclusion and Radiomorphometric Indices of the Mandible in Long Face Patients. Diagnostics 2024, 14, 459. [Google Scholar] [CrossRef] [PubMed]
- Ghodasra, R.; Brizuela, M. Orthodontics, Malocclusion. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kurniawan, A.; Chusida, A.; Margaretha, M.S.; Rizky, B.N.; Prakoeswa, B.F.W.R.; Jethani, P.S.; Ramadani, I.P.; Yudianto, A.; Marya, A. Tooth Evolution and Its Effect on the Malocclusion in Modern Human Dentition. Bull. Int. Assoc. Paleodont. 2022, 16, 262–266. [Google Scholar]
- Holmes, M.A.; Ruff, C.B. Dietary Effects on Development of the Human Mandibular Corpus. Am. J. Phys. Anthropol. 2011, 145, 615–628. [Google Scholar] [CrossRef]
- Stansfield, E.; Evteev, A.; O’Higgins, P. Can Diet Be Inferred from the Biomechanical Response to Simulated Biting in Modern and Pre-Historic Human Mandibles? J. Archaeol. Sci. Rep. 2018, 22, 433–443. [Google Scholar] [CrossRef]
- Xu, M.C.; Jeong, J.-S.; Chen, Z.H.; Perinpanayagam, H.; Liu, C.R.; Zhao, Y.S.; Wang, F.; Fang, H.; Kum, K.-Y.; Gu, Y. Evolutionary Trends in Human Mandibles and Dentition from Neolithic to Current Chinese. Arch. Oral Biol. 2022, 142, 105512. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.F.; Riga, A.; Bondioli, L.; Rubini, M.; Belcatsro, M.G.; Manzi, G.; Acconcia, V.; Mancinelli, M.L. I Resti Scheletrici Umani: Dallo Scavo al Laboratorio al Museo-Ministero Della Cultura ICCD-Istituto Centrale per Il Catalogo e La Ministero Della Cultura ICCD -Istituto Centrale per Il Catalogo e La Documentaz; Ministero della Cultural: Rome, Italy, 2022. [Google Scholar]
- Squires, K.; Roberts, C.A.; Márquez-Grant, N. Ethical Considerations and Publishing in Human Bioarcheology. Am. J. Biol. Anthropol. 2022, 177, 615–619. [Google Scholar] [CrossRef]
- Grassi, R.; Cappabianca, S.; Urraro, F.; Granata, V.; Giacobbe, G.; Magliocchetti, S.; Cozzi, D.; Fusco, R.; Galdiero, R.; Picone, C.; et al. Evolution of CT Findings and Lung Residue in Patients with COVID-19 Pneumonia: Quantitative Analysis of the Disease with a Computer Automatic Tool. J. Pers. Med. 2021, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Russo, A.; Parisi, V.; Patanè, V.; Cappabianca, S.; Fusco, L.; Tomaino, F.; Averna, A.; Rescigno, C.; Iolascon, G.; et al. Multidisciplinary Analysis of Ancient Human Skeletal Remains Using Computed Tomography: A Case Study from Cumae, Italy. J. Forensic Leg. Med. 2025, 111, 102832. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Russo, A.; Pinto, A.; Stanzione, F.; Martiniello, C.; Cappabianca, S.; Brunese, L.; Squillaci, E. The Role of Computed Tomography in the Preoperative Assessment of Gastrointestinal Causes of Acute Abdomen in Elderly Patients. Int. J. Surg. 2014, 12 (Suppl. 2), S181–S186. [Google Scholar] [CrossRef]
- Reginelli, A.; Russo, A.; Micheletti, E.; Picascia, R.; Pinto, A.; Giovine, S.; Cappabianca, S.; Grassi, R. Imaging Techniques for Forensic Radiology in Living Individuals. In Radiology in Forensic Medicine: From Identification to Post-Mortem Imaging; Lo Re, G., Argo, A., Midiri, M., Cattaneo, C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 19–27. ISBN 978-3-319-96737-0. [Google Scholar]
- Reginelli, A.; Del Canto, M.; Clemente, A.; Gragnano, E.; Cioce, F.; Urraro, F.; Martinelli, E.; Cappabianca, S. The Role of Dual-Energy CT for the Assessment of Liver Metastasis Response to Treatment: Above the RECIST 1.1 Criteria. J. Clin. Med. 2023, 12, 879. [Google Scholar] [CrossRef]
- R Core Team. European Environment Agency. 2020. Available online: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 (accessed on 22 August 2022).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the p Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 2013; ISBN 978-0-203-77158-7. [Google Scholar]
- Bonferroni, C.E. Teoria Statistica Delle Classi e Calcolo Delle Probabilità; Seeber: Remseck am Neckar, Germany, 1936. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- von Cramon-Taubadel, N. Global Human Mandibular Variation Reflects Differences in Agricultural and Hunter-Gatherer Subsistence Strategies. Proc. Natl. Acad. Sci. USA 2011, 108, 19546–19551. [Google Scholar] [CrossRef]
- Krishan, K.; Chatterjee, P.M.; Kanchan, T.; Kaur, S.; Baryah, N.; Singh, R.K. A Review of Sex Estimation Techniques during Examination of Skeletal Remains in Forensic Anthropology Casework. Forensic Sci. Int. 2016, 261, 165.e1–165.e8. [Google Scholar] [CrossRef]
- Mello-Gentil, T.; Souza-Mello, V. Contributions of Anatomy to Forensic Sex Estimation: Focus on Head and Neck Bones. Forensic Sci. Res. 2022, 7, 11–23. [Google Scholar] [CrossRef]
- Cappella, A.; Bertoglio, B.; Di Maso, M.; Mazzarelli, D.; Affatato, L.; Stacchiotti, A.; Sforza, C.; Cattaneo, C. Sexual Dimorphism of Cranial Morphological Traits in an Italian Sample: A Population-Specific Logistic Regression Model for Predicting Sex. Biology 2022, 11, 1202. [Google Scholar] [CrossRef]
- Milella, M.; Franklin, D.; Belcastro, M.G.; Cardini, A. Sexual Differences in Human Cranial Morphology: Is One Sex More Variable or One Region More Dimorphic? Anat. Rec. 2021, 304, 2789–2810. [Google Scholar] [CrossRef]
- Acsadi, G.; Nemeskeri, J. Review of History of Human Life Span and Mortality. Curr. Anthropol. 1974, 15, 495–507. [Google Scholar]
- Bruzek, J. A Method for Visual Determination of Sex, Using the Human Hip Bone. Am. J. Phys. Anthropol. 2002, 117, 157–168. [Google Scholar] [CrossRef]
- Buikstra, J.E. Bioarchaeologists Speak Out: Deep Time Perspectives on Contemporary Issues; Bioarchaeology and Social Theory; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-319-93011-4. [Google Scholar]
- Buikstra, J.; Ubelaker, D. Standards for Data Collection from Human Skeletal Remains; Arkansas Archeological Survey: Fayetteville, AR, USA, 2021. [Google Scholar]
- Boo Gordillo, P.; Marqués Martínez, L.; Borrell García, C.; García Miralles, E. Relationship between Nutrition and Development of the Jaws in Children: A Pilot Study. Children 2024, 11, 201. [Google Scholar] [CrossRef]
- Karamani, I.I.; Tsolakis, I.A.; Makrygiannakis, M.A.; Georgaki, M.; Tsolakis, A.I. Impact of Diet Consistency on the Mandibular Morphology: A Systematic Review of Studies on Rat Models. Int. J. Environ. Res. Public Health 2022, 19, 2706. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.E.; Krovitz, G.E.; Yates, F.W.; Devlin, M.; St Claire, M. Effects of Food Processing on Masticatory Strain and Craniofacial Growth in a Retrognathic Face. J. Hum. Evol. 2004, 46, 655–677. [Google Scholar] [CrossRef]
- Inoue, M.; Ono, T.; Kameo, Y.; Sasaki, F.; Ono, T.; Adachi, T.; Nakashima, T. Forceful Mastication Activates Osteocytes and Builds a Stout Jawbone. Sci. Rep. 2019, 9, 4404. [Google Scholar] [CrossRef]
- De Stefano, M.; Ruggiero, A. A Critical Review of Human Jaw Biomechanical Modeling. Appl. Sci. 2024, 14, 3813. [Google Scholar] [CrossRef]
- Basit, H.; Tariq, M.A.; Siccardi, M.A. Anatomy, Head and Neck, Mastication Muscles. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ucar, F.I.; Ekizer, A.; Uysal, T. Comparison of Craniofacial Morphology, Head Posture and Hyoid Bone Position with Different Breathing Patterns. Saudi Dent. J. 2012, 24, 135–141. [Google Scholar] [CrossRef]
- Wang, G.; Saif, B.S.; Cheng, B.; Li, H.; Li, Y.; Liu, J.; Ren, X.; Zou, R.; Wang, F. Effect of Breathing Patterns on Mandibular Cortical Bone Quality in Children and Establishment of a Preliminary Screening Model. BMC Oral Health 2023, 23, 808. [Google Scholar] [CrossRef]
- Li, Q.; Faux, P.; Winchester, E.W.; Yang, G.; Chen, Y.; Ramírez, L.M.; Fuentes-Guajardo, M.; Poloni, L.; Steimetz, E.; Gonzalez-José, R.; et al. PITX2 Expression and Neanderthal Introgression in HS3ST3A1 Contribute to Variation in Tooth Dimensions in Modern Humans. Curr. Biol. 2025, 35, 131–144.e6. [Google Scholar] [CrossRef]
- De Angelis, F.; Vaccaro, S.; Romboni, M.; Di Cicco, M.R.; Mantile, N.; Altieri, S.; Mezzogiorno, A.; Lo Blundo, M.; Rickards, O.; Lubritto, C.; et al. Echoes from the Past: Bioarchaeological Insights into the Burial Grounds of Portus Romae. J. Archaeol. Sci. Rep. 2025, 61, 104931. [Google Scholar] [CrossRef]
- Jain, P.; Rathee, M. Stability in Mandibular Denture. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Natarajan Gavriilidou, N.; Jonasson, G.; Sundh, V.; Rothenberg, E.; Lissner, L. Does Mandibular Bone Structure Predict Subsequent Height Loss? A Longitudinal Cohort Study of Women in Gothenburg, Sweden. BMJ Open 2023, 13, e066844. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.; Watkinson, S.; Harrison, J.E.; Turner, S.; Worthington, H.V. Orthodontic Treatment for Prominent Lower Front Teeth (Class III Malocclusion) in Children. Cochrane Database Syst. Rev. 2024, 4, CD003451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-G.; Hans, M.G.; Palomo, J.M.; Lin, J.-X. Comparison of Chinese and White Bolton Standards at Age 13. Angle Orthod. 2013, 83, 809–816. [Google Scholar] [CrossRef]
- Godden, M.R. The Anglo-Saxons and the Goths: Rewriting the Sack of Rome. Anglo-Saxon Engl. 2002, 31, 47–68. [Google Scholar] [CrossRef]
- Gwynn, D.M. The Goths in Ancient History. In The Cambridge History of the Gothic: Volume 1: Gothic in the Long Eighteenth Century; Wright, A., Townshend, D., Eds.; The Cambridge History of the Gothic; Cambridge University Press: Cambridge, UK, 2020; Volume 1, pp. 22–43. ISBN 978-1-108-47270-8. [Google Scholar]
- Brabo, R.C. Coins of Crisis: Rome’s 3rd Century Instability. Myth. J. 2024. Available online: https://medium.com/mythology-journal/coins-of-crisis-exploring-the-roman-empires-turbulent-3rd-century-through-numismatics-5c19bb27f330 (accessed on 8 October 2025).
- De Angelis, F.; Varano, S.; Battistini, A.; Di Giannantonio, S.; Ricci, P.; Lubritto, C.; Facchin, G.; Brancazi, L.; Santangeli-Valenzani, R.; Catalano, P.; et al. Food at the Heart of the Empire: Dietary Reconstruction for Imperial Rome Inhabitants. Archaeol. Anthropol. Sci. 2020, 12, 244. [Google Scholar] [CrossRef]
- De Angelis, F.; Veltre, V.; Varano, S.; Romboni, M.; Renzi, S.; Zingale, S.; Ricci, P.; Caldarini, C.; Giannantonio, S.D.; Lubritto, C.; et al. Dietary and Weaning Habits of the Roman Community of Quarto Cappello Del Prete (Rome, 1st–3rd Century CE). Environ. Archaeol. 2020, 30, 156–170. [Google Scholar] [CrossRef]
- Varano, S.; De Angelis, F.; Battistini, A.; Brancazi, L.; Pantano, W.; Ricci, P.; Romboni, M.; Catalano, P.; Gazzaniga, V.; Lubritto, C.; et al. The Edge of the Empire: Diet Characterization of Medieval Rome through Stable Isotope Analysis. Archaeol. Anthropol. Sci. 2020, 12, 196. [Google Scholar] [CrossRef]
- Movassagh, E.Z.; Vatanparast, H. Current Evidence on the Association of Dietary Patterns and Bone Health: A Scoping Review123. Adv. Nutr. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Proia, P.; Amato, A.; Drid, P.; Korovljev, D.; Vasto, S.; Baldassano, S. The Impact of Diet and Physical Activity on Bone Health in Children and Adolescents. Front. Endocrinol. 2021, 12, 704647. [Google Scholar] [CrossRef]
- Palinkas, M.; Nassar, M.S.P.; Cecílio, F.A.; Siéssere, S.; Semprini, M.; Machado-de-Sousa, J.P.; Hallak, J.E.C.; Regalo, S.C.H. Age and Gender Influence on Maximal Bite Force and Masticatory Muscles Thickness. Arch. Oral Biol. 2010, 55, 797–802. [Google Scholar] [CrossRef]
- Thumati, P.; Thumati, R.P.; Radke, J. Gender Differences in Human Masticatory Function. Adv. Dent. Technol. 2022, 2022, 1–9. [Google Scholar]
- Moskowitsch, M.; Smith, P. A Note on Functional Implications of Morphological Variation in the Human Mandible. Int. J. Anthropol. 1993, 8, 53–60. [Google Scholar] [CrossRef]
- Hanegraef, H.; David, R.; Spoor, F. Integrating Mandibular Evidence to Assess Morphological Variation of the Australopithecus Afarensis Maxilla. Anat. Rec. 2025, 2025, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pei, S.; Cai, Y.; Tong, H.; Zhang, Z.; Yan, Y.; Xing, S.; Martinón-Torres, M.; Bermúdez de Castro, J.M.; Liu, W. Morphological and Morphometric Analyses of a Late Middle Pleistocene Hominin Mandible from Hualongdong, China. J. Hum. Evol. 2023, 182, 103411. [Google Scholar] [CrossRef] [PubMed]
- Nadal, L.; Lahr, M.M. Patterns of Sexual Variation in Hominoid Mandibular Morphology: A Framework for Interpreting the Hominin Fossil Record. bioRxiv 2022. bioRxiv:2022.06.15.496279. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).